Abstract
The asymmetric unit of the title compound, C20H18O6 (systematic name: 1β,3β-dihydroxy-2β-methoxyfuro[4′,3′,2′:4,5,6]-18-norandrosta-8,11,13-triene-7,17-dione), a dihydro derivative of the fungal steroid viridin, contains two molecules with similar conformations. The rings bearing the hydroxy groups adopt boat conformations. The absolute structure was assigned based on the known chirality of a precursor compound. In the crystal, molecules are linked by O—H⋯O hydrogen bonds, generating a three-dimensional network and weak C—H⋯O interactions consolidate the packing.
Related literature
For background to fungal metabolites, see: Brian & McGowan (1945 ▶); Moffatt et al. (1969 ▶); Jones & Hancock (1987 ▶); Hanson (1995 ▶); Cross et al. (1995 ▶); Przybyl (2002 ▶); Smith et al. (2009 ▶); Andersson et al. (2010 ▶); Queloz et al. (2011 ▶); Andersson (2012 ▶); Andersson et al. (2012 ▶, 2013 ▶). For related structures, see: Neidle et al. (1972 ▶); Lang et al. (2009 ▶). For other characterization methods, see: Brian et al. (1957 ▶); Aldridge et al. (1975 ▶); Blight & Grove (1986 ▶). For background to the assignment of the absolute structure of the title compound, see: MacMillan et al. (1972 ▶); Harrison (1990 ▶); Dewick (2002 ▶); Wipf & Kerekes (2003 ▶); Flack & Bernardinelli (2000 ▶).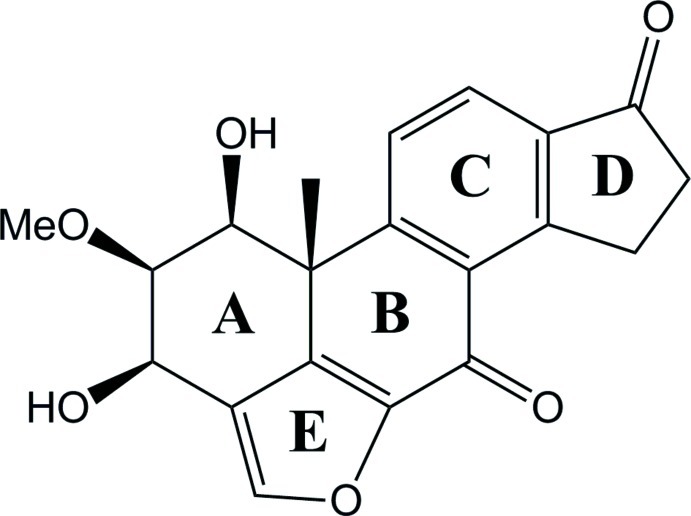
Experimental
Crystal data
C20H18O6
M r = 354.34
Orthorhombic,

a = 6.8285 (2) Å
b = 20.1939 (6) Å
c = 22.4344 (6) Å
V = 3093.57 (15) Å3
Z = 8
Mo Kα radiation
μ = 0.11 mm−1
T = 93 K
0.3 × 0.25 × 0.2 mm
Data collection
Oxford Diffraction XcaliburIII Sapphire-3 CCD diffractometer
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2007 ▶) T min = 0.891, T max = 1.000
26916 measured reflections
4874 independent reflections
3294 reflections with I > 2σ(I)
R int = 0.085
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.089
S = 0.91
4874 reflections
485 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.33 e Å−3
Δρmin = −0.25 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2007 ▶); cell refinement: CrysAlis RED (Oxford Diffraction, 2007 ▶); data reduction: CrysAlis RED; program(s) used to solve structure: SHELXD (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXL (Sheldrick, 2008 ▶); software used to prepare material for publication: DIAMOND (Brandenburg, 2001 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813005606/hb7028sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813005606/hb7028Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813005606/hb7028Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O19A—H19A⋯O19B i | 0.85 (3) | 2.08 (3) | 2.886 (3) | 160 (3) |
| O19B—H19B⋯O20A ii | 0.83 (3) | 2.30 (3) | 3.002 (3) | 143 (3) |
| O20A—H20A⋯O25B iii | 0.91 (3) | 1.85 (3) | 2.717 (3) | 160 (3) |
| O20B—H20B⋯O24A iv | 0.81 (3) | 2.05 (3) | 2.842 (3) | 166 (3) |
| C2A—H2A⋯O23B v | 1.00 | 2.45 | 3.325 (3) | 146 |
| C2B—H2B⋯O23A | 1.00 | 2.38 | 3.295 (3) | 151 |
| C11A—H11A⋯O19A | 0.95 | 2.43 | 3.084 (3) | 126 |
| C11B—H11B⋯O19B | 0.95 | 2.58 | 3.230 (3) | 126 |
| C18A—H18A⋯O20A | 0.98 | 2.38 | 3.227 (3) | 145 |
| C18B—H18D⋯O20B | 0.98 | 2.39 | 3.241 (4) | 145 |
| C21A—H21A⋯O24A vi | 0.95 | 2.37 | 3.282 (3) | 162 |
| C21B—H21B⋯O24B vii | 0.95 | 2.25 | 3.180 (3) | 167 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  ; (vii)
; (vii)  .
.
