IL-12Rβ2 is critical in parenteral and mucosal host resistance to primary Francisella tularensis LVS infection, and controls liver damage.
Keywords: cytokines, bacterial infections, adaptive immunity, vaccines, host-pathogen interactions
Abstract
Using a panel of vaccines that provided different degrees of protection, we previously identified the IL-12 receptor subunit β2 as a mediator, whose relative expression correlated with strength of protection against secondary lethal challenge of vaccinated mice with an intracellular bacterium, the LVS of Francisella tularensis. The present study therefore tested the hypothesis that IL-12Rβ2 is an important mediator in resistance to LVS by directly examining its role during infections. IL-12Rβ2 KO mice were highly susceptible to LVS primary infection, administered i.d. or i.n. The LD50 of LVS infection of KO mice were 2 logs lower than those of WT mice, regardless of route. Five days after infection with LVS, bacterial organ burdens were significantly higher in IL-12Rβ2 KO mice. IL-12Rβ2 KO mice infected with lethal doses of LVS had more severe liver pathology, including significant increases in the liver enzymes ALT and AST. Despite decreased levels of IFN-γ, LVS-vaccinated IL-12Rβ2 KO mice survived large lethal LVS secondary challenge. Consistent with in vivo protection, in vitro intramacrophage LVS growth was well-controlled in cocultures containing WT or IL-12Rβ2 KO LVS-immune splenocytes. Thus, survival of secondary LVS challenge was not strictly dependent on IL-12Rβ2. However, IL-12Rβ2 is important in parenteral and mucosal host resistance to primary LVS infection and in the ability of WT mice to clear LVS infection and serves to restrict liver damage.
Introduction
F. tularensis is a Gram-negative bacterium that causes the zoonotic disease tularemia. F. tularensis natural infections are rare; however, Francisella is considered a potential biological weapon because of its extreme infectivity, ease of intentional dissemination via aerosols, and capacity to cause illness and death. F. tularensis biotypes include subspecies tularensis (Type A), which comprises the most virulent strains studied to date, subspecies holarctica (Type B), from which the LVS was derived, and F. novicida, which is typically not pathogenic in humans [1]. F. tularensis LVS has emerged as a useful model organism to study host responses in animals to F. tularensis and to the broader class of intracellular pathogens [2]. The outcome of LVS infection of inbred mice depends on the route of inoculation, allowing studies of the immunological responses in primary and secondary infections. The i.d. LD50 in C57BL/6 mice is >106 bacteria, whereas the i.p. LD50 is <10 [2], and the i.n. LD50 is ∼103 [3]. Additionally, survival of sublethal i.d. or i.n. doses (<106 or <103, respectively) results in strong immune responses that protect against lethal LVS challenge of >106 i.p. and >107 i.n. [2]. Protection of mice against F. tularensis is T cell-dependent, particularly Th1 T cells, and involves production of IFN-γ [2, 4, 5], TNF-α [2], IL-12 [6], and NO [7].
IL-12 up-regulates expression of its own receptor on activated T, NK, and B cells, increasing IFN-γ production [8]. In addition to its impact on IFN-γ production and Th1 differentiation, IL-12 has several other biological activities, including activation of NK cells and enhancement of cytotoxic activity of CTLs. IL-12 is a heterodimeric (p70) molecule and consists of two subunits, p40 and p35. Previous studies have shown that IL-12 is induced in response to Francisella infection [6, 9]. However, the p40 chain in particular, but not p35 or IL-12p70, plays a critical role in clearance of LVS [6]. IL-12p40 KO mice, as well as mice treated with neutralizing anti-IL-12 antibodies, survived large doses of primary and secondary LVS infection but were unable to clear bacteria and displayed chronic infection [6]. In addition, IL-12Rβ1 signaling contributes to the migration of F. tularensis-activated lung DCs to the draining LN, and production of IL-12p40 by DCs may be an important step in generation of rapid immune responses following F. tularensis infection [10].
The two subunits of the IL-12R include IL-12Rβ1 and IL-12Rβ2, which together confer high-affinity binding of IL-12 and play an essential role in mediating its biological functions [11]. Each subunit participates in the formation of additional receptors; IL-12Rβ1 chain is also a subunit of the IL-23R, and the IL-12Rβ2 chain was identified recently as an IL-35R subunit [12]. IL-12Rβ2 is expressed mainly on T and NK cells [11], but expression has also been identified on DCs [13] and B cells [14]. IL-12Rβ2 expression is up-regulated by IFN-γ and plays an important role in Th1 cell differentiation.
Detailed understanding of the mechanisms by which Th1 T cells protect against intracellular bacteria, such as Francisella, may aid in identifying correlates of immune protection, which is important for vaccine development. Using qualitatively different vaccines, our laboratory recently identified a working panel of mediators that correlated with strength of protection against secondary challenge with LVS [15]. In that study, a panel of F. tularensis vaccine candidates, which induce quantitatively different levels of protection against F. tularensis challenge, was used to vaccinate C57BL/6J mice. Relative gene expression of a number of immune mediators in cells recovered from cocultures containing Francisella-immune lymphocytes with LVS-infected macrophages reflected the strength of in vivo protection [15]. IFN-γ and TNF-α were identified among these mediators, and have previously been shown to be important for host resistance to LVS [5, 16]. IL-12Rβ2 was also identified as a mediator, and was strongly up-regulated to a greater extent in spleens and livers of LVS-immune lymphocytes compared to the less-protective immune lymphocytes. In the current study, we therefore investigate the direct role that IL-12Rβ2 plays in host resistance to F. tularensis.
MATERIALS AND METHODS
Experimental animals
Male C57BL/6J mice and B6.129S1-Il12rb2tm1Jm/J-deficient mice (referred to here as IL-12Rβ2 KO mice) on a C57BL/6J background were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Animals were housed in a barrier environment at the CBER/U.S. Food and Drug Administration and were fed autoclaved food and water ad libitum. Procedures were performed according to approved protocols under Animal Care and Use Committee guidelines. Mice between the ages of 6 and 12 weeks old were used throughout the study, and were age-matched within each experiment.
Bacteria and growth conditions
F. tularensis LVS (ATCC 29,684; American Type Culture Collection, Manassas, VA, USA) was cultured on modified MH agar plates, supplemented with ferric pyrophosphate, glucose, 2.5% FCS, and IsoVitaleX. Active midlog-phase bacteria grown in MH broth were harvested and stored at −70°C; 0.5 ml aliquots were thawed for single use. Bacterial cell viability was measured using the LIVE/DEAD Bac Light Bacterial Viability Kit (Invitrogen, Grand Island, NY, USA), following the manufacturer's protocol. Viable bacteria were quantified by plating serial dilutions on MH agar plates that were incubated for 2–3 days at 37°C in 5% CO2.
In vivo bacterial infections
C57BL/6J and IL-12Rβ2 KO mice were infected with a range of doses of LVS delivered through various routes. Mice were given 0.5 ml i.p., 0.1 ml i.d., or 20 μl i.n. (in one nostril) of sterile, low-endotoxin PBS, containing the indicated CFUs of F. tularensis LVS. For i.n. infections, mice were anesthetized with 0.1 ml of a cocktail of Ketaject ketamine HCl (1.5 mg/0.1 ml; Phoenix Pharmaceuticals, St. Joseph, MO, USA) and AnaSed (0.3 mg/0.1 ml; Lloyd Laboratories, Shenandoah, IA, USA), diluted in sterile PBS and given i.p. Actual doses of inoculated bacteria were determined by plate count. Bacteria were diluted in PBS containing <0.01 ng endotoxin/ml. The numbers of CFUs in the organs of infected mice were determined at various time-points. Mice were killed, and spleens, livers, and lungs were removed aseptically and homogenized in a Stomacher in 5 ml sterile PBS. Appropriate dilutions were plated on MH plates, and the bacterial loads were enumerated 2–3 days later.
Blood and serum chemistry
Blood from male naïve C57BL/6J and LVS-infected C57BL/6J and IL-12Rβ2 KO mice was collected from the femoral artery and heart. Whole blood was collected and diluted in 0.5 mg/ml EDTA to prevent clotting. Sera were also collected from whole blood using Sarstedt serum gel microtubes (Fisher Scientific, Pittsburgh, PA, USA). Whole blood and serum samples were sent to the Department of Laboratory Medicine, Clinical Center, National Institutes of Health in Bethesda, MD, USA, for analyses. Whole blood CBC was performed on a CELL-DYNN 3700 analyzer (Abbott Diagnostics, Abbott Park, IL, USA). Serum chemistry analyses were performed on a Siemens Dimension Vista 1500 analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA); analytes tested in the serum are listed in Table 1.
