Abstract
Objective
To provide cost guidance for developing a locally adaptable and nationally scalable community health worker (CHW) system within primary-health-care systems in sub-Saharan Africa.
Methods
The yearly costs of training, equipping and deploying CHWs throughout rural sub-Saharan Africa were calculated using data from the literature and from the Millennium Villages Project. Model assumptions were such as to allow national governments to adapt the CHW subsystem to national needs and to deploy an average of 1 CHW per 650 rural inhabitants by 2015. The CHW subsystem described was costed by employing geographic information system (GIS) data on population, urban extents, national and subnational disease prevalence, and unit costs (from the field for wages and commodities). The model is easily replicable and configurable. Countries can adapt it to local prices, wages, population density and disease burdens in different geographic areas.
Findings
The average annual cost of deploying CHWs to service the entire sub-Saharan African rural population by 2015 would be approximately 2.6 billion (i.e. 2600 million) United States dollars (US$). This sum, to be covered both by national governments and by donor partners, translates into US$ 6.86 per year per inhabitant covered by the CHW subsystem and into US$ 2.72 per year per inhabitant. Alternatively, it would take an annual average of US$ 3750 to train, equip and support each CHW.
Conclusion
Comprehensive CHW subsystems can be deployed across sub-Saharan Africa at cost that is modest compared with the projected costs of the primary-health-care system. Given their documented successes, they offer a strong complement to facility-based care in rural African settings.
Résumé
Objectif
Fournir des conseils en termes de coûts pour le développement d'un système d'agents de santé communautaires (ASC) localement adaptable et évolutif à l'échelle nationale, au sein des systèmes de soins de santé primaires en Afrique sub-saharienne.
Méthodes
Les coûts annuels de formation, d'équipement et de déploiement des ASC dans les régions rurales d'Afrique subsaharienne ont été calculés en utilisant les données publiées et celles du projet Villages du Millénaire. Les hypothèses du modèle sont de nature à permettre aux gouvernements nationaux d'adapter le sous-système d'ASC aux besoins nationaux et de déployer une moyenne d'un ASC pour 650 habitants de milieux ruraux d'ici 2015. Le sous-système des ASC a été chiffré à l'aide des données d'un système d'information géographique (SIG) sur la population, les étendues urbaines, la prévalence de maladies au niveau national et infranational,et les coûts unitaires (sur le terrain, pour les salaires et le matériel). Le modèle est facile à reproduire et à configurer. Les pays peuvent l'adapter aux prix locaux, aux salaires, à la densité de population et aux charges de morbidité dans différentes zones géographiques.
Résultats
Le coût annuel moyen pour déployer des ASC prenant en charge l'ensemble de la population rurale d’Afrique subsaharienne d'ici 2015 serait d'environ 2,6 milliards (c.à.d. 2 600 millions) de dollars. Cette somme, qui doit être fournie à la fois par les gouvernements nationaux et les bailleurs de fonds, correspond à 6,86 dollars par an et par habitant couvert par le sous-système d'ASC, et à 2,72 dollars par an et par habitant. Il faudrait en outre compter un montant moyen annuel de 3 750 dollars pour former, équiper et assister chaque ASC.
Conclusion
Des sous-systèmes complets d'ASC peuvent être déployés en Afrique sub-saharienne pour un coût modeste par rapport aux coûts prévus du système de soins de santé primaires. Compte tenu de leurs succès attestés, ils constituent un complément solide aux structures de soins dans les contextes ruraux africains.
Resumen
Objetivo
Facilitar asesoramiento sobre los costes necesarios para desarrollar un sistema de trabajadores comunitarios de la salud (TCS) con capacidad para adaptarse a ámbitos locales y con flexibilidad a nivel nacional, en el marco de los sistemas sanitarios de atención primaria en el África subsahariana.
Métodos
Se estimaron los gastos anuales para la capacitación, el equipamiento y el despliegue de los trabajadores comunitarios de la salud en las zonas rurales del África subsahariana mediante el análisis de datos procedentes de la literatura, así como del Proyecto Aldeas del Milenio. Los supuestos del modelo son adecuados para permitir a los gobiernos nacionales adaptar el subsistema de los trabajadores comunitarios de la salud a las necesidades nacionales, así como para realizar un despliegue medio de un trabajador comunitario de la salud por cada 650 habitantes en las zonas rurales antes de 2015. El subsistema de trabajadores comunitarios de la salud descrito se calculó mediante el análisis de datos del sistema de información geográfica (GIS, por sus siglas en inglés) sobre la población, los territorios urbanos, la incidencia de enfermedades a nivel nacional y subnacional, así como los costes unitarios (en el campo de salarios y necesidades básicas). El modelo puede configurarse y reproducirse con facilidad. Los países pueden adaptarlo a los precios, los salarios, la densidad demográfica, así como a la carga de enfermedades locales en distintas áreas geográficas.
Resultados
Se estima que el coste medio anual por el despliegue de trabajadores comunitarios de la salud para prestar atención a toda la población de las zonas rurales del África subsahariana antes de 2015 sería de unos 2,6 billones (es decir, 2 600 millones) de dólares estadounidenses (US$). Dicha suma, que será cubierta tanto por los gobiernos nacionales como por los socios donantes, se traduce en US$ 6,86 anuales por habitante, cubierta por el subsistema de trabajadores comunitarios de la salud, y en US$ 2,72 anuales por habitante. Asimismo, la capacitación, el equipamiento y el apoyo a cada TCS supondría una media anual de US$ 3750.
Conclusión
Se pueden desplegar subsistemas integrales de trabajadores comunitarios de la salud en todo el África subsahariana por un coste modesto, si se compara con los costes previstos para un sistema de atención sanitaria primaria. A juzgar por los éxitos documentados, estos ofrecen un sólido complemento para la atención en servicios sanitarios en entornos rurales de África.
ملخص
الغرض
تقديم توجيهات التكلفة لوضع نظام للعاملين الصحيين المجتمعيين (CHW) قابل للتكيف على الصعيد المحلي وقابل للقياس على الصعيد الوطني ضمن نظم الرعاية الصحية الأولية في أفريقيا جنوب الصحراء الكبرى.
الطريقة
تم حساب التكاليف السنوية لتدريب العاملين الصحيين المجتمعيين وتزويدهم بالمعدات اللازمة ونشرهم في جميع أرجاء المناطق الريفية في أفريقيا جنوب الصحراء الكبرى باستخدام البيانات المستقاة من الأبحاث الرسمية ومن مشروع قرى الألفية. وتم وضع افتراضات النموذج على نحو يتيح للحكومات الوطنية تكييف النظام الفرعي للعاملين الصحيين المجتمعيين وفق الاحتياجات الوطنية ونشر عامل صحي مجتمعي لكل 650 ساكناً قروياً في المتوسط بحلول عام 2015. وتم حساب تكلفة النظام الفرعي للعاملين الصحيين المجتمعيين من خلال استخدام بيانات نظام المعلومات الجغرافية (GIS) حول السكان والمساحات الحضرية ومدى انتشار الأمراض على الصعيد الوطني ودون الوطني وتكاليف الوحدات (بالنسبة للأجور والسلع ميدانياً). ويمكن نسخ هذا النموذج وتكوينه بسهولة. وتستطيع البلدان تكييفه وفق الأسعار والأجور والكثافة السكانية وأعباء المرض على الصعيد المحلي في مناطق جغرافية مختلفة.
