Abstract
Background:
Recent therapeutic developments demand for an update of information on natural history, risk factors and prognosis of peritoneal carcinomatosis (PC) of colorectal origin. Therefore, prospective registry data should provide information about incidence, predictors and outcome.
Methods:
From a prospectively expanded single-institutional database with 2406 consecutive patients with colorectal cancer (CRC), clinical, histological and survival data were analysed for independent risk factors and prognosis. Findings were then stratified to the era of treatment without chemotherapy, 5-Fluorouracil-only and contemporary systemic chemotherapy, respectively.
Results:
Overall, 256 (10.6%) patients were diagnosed with PC thereof 141 (5.85%) with metachronous PC. Independent risk factors for the development of metachronous PC were age <62 years, N2-status, T4-status, location of the primary in the left colon or appendix. In the era of contemporary systemic chemotherapy, prognosis for PC improved only not-significantly (median survival of 17.9 months vs 7.03 months, P=0.054).
Conclusion:
Despite improvement in the overall outcome with prolonged median survival for the complete patient cohort with CRC, those patients with PC have not experienced the same benefit. In the era of contemporary systemic chemotherapy, progress in treatment resulted in only limited survival benefit. Thus, continuous efforts for further therapeutic advancements should be undertaken in these patients diagnosed with PC.
Keywords: risk factors, colorectal cancer, peritoneal carcinomatosis, metastasis, HIPEC
About 10% of patients with colorectal cancer (CRC) develop peritoneal carcinomatosis (PC) during the course of their disease (Jayne et al, 2002; Lemmens et al, 2011; Segelman et al, 2012). Peritoneal carcinomatosis from CRC (pcCRC) is associated with significant shorter overall survival (OS) as compared with non-PC manifestations of metastatic CRC (Franko et al, 2012). In a large-scaled study about risk factors for pcCRC with 3019 patients, Jayne et al (2002) reported a median survival of 7 months. In the last two decades, prognosis of patients with metastatic CRC dramatically improved with a median OS increasing from <6 months to >20 months due to development of optimised systemic therapy and increased resection rates of hepatic and pulmonary metastases (André et al, 2004; Falcone et al, 2007; Golfinopoulos et al, 2007; Cassidy et al, 2008; Saltz et al, 2008). However, at present it is not clear, if the reported survival benefit in overall metastatic CRC population includes the subset of patients with isolated (or predominant) PC since PC is often classified as ‘non-measurable disease' by imaging techniques and these PC patients are usually excluded from randomised chemotherapy trials. It is also not clear if further evolvement of first-line treatment of CRC as well as improvement in preventive care and diagnostic equipment has changed prevalence and incidence of pcCRC. This, however, affects the risk factors for development of PC, demanding for a re-assessment of recent outcome data and resulting therapeutic considerations (Klaver et al, 2011). These changes and improvements in the treatment of CRC were considered for differentiated interpretation of potential findings in this study. Assuming that systemic chemotherapy has underwent the most dynamic development, it was differentiated between three eras of systemic chemotherapy according to the availability and routine use at the Wuerzburg Medical Center.
Considering recent development in therapeutic options of chemotherapy and surgical treatment, the specific aims of this study were first to determine the incidence, risk factors and outcome of PC from CRC. In addition, a comprehensive overview of epidemiological, histopathological and clinical features of three groups of patients with synchronous (synPC), metachronous (metaPC) and with no evidence of PC (noPC) will be given. Upon comprehensive data analysis, we will define risk factors that are correlated with the development of metachronous PC. As a result, better knowledge about all of these factors may help to define subgroups of patients at risk who might benefit from close observation or aggressive multimodal treatment.
Materials and Methods
Cohort definition
For this study, patients with CRC treated at the University of Wuerzburg Medical Center between September 1986 and December 2009 were identified from the Wuerzburg Institutional Database (WID). Patients were grouped into three categories: those who never developed carcinomatosis (noPC), those with synchronous PC (synPC) and those who developed metachronous disease (metaPC). Patients with synPC were diagnosed at the time of presentation with CRC, either on routine staging, computed tomography or at laparotomy. Patients with metaPC were considered to be clear of peritoneal disease at the initial colorectal resection, but subsequently became symptomatic on follow-up and were diagnosed with peritoneal metastases on computed tomography or at the time of another surgical exploration. The noPC group consisted of patients without peritoneal disease at initial colorectal resection and no evidence of PC on clinical or radiological follow-up. Patients were also collated to the ‘noCTx', 5-Flurouracil (‘5-FU') and ‘contempCTx' group based on the availability and routine use of systemic chemotherapy according to the international guidelines at the University of Wuerzburg Medical Center at the time of diagnosis of the primary cancer. In the first ‘noCTx' group starting from January 1986 to August 1994, usually no systemic chemotherapy was provided to the patients, in the second ‘5-FU' group beginning in September 1994 until August 2004, 5-FU was available for treatment. In the third ‘contempCTx' group from September 2004 to December 2009, 5-FU were administered either alone or in combination with Oxaliplatin or Irinotecane when available for advanced CRC and later on as first-line treatment. In some cases, combinations with biologicals against growth factors like Cetuximab and Bevacizumab were administered. Being in one of the groups did not mean that the patients were treated obligatory with systemic chemotherapy (Table 1).