Acknowledgments
We are grateful to Dr Lars Eriksson for skillful technical assistance and fruitful discussions. The isolate Ö3 from which the viridiol was obtained was from a study which was, in part, supported by the Swedish research council FORMAS grant No. 2010–1344.
supplementary crystallographic information
Comment
The disease known as dieback of ash on European ash (Fraxinus excelsior) was first observed in Poland in 1995, but has spread rapidly over most of the European subcontinent (Przybyl, 2002). During studies of the secondary metabolite production of the fungus Hymenoscyphus pseudoalbidus, the pathogen responsible for dieback of ash (Queloz et al., 2011), a number of steroidal compounds have been isolated (Andersson et al., 2010; Andersson et al. 2013; Andersson et al. 2012). These compounds belong to a family of fungal steroids (Hanson, 1995), of which some have been shown to have interesting bioactivities (Cross et al. 1995; Smith et al. 2009; Andersson 2012).
The first reported compound of this family was viridin (Brian & McGowan, 1945), with its crystal structure published nearly 30 years later (Neidle et al., 1972). The absolute structures of a few other members have been reported through successful osmylation (Lang et al. 2009). Other members of the same family have also been characterized through other methods than crystallography, including wortmannin (Brian et al., 1957), demethoxyviridin and demethoxyviridiol (Aldridge et al., 1975), and virone and wortmannolone (Blight & Grove, 1986). The phytotoxin viridiol (Moffatt et al., 1969), which has also been suggested to be part of the pathogenicity of H. pseudoalbidus (Andersson et al. 2010), can be produced in Gliocladium virens from viridin (Jones & Hancock, 1987). The previously reported absolute configuration of these compounds are based on the evidence of their steroidal origin i. e. the configuration of the C10 carbon is based on lanosterol (Dewick, 2002; Harrison, 1990). Here, we present the crystal structure of viridiol (I) confirming the previously presented structure, both relative and absolute (MacMillan et al., 1972; Moffatt et al., 1969; Wipf & Kerekes, 2003) (Scheme 1, Fig. 1)
Compound (I) crystallizes in the orthorhombic space group P212121 (No. 19), with two crystallographically independent viridiol molecules with a total of eight in the unit cell. (Fig. 2) The nearly flat furanosteroid skeleton lies in the bc plane, with the A ring and its methoxy group bending away from the plane (Fig. 1). In the crystal, O—H···O and C—H···O interactions link the molecules (Table 1, Fig. 3).
Experimental
The viridiol containing fraction from a previous study (Andersson et al., 2013) was subjected to rotatory evaporation, which lead to crystal formation. The crystals, too small for crystallography, were harvested by filtration and dried (approx. 3 mg). The crystals were subsequently dissolved in 80 °C toluene (4 ml) in a 5 ml test tube. The solution was left at room temperature and the toluene was evaporated slowly by a gentle stream of nitrogen gas. Large enough crystals formed at the bottom of the test tube after stepwise precipitation of impurities on the inner test tube wall. A colourless block was mounted on a glass capillary and a data set was measured under cold conditions (93 K).
Refinement
After initial integration, the furanosteroid backbone was found through refinements using SHELXD. After additional cycles in SHELXL, the remaining atoms were found. No restraints were applied to the carbon skeleton. All non-H atoms were refined anisotropically. Hydrogen atoms on carbons were refined as riding on their respective carbon, while the two hydroxy hydrogen were fully refined. In the absence of any significant anomalous scattering, the Flack parameter was indeterminate (Flack & Bernardinelli, 2000). Hence, the Friedel equivalents were merged prior to the final refinements, and the absolute structure was set by reference to the known chirality of the pathway for the previously reported precursor lanosterol (Dewick, 2002; Harrison, 1990) (Fig. 4).
Figures
Fig. 1.
Labelling of (I) follows the system used previously (Aldridge et al., 1975). H atoms on alkyl and aryl carbons have been removed for clarity. Displacement ellipoids are set at 50%.
Fig. 2.
Unit cell packing of (I), viewed along the a axis.
Fig. 3.
One intramolecular and many intermolecular hydrogen bonds in the range 1.85–2.58 Å are present in (I), including both conventional (O–H···O) and non-conventional (C–H···O) ones.
Fig. 4.
ORTEP plot of (I).