النتائج
سوف يكون متوسط التكلفة السنوية لنشر العامليين الصحيين المجتمعيين لخدمة سكان المناطق الريفية في أفريقيا جنوب الصحراء الكبرى بالكامل بحلول عام 2015 نحو 2.6 مليار (أي 2600 مليون) دولار أمريكي تقريباً. وتتم ترجمة هذا المبلغ، الذي ستوفره الحكومات الوطنية والجهات المانحة الشريكة إلى 6.86 دولاراً أمريكياً سنوياً لكل ساكن يشمله النظام الفرعي للعاملين الصحيين المجتمعيين وإلى 2.72 دولاراً أمريكياً سنوياً لكل ساكن. وعلاوة على ذلك، سيتم تخصيص 3750 دولاراً أمريكياً سنوياً في المتوسط لتدريب كل عامل من العامليين الصحيين المجتمعيين وتزويده بالمعدات اللازمة ودعمه.
الاستنتاج
يمكن نشر النظم الفرعية الشاملة للعاملين الصحيين المجتمعيين في أفريقيا جنوب الصحراء الكبرى بتكلفة متواضعة مقارنة بالتكاليف المتوقعة لنظام الرعاية الصحية الأولية. وبالنظر إلى النجاحات الموثقة لهذه النظم، فإنها توفر مكملاً قوياً للرعاية المستندة على المرافق في البيئات الأفريقية الريفية.
摘要
目的
提供在撒哈拉以南非洲初级卫生保健系统内发展具有本地适应性和全国扩展性的社区卫生工作者(CHW)的成本指导。
方法
使用文献和千禧村项目的数据计算在撒哈拉以南非洲农村地区进行CHW培训、装备和部署的年成本。模型的假设大致是允许各国政府调整CHW子系统以适应国家需求,并在2015 年结束之前平均在每650 个农村居民中部署1 个CHW。描述的CHW子系统采用地理信息系统(GIS)人口及城市范围的数据、国家和地方疾病的发病率、单位成本(工资和商品)进行成本估算。模型很容易复制和配置。各个国家可以调整模型以适应不同的地理区域的地方价格、工资、人口密度和疾病负担。
结果
在2015 年结束之前部署为整个撒哈拉以南非洲农村人口提供服务的CHW的平均年费用约为26 亿美元(US$)。 这笔款项由各国政府和捐助合作伙伴共同提供,换算到CHW子系统覆盖的每个居民为每年6.86 美元,换算到每个居民为每年2.72 美元。此外,培训、装备和支持每名CHW每年平均需要3750 美元。
结论
综合的CHW子系统可以在整个撒哈拉以南非洲地区部署,其成本与初级卫生保健系统的预计支出相比较为适中。鉴于其有案可查的成功先例,这些系统为非洲农村环境中以设施为基础的卫生护理提供了强有力的补充。
Резюме
Цель
Обеспечить экономическое руководство по разработке локально адаптируемой и национально масштабируемой сети медико-санитарных работников (МСР) в рамках систем первичной медико-санитарной помощи в странах Африки южнее Сахары.
Методы
Ежегодные затраты на обучение, оснащение и развертывание сети МСР во всех сельских районах к югу от Сахары были рассчитаны с использованием данных из литературы и проекта «Деревни тысячелетия» (Millennium Villages). Допущения модели таковы, что позволяют национальным правительствам адаптировать подсистему МСР для национальных потребностей и обеспечить в среднем 1 МСР на 650 сельских жителей к 2015 г. Была оценена стоимость описанной подсистемы МСР на основе использования географических информационных систем (ГИС), данных о численности населения, степени развития городов, распространенности заболеваний на национальном и субнациональном уровнях, а также удельных затрат на содержание (таких как заработная плата и материальное снабжение). Модель является легко воспроизводимой и настраиваемой. Страны могут адаптировать ее к местным ценам, заработной плате, плотности населения и бремени болезней в различных географических районах.
Результаты
Среднегодовая стоимость развертывания сети МСР для обслуживания сельского населения всей Африки южнее Сахары к 2015 г. составит около 2,6 млрд. (т.е. 2 600 млн.) долл. США. Эта сумма, которая должна быть выделена как национальными правительствами, так и партнерами-донорами, составляет 6,86 долл. США в год на одного жителя в районах, охваченных подсистемой МСР, и 2,72 долл. США в год на одного жителя страны. Кроме того, в среднем потребуется 3 750 долл. США в год на подготовку, оснащение и поддержку каждого МСР.
Вывод
По всей Африке южнее Сахары могут быть развернуты всеобъемлющие подсистемы МСР по стоимости, более скромной по сравнению с прогнозируемыми затраты на систему первичной медико-санитарной помощи. Учитывая документированные успехи систем МСР, они являются значительным усилением медицинской помощи на основе лечебных учреждений, расположенных в сельских районах Африки.
Introduction
The evidence is overwhelming that community-based interventions are an effective platform for extending health care delivery and improving health outcomes. Such evidence indicates that a well-implemented community-based health programme can: (i) reduce infant and child mortality and morbidity; (ii) improve health-care-seeking behaviour (e.g. increase rates of institutional delivery and immunization); and (iii) provide low-cost interventions for common maternal and paediatric health problems while improving the continuum of care.1–3 Such community-level programmes can be particularly effective for addressing the most common causes of paediatric mortality and morbidity, such as pneumonia, diarrhoea, undernutrition, malaria, human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) and measles.4–11 These community-based health programmes are often successfully executed through community health workers (CHWs). These are lay people who live in the communities they serve and who function as a critical link between those communities and the primary-health-care system.12 The well-documented success of CHW programmes over the last few decades has increasingly pushed investment in CHW subsystems to national and international policy platforms as part of coordinated efforts to improve health-care systems.13
Although several reviews have documented the positive impact of CHWs on health outcomes, CHW programme costs have barely been examined, probably because cost data are much less widely available than data on programme outcomes. In one revealing review of 53 studies on CHW programmes in the United States of America, only six studies referenced costs and the authors considered such data to be insufficient to draw any conclusions.14 When included, costs are generally associated with interventions rather than with comprehensive CHW programmes; furthermore, estimates do not account for economies of scale or year-on-year efficiencies.15 To date, the official costs of national CHW programmes in pioneering countries such as Ethiopia, Malawi or Rwanda have not been estimated, partly because tracking unit costs is difficult and because methods for isolating the CHW subsystem from an integrated primary-health-care system have been elusive.
This paper provides cost guidance for one adaptable configuration of a CHW “subsystem”: a provider system housed within a larger primary-health-care system that includes clinics and referral hospitals. Costing is done by function (e.g. diagnosing and treating malaria) and by local epidemiologic characteristics (e.g. each country’s prevalence of HIV infection), so that components and assumptions can be easily modified. National scale-up of CHW programmes and of primary-health-care systems more broadly is likely to reduce the incidence of many of the diseases discussed in this paper. This model allows costs to be easily recalculated as incidence rates change. New functions, such as the care of patients with chronic conditions, could be added and costed once a vetted CHW protocol for these functions has emerged.
Methods
Model community health worker system
We designed the model CHW system presented in this paper using the health system building blocks of the World Health Organization (WHO) (Table 1), empirical evidence and lessons learnt from several global CHW programmes, and data from the Millennium Villages Project. In this costing model we focus on full-time, paid public sector CHWs who are formally recognized as an integral part of the rural primary-health-care system and who perform their work primarily in the community and through household visits.