Table 1. Number of patients in the three groups with no (no)PC, synchronous (syn)PC and metachronous (meta)PC stratified for the era of treatment before September 1994 (no systemic chemotherapy available – ‘noCTx') before September 2004 (5-Fluoruracil available – ‘5-FU') and after September 2004 (Oxaliplatin/Irinotecane available – ‘contempCTx').
| Total | noPC | synPC | metaPC | |
|---|---|---|---|---|
|
Total |
2406 (100%) |
2150 (89.4%) |
115 (4.8%) |
141 (5.9%) |
| January 1986 – August 1994 (noCTx group) |
733 (30.5%) |
656 (89.5%) |
33 (4.5%) |
44 (6.0%) |
| September 1994 – August 2004 (5-FU group) |
1015 (42.2%) |
899 (88.6%) |
48 (4.7%) |
68 (6.7%) |
| September 2004 – December 2009 (contempCTx group) | 658 (27.3%) | 595 (90.4%) | 34 (5.2%) | 29 (4.4%) |
Data source
The WID is a central data repository that is expanded prospectively on a daily basis since 1984 with clinical, operative and research data of patients who were evaluated and treated at the University of Wuerzburg Medical Center. Wuerzburg Medical Centre is one of the three equally capable institutions covering most patients in an area of about 515 000 people in the treatment of CRC. Data available within the WID include patient demographics, histological diagnoses that are based on International Classification of Diseases coding standards, physician data, inpatient admission and outpatient registration data, operating room procedures, laboratory results and computerised pharmacy records. The WID undergoes continuous cross platform integration with the Comprehensive Cancer Registry to ensure updated follow-up information for identification of deceased patients. Inpatient and outpatient records of all identified patients were reviewed retrospectively to extract information regarding type and duration of chemotherapy, sites of metastatic disease at presentation and disease status at last follow-up. Missing data were retrieved from patient case notes where possible. Autopsy was not performed routinely and rates were not documented.
Demographic details of the three groups were compiled, along with clinical variables recorded at the time of primary diagnosis as well as at initial operation (tumour site and the presence of any metastases) and histological details of the resected specimen (tumour (T) stage, nodal (N) stage, tumour differentiation (G) and evidence of microscopic venous (V) and lymphatic vessel invasion (L)). These data were collated with survival data obtained from prospective follow-up. In patients with multiple CRCs, data were analysed for the first cancer. Metastases diagnosed within <30 days after the primary tumour were also defined as synchronous. Peritoneal carcinomatosis was diagnosed usually intraoperatively and confirmed histopathologically and in the other cases by computed tomography.
Follow-up
Postoperative follow-up consisted of 3-monthly outpatient assessment or by gathering complete information from the patient's family doctor also in a 3-monthly interval for at least 10 years. After 10 years, information was gathered retrospectively every year. Aftercare was performed on protocols according to entity and tumour stage with abdominal ultrasound after 3, 6, 12 and 18 months and after that on a yearly basis. According to the guidelines, computed tomography and surveillance colonoscopy were intended to be performed routinely 3 or 6 months after operation and repeated every year. No complete data are given about actually applied examinations. After 5 years, aftercare was performed as appropriate for the individual. In all, 2.3% of patients were lost to follow-up within a follow-up time of 10 years. If not stated otherwise, OS was estimated from diagnosis of primary tumour.
Statistical analysis
The data were analysed with a statistical software package (‘SPSS', Statistical Package for Social Sciences, version 16, SPSS, Chicago, IL, USA). Clinical and histological parameters of the three groups were compared with the Mann–Whitney U or Kruskal–Wallis test for continuous data and with the χ2 test for categorical variables. P<0.05 was considered statistically significant. Cox proportional hazard modelling or ‘Cox regression' was used to determine predictors for the development of metachronous PC by comparing the noPC and the metaPC group whenever univariate analysis showed any significance (Cox, 1972; Hosmer, 1990). Survival curves were drawn according to Kaplan–Meier methods.
Results
Survival data
There were 2406 patients identified from the database who presented with primary CRC for treatment at Wuerzburg University Hospital from September 1986 to December 2009. In all, 2150 (89.4%) of these patients were never diagnosed with PC, 115 (4.78%) patients presented with synPC and 141 (5.85%) patients were diagnosed with metaPC (Table 1). Primary end point was tumour-related death, which occurred in 40.1% (n=964) of all cases and occurred in the three groups with noPC, metaPC and synPC in 35.3% (n=760), 75.7% (n=87) and 83.0% (n=117), respectively (P<0.001). Median follow-up time was 39.1 months, and the overall follow-up period ended in October 2010; the median tumour-related survival was 107.1 months. In the noPC group, median follow-up time was 43.7 months and median tumour-related survival was 141.0 months, in the synPC group median follow-up time was 6.9 months and median tumour-related survival was 8.0 months and in the metaPC group median follow-up time was 28.0 months with a median OS of 30.0 months. The estimated tumour-related survival at 5 years was 60.0% with 65.1% in the noPC group, 8.1% in the synPC group and 25.4% in the metaPC group (Table 2). Peritoneal carcinomatosis was the only site of metastasis in 152 patients during the course of their disease (59% of all patients with PC). In patients who developed metaPC without any other distant metastases or local recurrence median tumour-related survival was 10.0 months vs 5.9 months with simultaneous distant metastases (P=0.001). Tumour-related 5-year survival for patients with PC and distant metastasis was 0%. In all, 21.2% of patients who succumbed to CRC had developed synPC or metaPC.