Crystal data
| C20H18O6 | F(000) = 1488 |
| Mr = 354.34 | Dx = 1.522 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 5568 reflections |
| a = 6.8285 (2) Å | θ = 2.9–32.3° |
| b = 20.1939 (6) Å | µ = 0.11 mm−1 |
| c = 22.4344 (6) Å | T = 93 K |
| V = 3093.57 (15) Å3 | Block, colourless |
| Z = 8 | 0.3 × 0.25 × 0.2 mm |
Data collection
| Oxford Diffraction XcaliburIII Sapphire-3 CCD diffractometer | 4874 independent reflections |
| Radiation source: fine-focus sealed tube | 3294 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.085 |
| Detector resolution: 16.5467 pixels mm-1 | θmax = 29.6°, θmin = 3.1° |
| ω scans at different φ | h = −9→8 |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2007) | k = −27→28 |
| Tmin = 0.891, Tmax = 1.000 | l = −31→27 |
| 26916 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.049 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.089 | w = 1/[σ2(Fo2) + (0.0383P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.91 | (Δ/σ)max < 0.001 |
| 4874 reflections | Δρmax = 0.33 e Å−3 |
| 485 parameters | Δρmin = −0.25 e Å−3 |
| 0 restraints | Absolute structure: syn |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1A | 0.6537 (4) | 0.27709 (14) | 0.36272 (12) | 0.0138 (6) | |
| H1A | 0.7696 | 0.2648 | 0.3378 | 0.017* | |
| C2A | 0.7233 (4) | 0.33366 (13) | 0.40850 (12) | 0.0126 (6) | |
| H2A | 0.8510 | 0.3506 | 0.3928 | 0.015* | |
| C3A | 0.5943 (4) | 0.39445 (13) | 0.41746 (12) | 0.0133 (6) | |
| H3A | 0.6728 | 0.4296 | 0.4379 | 0.016* | |
| C4A | 0.5392 (4) | 0.41850 (13) | 0.35652 (12) | 0.0130 (6) | |
| C5A | 0.5063 (4) | 0.37163 (12) | 0.31066 (11) | 0.0112 (5) | |
| C6A | 0.4956 (5) | 0.40461 (12) | 0.25876 (11) | 0.0144 (6) | |
| C7A | 0.4926 (4) | 0.37424 (13) | 0.20055 (11) | 0.0142 (6) | |
| C8A | 0.4930 (5) | 0.29928 (12) | 0.20388 (11) | 0.0129 (6) | |
| C9A | 0.4948 (4) | 0.26436 (12) | 0.25865 (11) | 0.0111 (6) | |
| C10A | 0.4855 (5) | 0.29948 (12) | 0.31901 (11) | 0.0125 (6) | |
| C11A | 0.4926 (5) | 0.19481 (13) | 0.25915 (11) | 0.0154 (6) | |
| H11A | 0.4941 | 0.1721 | 0.2962 | 0.019* | |
| C12A | 0.4883 (5) | 0.15934 (13) | 0.20748 (11) | 0.0142 (6) | |
| H12A | 0.4869 | 0.1123 | 0.2082 | 0.017* | |
| C13A | 0.4860 (5) | 0.19347 (13) | 0.15357 (11) | 0.0128 (6) | |
| C14A | 0.4907 (5) | 0.26182 (13) | 0.15062 (11) | 0.0128 (6) | |
| C15A | 0.4908 (5) | 0.28556 (13) | 0.08641 (11) | 0.0170 (6) | |
| H15A | 0.6103 | 0.3115 | 0.0776 | 0.020* | |
| H15B | 0.3744 | 0.3133 | 0.0781 | 0.020* | |
| C16A | 0.4857 (5) | 0.22141 (13) | 0.04961 (11) | 0.0169 (6) | |
| H16A | 0.3696 | 0.2209 | 0.0232 | 0.020* | |
| H16B | 0.6048 | 0.2177 | 0.0247 | 0.020* | |
| C17A | 0.4762 (4) | 0.16537 (14) | 0.09340 (11) | 0.0144 (6) | |
| C18A | 0.2815 (4) | 0.28525 (15) | 0.34602 (12) | 0.0152 (6) | |
| H18A | 0.2704 | 0.3073 | 0.3848 | 0.023* | |
| H18B | 0.2650 | 0.2374 | 0.3512 | 0.023* | |
| H18C | 0.1798 | 0.3021 | 0.3192 | 0.023* | |
| O19A | 0.5889 (3) | 0.21883 (10) | 0.39170 (9) | 0.0183 (5) | |
| H19A | 0.690 (5) | 0.1979 (16) | 0.4026 (14) | 0.027* | |
| O20A | 0.4303 (3) | 0.37778 (10) | 0.45422 (9) | 0.0175 (5) | |
| H20A | 0.360 (5) | 0.4134 (16) | 0.4665 (13) | 0.026* | |
| C21A | 0.5428 (4) | 0.47768 (14) | 0.32940 (12) | 0.0169 (7) | |