Table 1. Model framework for a community health worker (CHW) subsystem within the health system.
| Model parameter | Inputs | Processes | Outputs |
|---|---|---|---|
| Service delivery |
Activities and skills: – CCM for malaria, pneumonia, malnutrition, diarrhoea – deworming – TB screening – assistance in adherence to drugs for HIV infection and TB – health promotion and disease prevention |
Changing household health behaviour; improving access to disease control/prevention; improving access to basic curative health services at the household/community level | Improved community health status through increased coverage of high-impact interventions |
| Referral to primary health clinics and follow-up; availability of emergency transport |
Linking to broader health system (advanced care) |
Increased use of advanced care and institutional delivery |
|
| Health workforce |
CHWs selected through community/facility partnership | Improving access to health services at the household/community level | Improved health through increased coverage of high-impact interventions |
| Senior CHWs (experienced CHWs, selected for supportive supervision) | Monitoring of quality of care | Improved service quality, data reporting and CHW retention | |
| Supportive supervision of CHWs | |||
| CHW managers (facility-based workers with training in management skills) |
Monitoring CHW programme, including system performance | Improved links to referral facilities and community governance structures | |
| Linking to both facilities and communities |
Improved quality of CHW subsystem |
||
| Information |
Health data reporting, and vital statistics tracking by CHW | Monitoring and discussing community health indicators | Data used to inform programme strategy, engage communities, and improve health status |
| Utilization of information by CHW programme managers stakeholders (CHWs, supervisors, district health management team, facility staff, etc.) | Using health indicators to inform management | Data used to guide community actions for health and programme improvements | |
| Information feedback mechanisms | Informing communities about epidemiology, health status, service delivery and quality of care | Data used to identify service delivery weaknesses and track quality improvement results | |
| Giving prompt feedback to community | |||
| Mobile technology suite (cell phone, text messaging, voice) |
Collecting real-time data | Improved quality of services |
|
| Facilitating alerts for emergency care | |||
| Providing decision support at point-of-care | |||
| Medical products, POC diagnosis and technology |
Equipment, consumables and tools to assess sickness and commodities for diagnosis and treatment (ORS, zinc, ACTs, RDTs, sputum containers for TB, deworming drugs [albendazol, praziquantel], antibiotics for pneumonia) |
Delivering health service | Improved health status of community members through increased coverage of high-impact interventions |
| Conducting surveillance for danger signs | |||
| Providing community-based treatment of specified diseases/conditions | |||
| Financing |
Remuneration of CHWs | Supporting CHWs as full-time professionals | Development of a professional, paid workforce with well-defined terms of reference |
| Financing for professionalization (training, uniforms, equipment, commodities, professional development/career advancement) |
Regularly training CHWs; | Improved CHW motivation and capabilities to provide quality care (with appropriate tools) |
|
| Incentivizing CHWs for high performance and retention | |||
| Leadership and governance | Community governance structures | Supporting local specification and community-based selection and oversight | CHW workforce efficiently supervised and managed and trained in skills necessary to improve maternal and child health indicators; |
| Governance structures from ministries of health and partner organizations | Ensuring CHW programme adherence to government policy | Community mobilized and engaged in CHW subsystem. | |
| Quality improvement processes and organizational culture | Developing culture of quality improvement within the workforce | ||
| Career ladder, professional training curricula and national certification | Attracting strong CHW candidates; | Improved CHW motivation and better candidates | |
| Motivating CHWs to execute roles and responsibilities | |||
ACT, artemisinin-based combination therapy; AZT, zidovudine; CCM, community case management; HIV, human immunodeficiency virus; ORS, oral rehydration salts; POC, point-of-care; RDT, rapid diagnostic test; TB, tuberculosis.
The CHWs in this subsystem visit households to provide community case management for diarrhoea, malaria and malnutrition, management of pregnancy and health promotion. This subsystem is supported by robust supervision, information-driven management and a provider network within the health system. This model is also based on the assumption that CHWs receive short training and strong supervision and that the health system is strong. In the particular model costed, we also assumed that an mHealth platform was used to collect data and provide decision support, given the increasing popularity of mHealth programmes in primary care. Detailed inputs, processes and outputs from this framework can be found in the One Million Community Health Workers report.16
This costing exercise also illustrates one possible additional configuration in which a subset of “generalist” CHWs is further trained to work closely with skilled birth attendants to enhance maternal care. Although this CHW subset relies on a skilled clinical team member for supporting pregnancy and childbirth, for the sake of discussion we herein refer to these CHWs as “childbirth specialists”.
Cost inputs for household services
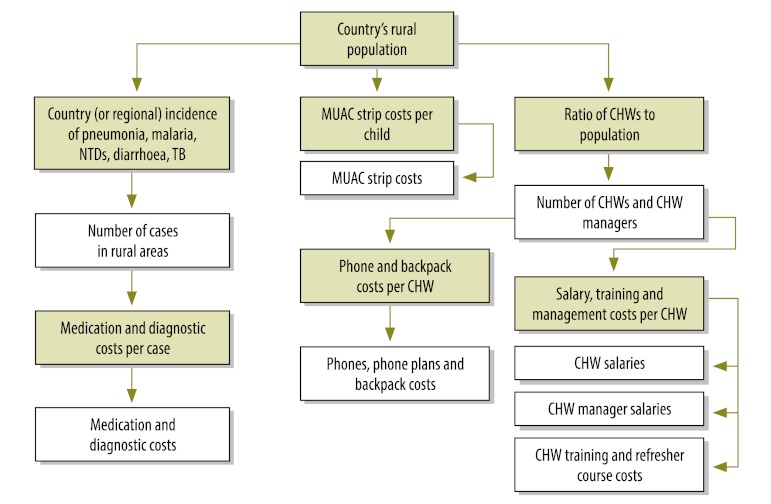
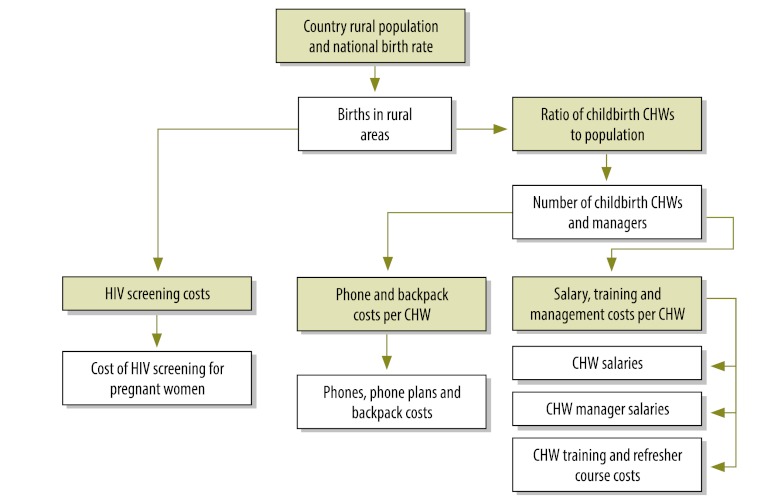
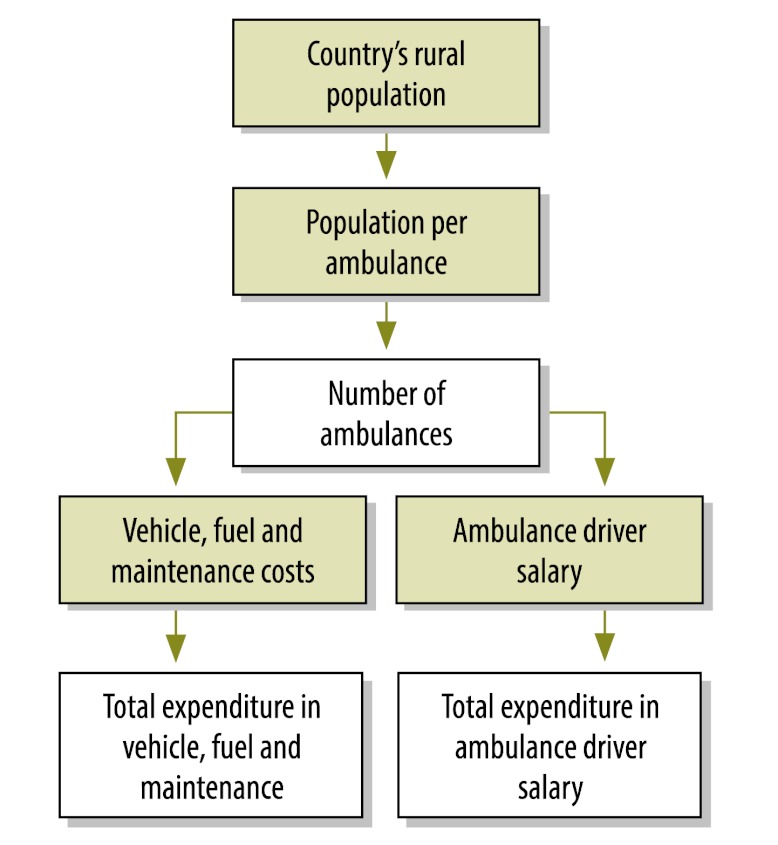
We estimated the cost of fully deploying CHW subsystems across the rural areas of sub-Saharan Africa and develop a costing framework for countries to refine and adapt in their own national planning. Fig. 1, Fig. 2 and Fig. 3 illustrate the algorithm for costing, including junctures (represented by green boxes) requiring data on population, disease incidence and supply and commodity costs. Unless otherwise indicated, unit costs were provided by the Millennium Villages Project.