Table 2. The tumour-related 2-year and 5-year survival rates of patients from the groups with no (no)PC, synchronous (syn)PC and metachronous (meta)PC stratified for the era of treatment (noCTx/5-FU before September 2004 and contemporary systemic chemotherapy [contempCTx] protocols after September 2004).
| Total | noPC | synPC | metaPC | |||||
|---|---|---|---|---|---|---|---|---|
|
PC-group |
2-year |
5-year |
2-year |
5-year |
2-year |
5-year |
2-year |
5-year |
|
Era |
Survival % |
Survival % |
Survival % |
Survival % |
||||
| Total |
77.6 |
60.0 |
81.5 |
65.1 |
22.7 |
8.1 |
61.0 |
25.4 |
| noCTx/5-FU |
75.9 |
58.1 |
80.1 |
63.3 |
19.1 |
5.8 |
58.5 |
24.4 |
| contempCTx |
82.8 |
66.3 |
86.0 |
71.6 |
31.1 |
20.8 |
71.5 |
28.1 |
| P-value | P=0.002 | P<0.000 | P=0.008 | P=0.001 | P=0.092 | P=0.081 | P=0.329 | P=0.411 |
Abbreviation: 5-FU=5-Flurouracil.
Treatment eras
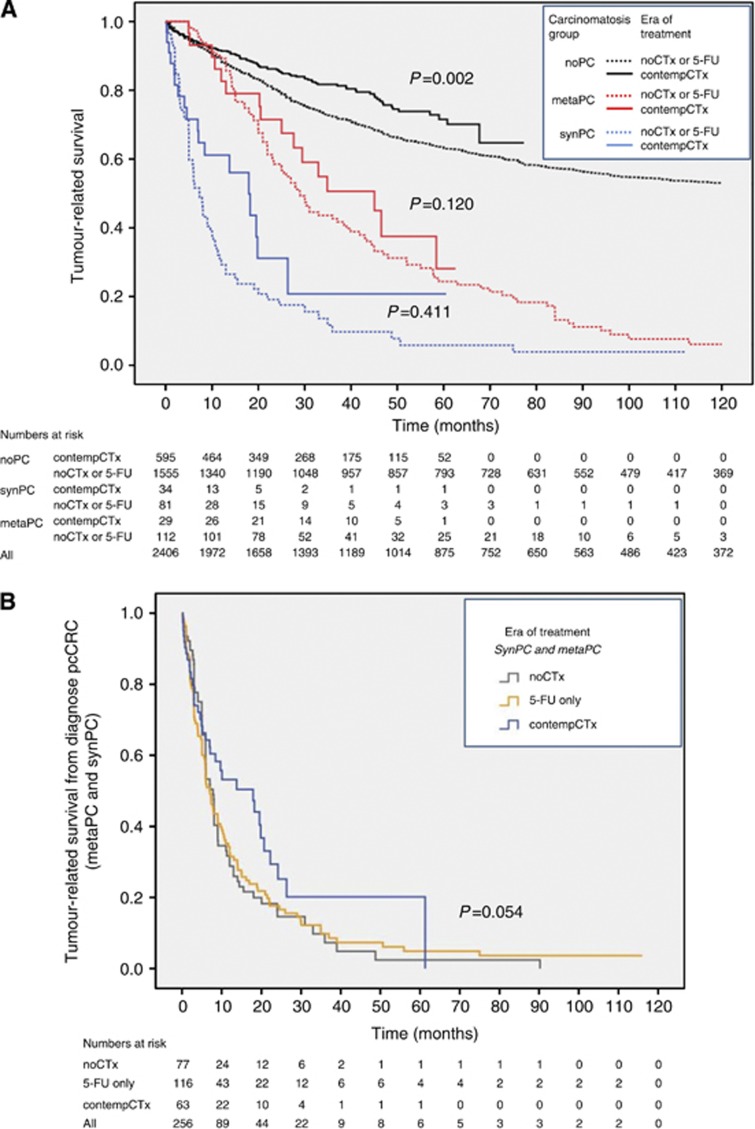
Of the 2406 patients, 733 patients (30.5%) were treated in the era ‘noCTx' (1984–1994), 1015 patients (42.2%) were treated in the era of 5-FU-only (1994–2004) and 658 (27.3%) were treated in the era ‘contempCTx' (Table 1). In the era ‘noCTx', 9.8% (n=72) of all patients received chemotherapy, in the era ‘5-FU' 32.7% (n=332) and in the era ‘contempCTx' 38% (n=250, Table 3). The subgroup of patients with PC was stratified for the three treatment eras. Between the treatment eras ‘noCTx' and ‘5-FU', there was no significant difference in terms of median tumour-related survival (synPC and metaPC: 17.9 months vs 21.9 months, P=0.52) nor in time to development of PC (metaPC: 17.5 months vs 18.0 months, P=0.64). Also, between the combined treatment eras ‘noCTx'/‘5-FU' and the era ‘contempCTx', there was no significant difference in terms of median tumour-related survival (synPC and metaPC: 20.0 months vs 26.4 months, P=0.33) nor in time to development of PC (metaPC: 17.9 months vs 22.1 months, P=0.19). Median tumour-related survival from time of diagnose of PC was 7.03 months (‘noCTx'/‘5-FU') vs 17.9 months (‘contempCTx', P=0.054; Figure 1b). While in our CRC patients without PC, 5-year survival improved from 63% without chemotherapy/5-FU only (before September 2004) to 71.6% in the era of ‘contempCTx' (P<0.001), 5-year survival of patients with PC showed no significant improvement between the two eras (Table 2).