| H21A | 0.5620 | 0.5185 | 0.3495 | 0.020* | |
| O22A | 0.5152 (3) | 0.47204 (8) | 0.26879 (7) | 0.0163 (4) | |
| O23A | 0.4968 (3) | 0.40411 (9) | 0.15318 (8) | 0.0202 (5) | |
| O24A | 0.4658 (3) | 0.10611 (9) | 0.08101 (8) | 0.0197 (5) | |
| O25A | 0.7644 (3) | 0.30680 (10) | 0.46491 (8) | 0.0155 (5) | |
| C26A | 0.9470 (5) | 0.27292 (15) | 0.46869 (13) | 0.0198 (7) | |
| H26A | 0.9599 | 0.2425 | 0.4349 | 0.030* | |
| H26B | 0.9525 | 0.2478 | 0.5060 | 0.030* | |
| H26C | 1.0542 | 0.3052 | 0.4678 | 0.030* | |
| C1B | 0.1585 (4) | 0.56087 (14) | 0.11712 (12) | 0.0138 (6) | |
| H1B | 0.2773 | 0.5707 | 0.1416 | 0.017* | |
| C2B | 0.2209 (4) | 0.50356 (13) | 0.07249 (11) | 0.0122 (6) | |
| H2B | 0.3410 | 0.4826 | 0.0897 | 0.015* | |
| C3B | 0.0753 (4) | 0.44728 (14) | 0.06042 (12) | 0.0142 (6) | |
| H3B | 0.1451 | 0.4112 | 0.0385 | 0.017* | |
| C4B | 0.0186 (5) | 0.42186 (13) | 0.12046 (12) | 0.0148 (6) | |
| C5B | 0.0000 (5) | 0.46678 (12) | 0.16840 (11) | 0.0122 (6) | |
| C6B | −0.0095 (5) | 0.43195 (12) | 0.21909 (12) | 0.0153 (6) | |
| C7B | 0.0047 (5) | 0.45916 (13) | 0.27823 (12) | 0.0147 (6) | |
| C8B | 0.0128 (5) | 0.53407 (12) | 0.27772 (11) | 0.0131 (6) | |
| C9B | 0.0140 (4) | 0.57193 (13) | 0.22427 (11) | 0.0124 (6) | |
| C10B | −0.0063 (5) | 0.54023 (12) | 0.16285 (11) | 0.0122 (6) | |
| C11B | 0.0197 (4) | 0.64112 (12) | 0.22764 (11) | 0.0130 (6) | |
| H11B | 0.0221 | 0.6661 | 0.1917 | 0.016* | |
| C12B | 0.0220 (5) | 0.67419 (13) | 0.28128 (11) | 0.0142 (6) | |
| H12B | 0.0244 | 0.7212 | 0.2827 | 0.017* | |
| C13B | 0.0207 (4) | 0.63669 (13) | 0.33360 (11) | 0.0111 (6) | |
| C14B | 0.0183 (4) | 0.56769 (12) | 0.33227 (11) | 0.0126 (6) | |
| C15B | 0.0213 (5) | 0.53980 (13) | 0.39498 (11) | 0.0143 (6) | |
| H15C | 0.1383 | 0.5116 | 0.4011 | 0.017* | |
| H15D | −0.0974 | 0.5130 | 0.4027 | 0.017* | |
| C16B | 0.0272 (5) | 0.60024 (13) | 0.43590 (11) | 0.0152 (6) | |
| H16C | −0.0855 | 0.5998 | 0.4636 | 0.018* | |
| H16D | 0.1496 | 0.6006 | 0.4596 | 0.018* | |
| C17B | 0.0180 (5) | 0.66011 (13) | 0.39581 (11) | 0.0148 (6) | |
| C18B | −0.2121 (5) | 0.55864 (15) | 0.13852 (13) | 0.0175 (7) | |
| H18D | −0.2304 | 0.5386 | 0.0991 | 0.026* | |
| H18E | −0.2231 | 0.6069 | 0.1353 | 0.026* | |
| H18F | −0.3128 | 0.5420 | 0.1658 | 0.026* | |
| O19B | 0.1100 (3) | 0.62125 (10) | 0.08747 (9) | 0.0187 (5) | |
| H19B | 0.052 (5) | 0.6121 (16) | 0.0560 (14) | 0.028* | |
| O20B | −0.0878 (3) | 0.46870 (10) | 0.02504 (9) | 0.0194 (5) | |
| H20B | −0.090 (5) | 0.4507 (15) | −0.0072 (14) | 0.029* | |
| C21B | 0.0133 (5) | 0.36090 (14) | 0.14567 (12) | 0.0180 (6) | |
| H21B | 0.0199 | 0.3206 | 0.1240 | 0.022* | |
| O22B | −0.0029 (3) | 0.36481 (8) | 0.20658 (8) | 0.0182 (4) | |
| O23B | 0.0138 (4) | 0.42681 (9) | 0.32411 (8) | 0.0211 (5) | |
| O24B | 0.0124 (4) | 0.71751 (9) | 0.41237 (8) | 0.0244 (5) | |
| O25B | 0.2759 (3) | 0.53000 (9) | 0.01613 (8) | 0.0145 (4) | |
| C26B | 0.4663 (4) | 0.55951 (14) | 0.01558 (12) | 0.0165 (6) | |
| H26D | 0.4740 | 0.5934 | 0.0468 | 0.025* | |
| H26E | 0.4899 | 0.5800 | −0.0233 | 0.025* | |
| H26F | 0.5654 | 0.5254 | 0.0229 | 0.025* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1A | 0.0182 (16) | 0.0109 (15) | 0.0122 (14) | 0.0002 (12) | 0.0016 (12) | 0.0007 (12) |