Table 1. Comparison of Serum Chemistry in LVS-Infected WT and IL-12Rβ2 KO Mice.
| Serum | Units | Naive | WT | IL-12Rβ2 KO |
|---|---|---|---|---|
| Glucose | mg/dl | 333.7 ± 21.1 | 151.0 ± 2.8 | 71 ± 4.6a |
| Cholesterol | Mg/dl | 71.7 ± 8.5 | 37.3 ± 3.7 | 42 ± 3.5 |
| Triglycerides | Mg/dl | 126 ± 21.8 | 54.7 ± 2.9 | 34 ± 8.9 |
| Sodium | mmol/L | 148.3 ± 9.7 | 159.3 ± 0.5 | 161 ± 0 |
| Potassium | mmol/L | 8.3 ± 1.8 | 7.6 ± 0.6 | 6.9 ± 0.2 |
| Chloride | mmol/L | 115 ± 12.7 | 128.7 ± 0.9 | 130 ± 1 |
| Creatinine | mg/dl | <0.1b | <0.1b | <0.1b |
| Blood urea nitrogen | Mg/dl | 24.7 ± 3.2 | 11 ± 1.4 | 11 ± 1.0 |
| Albumin | g/l | 3 ± 0.2 | 1.1 ± 0 | 1.0 ± 0 |
| Calcium | mmol/L | 2.5 ± 0.2 | <1.25b | 1.3 ± 0.04 |
| Magnesium | mmol/L | 1 ± 0.5 | 0.7 ± 0.03 | 0.6 ± 0.05 |
| Phosphorus | mg/dl | >9b | >9b | >9b |
| Alkaline phosphatase | U/L | 129 ± 34.7 | 46 ± 5.7 | 54 ± 18 |
| ALT | U/L | 80 ± 4.2 | 72.3 ± 23.2 | 605 ± 300a |
| AST | U/L | 327 ± 56 | 155 ± 16.3 | 866 ± 116a |
| Amylase | U/L | 917 ± 247 | 462.7 ± 27.7 | 361 ± 23.1 |
| Creatine kinase | U/L | >1000b | >1000b | >1000b |
| Lactate dehydrogenase | U/L | 911 ± 53.7 | 464 ± 54.9 | 764 ± 65.8 |
| Protein (total) | g/l | 5.3 ± 0.4 | 2.4 ± 0.05 | 2.6 ± 0.1 |
| Uric acid | mg/dl | 4.8 ± 0.3 | 3.6 ± 0.2 | 1.3 ± 0.4 |
WT and IL-12Rβ2 KO mice were infected with LVS 105 i.d., a dose that is lethal for KO mice and sublethal for WT mice. Sera were collected on Day 7 following infection and analyzed. Numbers represent the mean ± sd values of four individual mice from two independent experiments.
P ≤ 0.05 by Student's t-test in a comparison between WT and KO.
These data yielded values that were greater than or less than the range measured, as indicated.
Preparation of lymphocytes
Spleens were aseptically removed and transferred to a sterile petri dish with PBS 2% FCS (Hyclone, Logan, CT, USA). The plunger of a 3-ml sterile syringe was used to homogenize the spleens, and the homogenate was passed through 40 μM cell strainers. Cells were washed in PBS 2% FCS and then resuspended in ACK lysing buffer (Invitrogen) for lysis of RBCs. Cells were again washed in PBS 2% FCS, and live cells were enumerated using trypan blue and a hemocytometer. Livers were aseptically removed and transferred to a filter bag containing sterile PBS. Livers were homogenized in a Stomacher for 30 s, transferred to 50-ml tubes containing PBS, and washed three times at room temperature. Following the third wash, cells were resuspended in 40% Percoll (Amersham Pharmacia, Piscataway, NJ, USA) and centrifuged to generate a gradient. The lymphocyte layer was recovered from the gradient, washed in PBS 2% FCS, and then resuspended in ACK lysing buffer for lysis of RBCs. Cells were washed in PBS 2% FCS at 4°C, and live cells were enumerated using trypan blue and a hemocytometer.
Flow cytometry analyses
Single-cell suspensions prepared from spleens or livers were stained for a panel of murine cell-surface markers and analyzed using a Becton Dickinson LSR II flow cytometer (San Jose, CA, USA) and FlowJo software (Tree Star, Ashland, OR, USA), as described previously [15, 17]. Briefly, cells were washed and resuspended in PBS 2% FCS. Nonspecific binding of antibodies was inhibited by blocking FcRs with anti-CD16 (Fc Block; BD Biosciences Pharmingen, Franklin Lakes, NJ, USA) for 10 min on ice. LIVE/DEAD staining was performed using a commercially available reagent and following the manufacturer's protocol (LIVE/DEAD staining kit; Invitrogen). Following LIVE/DEAD staining, cells were washed and stained for cell-surface markers. Antibody concentrations were optimized separately for use in nine-color staining protocols, using appropriate fluorochrome-labeled isotype-matched control antibodies. The following antibodies were used: anti-B220 (clone RA3-6B2), anti-CD19 (clone 1D3), anti-TCR-β (clone H57-597), anti-CD4 (clone RM4-5), anti-CD8α (H35-17.2), anti-NK1.1 (clone PK136), anti-CD11b (clone M1/70), anti-Gr-1 (clone RB6-8C5), and anti-CD11c (cloneHL3), each labeled with a variety of fluorochromes as needed (above antibodies were purchased from BD Biosciences Pharmingen). Side-scatter (width and height) and forward-scatter (width and height) plots were used to gate on singlet events prior to all subsequent analyses. A representative example of the gating scheme applied is shown in Supplemental Fig. 1.
Overlay assay
BMDMs were prepared and cultured as described previously [18, 19]. Briefly, bone marrow was flushed from femurs of mice using complete DMEM, supplemented with 10% heat-inactivated, FBS (Hyclone), 10% L-929 conditioned medium, 0.2 mM l-glutamine, 10 mM HEPES buffer, and 0.1 mM nonessential amino acids. Cells were seeded in 24- or 48-well plates and incubated for 7 days 37°C in 5% CO2. Medium was replaced every 2–3 days. BMDMs were infected with F. tularensis LVS at a multiplicity of infection of 1:20 (bacteria:macrophages). Two hours following infection, medium containing the bacteria was removed and replaced with media containing 100 μg/ml gentamicin. Cells were incubated in the presence of gentamicin for 1 h to kill extracellular bacteria. Following incubation, cells were washed three times with sterile PBS. Single-cell suspensions of lymphocytes derived from vaccinated mice (5×106/ml or as indicated) were added to LVS-infected macrophages. Neutralizing antibodies in a low-endotoxin format were purchased from BD Biosciences Pharmingen and were added to cocultures with the lymphocyte suspensions at a concentration of 25 μg/ml where indicated. At 72 h after infection, supernatants were harvested and stored at −70°C. Adherent, infected macrophages were lysed with sterile water, lysates serially diluted in sterile PBS, and dilutions plated on modified MH plates for bacterial enumeration.
Cytokine measurements
Culture supernatants were assayed for IFN-γ, IL-12p40, IL-12p70, IL-6, IL-4, and TNF-α using standard sandwich ELISAs. Antibody pairs and standards were purchased from BD Biosciences Pharmingen, and assays were performed according to the manufacturer's instructions. The absorbance was read at 405 nm on a VersaMax tunable microplate reader with a reference wavelength of 630 nm. Cytokine levels were measured by comparison with recombinant standard proteins using four-parameter fit regression in the SoftMax Pro ELISA analysis software (Molecular Devices, Sunnyvale, CA, USA).
Characterization of antibody responses
Titers of specific anti-LVS serum antibodies were determined by ELISA as described previously [20]. Briefly, Immulon 1 plates were coated with live LVS, washed, and blocked with 10% calf serum, and sera samples were serially diluted. In each assay, a pool of sera from naïve mice was used as a negative control, and a pool of sera from LVS-hyperimmune mice was used as a positive control. HRP-labeled antibodies (anti-IgM; anti-IgG that detects IgG1, IgG2c, and IgG3; or subtype-specific IgG2c and subtype IgG1; SouthernBiotech, Birmingham, AL, USA) were added, and ABTS peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA) was used for color development. The end-point titer was defined as the lowest dilution of serum that gave an OD of 405 nm greater than the OD + 3 sd of the mean added to the OD value of the matched dilution of normal, prebleed mouse serum, and also >0.025.