Table 3. Numbers of patients receiving systemic chemotherapy (CTx) or no chemotherapy (noCTx) stratified for treatment eras and type of systemic chemotherapy (‘5-FU'=5-Fluoruracil only, ‘Ox/Irino'=Oxaliplatin or Irinotecane-based CTx, ‘Beva/Cetux'=Bevacizumab or Cetuximab).
| noCTx | CTx | |||||
|---|---|---|---|---|---|---|
|
All eras
n=2406 (100%) |
n=1752 (72.8%) |
n=654 (27.2%) |
||||
| noPC | synPC | metaPC | noPC | synPC | metaPC | |
| |
n=1597 |
n=74 |
n=81 |
n=553 |
n=41 |
n=60 |
| |
noCTx |
CTx |
||||
|
Era of noCTx
n=733 (100%) |
n=661 (90.2%) |
n=72 (9.8%)
– 5-FU, n=72 (9.8%)
– Ox/Irino, n=0 (0%)
– Beva/Cetux, n=0 (0%) |
||||
| noPC | synPC | metaPC | noPC | synPC | metaPC | |
| |
n= 600 |
n= 25 |
n= 36 |
n=56 |
n=8 |
n=8 |
| |
noCTx |
CTx |
||||
|
Era of 5-FU only
n=1015 (100%) |
n=683 (67.3%) |
n=332 (32.7%)
– 5-FU, n=332 (32.4%)
– Ox/Irino, n=3 (0.3%)
– Beva/Cetux, n=0 (0%) |
||||
| noPC | synPC | metaPC | noPC | synPC | metaPC | |
| |
n=614 |
n=36 |
n=33 |
n=285 |
n=12 |
n=35 |
| |
noCTx |
CTx |
||||
|
Era of
contempCTx
n=658 (100%) |
n=408 (62.0%) |
n=250 (38.0%)
– 5-FU, n=144 (21.9%)
– Ox/Irino, n=92 (14.0%)
– Beva/Cetux, n=14 (2.1%) |
||||
| noPC | synPC | metaPC | noPC | synPC | metaPC | |
| n=383 | n=13 | n=12 | n=212 | n=21 | n=17 | |
Treatment eras were also stratified for patients with no (no)PC, synchronous (syn)PC and metachronous (meta)PC.
Figure 1.
(A) Tumour-related survival of the three groups with synchronous (syn), metachronous (meta) and without peritoneal carcinomatosis (noPC) stratified for the era of treatment: noCTx or 5-FU vs 5-FU+Oxaliplatin/Irinotecane (contemporary (contemp)CTx). (B) Tumour-related survival after diagnosis of peritoneal carcinomatosis (synchronous (syn)PC and metachronous (meta)PC) of colorectal cancer (pcCRC). Tumour-related survival was 17.9 months for treatment in the era of contemporary chemotherapy vs 7.03 months in the eras before (P=0.054).
Risk factors: clinical, therapy and histological features
All demographical, clinical and therapy characteristics are displayed in Table 4, all histopathological characteristics for primary tumours are displayed in Table 5. In the multivariate analysis of epidemiological features only age younger than 62 years (hazard ratio (HR)=1.23, confidence interval (CI): 1.07–1.50, P=0.006), tumour localisation in the appendix (HR=3.70, CI=1.62–8.47, P<0.001) and left-sided colon (HR=1.53, CI: 1.07–2.19, P=0.018) were independent predictors for the development of metaPC whereas localisation in the upper rectum (HR=0.32, CI: 0.13–0.80, P=0.015) was associated with a lower risk of developing metaPC.
