A role for regulatory B cells is shown in HIV-pathogenesis, potentially impacting HIV cell-mediated control.
Keywords: immune activation, immune exhaustion, IL-10
Abstract
HIV infection is associated with elevated expression of IL-10 and PD-L1, contributing to impairment of T cell effector functions. In autoimmunity, tumor immunology, and some viral infections, Bregs modulate T cell function via IL-10 production. In this study, we tested the hypothesis that during HIV infection, Bregs attenuate CD8+ T cell effector function, contributing to immune dysfunction. We determined that in vitro, TLR2-, TLR9-, and CD40L-costimulated Bregs from HIV− individuals exhibited a high frequency of cells expressing IL-10 and PD-L1. Compared with Bregs from HIV− individuals, a significantly higher percentage of Bregs from HIV+ individuals spontaneously expressed IL-10 (P=0.0218). After in vitro stimulation with HIV peptides, Breg-depleted PBMCs from HIV+ individuals exhibited a heightened frequency of cytotoxic (CD107a+; P=0.0171) and HIV-specific CD8+ T cells compared with total PBMCs. Furthermore, Breg depletion led to enhanced proliferation of total CD8+ and CD107a+CD8+ T cells (P=0.0280, and P=0.0102, respectively). In addition, augmented CD8+ T cell effector function in vitro was reflected in a 67% increased clearance of infected CD4+ T cells. The observed Breg suppression of CD8+ T cell proliferation was IL-10-dependent. In HIV+ individuals, Breg frequency correlated positively with viral load (r=0.4324; P=0.0095), immune activation (r=0.5978; P=0.0005), and CD8+ T cell exhaustion (CD8+PD-1+; r=0.5893; P=0.0101). Finally, the frequency of PD-L1-expressing Bregs correlated positively with CD8+PD-1+ T cells (r=0.4791; P=0.0443). Our data indicate that Bregs contribute to HIV-infection associated immune dysfunction by T cell impairment, via IL-10 and possibly PD-L1 expression.
Introduction
Robust T cell effector functions are crucial for effective clearance of viral infections. Nonetheless, during some chronic viral infections, including HIV infection, T cells lose the ability to mount effective antiviral responses, resulting in a reversible state described as “immune exhaustion” [1], culminating in viral persistence. During HIV infection, these exhausted T cells are characterized by the elevated expression of the coinhibitory protein PD-1 [2, 3], which correlates with viral persistence and disease progression. Furthermore, IL-10, secreted by monocytes, has been shown to contribute to viral persistence [4], and antibody blockade of IL-10 and PD-1 have been shown to reverse T cell exhaustion and reduce viral replication [4, 5]. IL-10 expression is up-regulated in multiple cell types during HIV infection [6], but animal model studies indicate that B cells are a key source of IL-10 [7], and these IL-10-expressing B cells are enriched in the Breg (CD19+CD24hiCD38hi) subset [8, 9]. Data from multiple studies indicate that Bregs, in an IL-10-dependent manner, suppress T cell effector functions in autoimmune, malignant diseases and recently during chronic viral infections [10–15]. HIV infection is associated with microbial translocation and the systemic prevalence of multiple TLR ligands [16] and CD40L [17], stimuli required for Breg activation in vitro. We therefore hypothesize that during HIV infection, Bregs attenuate anti-HIV CD8+ T cell effector functions, contributing to immune dysfunction. To test our hypothesis, we characterized Bregs in HIV− healthy controls and HIV+ individuals. In HIV+ individuals, we evaluated if Bregs suppressed proliferation of anti-HIV effector CD8+ T cell subsets. We further assessed Breg frequency in viremic (HIVvir+) and aviremic (HIVavir+) HIV-infected individuals and investigated associations with markers of disease progression, including viral load, chronic immune activation, and T cell exhaustion. Our data indicate a novel role for Bregs in immune dysfunction during HIV pathogenesis.
MATERIALS AND METHODS
Study participants
All studies were performed after signed, informed written research consent by each study subject. The study was reviewed and approved by the Institutional Review Board of Rush University Medical Center, Cook County Health and Hospitals System, and the University of Iowa City Veterans Affairs Medical Center and University of Iowa. Healthy controls had a mean age of 40 years (range, 22–70), and HIV+ individuals had a mean age of 45 years (22–65 years). HIVavir individuals had an undetectable viral load (<48 copies/ml) and mean CD4 count of 597 cells/μl (range, 223–1236); HIVvir-untreated individuals had a mean viral load of 38,984 copies/ml (range, 330–214,000) and mean CD4 count of 414 cells/μl (range, 130–515).