Histopathology
Tissue sections from livers, lungs, and spleens were fixed with 10% buffered formalin. Samples were then sent to American Histolabs (Gaithersburg, MD, USA), where the tissues were embedded in paraffin and sectioned for slides and the slides stained with H&E. Pathology slides were analyzed by a board-certified pathologist in a blinded fashion.
Statistical analyses
All analyses, using Student's t-test or log-rank test for survival curves, were performed using InStat software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Characterization of primary F. tularensis LVS infection in IL-12Rβ2 KO mice
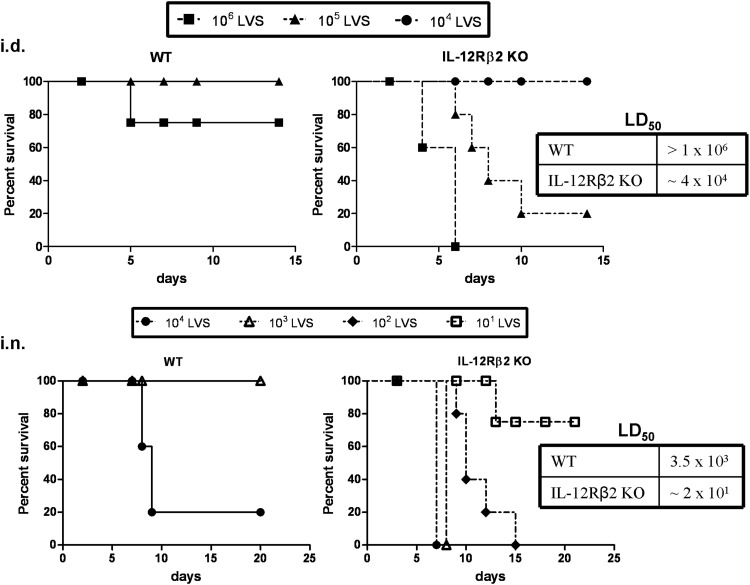
To examine the role of the IL-12Rβ2 in host resistance to primary F. tularensis LVS infection, IL-12Rβ2 KO mice were inoculated with various doses of LVS via the i.d. or i.n. routes. IL-12Rβ2 KO mice were considerably more susceptible to F. tularensis LVS compared to WT mice, regardless of the route of infection (Fig. 1). IL-12Rβ2 KO mice succumbed to i.d. infection between Days 4 and 10 when administered 106 or 105 LVS, whereas all but one WT mice survived these doses. The calculated LD50 following i.d. infection for the IL-12Rβ2 KO mice was ∼4 × 104, nearly two logs less than that of WT mice (>1×106; Fig. 1, upper panels). IL-12Rβ2 KO mice also exhibited increased susceptibility to i.n. infection (Fig. 1, lower panels). IL-12Rβ2 KO mice succumbed to i.n. infection between Days 6 and 15 when administered 101–104 LVS. The IL-12Rβ2 KO mice i.n. LD50 (∼2×101 LVS) was approximately two logs less than that of the WT mice (3.5×103). In both experimental scenarios, the remaining WT and IL-12Rβ2 KO mice survived for over 30 days after infection.
Figure 1. Susceptibility of IL-12Rβ2 KO mice to F. tularensis LVS infection.
Groups of five WT or IL-12Rβ2 KO mice were given 106, 105, or 104 F. tularensis LVS i.d. (upper panels) or 104, 103, 102, or 101 F. tularensis LVS i.n. (lower panels). The dose of infection was confirmed by simultaneous plate counts. The LD50s were calculated for WT and IL-12Rβ2 KO following i.d. or i.n. infections and are displayed in the table next to the graphs. These data are representative of four experiments (i.d.) or three experiments (i.n.) of similar design, in which additional doses of 104 LVS i.d. and 101 and 102 LVS doses i.n. were tested in WT mice, but are not displayed here for clarity. The results are expressed as Kaplan-Meier survival curves, and the data were analyzed using the log-rank test. The median time to death was significantly longer in WT mice infected with LVS 106 i.d. (P≤0.01), LVS 105 i.d. (P≤0.05), LVS 103 i.n. (P≤0.01), and LVS 102 i.n. (P≤0.01) compared with IL-12Rβ2 KO mice.
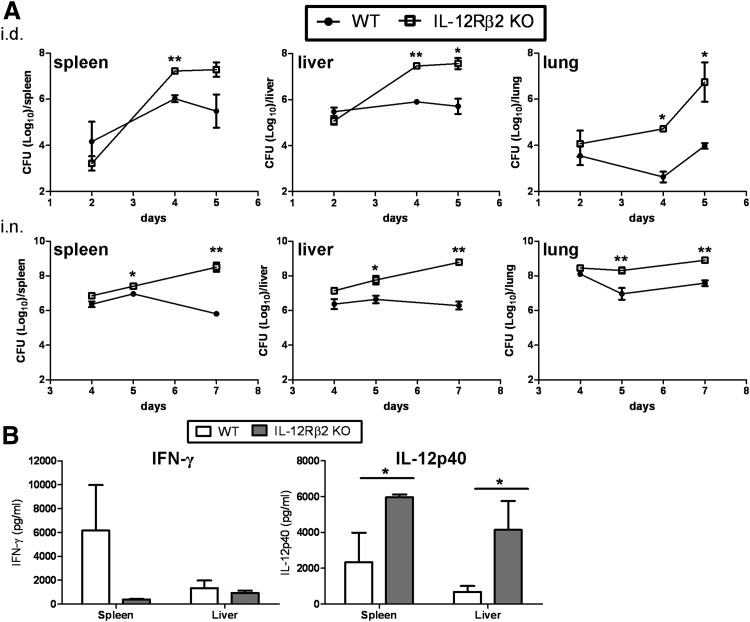
Next, we sought to determine if the cause of death in the IL-12Rβ2 KO mice was related to uncontrolled bacterial growth. Bacterial burdens were measured in the spleens, livers, and lungs of WT and IL-12Rβ2 KO mice infected with F. tularensis LVS. Mice were infected i.d. with 105 LVS, a dose that is lethal for IL-12Rβ2 KO mice but sublethal for WT mice. By Days 4–5 after infection, IL-12Rβ2 KO mice exhibited significant increases in bacterial burden in all three organs examined; increases of two to three log CFUs of LVS were observed in IL-12Rβ2 KO mice compared to WT (Fig. 2A, upper panels). Similarly, when WT and IL-12Rβ2 KO mice were infected i.n. with 103 LVS (a lethal dose for the IL-12Rβ2 KO), we observed increased bacterial burdens following i.n. infection. IL-12Rβ2 KO mice exhibited significant increases in bacterial organ burden by Day 5 after infection when compared with WT mice (Fig. 2A, lower panels). In addition, cytokine levels were measured in the spleens and livers homogenates on Day 5 after i.d. infection. IFN-γ levels were reduced substantially in the spleens of the IL-12Rβ2 KO mice compared with WT mice (Fig. 2B), although a large range was observed in LVS-infected WT mice, and differences were not consistently significant (here, P=0.0579). IFN-γ levels were lower in the WT and IL-12Rβ2 KO liver samples compared with spleens. Levels of IL-12p40 were elevated in the IL-12Rβ2 KO mice, especially in the livers (Fig. 2B). These results indicate that IL-12Rβ2 KO mice are more susceptible to LVS infection by either route compared to their WT counterparts, and exhibit reduced IFN-γ production but increased levels of IL-12p40.
Figure 2. Bacterial organ burden levels in WT and IL-12Rβ2 KO mice following primary lethal F. tularensis LVS infection.
(A) WT and IL-12Rβ2 KO mice were infected with 105 F. tularensis LVS i.d. (upper panels) or 103 i.n. (lower panels), doses that are sublethal for WT mice but lethal for KO mice. Three mice/group were sacrificed at Days 2, 4, and 5 following i.d. infection or Days 4, 5, and 7 following i.n. infection, and total CFU/organ were determined. The dose of infection was confirmed by simultaneous plate counts. These data are representative of three independent experiments for i.d. and i.n. of similar design. (B) IFN-γ and IL-12p40 levels were measured on Day 5 after infection in the spleens and livers of WT and IL-12Rβ2 KO mice following i.d. LVS infection. These data are representative of two independent experiments. *P ≤ 0.05; **P ≤ 0.01 by Student's t-test in a pair-wise comparison between samples from WT and KO mice.