Fig. 1.
Costing algorithm for generalist community health workers (CHWs)
MUAC, mid upper arm circumference; NTD, neglected tropical disease; TB, tuberculosis.
Green boxes indicate junctures requiring data on requiring data on population, disease incidence and supply and commodity costs.
Fig. 2.
Costing algorithm for childbirth specialist community health workers (CHWs)
HIV, human immunodeficiency virus.
Green boxes indicate junctures requiring data on requiring data on population, disease incidence and supply and commodity costs.
Fig. 3.
Costing algorithm for community health worker (CHW) referral system costs
Green boxes indicate junctures requiring data on requiring data on population, disease incidence and supply and commodity costs.
Results
Costs per community health worker
Backpack and mobile phone: Each CHW is given a mobile smartphone costing US$ 150, a solar charger costing US$ 40 and a data plan costing US$ 480 per year for supervisory support, communication and data collection. To reduce costs, national CHW programmes can establish closed user groups – a nationwide network that allows free calls and short message service between identified health staff. Each CHW is also given a backpack costing US$ 10 to carry supplies.
Training: CHWs receive one year of training: 3 months in a classroom and 9 months while in service, plus supervision and feedback. They also receive a yearly refresher course lasting 20 hours. In the Millennium Villages Project, training 50 CHWs for 80 hours costs US$ 300. This includes transport, meals, supplies and trainer’s honorarium, as necessary. We used this figure to compute an hourly cost. Following the experience of Pakistan’s national Lady Health Worker programme, we assumed a 5% student attrition rate during training.17
Salaries: In the costing model, each CHW receives a monthly salary of US$ 80. This is the average CHW salary across Millennium Village Project settings in 10 countries. This figure will fluctuate depending on national rural wage levels.
Management: Each CHW Manager is assumed to manage 30 CHWs and receives an annual salary of US$ 9600. This 10-fold difference between CHW and CHW manager salaries represents the average difference across Millenium Village sites. Note that CHW managers could be physicians in primary-health-care facilities with supplemental training in management, which would parlay some of this cost into existing primary-health-care system budgets.
Overhead: We added 15% to total programme costs in order to account for the overhead costs at the local, national and international levels of a global effort to reach full coverage, or around 1 million CHWs across low-income sub-Saharan Africa by 2015. This includes components of the operational design not listed as segregated costs, such as community engagement and information management.
Generalist CHWs are expected to generate various costs. The cost modelling we have conducted is based on country-specific disease prevalence rates (and subnational endemicity data in the case of malaria). If the prevalence of any disease drops, the resulting commodity expenditures will decrease accordingly.
Monitoring for undernutrition: CHWs will use mid-upper arm circumference (MUAC) strips to monitor children between the ages of 1 and 5 years for undernutrition at least once every 90 days. We estimate a requirement of one MUAC strip per child per year, at a cost per strip of US$ 0.05.18
Treating diarrhoea: CHWs will treat episodes of diarrhoea with oral rehydration salts (ORS) and zinc tablets. They should teach caretakers how to administer ORS and leave two packets in the household, along with 20-mg tablets, to be taken once daily for 14 days (half a tablet in the case of children younger than 6 months).19 We calculate the average cost per episode to be around US$ 0.42.18 Following published data,20 we assume that children under 5 years of age experience 5 diarrhoeal episodes annually, and that people older than 5 years’ experience 1.28. This leads to an estimated 1.1 billion diarrhoeal episodes per year in rural Africa. Notably, diarrhoeal disease is projected to be the most expensive health condition covered by CHWs: US$ 0.79 out of US$ 2.88 in supply and commodity costs per person served by the CHW subsystem. A reduction in diarrhoeal disease incidence would have important cost implications for CHW programmes.
Testing for and treating malaria: In the absence of microscopy, WHO protocol dictates that all people with fever in malarious areas should undergo a rapid diagnostic test (RDT) before being treated with antimalarials. We used the United Nations Population Division’s World Population Prospects’ demographic breakdown for 2010 to divide the population in malarious zones into groups aged 0–4, 5–9, 10–14 and 14+ years. We assume that the 0–4 age group will experience two fever episodes per year; the 5–9 and 10–14 age groups, one fever episode per year, and the 14+ age group, 0.5 fever episodes per year. These figures are the average annual number of fever episodes from all causes, across all malaria endemicity levels. One RDT kit costing US $0.50 is needed for each of these fever episodes.18,21 We also calculate the costs of treating malaria with artemisinin-based combination therapy (ACT). We assume that RDT will reveal that around 30% of the fevers are due to malaria and require ACT. The total number of fever episodes requiring treatment for malaria by 2015 is estimated at 150 million, which is within the range of other published estimates. Each case can be treated with either artemether–lumefantrine or artesunate–amodiaquine, so we use the average cost. The costs per treatment course are as follows: for artemether–lumefantrine, US$ 0.45 for the 0–4 age group, US$ 0.90 for the 5–9 age group, US$ 1.35 for the 10–14 age group and US$ 1.80 for the 14+ age group; for artesunate–amodiaquine, US$ 0.23 for infants, US$ 0.45 for children aged 1–6 years, US$ 0.80 for children aged 7–13 years and US$ 1.48 for people older than 13. We used the dosages recommended by the Roll Back Malaria Partnership and average drug prices obtained from Sanofi-Aventis (Paris, France), Ipca (Mumbai, India) and Cipla (Mumbai), with adjustment to match the age groups in our analysis. The total projected expenditure is US$ 153 million in RDTs and US$ 87 million in ACTs, with national per capita expenditure varying in accordance with each country’s endemicity levels.