Analysis of IL-10 production by Bregs and immunophenotyping of PBMCs
PBMCs were isolated from whole blood using a Ficoll gradient (Lymphocyte Cell Separation Medium, Mediatech, Manassas, VA, USA). To characterize TLR-activated Bregs, MACS-purified (Miltenyi Biotec, Auburn, CA, USA; according to the manufacturer's instructions) B cells from healthy controls were stained with antibodies against CD19-PE-CF594 (Beckman Coulter, Brea, CA, USA), CD24-PerCP-Cy5.5 (BD Biosciences, San Jose, CA, USA), and CD38-FITC (BD Biosciences). CD19+CD24hiCD38hi (Bregs) and CD19+CD24loCD38lo cells (“non-Bregs”; mature B cells) were sorted (FACSAria; BD Biosciences) and cultured for 48 h in the presence of 10 μg/ml CpG-B oligodeoxynucleotide-2006, 2 μg/ml palmitoyl-3-cysteine-serine-lysine-4 (InvivoGen, San Diego, CA, USA), and 2 μg/ml CD40L (InvivoGen). During the final 5 h of incubation, the cultures were supplemented with PIB (PMA, (50ng/ml) Ionomycin (1μg/ml) and Brefeldin A (1:1000); InvivoGen and BD Biosciences). After incubation, the cells were washed, stained for viable cells (LIVE/DEAD fixable aqua dead cell stain kit; Invitrogen, Life Technologies, Carlsbad, CA, USA), surface-stained, fixed/permeabilized (Fix/Perm kit; BD Biosciences), and stained for intracellular IL-10 (IL-10-AF-647; eBioscience, San Diego, CA, USA). To determine spontaneous expression of IL-10 by Bregs from HIV+ individuals and healthy controls, PBMCs were incubated overnight, stimulated for the final 5 h, and stained as described for healthy controls. The following antibodies were used for immunophenotyping of PBMCs: CD38-AF-700, HLA-DR-PE-Cy7, CD4-Pacific Blue, CD8-APC-H7, and PD-L1-PE-Cy7 (eBioscience). All samples were acquired on an LSR II (BD Biosciences) flow cytometer and the data analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Proliferation assay
Proliferation dye VPD450 (BD Biosciences)-labeled total or Breg-depleted PBMCs from HIV+ individuals were stimulated with an HIV peptide (NIH AIDS Repository) pool, spanning nef, env, gag, and pol (2 μg/ml each). After 96 h, the frequencies of CD8+CD107a+ (cytotoxic CD8+ T cells) and infected CD4+ T cells (using KC57-Rd1; Beckman Coulter; antibody that binds HIV-1 core proteins 55, 39, 33, and 24 KDa) were determined by flow cytometry. Proliferation of CD8+CD107a+ T cells was determined after 96 h, and proliferation of total CD8+ T cells was assessed after 7 days in culture. Bregs and CD8+ T cells from HIV+ individuals were cocultured and supplemented with blocking IL-10R antibody (BioLegend, San Diego, CA, USA), and proliferation of CD8+ T cells was assessed after 7 days.
Staining of HIV-specific CD8+ T cells with the HLA-A*0201-restricted peptide complex
The frequency of antigen-specific CD8+ T cells was determined by binding to APC-labeled, HLA-A2-restricted SL9 (SLYNTVATL) HIV-Gag epitope MHC-I-Dextramer (Immudex, Copenhagen, Denmark). Cells of HLA-A2-typed HIV+ individuals were washed twice with PBS and incubated with 10 μl Dextramer for 10 min at room temperature, stained with antibodies, and analyzed by flow cytometry.
Statistical analysis
Results are expressed as mean ± sem or as indicated. GraphPad Prism software, version 5.03, was used for all statistical analysis. The statistical significance P value between group parameters was determined using unpaired or paired Student's t-test (with a confidence level of 95%). The statistical dependence between variables was calculated using the Spearman rank correlation analysis. P values of <0.05 were considered statistically significant.
RESULTS
TLR and CD40L costimulation of Bregs from healthy controls leads to a high frequency of cells expressing PD-L1 and IL-10
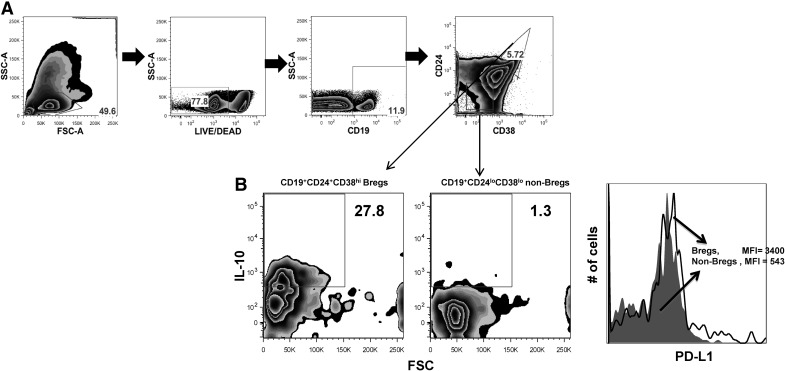
Antibody blockade of PD-L1 and IL-10 has been shown to reverse impaired T cell effector functions during HIV infection. The cellular sources of PD-L1 and IL-10 have not been fully identified, yet data from a recent study [10] indicate that during hepatitis B viral infection, IL-10-competent Bregs (CD19+CD24hiCD38hi) suppress CD8+ effector functions. Therefore, we sought to determine a potential contribution of Bregs to T cell impairment during HIV infection, possibly involving the synergistic expression of IL-10 and PD-L1. We evaluated the association between IL-10 expression and levels of PD-L1 on activated Bregs of HIV− individuals. After TLR2, TLR9, and CD40L costimulation, we found that a higher frequency of Breg cells (CD19+CD24hiCD38hi; Fig. 1A) was positive for IL-10 (15-fold; Fig. 1B, left) and PD-L1 (Fig. 1B, right) compared with non-Bregs cells (CD19+CD24loCD38lo; mature B cells). This indicates that TLR/CD40L-costimulated Bregs might contribute to suppression of T cell effector functions via IL-10 and PD-L1 pathways.