Previous studies have shown that WT mice clear i.d. or i.n. LVS infection between 14 and 21 days, depending on dose, but IL-12p40 KO mice are unable to resolve LVS infection for months [6]. Therefore, we examined the ability of IL-12Rβ2 KO mice that survived sublethal, primary LVS infection to clear bacteria over the long term. Despite a notable increase in the size of spleens from LVS-infected IL-12Rβ2 KO mice compared with those from WT mice (∼24 mm and ∼16 mm, respectively), ∼40 days after sublethal i.d. LVS infection, minimal numbers of bacteria were detected in the organs of IL-12Rβ2 KO mice (data not shown). Thus, loss of IL-12Rβ2 does not lead to persistent infection of mice, which was seen in the IL12p40 KO mice.
LVS-infected IL-12Rβ2 KO mice exhibit differential cell recruitment and enhanced liver damage
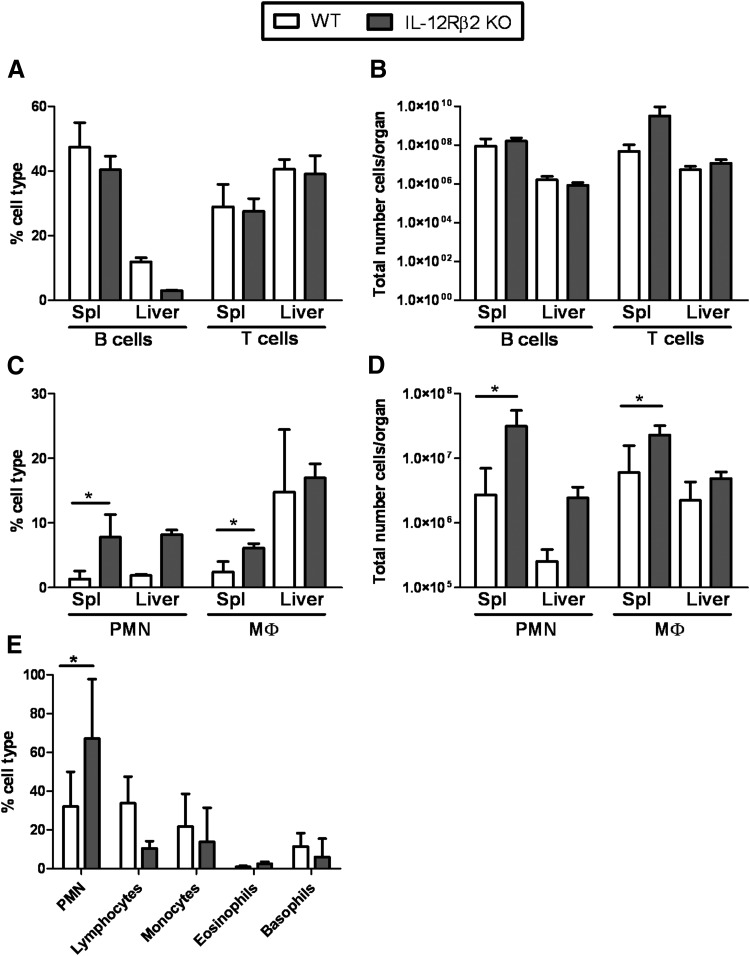
Because IL-12Rβ2 KO mice failed to control bacterial burdens, we next examined the composition of immune cells in tissues during the early phase of active infection. WT and IL-12Rβ2 KO mice were infected with 105 LVS i.d., and spleen and liver lymphocytes were isolated and analyzed by multiparameter flow cytometry. No significant differences were noted between any cell populations on Day 3 (data not shown). The total numbers of B cells and T cells were similar between spleens and livers of LVS-infected WT and IL-12Rβ2 KO mice on Days 3 (data not shown) and 7 after infection (Fig. 3B); however, the proportion of B cells in the livers of LVS-infected IL-12Rβ2 KO mice was decreased compared with LVS-infected WT mice (Fig. 3A). However, the proportions and total cell numbers of neutrophils (identified as CD45+TCR-β−CD19−CD11b+Gr1hi cells) present in the spleens and livers of LVS-infected IL-12Rβ2 KO mice on Day 7 were substantially greater than that of LVS-infected WT mice (Fig. 3C and D). In addition, there were increased levels of macrophages (identified as CD45+TCR-β−CD19−CD11b+Gr1−) in the spleens of LVS-infected IL-12Rβ2 KO mice compared with LVS-infected WT mice (Fig. 3C and D). No differences in the proportions or total numbers of DCs (identified as CD45+TCR-β−CD19−CD11c+) or NK cells (identified as CD45+TCR-β−CD19−NK1.1+) were noted between the two groups at either time-point (data not shown).
Figure 3. Cell recruitment to the spleens and livers of WT and IL-12Rβ2 KO following i.d. LVS infection.
WT and IL-12Rβ2 KO mice were infected with 105 LVS i.d. on Day 7 following infection. Spleen (Spl) and liver cells were harvested and assessed by flow cytometry for (A and B) B cells (CD45+B220+CD19+) and T cells (CD45+B220−TCR-β+) and (C and D) neutrophils (PMN; CD45+CD19−TCR-β−CD11b+Gr1hi cells) and macrophages (Mϕ; CD45+CD19−TCR-β−CD11b+Gr1−). Values shown are the mean percentages of cell type ± sd of three independent experiments (for spleens) and two independent experiments (for livers). Each individual experiment was performed with three mice/group. (E) Sera were obtained from mice infected with 105 LVS on Day 7 and analyzed for CBC. CBC data are comprised of a combination of four individual mice from two independent experiments. *P ≤ 0.05 by Student's t-test in a pair-wise comparison of samples from WT and KO mice.
In addition to cellular analysis in the organs, blood from WT and IL-12Rβ2 KO mice infected with 105 LVS i.d. was analyzed by a traditional clinical CBC on Day 7 after infection (Fig. 3E). Both groups had similar proportions of monocytes, eosinophils, and basophils in the blood; the proportion of lymphocytes was decreased slightly in the blood of KO mice compared with WT mice, although this difference was not significant (P>0.05). Similar to the livers and spleens, IL-12Rβ2 KO mice had a significantly higher percentage of polymorphonuclear leukocytes in the blood compared with WT mice, under circumstances in which the total number of cells/μl was similar between WT and IL-12Rβ2 KO mice.
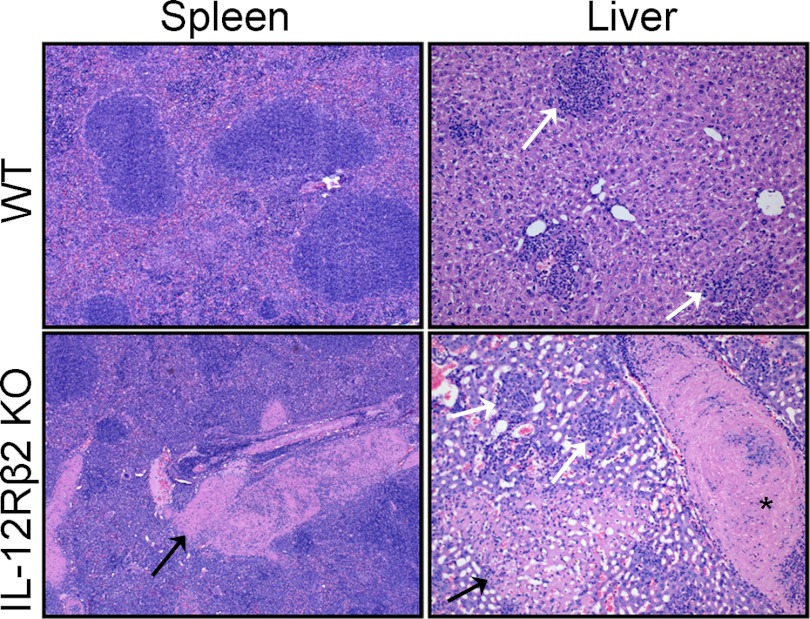
To further assess the effects of differential bacterial burdens and cell recruitment between WT and IL-12Rβ2 KO mice, tissue pathology was examined. WT and IL-12Rβ2 KO mice were infected with 105 LVS i.d., a dose that was lethal for IL-12Rβ2 KO mice but sublethal for WT mice. Lungs, livers, and spleens were obtained at Days 2 and 7 after infection, and pathology was analyzed. There were no obvious differences between the tissues from LVS-infected WT and IL-12Rβ2 KO mice on Day 2 after infection (data not shown). However, by Day 7, there was substantial tissue damage to the spleens and the livers of LVS-infected IL-12Rβ2 KO mice compared to WT mice (Fig. 4). LVS-infected IL-12Rβ2 KO spleens had multiple large vein thrombi and signs of mild multifocal splenitis, whereas the LVS-infected WT spleen appeared less affected and did not exhibit thrombosis. IL-12Rβ2 KO LVS-infected livers exhibited severe multifocal hepatocellular necrosis, also associated with vascular thrombosis and granulomatous inflammation. Pathology in liver sections from LVS-infected WT mice was less severe than that of IL-12Rβ2 KO mice; however, WT liver sections exhibited multifocal aggregates of macrophages and lymphocytes. The histology of the lungs of the LVS-infected WT and the IL-12Rβ2 KO appeared normal at this time-point (data not shown).