Deworming: Using country-level data on the prevalence of ascariasis, trichuriasis and hookworm,22 we applied the following protocol: if the country-level prevalence of any of the three infestations is over 50%, all individuals receive three doses of albendazole (costing US$ 0.02 per dose) per year.18 If the prevalence is above 20% and below 50%, then everyone receives one dose of albendazole. For schistosomiasis, if the prevalence is above 50%, everyone older than 4 years is treated with praziquantel once a year.23 If the prevalence is above 10%, every eligible person is treated once every two years. If the prevalence is below 10%, only school-age children are treated twice during their school-aged years (once on entry and once on exit). Praziquantel costs US$ 0.22 a tablet,18 and since the number of tablets in the treatment varies from 1 to 5 depending on body weight, we assume an average of 2.5 tablets per treatment.
Pneumonia: WHO reported 131.3 million pneumonia episodes in Africa in 2004.24 From this we calculate the number of episodes per person and, on the assumption that incidence in a given country remains constant, we estimate the number of pneumonia episodes in the population covered by CHWs each year. We include timers for each CHW to assess children’s respiratory rate; respiratory timers from the United Nations Children’s Fund cost US$ 3.50.18 Since they are good for 10 000 uses, we assume one timer per CHW per year. The average cost of antibiotics is US$ 0.27 per case.24
Screening for tuberculosis: CHWs should collect sputum samples from suspected tuberculosis cases. We estimate the number of screenings using each country’s incidence of tuberculosis and assume a positivity rate of 10%. The cost of screening one person covers the collection of three sputum samples in containers (one on the first day and two on the second day). The containers are labelled with a marker and put in a reusable sealable plastic bag. The cost of each container is US$ 0.054. Each CHW is given two markers for the year and three reusable sealable plastic bags every 3 months. Finally, CHWs use two new pairs of surgical gloves for each screening, since the screening is conducted over two days. In the Bonsaaso Millennium Village in Ghana, markers are priced at US$ 10 for a pack of 8, plastic bags are priced at US$ 8 for a pack of 25 and surgical gloves are priced at US$ 0.60 a pair.
The childbirth specialist CHW would generate the same human resource expenditures (training, salary, management) as the generalist CHW, but different commodity costs:
Screening pregnant women for HIV infection: All pregnant women will undergo at least one HIV test. Following Joint United Nations Programme on HIV/AIDS cost estimates for the prevention of mother-to-child transmission of HIV, we estimate a cost of US$ 3.90 for each HIV serological test. If a pregnant woman tests positive for HIV, the CHW will also test her husband and children (assumed to be 2, on average). HIV-positive individuals will be referred to the national AIDS programme for counselling and treatment.
Ambulances are included in the costing model as a necessary link between the CHWs and the primary-health-care system.
Ambulances: One ambulance is assumed to cover a population of 50 000 people in a rural area. Based on experience in Millennium Villages, an ambulance costs an average of US$ 36 000. The ambulance driver receives an annual salary of US$ 4782. Ambulance fuel and maintenance costs average US$ 4825 per year across Millennium Villages.
In total, it will take an average of US$ 3750 annually to train, equip and support each CHW between 2012 and 2015. Maintenance of the CHW programme after 2015 will cost US$ 3150 per CHW. These costs do not include two potential CHW services: family planning and HIV screening for the general population. Since public health specialists have not reached consensus on whether CHWs should be tasked with these services, we have separated them from the costing framework above and consider them below:
Family planning: The average cost of family planning in Africa, including contraceptives, has been estimated at around US$ 26.90 annually per woman of reproductive age (15–49 years).25 If generalist CHWs offer family planning services to women in this age group, the total cost comes to an average of US$ 2.4 billion per year, or US$ 6.36 per person serviced by the CHW programme.
HIV screening in the general population: Studies have begun documenting the benefits of active case finding and outreach by CHWs.26 Health service provision is moving towards increased decentralization and at some point CHWs may be tasked with testing the general population for HIV. (Note that in the costing presented earlier, pregnant women and their families are already tested through childbirth specialist CHWs). Although the appropriate frequency of testing remains to be determined, we assume one annual test per person to get an idea of costs. Extending HIV testing once a year to all HIV-negative people older than 14 years costs an average of US$ 791 million per year, or US$ 2.12 per rural inhabitant.
Estimated costs of scale-up by 2015
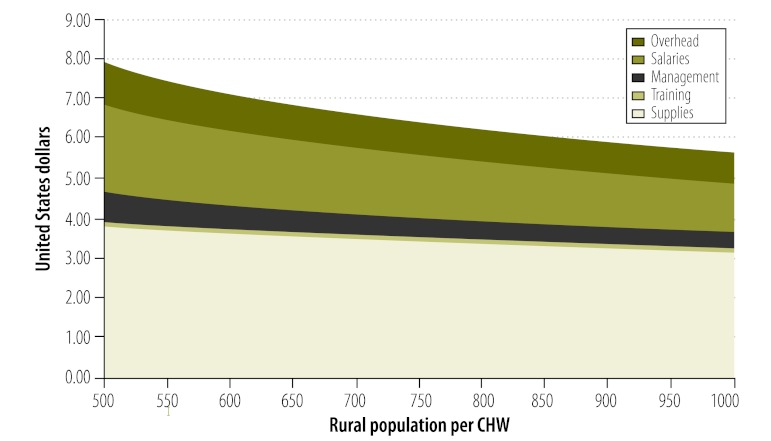
The model ramps up CHW coverage evenly from 2012 to 2015: in 2012 one quarter of the rural population receives services, but by 2015 the entire rural population will be serviced. Note that in the costing model rural population and population growth are based on United Nations Population Division data, which come from national statistics offices with varying definitions of urban and rural areas. Thus, our model is based on each country’s definition of “rural” and “urban”. The number of CHWs per inhabitant will be different for each country, especially in light of differences in rural population density. For this reason, Fig. 4 shows programme costs by major category as a function of the number of rural residents per CHW, which varies from 500 to 1000 people.
Fig. 4.
Average annual CHW costs as a function of population served
CHW, community health worker.
Note: Costs are for generalist and childbirth specialist CHWs combined.
Table 2 (available at: http://www.who.int/bulletin/volumes/91/4/12-109660) shows the country-by-country breakdown of the number of CHWs required by 2015 and the average yearly costs for supplies, training, salaries, management and overhead for the combined workforce of generalist CHWs and childbirth specialist CHWs. At a rate of 1 generalist for every 650 rural Africans and of 1 childbirth specialist for every 3500 Africans, the average annual cost of CHW system ramp-up across Africa is around US$ 2.6 thousand million (billion), or US$ 6.86 per person covered by CHW services, or US$ 2.72 per inhabitant. The cost of training, equipping and supporting each CHW amounts to US$ 3750 per year.