Figure 1. TLR and CD40 costimulation of Bregs lead to a higher frequency of IL-10+ cells and up-regulation of PD-L1 expression.
CD19+CD24hiCD38hi (Bregs)- and CD19+CD24loCD38lo (non-Bregs; mature B cell subset; A)-purified cells from healthy controls were costimulated with TLR2, TLR9, and CD40L for 48 h; for the final 5 h, the cultures were supplemented with PIB, and expression of intracellular IL-10 (B, left) and surface PD-L1 (B, right) was determined by flow cytometry. Representative diagrams from at least two independent experiments are shown. Numbers in quadrants indicate percentages. SSC-A, Side-scatter-area; FSC-A, forward-scatter-area; MFI, mean fluorescence intensity; PIB = PMA, (50ng/ml) Ionomycin (1μg/ml) and Brefeldin A (1:100).
Ex vivo compared with Bregs from HIV− individuals, Bregs from HIV+ individuals exhibit a high frequency of IL-10-positive cells
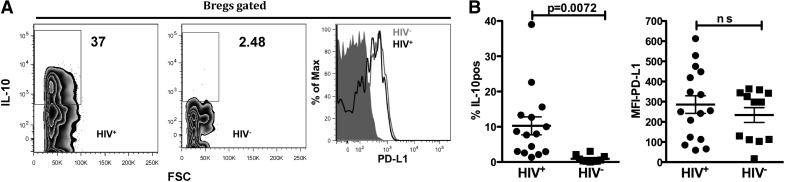
To determine if during HIV infection, Bregs express high levels of IL-10 and PD-L1, we evaluated the frequency of IL-10 and PD-L1-positive Bregs from HIV+ and HIV− individuals ex vivo. We determined that compared with Bregs from HIV− individuals, Bregs from HIV+ individuals exhibit a significantly higher frequency of IL-10-positive cells (P=0.0072; Fig. 2A and B). We determined no statistically significant difference in Breg PD-L1 expression between HIV− and HIV+ individuals (Fig. 2A and B).
Figure 2. Compared with Bregs from HIV− individuals, Bregs from HIV+ individuals exhibit a higher percentage of IL-10-positive cells.
(A) Bregs—intracellular IL-10 (left) and surface PD-L1 (right)—expression was determined by flow cytometry after 48 h in culture with PIB. (Representative plots are shown.) (B) IL-10 (left) and PD-L1 (right) summary of results from HIV+ (n=15) and HIV− (n=12) individuals. P values (unpaired two-tailed t-test; confidence interval of 95%) are indicated.
In vitro Bregs attenuate proliferation of anti-HIV CD8+ T cell effector subsets
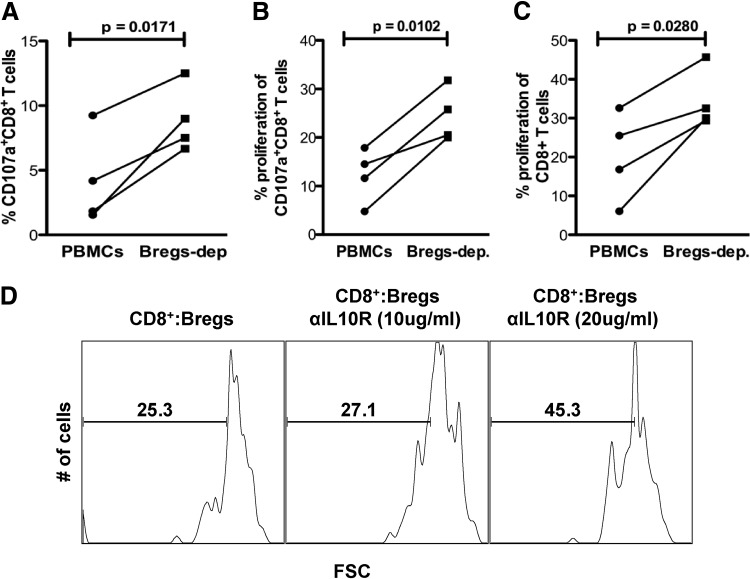
In disease settings, Bregs have been reported to negatively regulate T cell proliferation and effector functions [8, 10]; therefore, we next assessed if Bregs suppress T cell functions during HIV infection. To test this, Bregs were depleted from PBMCs of HIV+ individuals by FACS sorting, and VPD450-labeled total or Breg-depleted PBMCs were stimulated with HIV peptides (spanning gag, nef, env, and pol, as described in ref. [18]). After 96 h in culture, we determined via flow cytometric analysis that Breg depletion led to a significant increase in frequency and proliferation of cytotoxic (CD107a+) CD8+ T cells (Fig. 3A, P=0.0171; and B, P=0.0102, respectively). Similarly, a significant increase in proliferation of total CD8+ T cells was observed after 7 days in culture (Fig. 3C; P=0.0280).
Figure 3. Depletion of Bregs leads to increased proliferation of effector CD8+ T cells in an IL-10-dependent manner.