| C2A | 0.0151 (16) | 0.0110 (14) | 0.0118 (15) | 0.0000 (12) | −0.0030 (12) | 0.0008 (12) |
| C3A | 0.0185 (16) | 0.0079 (14) | 0.0136 (14) | −0.0002 (12) | 0.0016 (12) | −0.0013 (12) |
| C4A | 0.0125 (16) | 0.0121 (14) | 0.0145 (14) | 0.0004 (12) | −0.0001 (12) | −0.0001 (11) |
| C5A | 0.0091 (14) | 0.0125 (13) | 0.0119 (13) | 0.0005 (13) | 0.0025 (13) | −0.0004 (10) |
| C6A | 0.0152 (15) | 0.0095 (14) | 0.0185 (14) | 0.0027 (14) | 0.0001 (14) | 0.0001 (11) |
| C7A | 0.0112 (15) | 0.0141 (13) | 0.0174 (14) | −0.0001 (14) | −0.0007 (14) | 0.0025 (12) |
| C8A | 0.0134 (15) | 0.0120 (13) | 0.0134 (13) | 0.0012 (14) | −0.0038 (13) | 0.0007 (11) |
| C9A | 0.0083 (14) | 0.0108 (13) | 0.0141 (13) | −0.0001 (13) | 0.0005 (13) | −0.0004 (10) |
| C10A | 0.0162 (16) | 0.0113 (13) | 0.0101 (12) | −0.0001 (14) | 0.0004 (13) | 0.0007 (10) |
| C11A | 0.0211 (16) | 0.0144 (14) | 0.0108 (13) | −0.0001 (15) | 0.0002 (14) | 0.0033 (11) |
| C12A | 0.0188 (16) | 0.0085 (12) | 0.0152 (14) | −0.0021 (14) | −0.0013 (14) | 0.0011 (11) |
| C13A | 0.0117 (15) | 0.0142 (13) | 0.0125 (13) | −0.0026 (14) | 0.0023 (13) | −0.0013 (11) |
| C14A | 0.0109 (15) | 0.0159 (14) | 0.0116 (13) | 0.0009 (14) | 0.0004 (13) | 0.0017 (10) |
| C15A | 0.0234 (17) | 0.0141 (14) | 0.0134 (13) | −0.0011 (15) | −0.0019 (14) | 0.0036 (11) |
| C16A | 0.0231 (17) | 0.0176 (14) | 0.0099 (13) | −0.0016 (15) | 0.0023 (14) | 0.0009 (11) |
| C17A | 0.0124 (16) | 0.0169 (14) | 0.0139 (14) | 0.0008 (14) | 0.0010 (13) | −0.0039 (12) |
| C18A | 0.0186 (16) | 0.0125 (15) | 0.0145 (15) | −0.0011 (13) | 0.0036 (12) | −0.0005 (12) |
| O19A | 0.0266 (14) | 0.0097 (11) | 0.0186 (11) | −0.0012 (10) | −0.0061 (9) | 0.0043 (9) |
| O20A | 0.0188 (12) | 0.0145 (11) | 0.0191 (11) | 0.0039 (9) | 0.0067 (9) | −0.0023 (9) |
| C21A | 0.0189 (18) | 0.0146 (15) | 0.0172 (15) | 0.0015 (13) | −0.0044 (13) | −0.0010 (12) |
| O22A | 0.0256 (12) | 0.0077 (9) | 0.0156 (10) | −0.0006 (10) | −0.0014 (10) | 0.0008 (7) |
| O23A | 0.0269 (12) | 0.0153 (10) | 0.0185 (10) | −0.0001 (11) | −0.0040 (11) | 0.0042 (8) |
| O24A | 0.0285 (13) | 0.0172 (11) | 0.0134 (10) | −0.0004 (10) | 0.0014 (9) | −0.0024 (8) |
| O25A | 0.0172 (12) | 0.0160 (11) | 0.0133 (10) | 0.0024 (9) | −0.0013 (8) | 0.0035 (9) |
| C26A | 0.0192 (18) | 0.0183 (16) | 0.0220 (16) | 0.0018 (14) | −0.0027 (13) | 0.0008 (13) |
| C1B | 0.0179 (17) | 0.0129 (16) | 0.0106 (14) | 0.0027 (13) | −0.0007 (12) | −0.0002 (12) |
| C2B | 0.0188 (16) | 0.0107 (14) | 0.0070 (13) | 0.0024 (12) | −0.0005 (12) | 0.0002 (11) |
| C3B | 0.0170 (17) | 0.0102 (14) | 0.0153 (15) | 0.0035 (13) | −0.0002 (12) | −0.0018 (12) |
| C4B | 0.0114 (15) | 0.0133 (14) | 0.0196 (15) | −0.0031 (14) | 0.0018 (13) | −0.0041 (11) |
| C5B | 0.0114 (15) | 0.0102 (13) | 0.0151 (14) | 0.0002 (14) | −0.0001 (13) | −0.0042 (10) |
| C6B | 0.0190 (16) | 0.0067 (13) | 0.0202 (14) | −0.0009 (14) | 0.0039 (14) | −0.0019 (11) |
| C7B | 0.0137 (15) | 0.0125 (13) | 0.0178 (14) | 0.0023 (13) | 0.0022 (14) | 0.0019 (11) |
| C8B | 0.0148 (15) | 0.0104 (13) | 0.0142 (13) | 0.0035 (13) | −0.0013 (13) | 0.0000 (11) |
| C9B | 0.0123 (15) | 0.0126 (14) | 0.0124 (13) | −0.0020 (13) | 0.0000 (13) | −0.0003 (10) |
| C10B | 0.0155 (15) | 0.0082 (13) | 0.0131 (13) | 0.0033 (14) | −0.0008 (13) | −0.0012 (10) |