Table 2. Average annual expenditure on the community health worker (CHW) programme (with 1 CHW for every 650 rural inhabitants) .
| Country | No. of CHWs in 2015 | Average annual expenditure in 2012–2015 (millions of US$) |
Average annual expenditure after 2015 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supplies | Training | Salaries | Management | Overhead | Total | Per population served | Total | Per rural population | Per total population | |||
| Angola | 15 209 | 18.75 | 0.61 | 9.13 | 3.04 | 4.72 | 36.24 | 6.97 | 48.90 | 5.88 | 2.25 | |
| Benin | 9 714 | 11.61 | 0.39 | 5.83 | 1.94 | 2.96 | 22.73 | 6.85 | 30.62 | 5.77 | 2.87 | |
| Botswana | 1 401 | 1.54 | 0.06 | 0.84 | 0.28 | 0.41 | 3.12 | 6.53 | 4.17 | 5.44 | 1.97 | |
| Burkina Faso | 23 623 | 28.95 | 0.95 | 14.17 | 4.72 | 7.31 | 56.10 | 6.95 | 75.63 | 5.86 | 3.97 | |
| Burundi | 15 611 | 17.98 | 0.63 | 9.37 | 3.12 | 4.65 | 35.75 | 6.70 | 48.11 | 5.64 | 5.09 | |
| Cameroon | 20 536 | 24.89 | 0.82 | 12.32 | 4.11 | 6.31 | 48.45 | 6.90 | 65.41 | 5.83 | 2.94 | |
| Cape Verde | 3 25 | 0.27 | 0.01 | 0.20 | 0.07 | 0.08 | 0.63 | 5.66 | 0.83 | 4.64 | 1.49 | |
| Central African Republic | 5 324 | 6.38 | 0.21 | 3.19 | 1.06 | 1.62 | 12.47 | 6.86 | 16.82 | 5.78 | 3.40 | |
| Chad | 19 255 | 23.53 | 0.77 | 11.55 | 3.85 | 5.94 | 45.65 | 6.94 | 61.44 | 5.84 | 4.67 | |
| Comoros | 1 104 | 0.96 | 0.04 | 0.66 | 0.22 | 0.28 | 2.17 | 5.76 | 2.83 | 4.69 | 3.68 | |
| Congo | 2 875 | 3.28 | 0.12 | 1.73 | 0.58 | 0.85 | 6.55 | 6.67 | 8.79 | 5.59 | 2.07 | |
| Congo, Democratic Republic | 89 555 | 111.25 | 3.59 | 53.73 | 17.91 | 27.92 | 214.40 | 7.01 | 289.55 | 5.91 | 3.73 | |
| Djibouti | 658 | 0.68 | 0.03 | 0.40 | 0.13 | 0.18 | 1.42 | 6.30 | 1.90 | 5.29 | 1.99 | |
| Equatorial Guinea | 887 | 1.01 | 0.04 | 0.53 | 0.18 | 0.26 | 2.02 | 6.66 | 2.70 | 5.58 | 3.45 | |
| Eritrea | 8 721 | 9.75 | 0.35 | 5.23 | 1.74 | 2.56 | 19.63 | 6.59 | 26.34 | 5.52 | 4.37 | |
| Ethiopia | 139 837 | 157.66 | 5.60 | 83.90 | 27.97 | 41.19 | 316.32 | 6.62 | 425.57 | 5.57 | 4.40 | |
| Gabon | 370 | 0.43 | 0.01 | 0.22 | 0.07 | 0.11 | 0.86 | 6.78 | 1.16 | 5.73 | 0.70 | |
| Gambia | 1 481 | 1.80 | 0.06 | 0.89 | 0.30 | 0.46 | 3.50 | 6.91 | 4.72 | 5.83 | 2.38 | |
| Ghana | 22 925 | 26.88 | 0.92 | 13.76 | 4.59 | 6.91 | 53.04 | 6.77 | 71.63 | 5.72 | 2.65 | |
| Guinea | 12 719 | 15.40 | 0.51 | 7.63 | 2.54 | 3.91 | 29.99 | 6.90 | 40.48 | 5.82 | 3.41 | |
| Guinea-Bissau | 1 404 | 1.68 | 0.06 | 0.84 | 0.28 | 0.43 | 3.29 | 6.86 | 4.44 | 5.78 | 2.39 | |
| Ivory Coast | 18 172 | 21.80 | 0.73 | 10.90 | 3.63 | 5.55 | 42.61 | 6.86 | 57.52 | 5.79 | 2.37 | |
| Kenya | 62 048 | 74.85 | 2.48 | 37.23 | 12.41 | 19.01 | 145.98 | 6.89 | 196.52 | 5.79 | 4.22 | |
| Lesotho | 2 912 | 2.62 | 0.12 | 1.75 | 0.58 | 0.76 | 5.83 | 5.86 | 7.56 | 4.75 | 3.46 | |
| Liberia | 5 851 | 7.11 | 0.23 | 3.51 | 1.17 | 1.80 | 13.82 | 6.91 | 18.74 | 5.86 | 4.00 | |
| Madagascar | 28 412 | 34.42 | 1.14 | 17.05 | 5.68 | 8.73 | 67.01 | 6.90 | 90.74 | 5.84 | 3.95 | |
| Malawi | 27 243 | 33.49 | 1.09 | 16.35 | 5.45 | 8.44 | 64.81 | 6.96 | 86.80 | 5.83 | 4.80 | |
| Mali | 20 364 | 24.53 | 0.82 | 12.22 | 4.07 | 6.23 | 47.86 | 6.88 | 64.31 | 5.78 | 4.28 | |
| Mauritania | 4 160 | 4.86 | 0.17 | 2.50 | 0.83 | 1.25 | 9.60 | 6.76 | 12.96 | 5.70 | 3.47 | |
| Mauritius | 1 418 | 1.13 | 0.06 | 0.85 | 0.28 | 0.35 | 2.67 | 5.52 | 3.51 | 4.53 | 2.59 | |
| Mozambique | 34 966 | 45.75 | 1.40 | 20.98 | 6.99 | 11.25 | 86.37 | 7.23 | 117.03 | 6.12 | 4.49 | |
| Namibia | 2 730 | 3.26 | 0.11 | 1.64 | 0.55 | 0.83 | 6.38 | 6.84 | 8.62 | 5.77 | 3.55 | |
| Niger | 27 399 | 34.03 | 1.10 | 16.44 | 5.48 | 8.54 | 65.59 | 7.01 | 88.40 | 5.90 | 4.61 | |
| Nigeria | 158 659 | 191.05 | 6.35 | 95.20 | 31.73 | 48.56 | 372.89 | 6.88 | 501.91 | 5.79 | 2.84 | |
| Rwanda | 17 900 | 20.62 | 0.72 | 10.74 | 3.58 | 5.34 | 40.99 | 6.70 | 54.93 | 5.61 | 4.66 | |
| São Tome and Principe | 1 13 | 0.12 | 0.00 | 0.07 | 0.02 | 0.03 | 0.25 | 6.54 | 0.34 | 5.49 | 1.87 | |
| Senegal | 14 833 | 17.81 | 0.59 | 8.90 | 2.97 | 4.53 | 34.80 | 6.87 | 47.01 | 5.80 | 3.23 | |
| Sierra Leone | 7 217 | 8.86 | 0.29 | 4.33 | 1.44 | 2.23 | 17.16 | 6.96 | 23.20 | 5.88 | 3.54 | |
| Somalia | 11 556 | 13.35 | 0.46 | 6.93 | 2.31 | 3.45 | 26.51 | 6.71 | 35.52 | 5.62 | 3.30 | |
| South Africa | 35 191 | 40.13 | 1.41 | 21.11 | 7.04 | 10.43 | 80.13 | 6.66 | 107.87 | 5.61 | 2.07 | |
| South Sudan | 15 090 | 17.72 | 0.60 | 9.05 | 3.02 | 4.55 | 34.95 | 6.78 | 47.17 | 5.72 | 4.31 | |
| Sudan | 33 900 | 39.51 | 1.36 | 20.34 | 6.78 | 10.18 | 78.17 | 6.75 | 105.40 | 5.69 | 2.85 | |
| Swaziland | 1 635 | 2.03 | 0.07 | 0.98 | 0.33 | 0.51 | 3.91 | 7.00 | 5.25 | 5.88 | 4.06 | |
| Togo | 7 963 | 9.63 | 0.32 | 4.78 | 1.59 | 2.44 | 18.77 | 6.90 | 25.43 | 5.84 | 3.33 | |
| Uganda | 59 032 | 72.94 | 2.36 | 35.42 | 11.81 | 18.34 | 140.88 | 6.98 | 189.23 | 5.86 | 4.75 | |
| United Republic of Tanzania | 67 826 | 82.16 | 2.72 | 40.70 | 13.57 | 20.83 | 159.96 | 6.90 | 215.05 | 5.80 | 4.11 | |
| Zambia | 15 399 | 19.29 | 0.62 | 9.24 | 3.08 | 4.83 | 37.06 | 7.04 | 49.53 | 5.88 | 3.29 | |
| Zimbabwe | 13 731 | 16.76 | 0.55 | 8.24 | 2.75 | 4.24 | 32.53 | 6.93 | 43.88 | 5.85 | 3.11 | |
| Total | 1 089 334 | 1305 | 44 | 654 | 218 | 332 | 2552 | – | 3437 | – | – | |
| Cost per inhabitant served | – | 3.51 | 0.12 | 1.76 | 0.59 | 0.89 | 6.86 | – | 5.63 | – | – | |
US$, United States dollar.