(A–C) VPD450-labeled total or Breg-depleted (Bregs-dep.) PBMCs of HIV+ individuals (n=4) were stimulated with HIV-pooled peptides (gag, pol, env, and nef; 2 μg/ml each), the frequency and proliferation of CD107a+CD8+ T cells were evaluated by flow cytometry after 4 days, whereas total CD8+ T cell proliferation was evaluated after 7 days in culture. P values (paired two-tailed t-test; confidence interval of 95%) are indicated. (D) Autologous Bregs and VPD450-labeled CD8+ T cells from an HIV+ donor were stimulated with HIV-pooled peptides as described above; supplemented with blocking IL-10R antibody (αIL-10R), as indicated; and after 7 days in culture proliferation of CD8+ T cells, determined by flow cytometry. Representative histograms of two independent experiments are shown.
In vitro Bregs attenuation of anti-HIV CD8+ T cell proliferation is IL-10-dependent
Breg suppressor function has been shown to be largely IL-10-mediated (reviewed in refs. [8, 9]). After determining that depletion of Bregs leads to enhanced proliferation of anti-HIV CD8+ T cell effector subsets, we investigated if this Breg-mediated suppression was IL-10-dependent. We cocultured purified Bregs and VPD450-labeled CD8+ T cells (ratio of 1:5 was used as described) [13] from HIV+ individuals. The cells were stimulated as described above and supplemented with 10 μg/ml- or 20 μg/ml-blocking anti-IL10R antibody (BioLegend) and proliferation of CD8+ T cells investigated after 7 days. We determined that compared with the control, without IL-10R blocking antibody, Breg-mediated proliferation inhibition was reversed in an IL-10 concentration-dependent manner; supplementation with 10 μg/ml- or 20 μg/ml-blocking anti-IL10R antibody led to a 7% and 78% proliferation increase, respectively (Fig. 3D).
In vitro Breg depletion leads to enhanced HIV-specific, CD8+ T cell CTL activity
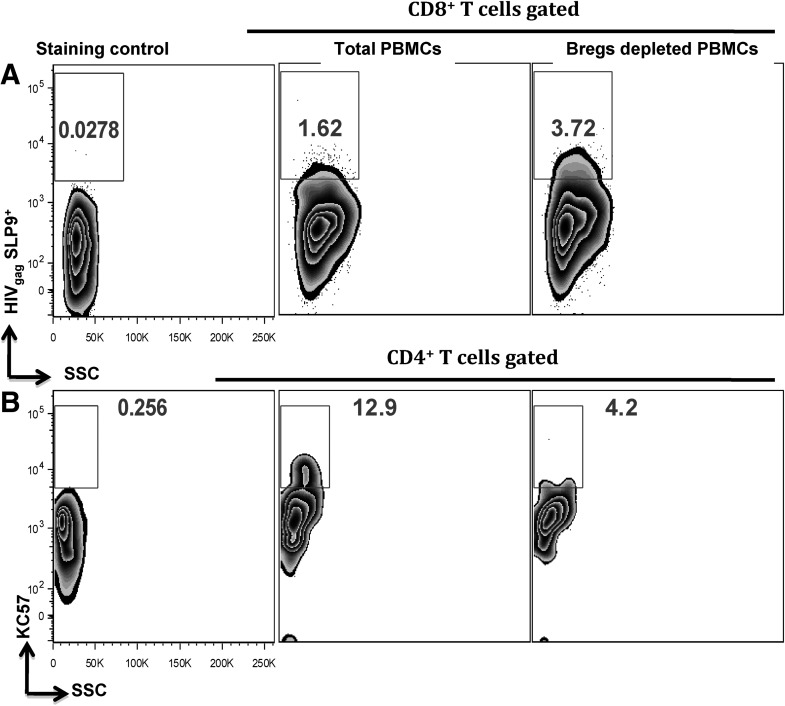
The observed proliferation of CD8+ T cell subsets in Breg-depleted cultures prompted us to investigate if this was associated with augmented HIV-specific CTL responses. After 4 days in culture, the cells described above were stained with APC-labeled MHC-I Dextramer (Immudex), specific for the HIVgag CTL-associated SL9 epitope [19–21] and analyzed by flow cytometry. We determined that Breg depletion led to a 129% increase in SL9+CD8+ T cells (Fig. 4A). This increase in HIV-specific, CTL-competent CD8+ T cells was reflected in a 67% decrease in frequency of infected CD4+ T cells in Breg-depleted cultures (Fig. 4B) compared with total PBMCs. Effective CTL responses have been shown to be required to clear infected CD4+ T cells in vitro after stimulation with HIV peptides [22, 23].
Figure 4. Depletion of Bregs leads to enhanced HIV-specific CTL responses.
Breg-depleted or total PBMCs from HLA-2A+ HIVavir+ individuals were stimulated with HIV-pooled peptides, as described above, and after 4 days in culture, the frequencies of (A) HIVgag-SLP9+CD8+ T cells (using MHC-I-Dextramers) and (B) KC57+ (anti-HIV core proteins) CD4+ T cells were determined by flow cytometry. Representative plots of two independent experiments are shown.
Breg frequency correlates positively with viral load and chronic T cell immune activation
Impairment of antiviral T cell effector functions leads to viral persistence associated with chronic immune activation [1–3, 24]. Chronic immune activation, as defined by the frequency of HLA-DR+CD38+ T cells, is a predictor of disease progression, independent of viral load [25]. After determining that in vitro, Bregs contribute to attenuation of anti-HIV effector CD8+ T cell functions, we investigated the potential in vivo relevance by evaluating the relationship between Breg frequency and markers of disease progression—viral load and immune activation. In HIV+ individuals, we found a significant, positive correlation between Breg frequency and viral load (Fig. 5A; r=0.4324; P=0.0095). We similarly determined a positive correlation between Breg frequency and immune activation (Fig. 5B, CD4+HLA-DR-CD38+: r=0.6202, P=0.0060; Fig. 5C, CD8+HLA-DR-CD38+: r=0.5878, P=0.0005), thereby indicating an association between Bregs and disease progression.