| C11B | 0.0161 (16) | 0.0105 (13) | 0.0125 (13) | 0.0002 (13) | −0.0015 (13) | 0.0032 (10) |
| C12B | 0.0181 (17) | 0.0073 (13) | 0.0173 (14) | −0.0008 (13) | 0.0001 (13) | −0.0024 (11) |
| C13B | 0.0078 (14) | 0.0156 (14) | 0.0101 (13) | 0.0014 (13) | −0.0008 (12) | −0.0015 (10) |
| C14B | 0.0090 (15) | 0.0132 (14) | 0.0157 (14) | 0.0013 (13) | −0.0007 (13) | 0.0045 (11) |
| C15B | 0.0186 (17) | 0.0111 (13) | 0.0132 (13) | 0.0009 (14) | 0.0009 (13) | 0.0024 (10) |
| C16B | 0.0173 (16) | 0.0174 (14) | 0.0109 (13) | 0.0006 (14) | −0.0021 (12) | 0.0025 (11) |
| C17B | 0.0142 (16) | 0.0133 (14) | 0.0170 (14) | −0.0006 (14) | −0.0030 (13) | −0.0029 (11) |
| C18B | 0.0209 (17) | 0.0149 (16) | 0.0167 (15) | 0.0024 (14) | −0.0062 (13) | −0.0018 (13) |
| O19B | 0.0324 (13) | 0.0112 (11) | 0.0125 (10) | 0.0024 (9) | −0.0019 (10) | 0.0015 (9) |
| O20B | 0.0196 (12) | 0.0227 (12) | 0.0160 (11) | 0.0050 (10) | −0.0058 (9) | −0.0068 (9) |
| C21B | 0.0187 (16) | 0.0165 (15) | 0.0187 (15) | −0.0018 (15) | 0.0013 (14) | −0.0054 (12) |
| O22B | 0.0256 (12) | 0.0097 (9) | 0.0195 (10) | −0.0004 (10) | 0.0039 (11) | −0.0013 (8) |
| O23B | 0.0325 (13) | 0.0138 (10) | 0.0169 (10) | 0.0022 (11) | 0.0044 (11) | 0.0042 (8) |
| O24B | 0.0435 (14) | 0.0147 (10) | 0.0151 (10) | −0.0009 (12) | −0.0042 (11) | −0.0013 (8) |
| O25B | 0.0178 (11) | 0.0138 (10) | 0.0118 (10) | 0.0004 (9) | −0.0003 (8) | 0.0019 (8) |
| C26B | 0.0149 (17) | 0.0177 (14) | 0.0169 (14) | 0.0017 (13) | −0.0008 (13) | −0.0007 (12) |
Geometric parameters (Å, º)
| C1A—O19A | 1.415 (3) | C1B—O19B | 1.428 (3) |
| C1A—C10A | 1.576 (4) | C1B—C10B | 1.579 (4) |
| C1A—C2A | 1.608 (4) | C1B—C2B | 1.589 (4) |
| C1A—H1A | 1.0000 | C1B—H1B | 1.0000 |
| C2A—O25A | 1.405 (3) | C2B—O25B | 1.423 (3) |
| C2A—C3A | 1.524 (4) | C2B—C3B | 1.534 (4) |
| C2A—H2A | 1.0000 | C2B—H2B | 1.0000 |
| C3A—O20A | 1.431 (3) | C3B—O20B | 1.434 (3) |
| C3A—C4A | 1.499 (4) | C3B—C4B | 1.492 (4) |
| C3A—H3A | 1.0000 | C3B—H3B | 1.0000 |
| C4A—C21A | 1.341 (4) | C4B—C21B | 1.355 (4) |
| C4A—C5A | 1.416 (3) | C4B—C5B | 1.413 (3) |
| C5A—C6A | 1.343 (3) | C5B—C6B | 1.339 (3) |
| C5A—C10A | 1.476 (4) | C5B—C10B | 1.489 (3) |
| C6A—O22A | 1.386 (3) | C6B—O22B | 1.385 (3) |
| C6A—C7A | 1.443 (4) | C6B—C7B | 1.439 (4) |
| C7A—O23A | 1.222 (3) | C7B—O23B | 1.221 (3) |
| C7A—C8A | 1.516 (3) | C7B—C8B | 1.514 (4) |
| C8A—C14A | 1.414 (3) | C8B—C14B | 1.400 (4) |
| C8A—C9A | 1.417 (3) | C8B—C9B | 1.422 (3) |
| C9A—C11A | 1.405 (4) | C9B—C11B | 1.400 (3) |
| C9A—C10A | 1.530 (3) | C9B—C10B | 1.526 (3) |
| C10A—C18A | 1.546 (4) | C10B—C18B | 1.553 (4) |
| C11A—C12A | 1.363 (3) | C11B—C12B | 1.377 (3) |
| C11A—H11A | 0.9500 | C11B—H11B | 0.9500 |
| C12A—C13A | 1.392 (3) | C12B—C13B | 1.397 (3) |
| C12A—H12A | 0.9500 | C12B—H12B | 0.9500 |
| C13A—C14A | 1.382 (4) | C13B—C14B | 1.394 (3) |
| C13A—C17A | 1.466 (4) | C13B—C17B | 1.474 (4) |
| C14A—C15A | 1.518 (3) | C14B—C15B | 1.515 (3) |
| C15A—C16A | 1.537 (3) | C15B—C16B | 1.528 (4) |
| C15A—H15A | 0.9900 | C15B—H15C | 0.9900 |
| C15A—H15B | 0.9900 | C15B—H15D | 0.9900 |
| C16A—C17A | 1.500 (4) | C16B—C17B | 1.508 (4) |
| C16A—H16A | 0.9900 | C16B—H16C | 0.9900 |
| C16A—H16B | 0.9900 | C16B—H16D | 0.9900 |
| C17A—O24A | 1.231 (3) | C17B—O24B | 1.218 (3) |
| C18A—H18A | 0.9800 | C18B—H18D | 0.9800 |