Table 4. Evaluation of the influence of demographical and clinical characteristics in patients with synchronous (synPC), metachronous (metaPC) and no peritoneal carcinomatosis (noPC).
| Characteristic | Total (N=2406) | noPC (N=2150) | synPC (N=115) | metaPC (N=141) | Univariate P | Multivariate P (Cox) | HR (95% confidence interval) |
|---|---|---|---|---|---|---|---|
| Sex ratio (M:F) | 1.46/1 (1428 : 978) | 1.5/1 (1293 : 857) | 1 : 1 (44 : 43) | 1.05 : 1 (60 : 57) | <0.001 | 0.302 | 0.827 (0.59–1.174) |
|
Age median (range) |
66.7 (17.8–93.6) |
66.9 (17.8–93.6) |
66.1 (32.8–88.4) |
62.2 (22.0–85.7) |
0.001a |
0.006 |
1.271 (1.07–1.508) |
|
Site (per cent) | |||||||
| Colon | 1398 (58.10) | 1203 (55.95) | 93 (80.87) | 102 (72.34) | <0.001 | — | |
| Appendix | 33 (1.37) | 11 (0.51) | 15 (13.04) | 7 (4.96) | <0.001 | 0.003 | 3.73 (1.58–8.78) |
| Right | 394 (16.38) | 336 (15.63) | 31 (26.96) | 27 (19.15) | 0.267 | — | |
| Transverse | 233 (9.68) | 217 (10.09) | 7 (6.09) | 9 (6.38) | 0.152 | — | |
| Left | 738 (30.67) | 639 (29.72) | 40 (34.78) | 59 (41.84) | 0.002 | 0.06 | 1.43 (0.98–2.09) |
| Rectum | 996 (41.40) | 935 (43.49) | 22 (19.13) | 39 (27.66) | <0.001 | — | |
| Upper | 323 (13.42) | 311 (14.47) | 7 (6.09) | 5 (3.55) | 0.001 | 0.033 | 2.778 (1.068–7.11) |
| Lower | 673 (27.97) | 624 (29.02) | 15 (13.04) | 34 (24.11) | 0.212 | — | |
| Other | 10 (0.42) | 10 (0.47) | 0 | 0 | — | — | |
|
NR |
2 (0.08) |
2 (0.09) |
0 |
0 |
— |
|
— |
|
Metastasis (per cent) | |||||||
| Sec. Carcinoma | 382 (15.88) | 347 (16.14) | 12 (10.43) | 23 (16.31) | 0.957 | — | |
| Sync. Metastasis | 534 (22.19) | 386 (17.95) | 72 (62.61) | 33 (23.40) | 0.105 | — | |
| hep | 403 (16.75) | 332 (15.44) | 48 (41.74) | 23 (16.31) | 0.001 | 0.121 | 1.51 (0.89–2.52) |
| pul | 86 (3.57) | 44 (2.05) | 13 (11.30) | 23 (16.31) | 0.036 | 0.355 | 0.69 (0.31–1.52) |
| None | 1872 (77.81) | 1764 (82.05) | 43 (37.39) | 108 (76.60) | 0.069 | — | |
| Perforation | 72 (3.0) | 55 (2.6) | 13 (11.3) | 4 (2.8) | 0.840 | — | |
| Era (per cent) | 0.392 | ||||||
| no Chemo | 733 (30.5) | 656 (30.5) | 33 (28.7) | 44 (31.2) | 0.862 | — | |
| 5-FU only | 1015 (42.2) | 899 (41.8) | 48 (41.7) | 68 (48.2) | 0.135 | — | |
| 5-FU+Oxali |
658 (27.3) |
595 (27.7) |
34 (29.6) |
29 (20.6) |
0.764 |
|
— |
|
Therapy (per cent) | |||||||
| Operation | 2312 (96.1) | 2069 (96.2) | 104 (90.4) | 139 (98.6) | 0.148 | ||
| noOR |
43 (1.8) |
35 (1.6) |
6 (5.2) |
2 (1.4) |
0.848 |
|
— |
|
Resection-status | |||||||
| 0 | 1560 (67.5) | 1496 (72.3) | 5 (4.3) | 59 (42.4) | <0.001 | <0.001 | 0.350 (0.227–0.540) |
| 1 | 48 (2.1) | 35 (1.7) | 7 (6.1) | 6 (4.3) | 0.023 | 0.447 | — |
| 2 | 378 (16.3) | 283 (13.7) | 63 (54.8) | 32 (23.0) | — | ||
| x | 46 (2.0) | 41 (2.0) | 2 (1.7) | 3 (2.2) | — | ||
| NR | 280 (12.1) | 214 (10.3) | 27 (23.5) | 39 (28.1) | — | ||
| Chemo adj | 665 (27.6) | 554 (25.8) | 41 (35.7) | 60 (42.6) | — | ||
| Radiation | 178 (7.4) | 156 (7.3) | 2 (1.7) | 11 (7.8) | — | ||
| NR | 51 (2.1) | 46 (2.1) | 5 (4.3) | 0 | |||
Abbreviations: NR=not reported; 5-FU=5-Flurouracil.
P-values and hazard ratios (HRs) concern noPC vs metaPC. If univariate analysis showed a significant influence a multivariate analysis (Cox regression) was performed and multivariate P-values along with HR were given.
Kruskal–Wallis test.