Figure 4. Representative organ sections from WT and IL-12Rβ2 KO mice.
WT and IL-12Rβ2 KO mice were infected with 105 LVS i.d. Spleens and livers were removed from two to three mice/group on Day 7 after infection, preserved in 10% formalin, embedded in paraffin, sectioned for slides, and stained with H&E. Black arrows indicate areas of necrosis; white arrows show granulomatous inflammation. *Large thrombus in hepatic central vein. These data are representative of two independent experiments of similar design.
To assess further the extent of liver damage, blood from 105 i.d. LVS-infected WT and IL-12Rβ2 KO mice was collected, and sera were obtained for chemistry analyses. Analytes tested in the serum are listed in Table 1; values obtained from sera from naïve C57BL/6J mice are shown for comparison. The most notable differences between sera from LVS-infected WT mice compared with LVS-infected IL-12Rβ2 KO mice were the significant increases of the liver enzymes ALT and AST, which were over three times higher in the LVS-infected IL-12Rβ2 KO mice compared with WT mice. Taken together, these results suggest that loss of IL-12Rβ2 coupled with LVS infection leads to increased liver damage, which may play a role in the inability of these mice to survive larger dose of LVS during primary infection.
Characterization of the role of IL-12Rβ2 in secondary immunity against F. tularensis LVS infection
Our laboratory recently identified IL-12Rβ2 as an immune mediator that was up-regulated to a greater extent in spleens and livers of LVS-immune lymphocytes compared with immune lymphocytes from mice vaccinated with the less-protective preparations [15]. Therefore, we examined the potential role of IL-12Rβ2 during secondary adaptive immune responses to LVS. WT and IL-12Rβ2 KO mice were vaccinated with 103 LVS i.d. or 101 i.n., challenged 6 weeks later with 2 × 106 i.p. or 5 × 107 i.n., and survival was monitored for 30 days. The results are summarized in Table 2. All naïve mice succumbed to i.p. challenge by Day 4, whereas all of the vaccinated WT and IL-12Rβ2 KO mice survived secondary i.p. challenge. We also examined the ability of vaccinated mice to survive i.n. secondary challenge. As shown in Table 2, vaccinated WT and IL-12Rβ2 KO mice challenged with 5 × 107 LVS i.n. ∼6 weeks following primary infection all survived, with the exception of one WT mouse, whereas naïve WT mice all succumbed to i.n. infection by Day 7. Thus, WT and IL-12Rβ2 KO mice, vaccinated through the i.d. route or i.n. route, all exhibited comparable survival after i.p or i.n. secondary challenge. Thus, survival of secondary challenge is not strictly dependent on IL-12Rβ2.
Table 2. Survival of Secondary LVS Challenge by WT and IL-12Rβ2 KO Mice.
| Mouse strain (C57BL/6J) | Primary LVS dose, Day 0 | Secondary challenge, Day 45 | Number of deaths/total | Mean time to death (days) |
|---|---|---|---|---|
| WT | PBS | 2 × 106 i.p. | 5/5 | 4 |
| WT | 1 × 103 i.d. | 2 × 106 i.p. | 0/5 | n/a |
| IL-12Rβ2 KO | 1 × 103 i.d. | 2 × 106 i.p. | 0/5 | n/a |
| WT | 1 × 101 i.n. | 2 × 106 i.p. | 0/5 | n/a |
| IL-12Rβ2 KO | 1 × 101 i.n. | 2 × 106 i.p. | 0/5 | n/a |
| WT | PBS | 5 × 107 i.n. | 5/5 | 7 |
| WT | 1 × 103 i.d. | 5 × 107 i.n. | 1/5 | 7 |
| IL-12Rβ2 KO | 1 × 103 i.d. | 5 × 107 i.n. | 0/5 | n/a |
| WT | 1 × 101 i.n. | 5 × 107 i.n. | 0/5 | n/a |
| IL-12Rβ2 KO | 1 × 101 i.n. | 5 × 107 i.n. | 0/5 | n/a |
WT and IL-12Rβ2 KO mice were vaccinated with indicated doses i.d. or i.n., followed by i.p. or i.n. secondary challenge. Actual vaccination and challenge doses were confirmed by simultaneous plate count. These data are representative of two independent experiments for i.p. and i.n. challenge. n/a, Not applicable.
Previous studies from our laboratory showed elevated serum antibody levels in C57BL/6 mice lacking the p40 subunit of IL-12 3 weeks after i.d. vaccination. Therefore, we examined the quantities of anti-LVS antibodies following vaccination of WT and IL-12Rβ2 KO mice. Approximately 3 weeks after sublethal i.d. or i.n. LVS infection, sera from LVS-vaccinated WT mice contained moderate amounts of IgM and IgG anti-LVS antibodies. However, there were substantially increased levels of anti-LVS total IgG, and the IgG2c and IgG1 subclasses, in all LVS-vaccinated IL-12Rβ2 KO mice when compared with LVS-vaccinated WT mice (Table 3). Infection via the i.d. route also led to increased levels of IgM in LVS-infected IL-12Rβ2 KO mice compared with WT mice.
Table 3. Comparison of Serum Antibody Levels following LVS Infection of WT or IL-12Rβ2 KO Mice.
| Mouse strain | Route | LVS dose | Antibody titer |
|||
|---|---|---|---|---|---|---|
| IgM | Total IgG | IgG1 | IgG2c | |||
| WT | i.d. | 1 × 103 | 1:1280 | 1:2560 | <1:20 | 1:2560 |
| IL-12Rβ2 KO | i.d. | 1 × 103 | 1:5120 | 1:10,240 | 1:5120 | 1:5120 |
| WT | i.n. | 1 × 101 | 1:1280 | 1:2560 | <1:20 | 1:1280 |
| IL-12Rβ2 KO | i.n. | 1 × 101 | 1:320 | 1:10,240 | 1:2560 | 1:5120 |
WT and IL-12Rβ2 KO mice were infected i.d. with 103 LVS or i.n. with 101 LVS. Sera were collected 32 days after infection, and end-point titers of specific anti-LVS antibodies were determined by ELISA.