| C18A—H18B | 0.9800 | C18B—H18E | 0.9800 |
| C18A—H18C | 0.9800 | C18B—H18F | 0.9800 |
| O19A—H19A | 0.84 (3) | O19B—H19B | 0.83 (3) |
| O20A—H20A | 0.91 (3) | O20B—H20B | 0.81 (3) |
| C21A—O22A | 1.377 (3) | C21B—O22B | 1.373 (3) |
| C21A—H21A | 0.9500 | C21B—H21B | 0.9500 |
| O25A—C26A | 1.425 (4) | O25B—C26B | 1.430 (3) |
| C26A—H26A | 0.9800 | C26B—H26D | 0.9800 |
| C26A—H26B | 0.9800 | C26B—H26E | 0.9800 |
| C26A—H26C | 0.9800 | C26B—H26F | 0.9800 |
| O19A—C1A—C10A | 107.2 (2) | O19B—C1B—C10B | 111.3 (2) |
| O19A—C1A—C2A | 112.9 (2) | O19B—C1B—C2B | 113.0 (2) |
| C10A—C1A—C2A | 114.1 (2) | C10B—C1B—C2B | 114.1 (2) |
| O19A—C1A—H1A | 107.4 | O19B—C1B—H1B | 105.9 |
| C10A—C1A—H1A | 107.4 | C10B—C1B—H1B | 105.9 |
| C2A—C1A—H1A | 107.4 | C2B—C1B—H1B | 105.9 |
| O25A—C2A—C3A | 107.9 (2) | O25B—C2B—C3B | 107.0 (2) |
| O25A—C2A—C1A | 111.1 (2) | O25B—C2B—C1B | 110.9 (2) |
| C3A—C2A—C1A | 119.0 (2) | C3B—C2B—C1B | 118.5 (2) |
| O25A—C2A—H2A | 106.0 | O25B—C2B—H2B | 106.6 |
| C3A—C2A—H2A | 106.0 | C3B—C2B—H2B | 106.6 |
| C1A—C2A—H2A | 106.0 | C1B—C2B—H2B | 106.6 |
| O20A—C3A—C4A | 113.9 (2) | O20B—C3B—C4B | 113.7 (2) |
| O20A—C3A—C2A | 109.8 (2) | O20B—C3B—C2B | 112.2 (2) |
| C4A—C3A—C2A | 106.6 (2) | C4B—C3B—C2B | 105.3 (2) |
| O20A—C3A—H3A | 108.8 | O20B—C3B—H3B | 108.5 |
| C4A—C3A—H3A | 108.8 | C4B—C3B—H3B | 108.5 |
| C2A—C3A—H3A | 108.8 | C2B—C3B—H3B | 108.5 |
| C21A—C4A—C5A | 105.6 (2) | C21B—C4B—C5B | 105.3 (2) |
| C21A—C4A—C3A | 134.3 (3) | C21B—C4B—C3B | 134.1 (3) |
| C5A—C4A—C3A | 119.1 (2) | C5B—C4B—C3B | 119.3 (2) |
| C6A—C5A—C4A | 107.9 (2) | C6B—C5B—C4B | 108.3 (2) |
| C6A—C5A—C10A | 126.5 (2) | C6B—C5B—C10B | 126.4 (2) |
| C4A—C5A—C10A | 125.7 (2) | C4B—C5B—C10B | 125.3 (2) |
| C5A—C6A—O22A | 109.9 (2) | C5B—C6B—O22B | 109.9 (2) |
| C5A—C6A—C7A | 125.1 (2) | C5B—C6B—C7B | 125.4 (2) |
| O22A—C6A—C7A | 124.4 (2) | O22B—C6B—C7B | 123.9 (2) |
| O23A—C7A—C6A | 125.2 (2) | O23B—C7B—C6B | 125.2 (2) |
| O23A—C7A—C8A | 122.4 (2) | O23B—C7B—C8B | 122.6 (2) |
| C6A—C7A—C8A | 112.3 (2) | C6B—C7B—C8B | 112.1 (2) |
| C14A—C8A—C9A | 117.8 (2) | C14B—C8B—C9B | 118.4 (2) |
| C14A—C8A—C7A | 119.5 (2) | C14B—C8B—C7B | 118.6 (2) |
| C9A—C8A—C7A | 122.7 (2) | C9B—C8B—C7B | 123.0 (2) |
| C11A—C9A—C8A | 120.3 (2) | C11B—C9B—C8B | 119.4 (2) |
| C11A—C9A—C10A | 117.1 (2) | C11B—C9B—C10B | 118.0 (2) |
| C8A—C9A—C10A | 122.5 (2) | C8B—C9B—C10B | 122.4 (2) |
| C5A—C10A—C9A | 110.0 (2) | C5B—C10B—C9B | 109.9 (2) |
| C5A—C10A—C18A | 108.7 (2) | C5B—C10B—C18B | 107.1 (2) |
| C9A—C10A—C18A | 107.3 (2) | C9B—C10B—C18B | 107.4 (2) |
| C5A—C10A—C1A | 107.0 (2) | C5B—C10B—C1B | 107.3 (2) |
| C9A—C10A—C1A | 112.8 (2) | C9B—C10B—C1B | 114.3 (2) |
| C18A—C10A—C1A | 111.1 (2) | C18B—C10B—C1B | 110.7 (2) |
| C12A—C11A—C9A | 121.3 (2) | C12B—C11B—C9B | 122.1 (2) |
| C12A—C11A—H11A | 119.4 | C12B—C11B—H11B | 118.9 |
| C9A—C11A—H11A | 119.4 | C9B—C11B—H11B | 118.9 |
| C11A—C12A—C13A | 118.6 (2) | C11B—C12B—C13B | 118.1 (2) |
| C11A—C12A—H12A | 120.7 | C11B—C12B—H12B | 120.9 |
| C13A—C12A—H12A | 120.7 | C13B—C12B—H12B | 120.9 |
| C14A—C13A—C12A | 122.4 (2) | C14B—C13B—C12B | 121.6 (2) |
| C14A—C13A—C17A | 110.1 (2) | C14B—C13B—C17B | 109.9 (2) |
| C12A—C13A—C17A | 127.5 (2) | C12B—C13B—C17B | 128.4 (2) |
| C13A—C14A—C8A | 119.6 (2) | C13B—C14B—C8B | 120.2 (2) |