Table 5. Evaluation of the influence of histopathological characteristics in patients with synchronous (synPC), metachronous (metaPC) and no peritoneal carcinomatosis (noPC).
| Characteristic | Total (N=2406) | noPC (N=2150 ) | synPC (N=115 ) | metaPC (N=141 ) | Univariate P | Multivariate P (Cox) | HR (95% confidence interval) |
|---|---|---|---|---|---|---|---|
|
Stage (UICC 7) |
|
|
|
|
<0.001 |
|
— |
|
I |
549 (22.8) |
530 (24.7) |
— |
9 (6.4) |
— |
|
|
|
II |
571 (23.7) |
549 (25.5) |
— |
18 (12.8) |
— |
|
|
|
III |
607 (25.2) |
546 (25.4) |
— |
54 (38.3) |
— |
|
|
|
IV |
522 (21.7) |
371 (17.3) |
115 (100) |
40 (28.4) |
— |
|
|
|
NR |
157 (6.5) |
154 (7.2) |
— |
20 (14.2) |
— |
|
|
|
T-stage | |||||||
|
T0 |
12 (0.5) |
12 (0.6) |
0 |
0 (0) |
— |
|
|
|
T1 |
223 (9.3) |
222 (10.3) |
0 |
1 (0.7) |
T12<0.001 |
0.016 |
0.45 (0.24–0.861) |
|
T2 |
495 (20.6) |
482 (22.4) |
2 (1.7) |
11 (7.8) |
|
|
|
|
T3 |
1170 (48.6) |
1077 (50.1) |
28 (24.3) |
65 (46.1) |
0.358 |
|
|
|
T4 |
378 (15.7) |
258 (12.0) |
72 (62.6) |
48 (34.0) |
<0.001 |
<0.001 |
3.03 (2.05–4.48) |
|
Tx |
128 (5.3) |
99 (4.6) |
13 (11.3) |
16 (11.3) |
— |
— |
— |
|
N-status | |||||||
|
N0 |
1211 (50.3) |
1169 (54.4) |
11 (9.6) |
31 (22.0) |
<0.001 |
0.044 |
0.48 (0.24–0.982) |
|
N1 |
523 (21.7) |
461 (21.4) |
23 (20.0) |
39 (27.7) |
0.044 |
— |
— |
|
N2 |
490 (20.4) |
383 (17.8) |
56 (48.7) |
51 (36.2) |
<0.001 |
0.058 |
1.95 (0.98–3.86) |
|
Nx |
182 (7.6) |
137 (6.4) |
25 (21.7) |
20 (14.2) |
— |
|
|
|
Histology | |||||||
|
AdenoCA |
1811 (75.3) |
1648 (76.7) |
64 (55.7) |
99 (70.2) |
0.082 |
|
|
|
Mucinous |
349 (14.5) |
295 (13.7) |
27 (23.5) |
27 (19.1) |
0.072 |
|
|
|
Signet Ring |
29 (1.2) |
15 (0.7) |
8 (7.0) |
6 (4.3) |
0.001 |
0.300 |
0.63 (0.26–1.51) |
|
Other |
210 (8.7) |
188 (8.7) |
13 (11.3) |
9 (6.4) |
— |
|
|
|
NR |
7 (0.3) |
4 (0.2) |
3 (2.6) |
0 |
— |
|
|
|
Grading | |||||||
|
G1 |
73 (3.0) |
67 (3.1) |
4 (3.5) |
2 (1.4) |
0.253 |
|
|
|
G2 |
1602 (66.6) |
1491 (69.3) |
36 (31.3) |
75 (53.2) |
0.047 |
0.83 |
0.95 (0.58–1.54) |
|
G3 |
346 (14.4) |
288 (13.4) |
32 (27.8) |
26 (18.4) |
0.047 |
0.67 |
1.21 (0.676–1.52) |
|
G4 |
15 (0.6) |
13 (0.6) |
2 (1.7) |
0 |
— |
|
|
|
Gx |
370 (15.4) |
291 (13.5) |
41 (35.7) |
38 (26.9) |
— |
|
|
|
L invasion |
658 (41) |
557 (37.6) |
60 (87.0) |
41 (78.8) |
0.406 |
|
|
| V invasion | 196 (13.1) | 168 (11.9) | 22 (45.8) | 37 (2.5) | 0.073 | ||
Abbreviation: NR=not reported.
P-values and hazard ratios (HRs) concern noPC vs metaPC. If univariate analysis showed significant influence, a multivariate analysis (Cox regression) was performed and multivariate P-values along with HR were given.
Nearly all patients underwent surgery (n=2312, 96.1%). Resection status was stated ‘R0' if no tumour was left, including resection of distant metastases. According to the tumour stage and current guidelines, chemotherapy was applied to patients where appropriate. The total amount of patients with systemic chemotherapy for primary cancer was low (27.2%) because 733 (30.5%) patients were treated in the era ‘noCTx' (Table 3). In the multivariate analysis, R0-status was a significant indicator for lower risk of the development of metaPC (HR=0.60, CI: 0.49–0.75, P<0.001). Tumour stage (UICC version 7, International Union Against Cancer (UICC), Geneva, Switzerland) was significant on univariate analysis but was excluded from multivariate analysis as it is a summary of histological features. On univariate analysis, the parameters T-stage, N-stage, histological type and differentiation showed a significant difference between the three groups, with patients in the synPC and metaPC groups having a higher proportion of tumours with features of advanced stage. The features of venous invasion and lymphatic vessel invasion were not significant. Cox regression comparison of metaPC and noPC groups demonstrated that T4-stage (HR=1.79, CI: 1.48–2.16, P<0.001) and N2-stage (HR=1.27, CI: 1.03–1.57, P=0.026) were the only independent histopathological predictors of PC. The characteristics R0-stage (HR=0.60, CI: 0.49–0.75, P<0.001, N0-stage (HR=0.64, CI: 0.50–0.80, P<0.001) and earlier primary tumours (T0, T1, T2) (HR=0.67, CI: 0.49–0.92, P=0.014) were factors that are of lower risk of developing metaPC.