Control of F. tularensis LVS replication in macrophages does not require IL-12Rβ2
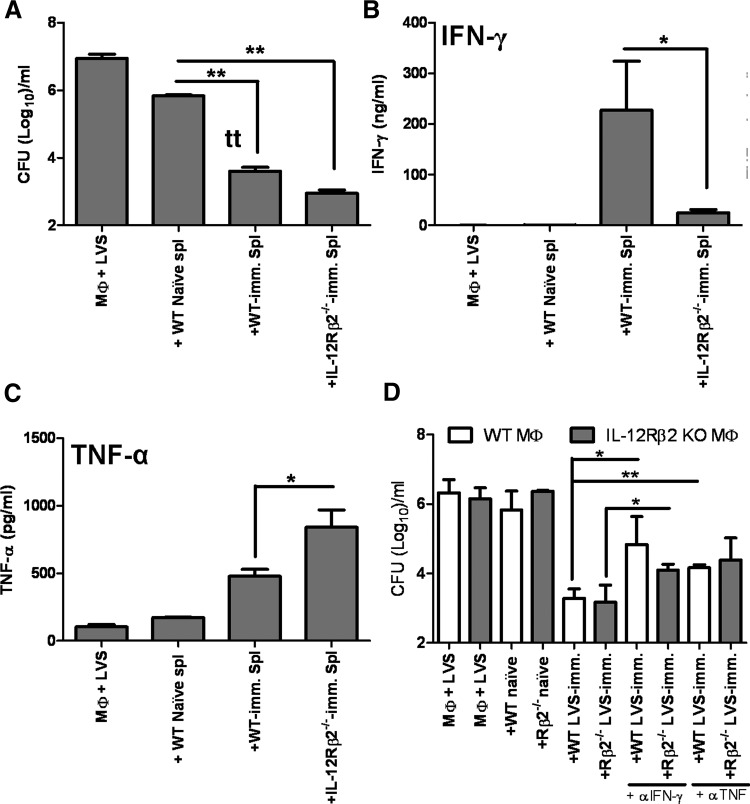
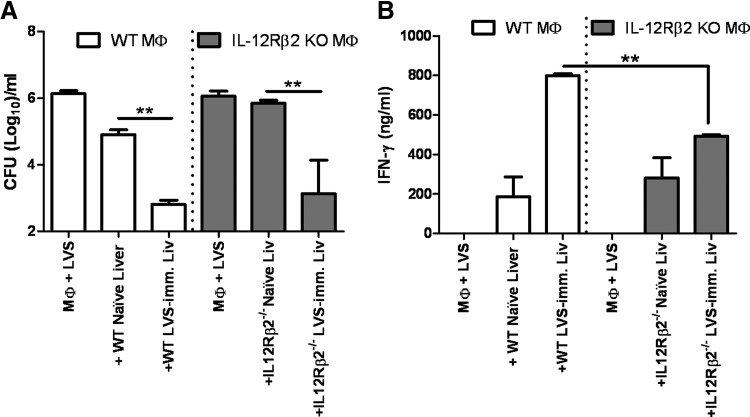
We next investigated the role of the IL-12Rβ2 in engendering LVS-immune T lymphocytes capable of controlling intramacrophage growth of F. tularensis LVS. WT and IL-12Rβ2 KO mice were vaccinated with sublethal doses of LVS, and at ∼6 weeks after infection splenocytes were isolated. The resulting splenocytes were cocultured with LVS-infected WT BMDM, and control of bacterial replication was monitored 72 h after infection. As shown in Fig. 5A, addition of splenocytes obtained from WT LVS-immune mice or IL-12Rβ2 KO LVS-immune mice to LVS-infected WT macrophages resulted in significant control of bacterial growth when compared to cultures containing naïve splenocytes or without splenocytes. We examined the production of various cytokines secreted in the supernatants of these cocultures, including IFN-γ, TNF-α, IL-6, IL-4, and IL-12p40. LVS-infected macrophages alone or LVS-infected macrophages that were cocultured with splenocytes from naïve mice produced no IFN-γ (Fig. 5B) and low amounts of TNF-α (Fig. 5C). Larger amounts of IFN-γ and TNF-α were generated when WT LVS-immune splenocytes were added to LVS-infected WT macrophages. In contrast, minimal amounts of IFN-γ were produced in cocultures containing IL-12Rβ2 KO LVS-immune splenocytes, whereas significant, increased amounts of TNF-α were produced in these wells (Fig. 5B and C). No significant differences were noted in levels of IL-6, IL-4, or IL-12p40 (data not shown).
Figure 5. Control of F. tularensis LVS intracellular replication by LVS-immune lymphocytes.
BMDMs from WT mice were infected with F. tularensis LVS, and splenocytes from WT naïve mice or the indicated WT or IL-12Rβ2 KO LVS-immune (imm.) mice were added. Immune mice were primed with 103 LVS i.d. At 72 h after initiation of cocultures, (A) Macrophage lysates plated to enumerate bacteria, and supernatants were removed and assayed for levels of (B) IFN-γ and (C) TNF-α. Results shown are the mean CFU/ml ± sd of triplicate wells or mean ng/ml or pg/ml cytokine ± sd, and are representative of four independent experiments. (D) BMDMs derived from WT or IL-12Rβ2 KO mice were infected with F. tularensis LVS and cocultured with indicated splenocytes, and murine IFN-γ- or TNF-α-neutralizing antibodies (αIFN-γ and αTNF; 25 μg/ml) were added to the indicated wells. Bacterial growth was assessed 72 h after infection. Results shown are the mean CFU/ml ± sd of triplicate wells of one experiment and are representative of two independent experiments. *P ≤ 0.05; **P ≤ 0.01 by Student's t-test in a pair-wise comparison of samples from WT and KO mice.
To directly examine the roles that IFN-γ and TNF-α played in cocultures, we added IFN-γ-neutralizing antibodies or TNF-α-neutralizing antibodies to wells in the presence of LVS-immune splenocytes (Fig. 5D). To eliminate contributions from IL-12-driven cytokine production, IL-12Rβ2 KO splenocytes were cocultured with IL-12Rβ2 KO BMDM for this series of experiments. Neutralization of IFN-γ significantly, but not completely, reversed the ability of WT LVS-immune splenocytes and IL-12Rβ2 KO LVS-immune splenocytes to control LVS intramacrophage growth. Thus, even the low levels of IFN-γ produced in the IL-12Rβ2 KO LVS-immune splenocyte cocultures impacted the control of bacterial growth. Neutralization of TNF-α also impacted LVS intramacrophage growth control in the presence of both types of LVS-immune splenocytes, although this difference was not statistically significant in the LVS-immune IL-12Rβ2 KO wells.
Because of the extent of liver damage in the IL-12Rβ2 KO mice following primary infection, we examined the ability of WT and IL-12Rβ2 KO liver lymphocytes to control bacterial replication. As shown in Fig. 6A, naïve liver lymphocytes had minimal impact on intramacrophage LVS replication, whereas liver lymphocytes obtained from WT LVS-immune mice or IL-12Rβ2 KO LVS-immune mice were equally able to control LVS growth when compared with each other. IL-12Rβ2 KO LVS-immune liver lymphocytes produced significantly less IFN-γ compared with WT controls, but levels of IFN-γ produced were still substantial (Fig. 6B). No significant differences were noted in levels of TNF-α, IL-6, or IL-12p40 (data not shown). Taken together, these data suggest that despite the high levels of liver damage following primary infection, IL-12Rβ2 KO mice that are able to survive low-dose primary infection have the ability to mount a sufficient immune response to defend against secondary LVS challenge.
Figure 6. Control of F. tularensis LVS intracellular replication by LVS-immune liver lymphocytes.
BMDMs derived from WT or IL-12Rβ2 KO mice were infected with F. tularensis LVS. Liver lymphocytes from naïve mice or from LVS-immune mice that were primed with 103 LVS i.d. were added. (A) Bacterial growth and (B) IFN-γ levels were assessed 72 h after infection. Results shown are the mean CFU/ml ± sd or ng/ml ± sd of triplicate wells in one experiment and are representative of two independent experiments. **P ≤ 0.01 by Student's t-test in a pair-wise comparison of samples from WT and KO mice.
DISCUSSION
Prompted by findings suggesting that differential expression of IL-12Rβ2 may correlate with strength of vaccine-induced protection, in this study we investigated its role in the development of innate and adaptive immune responses against F. tularensis LVS infection. We found that IL-12Rβ2 is important for in vivo control of primary bacterial infection and host survival for two routes of infection (i.d. or i.n.). LVS-infected IL-12Rβ2 KO mice exhibited increased neutrophil migration to infected organs and extensive liver damage, which likely was the cause of increased susceptibility to LVS primary infection. Previous data from our laboratory showed that IL-12p40 KO mice were chronically infected [6]; although low numbers of bacteria were detected in the organs of the IL-12Rβ2 KO over 1 month after infection, there were obvious differences between the phenotypes of LVS infection in IL-12p40 KO mice and IL-12Rβ2 KO mice. IL-12p40 KO mice did not die acutely at high doses of infection, but were chronically infected and exhibited higher bacterial burdens in all organs. This suggests different mechanisms of clearance between the two types of KO mice. Thus the mechanisms provided by each mediator may overlap, but have independent contributions as well. The role of IL-35 is an obvious area of future study.
IL-12 is an essential cytokine in the differentiation of Th cells into Th1 cells, resulting in the development of Th1 CD4+ T cell responses. Consistent with its role in Th1 T cell development, IL-12 has important roles in a variety of intracellular bacterial infections, including Listeria monocytogenes [21], Brucella aborus [22, 23], and Mycobacterium tuberculosis infection [24, 25]. IL-12 consists of two subunits, p40 and p35, which are linked covalently by disulfide bonds to give rise to a heterodimeric (p70) molecule. The IL-12p70 cytokine is considered the biologically active protein, although studies also suggest that p40 monomer and/or homodimer produced in excess of that used to form p70 can block IL-12 binding to its receptor, as well as having positive biological activity [26]. The p40 subunit can also form a heterodimer with the p19 subunit to form IL-23. Coexpression of the β1 and β2 subunits of IL-12R is required for the generation of high-affinity IL-12 binding sites. IL-12p40 is thought to interact with IL-12Rβ1, whereas IL-12p35 interacts mainly with the IL-12Rβ2 subunit [27]. The IL-12Rβ2 subunit is the signal-transducing chain of the receptor and acts through phosphorylation of STAT4, which is critical for CD4+ Th1 T cell development; thus, in many circumstances, STAT4 KO mice have phenotypes identical to IL-12p40 KO mice [28, 29]. Cells from uninfected IL-12Rβ2 KO mice have reduced NK cell activity, IFN-γ production and lack STAT4 phosphorylation and IL-12Rβ2 KO mice have increased circulating IL-12 (p70) levels [30]. The other known activators of STAT4 in T cells, in addition to IL-12, are IL-23 and type I IFN [31]; IL-2 can also activate STAT4 in NK cells but not T cells [32]. The inherent defects in IL-12Rβ2 KO mice do not appear to directly explain their susceptibility to LVS infection. However, IFN-γ production and presumably Th1 T cell development was reduced but not absent following LVS infection, suggesting alternative means of STAT4 activation play a critical role in the response against intracellular pathogens, including F. tularensis.
