Abstract
Discussion of dysregulation of the inflammatory response in tularemia, focusing on cytokines and the role of neutrophils.
Keywords: Francisella tularensis, IL-12, neutrophil, macrophage, dendritic cell, Th1
Tularemia is a disease caused by the facultative intracellular, Gram-negative bacterium Francisella tularensis [1]. Infection ensues after the organism is inhaled into the lungs or introduced into the skin by direct contact with an infected animal or the bite of an infected arthropod vector, and local bacterial replication is followed by dissemination to the liver and spleen. F. tularensis has been stockpiled as a bioweapon, and a distinguishing feature of the most highly virulent type A strains of this organism is the short interval between infection at the onset of symptoms and death, which can ensue prior to development of an adaptive immune response. In mouse and nonhuman primate models of infection, moribund status is characterized by an accumulation of neutrophils, live bacteria, and extensive necrotic tissue damage. In contrast, infection with type B F. tularensis strains is less severe, and an attenuated type B strain (designated LVS) is often used as a model organism for tularemia research, as it can cause a range of diseases in mice depending on the dose administered. With the use of this strategy, as well as models in which type A F. tularensis infection is combined with antibiotics, it has been established that cell-mediated immunity, particularly T cells and IFN-γ, are essential for survival of primary infection and an effective anti-F. tularensis immune response [1, 2], whereas other correlates of effective immunity or vaccination are less well-defined.
Studies using mutant mice, inhibitors, and blocking reagents have shown that IL-12 and IFN-γ are critical for effective immunity and survival of mice infected with LVS or the type A F. tularensis strain Schu S4 [1, 2], and the pivotal role of IL-12p70 in IFN-γ induction and control of infection was confirmed by Melillo et al. [3] in this issue of the Journal of Leukocyte Biology using mice that lack the IL-12R subunit IL-12Rβ2. Nevertheless, it is also clear that induction of an effective immune response is diminished and delayed by F. tularensis as part of its virulence strategy. Typically, DCs are the major source of IL-12, which is critical for T cell activation and IFN-γ production, and IL-12 synthesis is triggered by TLR or nucleotide-binding oligomerization-like receptor signaling, whereas IL-12p40 homodimers play a distinct role in stimulating DC maturation, activation, and migration to draining LNs. Unlike most Gram-negative bacteria, initial detection of F. tularensis is mediated, not by TLR4 and LPS but rather, by the interactions of bacterial lipoproteins with TLR2 [1]. Although NF-κB is activated, synthesis and secretion of proinflammatory cytokines (including TNF-α, IL-6, IL-1β, IFN-γ, and IL-12) by infected macrophages and DCs are diminished and delayed relative to control stimuli [1, 2] and may be undermined directly by rapid production of antiinflammatory cytokines, such as TGF-β and IL-10 [4]. DC trafficking may also be impaired by the negative effects of IFN-β on IL-12p40 homodimer production [5].
Direct evidence that IL-12 and/or IL-23 are also of critical importance in F. tularensis-infected humans is indicated by the recent report of tularemia in a 61-year-old Canadian man being treated with ustekinumab [6]. Ustekinumab is a highly specific IL-12p40-blocking antibody that was developed for treatment of plaque psoriasis [7]. Although long-term studies are not yet in-hand, and specific effects of ustekinumab on tularemia severity and progression remain obscure, this clinical case, together with evidence of hepatitis B virus reactivation in another patient receiving ustekinumab [8], suggests that risk of infection with at least some bacterial and viral pathogens is enhanced by this immunomodulatory therapy.
To control F. tularensis replication in macrophages, IFN-γ must be present very early in infection [1]. Typically, NK cells are a prominent, initial source of this important cytokine. However, NK cells are depleted during infection with type A F. tularensis and have a limited role in IFN-γ production [2, 9]. Consequently, CD4- and CD8-positive T cells are essential sources of IFN-γ, but the delayed timing of the adaptive immune response undermines its efficacy and favors pathogen replication.
Most studies of tularemia have focused on mononuclear phagocytes as vehicles for bacterial replication and dissemination from the site of infection to the liver and spleen. Nevertheless, a distinguishing feature of F. tularensis is its ability to productively infect many types of cells, including DCs, neutrophils, and epithelial cells, as well as monocytes and macrophages. Neutrophils can account for up to one-half of the infected cells in the lung, and there is strong evidence that PMNs contribute directly to disease progression rather than effective host defense. N-Acetyl Pro-Gly-Pro, a potent neutrophil chemoattractant that is generated when collagen in the ECM is cleaved by MMP-9, is essential for PMN recruitment to F. tularensis-infected tissues, and neutrophil accumulation correlates directly with the severity of tissue destruction [4, 10]. In keeping with this, neutrophilia is associated with increased susceptibility to this infection [1, 3]. Conversely, neutrophil recruitment to the lung is diminished profoundly in MMP-9 null mice, and these animals are able to survive infection with type A F. tularensis strains as well as LVS [10]. At the single-cell level, F. tularensis impairs neutrophil oxidative host defense via effects on NADPH oxidase assembly and activity and as in other cell types, escapes the phagosome to replicate in the cytosol [11, 12]. At the same time, F. tularensis profoundly prolongs neutrophil lifespan via effects on the intrinsic and extrinsic apoptotic pathways [12]. As defects in PMN turnover and clearance are emblematic of an ineffective and dysregulated inflammatory response that undermines control of infection and increases the risk of tissue damage by neutrophil progression to secondary necrosis [13], these data suggest a mechanism to account for the role of neutrophils in tularemia pathogenesis.