Shift of risk factors in the era of contemporary systemic chemotherapy
All previous analysis was done with the entirety of data. To exclusively identify risk factors in the era of contemporary chemotherapy for the development of metaPC, Cox regression modelling was used on available data from September 2004 to December 2009. In this setting, all previously significant risk factors were excluded leaving localisation of primary in the appendix (HR=11.85, CI: 2.73–51.379, P=0.001) and T4-status (HR=1.825, CI: 1.22–2.73, P=0.003) as independent risk factors in the era of contemporary chemotherapy. R0-status (HR=0.556, CI: 0.34–0.90, P=0.018) was the only significant factor of lower risk of developing metaPC in this era. Between the eras, there was no significant difference in the allocation of patients in terms of UICC-stage (P=0.906). Prevalence of risk factors for metaPC in the ‘contempCTx' group compared with ‘noCTx' and ‘5-FU' groups was lower in terms of age, R0-status, Appendix and N2-status. All other risk factors showed no significant differences in prevalence.
Discussion
Peritoneal carcinomatosis has always been a fatal stage in the course of the disease of CRC. Median OS of patients with pcCRC in the last decades remained dismal with 7–8 months (Sadeghi et al, 2000; Koppe et al, 2006). Unlike other sites of distant metastases like the liver, it did not improve substantially over time despite of achievements in the field of systemic chemotherapy (Klaver et al, 2011) and surgery, as well as in terms of diagnostic capabilities, perioperative management and best supportive care (Lemmens et al, 2011). Only most recent results show a slight improvement in survival for patients with pcCRC (Klaver et al, 2011). This may be due to arising targeted strategies and more effective combinations with biologicals against growth factors like Cetuximab and Bevacizumab (Jonker et al, 2000; Goldberg et al, 2004; Hurwitz et al, 2004).
Our results from a large patient cohort demonstrate a prolonged median tumour-related survival for the entirety of patients with CRC in the era of contemporary systemic chemotherapy. However, the patient subgroup with pcCRC could not benefit simultaneously with a significant gain in survival time, no matter whether we looked from the date of the primary cancer or from the date of the diagnosis of PC and also regardless of the metachronous or synchronous occurrence of PC. Nevertheless, with a P-value of 0.54 in our currently analysed patient cohort with PC treated with contemporary chemotherapy protocols a trend towards significance in tumour-related survival benefit is recognisable, that may become provable with rising numbers of patients in this group. Although this study represents one of the largest studies so far on pcCRC patients, the relatively small numbers of patients affected by PC in each era impede elaborating an effect of contemporary systemic chemotherapy on the development of PC and on OS. Furthermore, treatment eras are not identical to treatment groups and not all of the reported patients were affected by changes in systemic chemotherapy. Only 27% of all CRC patients in this study were actually treated with systemic chemotherapy, which might be the reason why the availability of certain protocols is of less impact on the 2-year and 5-year survival (Table 2). Even in the subgroups of patients with synPC and metaPC in the era of solely 5-FU treatment only 40.5% and in the era of contemporary chemotherapy only 60.3% of those patients received systemic chemotherapy. Nevertheless, a much higher impact of the availability of contemporary chemotherapy on survival time for these patients with PC would have been expected. However, the results do not reach significance because our study cohort with >2400 patients is still underpowered.
Our results demand for continuous efforts for surveillance and further therapeutic advancements that are needed for patients with pcCRC. Defining further risk factors for the development of metaPC is needed to better select patients at high risk for developing PC, which consequently might benefit from intensified adjuvant treatment regimens. It seems obvious that some risk factors that were defined in this study are reflected by the UICC staging system (N2, T4) and correlate with advanced CRC. However, other risk factors like younger age and specific localisation of the primary tumour as well as factors that are associated with lower risk of developing PC are not included in the UICC staging system. Specific risk factors can support decision-making on individual aftercare and may add important information for appropriate consultation of patients suffering from advanced CRC.
Management of patients with isolated (or predominant) pcCRC is a very controversial topic since the optimal systemic chemotherapy and locoregional treatment approach (cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC)) or combination of both has not been satisfactorily addressed in prospective, randomised studies (Verwaal et al, 2003, 2008). Patients that were carefully selected in subgroups of patients with pcCRC demonstrated quite impressively prolonged median survival when treated with multimodal aggressive therapy and cytoreductive surgery combined with HIPEC (Elias et al, 2009). While cytoreductive surgery combined with HIPEC may be associated with relevant morbidity and mortality, patient selection remains therefore crucial as well as a significant expertise in the field (Smeenk et al, 2007; Chua et al, 2009; Kerscher et al, 2010). Patients that potentially benefit from this advanced therapeutic approach should be assessed preoperatively by histological characteristics, imaging and clinical features (Pelz et al, 2009, 2010; Stojadinovic et al, 2011).