Figure 5. Breg frequency correlates positively with markers of HIV disease progression.
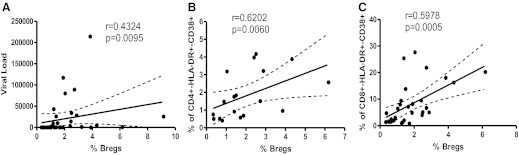
In HIV+ individuals (n=32), Breg frequency positively correlates with (A) viral load and(B and C) immune activation. Relationship variables were determined by nonparametric Spearman correlation.
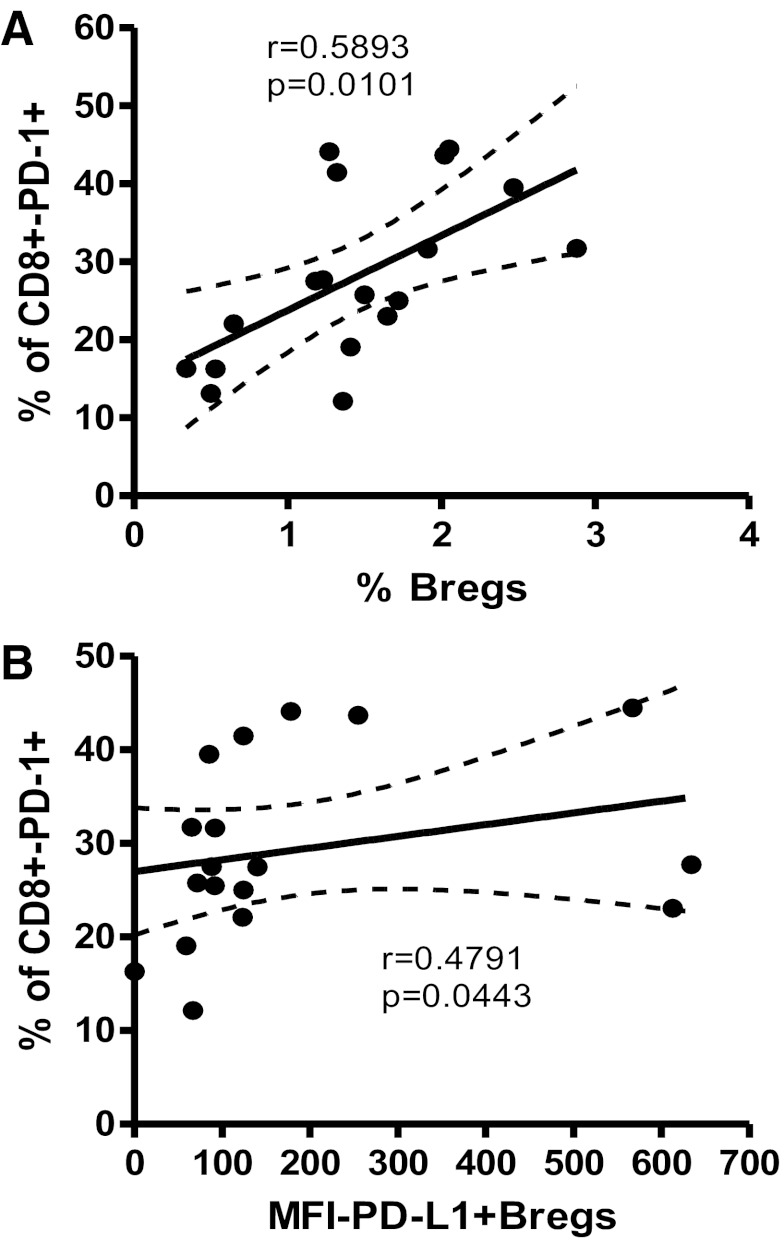
Breg frequency correlates positively CD8+ T cell exhaustion
During HIV infection, T cell exhaustion, characterized by heightened PD-1 expression, is a hallmark of T cell effector impairment, leading to viral persistence [3]. After determining that activated Bregs also express high levels of PD-L1 (Fig. 2B, right), we investigated a relationship between Breg frequency and CD8+ T cell exhaustion. We found a positive correlation between Breg frequency and exhausted CD8+PD-1+ cells (Fig. 6A; r=0.5893; P=0.0101), as well as between PD-L1+ Breg frequency and CD8+PD-1+ exhausted T cells (Fig. 6B; r=0.4791; P=0.0447).
Figure 6. Breg frequency correlates positively with exhausted CD8+ T cells.
In HIV+ individuals (n=18), Breg frequency correlates positively with (A) frequency of CD8+PD-1+ T cells. (B) The frequency of PD-L1-expressing Bregs also correlates positively with CD8+PD-1+ T cells. Relationship variables were determined by nonparametric Spearman correlation.