Steady-state system costs listed in the last three columns of Table 2 include the training of new CHWs to keep up with rural population growth and refresher courses for all CHWs in the system. Steady-state estimates include capital costs such as phones, backpacks and ambulances; these are amortized over 3, 3 and 7 years, respectively.
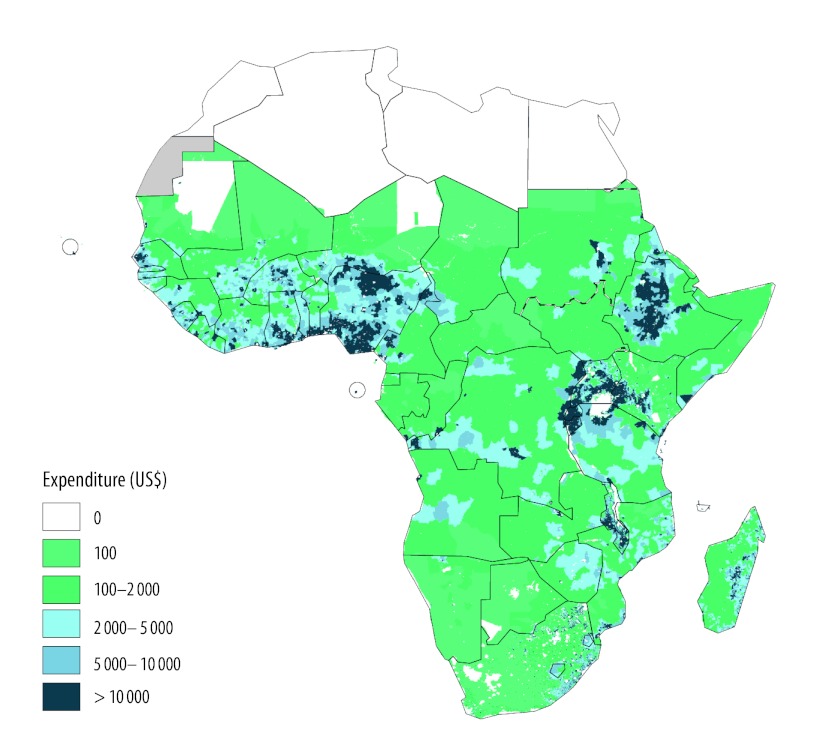
Fig. 5 shows how expenditure is spatially distributed across rural Africa. To obtain the distribution we used the costs per rural inhabitant given earlier and 2.5 arc-minute GIS data on population and urban extents in 2010.27 We eliminated the urban population and calculated the average annual cost per grid cell. The map allows international and national policy-makers to see how CHW programme expenditures would be distributed within and across sub-Saharan African countries.
Fig. 5.
Spatial distribution across sub-Saharan Africa of the annual cost of a community health worker programme
a These protocols are from the Millennium Villages’ deworming protocol.
Note: The map shows expenditure in every 2.5 minute cell (roughly 4.6 × 4.6 km). Costs are calculated as CHW programme cost per rural inhabitant at the country level, then multiplied by the rural population in each grid cell.
Discussion
According to studies published by the Commission on Macroeconomics and Health, the United Nations Millennium Project and the International Task Force on Innovative Financing for Health, in a low-income country a primary-health-care system should cost from US$ 50 to US$ 55 per capita per year in 2011 prices.28–30 According to this model, the CHW subsystem configured in this paper would cost approximately 5% of the total cost of a primary-health-care system.
Although this framework provides an estimate of CHW-related costs across sub-Saharan Africa, country-specific financing models reflecting the local costs of training and deploying the CHWs should emerge, along with country-specific strategies to suit different CHW programme characteristics. Nigeria is currently planning to deploy a CHW subsystem nationally.
The effectiveness of a CHW subsystem depends on how well the rest of the health system functions. Although we excluded hospitals, clinics and non-CHW health staff from our costing model, they provide vital logistical support to CHWs by making available to them adequate supplies of medicines and possibilities for patient referral. When national CHW initiatives have been taken to scale without supporting infrastructure, retrospective analysis reveals weaknesses in management, supply chain, financing and other components of the supporting system.31
Depending on existing policies or disease prevalence, national CHW strategies may be designed to train CHWs to provide supplemental services beyond those described in this model. As countries engage in the financial and operational planning of their CHW programmes, model inputs will differ in accordance with the local context, as exemplified in Table 3. The adaptation of the model to country-specific needs will become increasingly important as ministries of health prepare their community-based health workforces for the management of the increasing burden of non-communicable diseases. However, the management of patients with these diseases by CHWs requires further study and operational detailing in low-resource settings, as well as a dedicated supplemental source of financing.
Table 3. Factors to be modified when adapting operational design of model community health worker (CHW) costing system to local context.
| Factor | Assumption for example model | Condition |
||
|---|---|---|---|---|
| Low | Medium | High | ||
| Population density | Low | Rural | Periurban | Urban |
| Facility density | Low | Rural | Periurban | Urban |
| Task load | Low | General counselling tasks at household or outreach centres | General counselling and case management tasks at household level, with data reporting | General counselling and case management tasks at household and/or health post level, with data reporting and additional specialized tasks |
| Education and literacy | Medium–high | None | Primary school | Secondary school |
| CHW integration with health systema | High | CHW programme works in isolation from health system; links to health system weak | Health system recognizes CHW programme and provides support or guidelines. | Health system provides comprehensive support to CHW programme, including training, supervision, referral, equipment and supplies. |
| Country ownership and financial supporta | High | CHWs not recognized as part of national system; no financial support provided by government | CHWs recognized as part of health system but role not formalized; little or no financial support provided by government | CHWs recognized as part of health system; partial to full financing by government |
a Functionality definition provided by the Community Health Worker Assessment and Improvement Matrix.32
In summary, we recommend that countries wishing to develop a CHW strategy perform a similar costing exercise to design programme budgets. According to previous studies, investment in a well-organized and comprehensive CHW subsystem that is embedded in a primary-health-care system can reduce maternal and child morbidity and mortality. Our assessment suggests that the costs of the core elements of such a system are a fraction of the costs of primary-health-care services overall. This costing exercise sets the stage for a costing framework that can be used to determine the cost–benefit and cost–effectiveness of CHW programmes.