Second look surgery and HIPEC for CRC patients considered to be at high risk of developing PC is probably the most venturesome approach currently under evaluation. Two ongoing prospective randomised clinical trials are evaluating the concept of locoregional treatment approach vs standard-of-care surveillance in these patients (Ripley et al, 2010; Elias et al, 2011).
Conclusion
Prospective registry data, as reported in this manuscript, provide important information about incidence, predictors and outcome of patients with PC from CRC. Our data demonstrate that PC remains a fatal condition in patients with metastatic CRC. Despite the fact that there is a trend towards better outcome in the era of contemporary systemic chemotherapy for patients with pcCRC, continuous efforts for further therapeutic advancements should be undertaken. In the future, a synergism of intensified surveillance of risk groups together with a locoregional treatment approach in combination with contemporary systemic chemotherapy might result in a significantly improved prognosis for these patients.
Ethics
This study has been approved for full ethics waiver due to its retrospective nature by the University of Wurzburg ethics committee.
Acknowledgments
We thank Mrs Nielsson for excellent collection of data since 1984. This study has been supported by the ART (Arbeitsgemeinschaft Regionale Tumortherapie).
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, Gramont Ade. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang T, Rivera F, Couture F, Sirzén F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann Surg. 2009;249:900–907. doi: 10.1097/SLA.0b013e3181a45d86. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) J R Stat Soc Series B. 1972;1972:931–940. [Google Scholar]
- Elias D, Honoré C, Dumont F, Ducreux M, Boige V, Malka D, Burtin P, Dromain C, Goéré D. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254:289–293. doi: 10.1097/SLA.0b013e31822638f6. [DOI] [PubMed] [Google Scholar]
- Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D, Bonastre J. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27 (5:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Golfinopoulos V, Salanti G, Pavlidis N, Ioannidis JPA. Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: a meta-analysis. Lancet Oncol. 2007;8:898–911. doi: 10.1016/S1470-2045(07)70281-4. [DOI] [PubMed] [Google Scholar]
- Hosmer D. Applied Survival Analysis Regression Modelling of Time to Event Data. Vol. 1990 Wiley: New York; 1990. [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- Jonker DJ, Maroun JA, Kocha W. Survival benefit of chemotherapy in metastatic colorectal cancer: a meta-analysis of randomized controlled trials. Br J Cancer. 2000;82:1789–1794. doi: 10.1054/bjoc.1999.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher AG, Mallalieu J, Pitroff A, Kerscher F, Esquivel J. Morbidity and mortality of 109 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) performed at a community hospital. World J Surg. 2010;34:62–69. doi: 10.1007/s00268-009-0281-2. [DOI] [PubMed] [Google Scholar]
- Klaver YLB, Lemmens VEPP, Creemers GJ, Rutten HJT, Nienhuijs SW, Hingh IHJTde. Population-based survival of patients with peritoneal carcinomatosis from colorectal origin in the era of increasing use of palliative chemotherapy. Ann Oncol. 2011;22:2250–2256. doi: 10.1093/annonc/mdq762. [DOI] [PubMed] [Google Scholar]
- Koppe MJ, Boerman OC, Oyen WJG, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212–222. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JWW, Hingh IHde. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128:2717–2725. doi: 10.1002/ijc.25596. [DOI] [PubMed] [Google Scholar]
- Pelz JOW, Chua TC, Esquivel J, Stojadinovic A, Doerfer J, Morris DL, Maeder U, Germer C, Kerscher AG. Evaluation of best supportive care and systemic chemotherapy as treatment stratified according to the retrospective peritoneal surface disease severity score (PSDSS) for peritoneal carcinomatosis of colorectal origin. BMC Cancer. 2010;10:689. doi: 10.1186/1471-2407-10-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz JOW, Stojadinovic A, Nissan A, Hohenberger W, Esquivel J. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol. 2009;99:9–15. doi: 10.1002/jso.21169. [DOI] [PubMed] [Google Scholar]
- Ripley RT, Davis JL, Kemp CD, Steinberg SM, Toomey MA, Avital I. Prospective randomized trial evaluating mandatory second look surgery with HIPEC and CRS vs. standard of care in patients at high risk of developing colorectal peritoneal metastases. Trials. 2010;11:62. doi: 10.1186/1745-6215-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, Vignal J, Gilly FN. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang T, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699–705. doi: 10.1002/bjs.8679. [DOI] [PubMed] [Google Scholar]
- Smeenk RM, Verwaal VJ, Zoetmulder FAN. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94:1408–1414. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- Stojadinovic A, Nissan A, Eberhardt J, Chua TC, Pelz JOW, Esquivel J. Development of a Bayesian Belief Network Model for personalized prognostic risk assessment in colon carcinomatosis. Am Surg. 2011;77:221–230. [PubMed] [Google Scholar]
- Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- Verwaal VJ, van Ruth S, Bree Ede, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FAN. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]