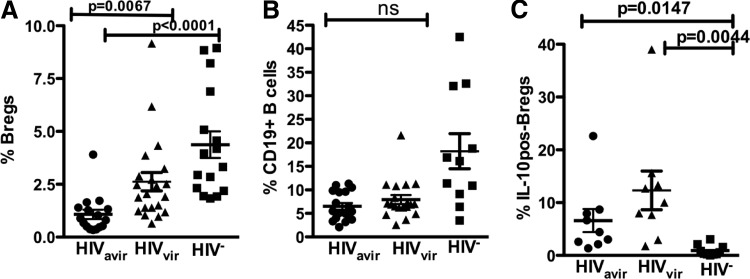
Bregs from HIVavir+ individuals exhibit a high percentage of IL-10-positive cells despite reduced Breg frequency
To determine the effects of ART on the Breg population, we characterized Bregs in viremic (HIVvir, n=18) and aviremic (HIVavir, n=16) HIV-infected individuals. Compared with HIV− subjects, Breg frequency was significantly lower in HIVvir (Fig. 7A; P=0.0235) and dramatically depleted in HIVavir (Fig. 7A; P<0.0001). We also observed a significant difference in Breg frequency between HIVavir and HIVvir individuals (Fig. 7A; P=0.0067). Interestingly, there was no difference in total B cell frequency between HIVavir and HIVvir individuals (Fig. 7B), indicating a selective loss of Bregs in HIVavir individuals. In Fig. 4, we show that Breg-mediated suppression of CD8+ T cell function is partially IL-10-dependent. We therefore investigated the levels of Breg-constitutive IL-10 expression in HIVavir, HIVvir, and HIV− individuals. We determined that despite the reduced Breg frequency in HIVavir individuals, their Bregs have a significantly higher frequency of IL-10-positive cells compared with Bregs from HIV− individuals (P=0.0147). Bregs from HIVvir individuals also had a twofold-higher frequency of IL-10-positive cells than Bregs of HIVavir individuals. Furthermore, Bregs of HIVvir individuals also exhibited a significantly higher frequency of IL-10-positive cells (P=0.0044) compared with Bregs from healthy controls (Fig. 7C).
Figure 7. Breg frequency is reduced dramatically in HIVavir+ individuals.
(A) HIVvir+ (n=17) and HIVavir+ (n=17) individuals exhibit significant differences in Breg frequency compared with HIV− individuals (n=19) (B) yet comparable B cell frequencies. (C) Despite low frequency, the Bregs of HIVavir+ individuals (n=9) exhibited a higher frequency of IL-10-positive cells than Bregs from healthy controls (n=10). P values (two-tailed t-test; confidence interval of 95%) are indicated.
DISCUSSION
During chronic viral infections, including HIV, effective antiviral responses are mediated by highly proliferating, virus-specific T cells. An ineffective antiviral T cell effector response is the main driver of viral persistence [1] and HIV disease progression [2]. Studies have demonstrated that during HIV infection, impairment of the T cell effector response is mediated by IL-10 and PD-L1 [5, 6]. Multiple groups have reported that B cells and specifically, Bregs are key sources of IL-10 [8, 9], and using a polyoma virus animal model Velupillai et al. [26] demonstrated that expression of IL-10 by TLR-stimulated B cells contributes significantly to impaired T cell effector function. Bregs have been ascribed various phenotypic markers, including CD19+CD24hiCD38hi immature translational [13] or CD19+CD27hiCD24hiB10 memory B cells [27]. It is likely that this discrepancy arises from diverse methods of in vitro stimulation used to achieve IL-10 expression. As a result of these factors, IL-10 expression remains the sole important criterion to identify Bregs. Nonetheless, by investigating endogenous IL-10 expression, thus circumventing in vitro stimulation-induced artifacts, Blair et al. [13] convincingly showed that CD19+CD24hiCD38hi B cells are highly IL-10-competent with regulatory properties. Data from a recent study corroborate the regulatory properties of CD19+CD24hiCD38hi B cells during hepatitis B viral infection [10]. Our findings extend these Breg-suppressive properties to HIV infection, which is associated with a systemic prevalence of TLR ligands and CD40L. In vitro, we demonstrate that TLR2-, TLR9-, and CD40L-costimulated Bregs from healthy controls lead to high PD-L1 expression and an increased frequency of IL-10-positive cells. This indicates that stimuli required for Breg activation and IL-10 expression prevail during HIV infection. We demonstrated that in HIV+ individuals, even after successful ART therapy, a high frequency of Bregs was constitutively IL-10-positive. Interestingly there was no statistically significant difference in Breg PD-L1 expression between HIV+ and HIV− individuals. In vitro, we show that depletion of Bregs from PBMCs of HIV+ individuals and subsequent stimulation with HIV-pooled peptides result in enhanced proliferation of HIV-specific CD8+ T cell effector subsets. In addition, Breg-mediated suppression of CD8+ T cell function was partially IL-10-dependent, corroborating published results [10, 13]. During HIV infection, effective CTL responses are pivotal in controlling viral replication [28–30], thus our finding that Breg frequency correlates positively with viral load indicates that during HIV infection, activated Bregs potentially contribute to attenuation of CTL functions, likely leading to viral persistence. Further evidence for this potential Breg role during HIV infection was provided by the significant, positive correlation between Breg frequency and chronic immune activation, as well as with T cell exhaustion—markers of disease progression that have been reported to correlate with residual viral replication [2, 31]. In published reports, Breg-suppressive activities are associated with an increase in Breg frequency [10, 13], Surprisingly, we found a reduction of Breg frequency in HIV+ individuals. This reduction was not related to HIV-associated lymphopenia, as we determined an inverse correlation between Breg frequency and CD4+ T cell count (data not shown). Despite this depletion, ex vivo-unstimulated Bregs of HIVvir+ individuals exhibited elevated frequencies of IL-10-positive cells compared with HIVavir+ individuals and healthy controls. Furthermore, the observed elevated, endogenous, IL-10-positive Breg population was reduced in HIVavir+ individuals and not normalized compared with Bregs from healthy controls. It is thus likely that despite the reduced Breg frequency in aviremic individuals, spontaneous IL-10 expression contributes to exhausted T cells observed, even in ART-treated subjects. Along similar lines, data from a recent study using a tumor animal model indicated that few Breg cells effectively suppress anti-tumor T cell effector function [12]. In vitro, we similarly determined that depletion of Bregs in ART-treated individuals resulted in enhanced, anti-HIV-specific CTL responses, as determined by clearance of infected CD4+ T cells.