An important role for the IL-23/Th17 pathway and IL-17A in recruiting neutrophils to sites of infection for phagocytosis and killing of extracellular bacteria and fungi is established. Typically, IL-17A synergizes with TNF-α, IL-1β, and IL-6 to enhance neutrophil activation and killing capacity. Recent data indicate that IL-17A is present relatively early during F. tularensis infection, even though accumulation of most proinflammatory cytokines is diminished and delayed for several days [1, 4]. It is attractive to predict that modulation of the cytokine milieu in this manner may synergize with F. tularensis virulence factors to drive neutrophil accumulation, while simultaneously undermining their killing capacity.
It is also clear that neutrophils play an important and previously unappreciated role in regulation of innate and adaptive immunity that is mediated by direct interactions with other leukocytes and by secretion of cytokines and lipid mediators [14]. In this manner, neutrophils influence the function of NK cells and B- and T-lymphocytes, as well as macrophages and DCs. For example, PMNs can directly affect production of IL-12p70 and IFN-γ by DCs and NK cells, respectively, and influence NK cell survival, while also acting via secreted cytokines to affect the function of various T cell subsets. Thus, the regulatory properties of neutrophils extend well beyond the ability of apoptotic cells to reprogram macrophages toward a proresolution phenotype. Whether neutrophils play an immunoregulatory role during tularemia remains to be determined. Nevertheless, this idea is attractive, as it is consistent with the phenotype of MMP-9 null mice, as inhibition of neutrophil influx markedly diminishes morbidity and mortality without significantly altering bacterial burden in the lung, liver, or spleen [10]. Recent data also show that PMNs contribute to cytokine synthesis and secretion in the lungs of F. tularensis-infected mice [4]. Of note, enhanced liver pathology and neutrophilia are characteristic features of IL-12Rβ2 null mice infected with LVS [3], but whether PMNs directly contribute to the enhanced hepatotoxicity is unclear.
Altogether, the data suggest that the infection of multiple leukocyte types by F. tularensis is an important aspect of virulence, with each cell type playing a distinct role in disease progression. Thus, whereas macrophages are major sites of F. tularensis replication, bacteria-induced defects in DC activation and migration undermine development of adaptive immunity to this organism. On the other hand, profound neutrophil accumulation, together with defects in cell activation and turnover, plays a central role in dysregulation of the inflammatory response, tissue destruction, and death. Recent studies have also substantially advanced our understanding of protective and detrimental aspects of the cytokine response. These data are summarized in Fig. 1. A major challenge in future studies will be to define whether and how direct interactions between leukocytes and their secreted products regulate tularemia progression to the benefit or detriment of the host.
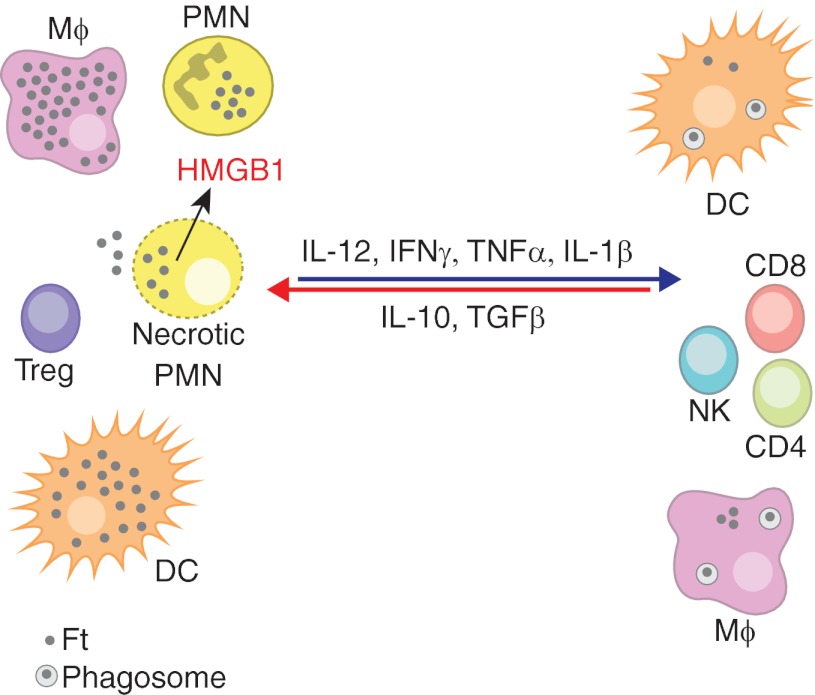
Figure 1. Protective and detrimental responses to F. tularensis.