Although there is substantial literature indicating the importance of IL-12 on the outcome of bacterial infections, there are few reports regarding the role of IL-12Rβ2 in similar models. One study showed that IL-12Rβ2 did not have an impact on VSV infection, as IL-12Rβ2 and IL-12Rβ1 KO mice survived VSV infection, despite the fact that IL-12 has been shown to help improve recovery following murine VSV infection [33]. Recently, absence of IL-12Rβ2 was reported to prevent the onset of experimental cerebral malaria, although mice deficient for IL-12p40 or IL-12p35 developed disease [34]. Resistance of IL-12Rβ2 KO mice to experimental cerebral malaria was attributed to reduced recruitment of activated T cells and decreased levels of lymphotoxin-α, TNF-α, and IFN-γ, rather than IL-12 binding per se. Another study examined IL-12Rβ2 and FoxP3 mRNA, and protein expression, in individuals with various stages of Lyme borreliosis [35]. The study observed that patients who displayed lower protein levels of Borrelia-specific IL-12Rβ2 on CD8+ cells also had a lower number of Borrelia-specific IFN-γ-secreting cells, suggesting that a substantial Th1 response is necessary for a positive outcome to Borrelia infection. These patients also had higher Borrelia-specific FoxP3 mRNA levels compared with healthy controls. With the exception of the Borrelia study, the other studies indicate that IL-12Rβ2 is dispensable for survival, or loss of IL-12Rβ2 actually benefits the host. These models are in contrast to the substantial role of IL-12Rβ2 in controlling F. tularensis LVS infection.
Previous studies from our laboratory suggested that differential expression of IL-12Rβ2 in splenocytes from Francisella-vaccinated mice correlated with increased protection against LVS lethal challenge [15]. Despite this association, LVS-immune IL-12Rβ2 KO mice survived secondary lethal LVS challenge. Although IFN-γ levels were decreased in the tissues or immune splenocytes from IL-12Rβ2 KO mice (Figs. 2 and 6), immune responses to LVS do not appear to be fully polarized toward a Th2 phenotype; levels of IL-4 or IL-5 were not different between IL-12Rβ2 KO and WT (data not shown). As shown in Table 3, large amounts IgG, IgG2c, and IgG1 anti-LVS antibodies were produced following sublethal LVS infection of the IL-12Rβ2 KO mice when compared to WT mice. These data are reminiscent of data from IL-12p40 KO LVS-infected mice [6]; in both cases, small amounts of IFN-γ are accompanied by production of large amounts of IgG2c anti-LVS antibodies. The production of anti-LVS antibodies of the IgG1 subclass may reflect some engagement of Th2 T cells, despite minimal shifts in cytokine production. It has been established previously that B cells and specific antibodies play important roles in immunity against F. tularensis [36, 37]. The observed increases in antibody levels may compensate for a partially impaired Th1 cell response. A 2005 study followed uninfected IL-12Rβ2 KO mice over time; although IL-12Rβ2 KO mice survived as long as WT mice, IL-12Rβ2 KO mice developed an autoimmune/lymphoproliferative disorder between 12 and 15 months of age that was characterized by immune-complex glomerulonephritis with mesangial deposition of IgG, increased levels of IL-6, and lymphoid infiltrates in the liver, kidney, and salivary glands [38]. Immune-complex glomerulonephritis has not been reported in IFN-γ KO mice, suggesting that this condition is independent of decreased IFN-γ levels in the IL-12Rβ2 KO mice. Thus, B cell hyperactivity in IL-12Rβ2 KO mice may contribute to the observed increases in anti-LVS antibodies.
IL-12Rβ2 KO mice have the ability to survive secondary LVS lethal challenge. Decreased IFN-γ production in the IL-12Rβ2 KO mice was accompanied by substantial increases in TNF-α, which may provide control in the face of decreased IFN-γ levels. In addition, neutrophils can accumulate in the liver in response to inflammatory cytokines, such as TNF-α [39]. Increased TNF-α production in IL-12Rβ2 KO mice may contribute to the increased number of neutrophils in the blood, spleens, and livers following lethal primary LVS infection. Neutrophils are recruited in response to acute liver damage, which themselves can cause various degrees of tissue damage and eventual liver failure [39].
Here, primary lethal LVS infection of IL-12Rβ2 KO mice was accompanied by substantial increases in the levels of liver enzymes ALT and AST. These enzymes are normally contained within hepatocytes but are released when the liver is damaged, resulting in elevated levels of the enzyme in the blood. The observed increases are consistent with the extensive damage to IL-12Rβ2 KO livers following primary LVS infection. A number of studies have demonstrated a correlation between increased levels of IL-12 and liver damage. One such study used a coinfection model with Schistosoma mansoni and Toxoplasma gondii, in which double-infected WT mice exhibited severe liver damage that was less severe in double-infected IL-12 KO mice [40]. Here, we observed substantial increases in the levels of IL-12p40 in the livers of the IL-12Rβ2 KO mice (Fig. 2B). Whether an excess level of IL-12 enhances liver damage has not been determined. In addition to aberrant B cell proliferation, aged IL-12Rβ2 KO mice manifested kidney and liver amyloidosis [38], indicating that IL-12Rβ2 plays a role in preventing liver pathology. Such observations, together with the data from the current study, suggest that infection may enhance this condition at an earlier time. Taken together, the results presented here show that survival of secondary LVS challenge was not strictly dependent on IL-12Rβ2, but IL-12Rβ2 plays an important role in defending against primary LVS infection and the ability of C57BL/6 mice to clear LVS infection by limiting liver damage. Future studies will continue to examine roles of this molecule other than engagement via IL-12 binding and the use of its differential expression as a predictive correlate.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by an interagency agreement with the U. S. National Institute of Allergy and Infectious Diseases (Y1-AI-6153-01/224-06-1322). This project was supported, in part, by an appointment to the Research Participation Program at the CBER, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
We are grateful to our CBER colleagues, Dr. Roberto De Pascalis, Sherry Kurtz, Dr. Jonathan Swoboda, Dr. Siobhán Cowley, Dr. Kris Kolibab, as well as Dr. Wendy Watford of the University of Georgia, for thoughtful and comprehensive reviews of the manuscript.
SEE CORRESPONDING EDITORIAL ON PAGE 641
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ACK
- ammonium-chloride-potassium
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- BMDM
- bone marrow-derived macrophage
- CBC
- complete blood count
- CBER
- Center for Biologics Evaluation and Research
- ECM
- experimental cerebral malaria
- FoxP3
- forkhead box P3
- i.n.
- intranasal
- KO
- knockout (deficient)
- LVS
- live vaccine strain
- MH
- Muller-Hinton
- RBC
- red blood cell
AUTHORSHIP
A.A.M. and K.L.E. provided the study's conception, design, and performance. O.F. performed experiments.
DISCLOSURES
The authors have declared that no competing interests exist.