Taken together, our data present the first report on B cell-mediated regulation of T cell function during HIV infection. Additionally, results from our study could lead to defining treatment strategies, enhancing antiviral T cell effector functions in ART-treated, HIV+ individuals. In these individuals, sustained, impaired CTL responses remain a major hurdle toward viral eradication. In an elegant in vivo model, therapeutic Breg depletion led to effective CTL-mediated tumor eradication [12]. Further studies are needed to delineate the impact of Breg loss in HIV+ individuals and to define further the mechanisms of Breg-mediated dysfunction of the T cell compartment in ART-treated patients.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Merit Review Grant to J.T.S.), U.S. National Institutes of Health (RO1 AI-58740 to J.T.S.), and U.S. National Institutes of Health, Developmental Center for AIDS Research, P30 AI-082151-01 and P01 AI-076174-01A1.
Footnotes
- APC
- allophycocyanin
- ART
- antiretroviral therapy
- Breg
- regulatory B cell
- CD40L
- CD40 ligand
- HIV−
- HIV-negative
- HIV+
- HIV-infected
- HIVavir
- HIV-aviremic
- HIVvir
- HIV-viremic
- PD-1
- programmed death 1
- PD-L1
- programmed death ligand 1
- PIB
- 50 ng/ml PMA, 1 μg/ml ionomycin, and 1:100 Brefeldin A
AUTHORSHIP
B.S. and A.L. conceived of the study. B.S., J.T.S., A.K., P.M.D., A.L.F., and A.L. designed the study and edited the manuscript. B.S., J.M., and N.K. performed experiments. B.S. and A.L. analyzed data and wrote the paper.
REFERENCES
- 1. Zajac A. J., Blattman J. N., Murali-Krishna K., Sourdive D. J., Suresh M., Altman J. D., Ahmed R. (1998) Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Day C. L., Kaufmann D. E., Kiepiela P., Brown J. A., Moodley E. S., Reddy S., Mackey E. W., Miller J. D., Leslie A. J., DePierres C., Mncube Z., Duraiswamy J., Zhu B., Eichbaum Q., Altfeld M., Wherry E. J., Coovadia H. M., Goulder P. J., Klenerman P., Ahmed R., Freeman G. J., Walker B. D. (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 [DOI] [PubMed] [Google Scholar]
- 3. Trautmann L., Janbazian L., Chomont N., Said E. A., Gimmig S., Bessette B., Boulassel M. R., Delwart E., Sepulveda H., Balderas R. S., Routy J. P., Haddad E. K., Sekaly R. P. (2006) Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12, 1198–1202 [DOI] [PubMed] [Google Scholar]
- 4. Brooks D. G., Trifilo M. J., Edelmann K. H., Teyton L., McGavern D. B., Oldstone M. B. (2006) Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12, 1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Said E. A., Dupuy F. P., Trautmann L., Zhang Y., Shi Y., El-Far M., Hill B. J., Noto A., Ancuta P., Peretz Y., Fonseca S. G., Van Grevenynghe J., Boulassel M. R., Bruneau J., Shoukry N. H., Routy J. P., Douek D. C., Haddad E. K., Sekaly R. P. (2010) Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16, 452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brockman M. A., Kwon D. S., Tighe D. P., Pavlik D. F., Rosato P. C., Sela J., Porichis F., Le Gall S., Waring M. T., Moss K., Jessen H., Pereyra F., Kavanagh D. G., Walker B. D., Kaufmann D. E. (2009) IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114, 346–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fillatreau S., Sweenie C. H., McGeachy M. J., Gray D., Anderton S. M. (2002) B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 [DOI] [PubMed] [Google Scholar]
- 8. Mauri C., Bosma A. (2012) Immune regulatory function of B cells. Annu. Rev. Immunol. 30, 221–241 [DOI] [PubMed] [Google Scholar]
- 9. Bouaziz J. D., Le Buanec H., Saussine A., Bensussan A., Bagot M. (2012) IL-10 producing regulatory B cells in mice and humans: state of the art. Curr. Mol. Med. 12, 519–527 [DOI] [PubMed] [Google Scholar]
- 10. Das A., Ellis G., Pallant C., Lopes A. R., Khanna P., Peppa D., Chen A., Blair P., Dusheiko G., Gill U., Kennedy P. T., Brunetto M., Lampertico P., Mauri C., Maini M. K. (2012) IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J. Immunol. 189, 3925–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inoue S., Leitner W. W., Golding B., Scott D. (2006) Inhibitory effects of B cells on antitumor immunity. Cancer Res. 66, 7741–7747 [DOI] [PubMed] [Google Scholar]
- 12. Horikawa M., Minard-Colin V., Matsushita T., Tedder T. F. (2011) Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J. Clin. Invest. 121, 4268–4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blair P. A., Norena L. Y., Flores-Borja F., Rawlings D. J., Isenberg D. A., Ehrenstein M. R., Mauri C. (2010) CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32, 129–140 [DOI] [PubMed] [Google Scholar]