The opposing effects of pro- and anti-inflammatory cytokines on the nature of the inflammatory infiltrate and bacterial growth in the cytosol of macrophages (Mϕ), DCs, and PMNs are shown. High levels of IL-12, IFN-γ, and other proinflammatory cytokines control F. tularensis (Ft) replication in macrophages and DCs at the level of phagosome escape and replication in the cytosol. F. tularensis grows less well in neutrophils, but delayed apoptosis of these cells favors their progression to secondary necrosis. Release of toxic cell contents and alarmins, including high-mobility group box 1 (HMGB1), from dying PMNs amplifies tissue destruction. Treg, Regulatory T cell.
ACKNOWLEDGMENTS
Research on this topic in the author's laboratory is supported by grants from the U.S. National Institutes of Health (R01 AI073835, P01 AI044642) and by funds (U54 AI057160) from the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (MRCE).
SEE CORRESPONDING ARTICLE ON PAGE 657
- LVS
- live vaccine strain
- MMP-9
- matrix metalloproteinase-9
REFERENCES
- 1. Cowley S., Elkins K. (2011) Immunity to Francisella. Front. Microbiol. 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crane D. D., Scott D. P., Bosio C. M. (2012) Generation of a convalescent model of virulent Francisella tularensis infection for assessment of host requirements for survival of tularemia. PLoS One 7, e33349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melillo A. A., Foreman O., Elkins K. L. (2013) IL-12Rβ2 is critical for survival of primary Francisella tularensis LVS infection J. Leukoc. Biol. 93, 677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Periasamy S., Singh A., Sahay B., Rahman T., Feustel P. J., Pham G. H., Gosselin E. J., Sellati T. J. (2011) Development of tolerogenic dendritic cells and regulatory T cells favors exponential bacterial growth and survival during early respiratory tularemia. J. Leukoc. Biol. 90, 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauler T. J., Chase J. C., Bosio C. M. (2011) IFN-β mediates suppression of IL-12p40 in human dendritic cells following infection with virulent Francisella tularensis. J. Immunol. 187, 1845–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saxinger L. (2012, November 19) Tularemia, pneumonic–Canada: (Alberta) biologic immunomodulator. ProMED Mail, Archive Number 20121119.1415382, International Society for Infectious Diseases, Brookline, MA, USA, from http://www.promedmail.org/?p=2400:1000 [Google Scholar]
- 7. Gandhi M., Alwawi E., Gordon K. B. (2010) Anti-p40 antibodies ustekinumab and briakinumab: blockade of interleukin-12 and interleukin-23 in the treatment of psoriasis. Semin. Cutan. Med. Surg. 29, 48–52 [DOI] [PubMed] [Google Scholar]
- 8. Koskinas J., Tampaki M., Doumba P. P., Rallis E. (2012) Hepatitis B virus reactivation during therapy with ustekinumab for psoriasis in an HBsAg negative anti-HBs positive patient. Br. J. Dermatol. doi: 10.1111/bjd.12120 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9. Schmitt D. M., O'Dee D. M., Brown M. J., Horzempa J., Russo B. C., Morel P. A., Nau G. J. Role of NK cells in host defense against pulmonary type A Francisella tularensis infection. Microbes Infect. (2012) http://dx.doi.org/10.1016/jmicinf.2012.11.008 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 10. Malik M., Bakshi C. S., McCabe K., Catlett S. V., Shah A., Singh R., Jackson P. L., Gaggar A., Metzger D. W., Melendez J. A., Blalock J. E., Sellati T. J. (2007) Matrix metalloproteinase 9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularensis. J. Immunol. 178, 1013–1020 [DOI] [PubMed] [Google Scholar]
- 11. McCaffrey R. L., Allen L-A. H. (2006) Pivotal Advance: Francisella tularensis evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J. Leukoc. Biol. 80, 1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz J. T., Barker J. H., Kaufman J., Fayram D. C., McCracken J. M., Allen L-A. H. (2012) Francisella tularensis inhibits the intrinsic and extrinsic pathways to delay constitutive apoptosis and prolong human neutrophil lifespan. J. Immmunol. 188, 3351–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nathan C. (2002) Points of control in inflammation. Nature 420, 846–852 [DOI] [PubMed] [Google Scholar]
- 14. Mantovani A., Cassatella M. A., Costantini C., Jaillon S. (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 [DOI] [PubMed] [Google Scholar]