REFERENCES
- 1. Sjöstedt A. (2004) Family XVII. Francisellaceae. In Bergey's Manual of Systematic Bacteriology (Brenner D. J., ed.), Springer, New York, NY, USA, 200–210 [Google Scholar]
- 2. Elkins K. L., Cowley S. C., Bosio C. M. (2003) Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5, 132–142 [DOI] [PubMed] [Google Scholar]
- 3. Metzger D. W., Bakshi C. S., Kirimanjeswara G. (2007) Mucosal immunopathogenesis of Francisella tularensis. Ann. N. Y. Acad. Sci. 1105, 266–283 [DOI] [PubMed] [Google Scholar]
- 4. Anthony L. S. D., Ghadirian E., Nestel F. P., Kongshavn P. A. L. (1989) The requirement for γ interferon in resistance of mice to experimental tularemia. Microb. Pathog. 7, 421–428 [DOI] [PubMed] [Google Scholar]
- 5. Elkins K. L., Colombini S. M., Meierovics A. I., Chu M. C., Chou A. Y., Cowley S. C. (2010) Survival of secondary lethal systemic Francisella LVS challenge depends largely on interferon γ. Microbes Infect. 12, 28–36 [DOI] [PubMed] [Google Scholar]
- 6. Elkins K. L., Cooper A., Colombini S. M., Cowley S. C., Kieffer T. L. (2002) In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12. p70. Infect. Immun. 70, 1936–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindgren H., Stenmark S., Chen W., Tarnvik A., Sjostedt A. (2004) Distinct roles of reactive nitrogen and oxygen species to control infection with the facultative intracellular bacterium Francisella tularensis. Infect. Immun. 72, 7172–7182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sinigaglia F., D'Ambrosio D., Panina-Bordignon P., Rogge L. (1999) Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol. Rev. 170, 65–72 [DOI] [PubMed] [Google Scholar]
- 9. Duckett N. S., Olmos S., Durrant D. M., Metzger D. W. (2005) Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 73, 2306–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slight S. R., Lin Y., Messmer M., Khader S. A. (2011) Francisella tularensis LVS-induced interleukin-12. p40 cytokine production mediates dendritic cell migration through IL-12 receptor β1. Cytokine 55, 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Presky D. H., Yang H., Minetti L. J., Chua A. O., Nabavi N., Wu C. Y., Gately M. K., Gubler U. (1996) A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA 93, 14002–14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collison L. W., Delgoffe G. M., Guy C. S., Vignali K. M., Chaturvedi V., Fairweather D., Satoskar A. R., Garcia K. C., Hunter C. A., Drake C. G., Murray P. J., Vignali D. A. (2012) The composition and signaling of the IL-35 receptor are unconventional. Nat. Immunol. 13, 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grohmann U., Belladonna M. L., Bianchi R., Orabona C., Ayroldi E., Fioretti M. C., Puccetti P. (1998) IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity 9, 315–323 [DOI] [PubMed] [Google Scholar]
- 14. Airoldi I., Gri G., Marshall J. D., Corcione A., Facchetti P., Guglielmino R., Trinchieri G., Pistoia V. (2000) Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J. Immunol. 165, 6880–6888 [DOI] [PubMed] [Google Scholar]
- 15. De Pascalis R., Chou A. Y., Bosio C. M., Huang C. Y., Follmann D. A., Elkins K. L. (2012) Development of functional and molecular correlates of vaccine-induced protection for a model intracellular pathogen, F. tularensis LVS. PLoS Pathog. 8, e1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elkins K. L., Rhinehart-Jones T. R., Culkin S. J., Yee D., Winegar R. K. (1996) Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64, 3288–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cowley S. C., Hamilton E., Frelinger J. A., Su J., Forman J., Elkins K. L. (2005) CD4−CD8− T cells control intracellular bacterial infections both in vitro and in vivo. J. Exp. Med. 202, 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elkins K. L., Cowley S. C., Conlan J. W. (2011) Measurement of macrophage-mediated killing of intracellular bacteria, including Francisella and Mycobacteria. Curr. Protoc. Immunol. 93, 14.25.1–14.25.13 [DOI] [PubMed] [Google Scholar]
- 19. Elkins K. L., Bosio C. M., Rhinehart-Jones T. R. (1999) Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the model intracellular bacterium, Francisella tularensis live vaccine strain. Infect. Immun. 67, 6002–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhinehart-Jones T. R., Fortier A. H., Elkins K. L. (1994) Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host γ interferon and T cells. Infect. Immun. 62, 3129–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tripp C. S., Gately M. K., Hakimi J., Ling P., Unanue E. R. (1994) Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-γ. J. Immunol. 152, 1883–1888 [PubMed] [Google Scholar]
- 22. Zhan Y., Liu Z., Cheers C. (1996) Tumor necrosis factor α and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect. Immun. 64, 2782–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhan Y., Cheers C. (1995) Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect. Immun. 63, 1387–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holscher C., Atkinson R. A., Arendse B., Brown N., Myburgh E., Alber G., Brombacher F. (2001) A protective and agonistic function of IL-12p40 in mycobacterial infection. J. Immunol. 167, 6957–6966 [DOI] [PubMed] [Google Scholar]
- 25. Cooper A. M., Magram J., Ferrante J., Orme I. M. (1997) Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J. Exp. Med. 186, 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gillessen S., Carvajal D., Ling P., Podlaski F. J., Stremlo D. L., Familletti P. C., Gubler U., Presky D. H., Stern A. S., Gately M. K. (1995) Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur. J. Immunol. 25, 200–206 [DOI] [PubMed] [Google Scholar]
- 27. Watford W. T., Moriguchi M., Morinobu A., O'Shea J. J. (2003) The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 14, 361–368 [DOI] [PubMed] [Google Scholar]
- 28. Kaplan M. H., Sun Y-L., Hoey T., Grusby M. J. (1996) Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382, 174–177 [DOI] [PubMed] [Google Scholar]
- 29. Thierfelder W. E., van Deursen J. M., Yamamoto K., Tripp R. A., Sarawar S. R., Carson R. T., Sangster M. Y., Vignali D. A., Doherty P. C., Grosveld G. C., Ihle J. N. (1996) Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382, 171–174 [DOI] [PubMed] [Google Scholar]
- 30. Airoldi I., Di Carlo E., Cocco C., Taverniti G., D'Antuono T., Ognio E., Watanabe M., Ribatti D., Pistoia V. (2007) Endogenous IL-12 triggers an antiangiogenic program in melanoma cells. Proc. Natl. Acad. Sci. USA 104, 3996–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen K. B., Watford W. T., Salomon R., Hofmann S. R., Pien G. C., Morinobu A., Gadina M., O'Shea J. J., Biron C. A. (2002) Critical role for STAT4 activation by type 1 interferons in the interferon-γ response to viral infection. Science 297, 2063–2066 [DOI] [PubMed] [Google Scholar]
- 32. Wang K. S., Ritz J., Frank D. A. (1999) IL-2 induces STAT4 activation in primary NK cells and NK cell lines, but not in T cells. J. Immunol. 162, 299–304 [PubMed] [Google Scholar]
- 33. Ireland D. D., Palian B. M., Reiss C. S. (2005) Interleukin (IL)-12 receptor β1 or IL-12 receptor β 2 deficiency in mice indicates that IL-12 and IL-23 are not essential for host recovery from viral encephalitis. Viral Immunol. 18, 397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fauconnier M., Palomo J., Bourigault M. L., Meme S., Szeremeta F., Beloeil J. C., Danneels A., Charron S., Rihet P., Ryffel B., Quesniaux V. F. (2012) IL-12Rβ2 is essential for the development of experimental cerebral malaria. J. Immunol. 188, 1905–1914 [DOI] [PubMed] [Google Scholar]
- 35. Jarefors S., Janefjord C. K., Forsberg P., Jenmalm M. C., Ekerfelt C. (2007) Decreased up-regulation of the interleukin-12Rβ2-chain and interferon-γ secretion and increased number of forkhead box P3-expressing cells in patients with a history of chronic Lyme borreliosis compared with asymptomatic Borrelia-exposed individuals. Clin. Exp. Immunol. 147, 18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bosio C. M., Elkins K. L. (2001) Susceptibility to secondary Francisella tularensis LVS infection in B cell deficient mice is associated with neutrophilia but not with defects in specific T cell mediated immunity. Infect. Immun. 69, 194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cole L. E., Yang Y., Elkins K. L., Fernandez E. T., Qureshi N., Shlomchik M. J., Herzenberg L. A., Vogel S. N. (2009) Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc. Natl. Acad. Sci. USA 106, 4343–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Airoldi I., Di Carlo E., Cocco C., Sorrentino C., Fais F., Cilli M., D'Antuono T., Colombo M. P., Pistoia V. (2005) Lack of Il12rβ2 signaling predisposes to spontaneous autoimmunity and malignancy. Blood 106, 3846–3853 [DOI] [PubMed] [Google Scholar]
- 39. Ramaiah S. K., Jaeschke H. (2007) Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol. Pathol. 35, 757–766 [DOI] [PubMed] [Google Scholar]
- 40. Araujo M. I., Bliss S. K., Suzuki Y., Alcaraz A., Denkers E. Y., Pearce E. J. (2001) Interleukin-12 promotes pathologic liver changes and death in mice coinfected with Schistosoma mansoni and Toxoplasma gondii. Infect. Immun. 69, 1454–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.