Competing interests:
None declared.
References
- 1.Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, van Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev. 2010;(3):CD004015. doi: 10.1002/14651858.CD004015.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehmann U, Sanders D. Community health workers: what do we know about them? The state of the evidence on programmes, activities, costs and impacts on health outcomes of using community health workers. In: Evidence and information for policy. Geneva: Department of Human Resources for Health, World Health Organization; 2007. [Google Scholar]
- 3.Perry H, Freeman P, Gupta S, Rassekh BM. How effective is community-based primary care in improving the health of children? Washington: Community-Based Primary Health Care Working Group, American Public Health Association; 2009. [Google Scholar]
- 4.Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(Suppl):519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]
- 5.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 6.Claeson M, Gillespie D, Mshinda H, Troedsson H, Victora CG, Bellagio Study Group on Child Survival Knowledge into action for child survival. Lancet. 2003;362:323–7. doi: 10.1016/S0140-6736(03)13977-3. [DOI] [PubMed] [Google Scholar]
- 7.Victora CG, Wagstaff A, Schellenberg JA, Gwatkin D, Claeson M, Habicht J-P. Applying an equity lens to child health and mortality: more of the same is not enough. Lancet. 2003;362:233–41. doi: 10.1016/S0140-6736(03)13917-7. [DOI] [PubMed] [Google Scholar]
- 8.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L, Lancet Neonatal Survival Steering Team Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 9.Knippenberg R, Lawn JE, Darmstadt GL, Begkoyian G, Fogstad H, Walelign N, et al. Lancet Neonatal Survival Steering Team Systematic scaling up of neonatal care in countries. Lancet. 2005;365:1087–98. doi: 10.1016/S0140-6736(05)71145-4. [DOI] [PubMed] [Google Scholar]
- 10.Martines J, Paul VK, Bhutta ZA, Koblinsky M, Soucat A, Walker N, et al. Lancet Neonatal Survival Steering Team Neonatal survival: a call for action. Lancet. 2005;365:1189–97. doi: 10.1016/S0140-6736(05)71882-1. [DOI] [PubMed] [Google Scholar]
- 11.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. Maternal and Child Undernutrition Study Group What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–40. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 12.Haines A, Sanders D, Lehmann U, Rowe AK, Lawn JE, Jan S, et al. Achieving child survival goals: potential contribution of community health workers. Lancet. 2007;369:2121–31. doi: 10.1016/S0140-6736(07)60325-0. [DOI] [PubMed] [Google Scholar]
- 13.Hongoro C, McPake B. How to bridge the gap in human resources for health. Lancet. 2004;364:1451–6. doi: 10.1016/S0140-6736(04)17229-2. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan M, Kraschnewski JL, Nishikawa B, Morgan LC, Honeycutt AA, Thieda P, et al. Outcomes and costs of community health worker interventions: a systematic review. Med Care. 2010;48:792–808. doi: 10.1097/MLR.0b013e3181e35b51. [DOI] [PubMed] [Google Scholar]
- 15.Bhutta ZA, Lassi ZS, Pariyo G, Huicho L. Global experiences of community health workers for delivering health related millennium development goals: a systematic review, country case studies, and recommendations for integration into national health systems. Geneva: WHO Global Health Workforce Alliance; 2010. [Google Scholar]
- 16.Singh Pa. One million community health workers: technical task force report. New York: Earth Institute, Columbia University; 2011. [Google Scholar]
- 17.Hunt S. Lady health worker programme: third party evaluation of performance. Oxford: Oxford Policy Management; 2009. [Google Scholar]
- 18.United Nations Children’s Fund [Internet]. Supply catalogue. New York: UNICEF; 2012. Available from: https://supply.unicef.org/unicef_b2c/app/displayApp/(layout=7.0-12_1_66_67_115&carea=%24ROOT)/.do?rf=y [accessed 29 January 2013].
- 19.United Nations Agency for International Development & United Nations Children’s Fund & World Health Organization. Diarrhoea treatment guidelines: including new recommendations for the use of ORS and zinc supplementation for clinic-based healthcare workers. Arlington: Management of Social Transformations Project; 2005. [Google Scholar]
- 20.Bern C. Diarrhoeal diseases. In: Global epidemiology of infectious diseases. Geneva: World Health Organization; 2004. [Google Scholar]
- 21.Teklehaimanot A, McCord GC, Sachs JD. Scaling up malaria control in Africa: an economic and epidemiological assessment. Am J Trop Med Hyg. 2007;77:138–44. [PubMed] [Google Scholar]
- 22.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization [Internet]. Neglected tropical diseases: PCT databank, schistosomiasis. Geneva: WHO; 2005. Available from: http://www.who.int/neglected_diseases/preventive_chemotherapy/sch/en/index.html [accessed 29 January 2013].
- 24.Pneumonia: the forgotten killer of children. New York & Geneva: United Nations Children’s Fund & World Health Organization; 2006. [Google Scholar]
- 25.Singh S, Darroch JE. Adding it up: costs and benefits of contraceptive services – estimates for 2012 Guttmacher Institute & United Nations Population Fund; 2012. Available from: http://www.guttmacher.org/pubs/AIU-2012-estimates.pdf [accessed 29 January 2013].
- 26.Mukherjee JS, Eustache FE. Community health workers as a cornerstone for integrating HIV and primary healthcare. AIDS Care. 2007;19:S73–82. doi: 10.1080/09540120601114485. [DOI] [PubMed] [Google Scholar]
- 27.Center for International Earth Science Information Network/Columbia University, International Food Policy Research Institute, The World Bank & Centro Internacional de Agricultura Tropical. Global Rural-Urban Mapping Project (GRUMP): urban/rural extents. Palisades: NASA Socioeconomic Data and Applications Center; 2004. Available from: http://sedac.ciesin.columbia.edu/data/set/grump-v1-urban-extents [accessed 29 January 2013]. [Google Scholar]
- 28.Taskforce on Innovative International Financing for Health Systems. More money for health, and more health for the money: final report. Geneva: International Health Partnership; 2009. [Google Scholar]
- 29.Millenium Project [Internet]. Investing in development: a practical plan to achieve the Millennium Development Goals. London: United Nations Development Programme; 2005: London. [Google Scholar]
- 30.Macroeconomics and health: investing in health for economic development. Geneva: World Health Organization; 2001. [Google Scholar]
- 31.Liu A, Sullivan S, Khan M, Sachs S, Singh P. Community health workers in global health: scale and scalability. Mt Sinai J Med. 2011;78:419–35. doi: 10.1002/msj.20260. [DOI] [PubMed] [Google Scholar]
- 32.Crigler L, Hill K, Furth R, Bjerregaard D. Community Health Worker Assessment and Improvement Matrix (CHW AIM): a toolkit for improving CHW programs and services Washington: United States Agency for International Development; 2011. [Google Scholar]