- 14. Mauri C., Gray D., Mushtaq N., Londei M. (2003) Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 197, 489–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dilillo D. J., Weinberg J. B., Yoshizaki A., Horikawa M., Bryant J. M., Iwata Y., Matsushita T., Matta K. M., Chen Y., Venturi G. M., Russo G., Gockerman J. P., Moore J. O., Diehl L. F., Volkheimer A. D., Friedman D. R., Lanasa M. C., Hall R. P., Tedder T. F. (2013) Chronic lymphocytic leukemia and regulatory B cells share IL-10-competence and immunosuppressive function. Leukemia 27, 170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brenchley J. M., Price D. A., Schacker T. W., Asher T. E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., Blazar B. R., Rodriguez B., Teixeira-Johnson L., Landay A., Martin J. N., Hecht F. M., Picker L. J., Lederman M. M., Deeks S. G., Douek D. C. (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 [DOI] [PubMed] [Google Scholar]
- 17. Imbeault M., Ouellet M., Giguere K., Bertin J., Bélanger D., Martin G., Tremblay M. J. (2011) Acquisition of host-derived CD40L by HIV-1 in vivo and its functional consequences in the B-cell compartment. J. Virol. 85, 2189–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zloza A., Schenkel J. M., Tenorio A. R., Martinson J. A., Jeziorczak P. M., Al-Harthi L. (2009) Potent HIV-specific responses are enriched in a unique subset of CD8+ T cells that coexpresses CD4 on its surface. Blood 114, 3841–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koup R. A., Safrit J. T., Cao Y., Andrews C. A., McLeod G., Borkowsky W., Farthing C., Ho D. D. (1994) Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68, 4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klein M. R., van Baalen C. A., Holwerda A. M., Kerkhof Garde S. R., Bende R. J., Keet I. P., Eeftinck-Schattenkerk J. K., Osterhaus A. D., Schuitemaker H., Miedema F. (1995) Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181, 1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang O. O., Kalams S. A., Trocha A., Cao H., Luster A., Johnson R. P., Walker B. D. (1997) Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71, 3120–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shan L., Deng K., Shroff N. S., Durand C. M., Rabi S. A., Yang H. C., Zhang H., Margolick J. B., Blankson J. N., Siliciano R. F. (2012) Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36, 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shete A., Thakar M., Singh D. P., Gangakhedkar R., Gaikwad A., Pawar J., Paranjape R. (2012) Short communication: HIV antigen-specific reactivation of HIV infection from cellular reservoirs: implications in the settings of therapeutic vaccinations. AIDS Res. Hum. Retroviruses 28, 835–843 [DOI] [PubMed] [Google Scholar]
- 24. Trautmann L., Said E. A., Halwani R., Janbazian L., Chomont N., El-Far M., Breton G., Haddad E. K., Sekaly R. P. (2007) Programmed death 1: a critical regulator of T-cell function and a strong target for immunotherapies for chronic viral infections. Curr. Opin. HIV AIDS 2, 219–227 [DOI] [PubMed] [Google Scholar]
- 25. Liu Z., Cumberland W. G., Hultin L. E., Prince H. E., Detels R., Giorgi J. V. (1997) Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol 16, 83–92 [DOI] [PubMed] [Google Scholar]
- 26. Velupillai P., Garcea R. L., Benjamin T. L. (2006) Polyoma virus-like particles elicit polarized cytokine responses in APCs from tumor-susceptible and -resistant mice. J. Immunol. 176, 1148–1153 [DOI] [PubMed] [Google Scholar]
- 27. Iwata Y., Matsushita T., Horikawa M., Dilillo D. J., Yanaba K., Venturi G. M., Szabolcs P. M., Bernstein S. H., Magro C. M., Williams A. D., Hall R. P., St Clair E. W., Tedder T. F. (2011) Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117, 530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matano T., Shibata R., Siemon C., Connors M., Lane H. C., Martin M. A. (1998) Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72, 164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuroda M. J., Schmitz J. E., Seth A., Veazey R. S., Nickerson C. E., Lifton M. A., Dailey P. J., Forman M. A., Racz P., Tenner-Racz K., Letvin N. L. (2000) Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac-infected rhesus monkeys. Blood 96, 1474–1479 [PubMed] [Google Scholar]
- 30. Jin X., Bauer D. E., Tuttleton S. E., Lewin S., Gettie A., Blanchard J., Irwin C. E., Safrit J. T., Mittler J., Weinberger L., Kostrikis L. G., Zhang L., Perelson A. S., Ho D. D. (1999) Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189, 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benito J. M., Lopez M., Lozano S., Martinez P., Gonzalez-Lahoz J., Soriano V. (2004) CD38 expression on CD8 T lymphocytes as a marker of residual virus replication in chronically HIV-infected patients receiving antiretroviral therapy. AIDS Res. Hum. Retroviruses 20, 227–233 [DOI] [PubMed] [Google Scholar]