| C13A—C14A—C15A | 111.1 (2) | C13B—C14B—C15B | 110.6 (2) |
| C8A—C14A—C15A | 129.3 (2) | C8B—C14B—C15B | 129.2 (2) |
| C14A—C15A—C16A | 104.1 (2) | C14B—C15B—C16B | 105.2 (2) |
| C14A—C15A—H15A | 110.9 | C14B—C15B—H15C | 110.7 |
| C16A—C15A—H15A | 110.9 | C16B—C15B—H15C | 110.7 |
| C14A—C15A—H15B | 110.9 | C14B—C15B—H15D | 110.7 |
| C16A—C15A—H15B | 110.9 | C16B—C15B—H15D | 110.7 |
| H15A—C15A—H15B | 109.0 | H15C—C15B—H15D | 108.8 |
| C17A—C16A—C15A | 106.6 (2) | C17B—C16B—C15B | 106.3 (2) |
| C17A—C16A—H16A | 110.4 | C17B—C16B—H16C | 110.5 |
| C15A—C16A—H16A | 110.4 | C15B—C16B—H16C | 110.5 |
| C17A—C16A—H16B | 110.4 | C17B—C16B—H16D | 110.5 |
| C15A—C16A—H16B | 110.4 | C15B—C16B—H16D | 110.5 |
| H16A—C16A—H16B | 108.6 | H16C—C16B—H16D | 108.7 |
| O24A—C17A—C13A | 125.9 (2) | O24B—C17B—C13B | 126.5 (2) |
| O24A—C17A—C16A | 126.0 (2) | O24B—C17B—C16B | 125.6 (2) |
| C13A—C17A—C16A | 108.0 (2) | C13B—C17B—C16B | 107.9 (2) |
| C10A—C18A—H18A | 109.5 | C10B—C18B—H18D | 109.5 |
| C10A—C18A—H18B | 109.5 | C10B—C18B—H18E | 109.5 |
| H18A—C18A—H18B | 109.5 | H18D—C18B—H18E | 109.5 |
| C10A—C18A—H18C | 109.5 | C10B—C18B—H18F | 109.5 |
| H18A—C18A—H18C | 109.5 | H18D—C18B—H18F | 109.5 |
| H18B—C18A—H18C | 109.5 | H18E—C18B—H18F | 109.5 |
| C1A—O19A—H19A | 107 (2) | C1B—O19B—H19B | 108 (2) |
| C3A—O20A—H20A | 114 (2) | C3B—O20B—H20B | 112 (2) |
| C4A—C21A—O22A | 111.8 (2) | C4B—C21B—O22B | 111.4 (2) |
| C4A—C21A—H21A | 124.1 | C4B—C21B—H21B | 124.3 |
| O22A—C21A—H21A | 124.1 | O22B—C21B—H21B | 124.3 |
| C21A—O22A—C6A | 104.8 (2) | C21B—O22B—C6B | 105.11 (19) |
| C2A—O25A—C26A | 114.4 (2) | C2B—O25B—C26B | 113.8 (2) |
| O25A—C26A—H26A | 109.5 | O25B—C26B—H26D | 109.5 |
| O25A—C26A—H26B | 109.5 | O25B—C26B—H26E | 109.5 |
| H26A—C26A—H26B | 109.5 | H26D—C26B—H26E | 109.5 |
| O25A—C26A—H26C | 109.5 | O25B—C26B—H26F | 109.5 |
| H26A—C26A—H26C | 109.5 | H26D—C26B—H26F | 109.5 |
| H26B—C26A—H26C | 109.5 | H26E—C26B—H26F | 109.5 |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O19A—H19A···O19Bi | 0.85 (3) | 2.08 (3) | 2.886 (3) | 160 (3) |
| O19B—H19B···O20Aii | 0.83 (3) | 2.30 (3) | 3.002 (3) | 143 (3) |
| O20A—H20A···O25Biii | 0.91 (3) | 1.85 (3) | 2.717 (3) | 160 (3) |
| O20B—H20B···O24Aiv | 0.81 (3) | 2.05 (3) | 2.842 (3) | 166 (3) |
| C2A—H2A···O23Bv | 1.00 | 2.45 | 3.325 (3) | 146 |
| C2B—H2B···O23A | 1.00 | 2.38 | 3.295 (3) | 151 |
| C11A—H11A···O19A | 0.95 | 2.43 | 3.084 (3) | 126 |
| C11B—H11B···O19B | 0.95 | 2.58 | 3.230 (3) | 126 |
| C18A—H18A···O20A | 0.98 | 2.38 | 3.227 (3) | 145 |
| C18B—H18D···O20B | 0.98 | 2.39 | 3.241 (4) | 145 |
| C21A—H21A···O24Avi | 0.95 | 2.37 | 3.282 (3) | 162 |
| C21B—H21B···O24Bvii | 0.95 | 2.25 | 3.180 (3) | 167 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) −x+1/2, −y+1, z−1/2; (iii) −x+1/2, −y+1, z+1/2; (iv) x−1/2, −y+1/2, −z; (v) x+1, y, z; (vi) −x+1, y+1/2, −z+1/2; (vii) −x, y−1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB7028).
References
- Aldridge, D. C., Turner, W. B., Geddes, A. J. & Sheldrick, B. (1975). J. Chem. Soc. Perkin Trans. 1, pp. 943–945.
- Andersson, P. F. (2012). PhD thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden. Available at http://pub.epsilon.slu.se/8996.
- Andersson, P. F., Bengtsson, S., Cleary, M. R., Stenlid, J. & Broberg, A. (2013). Phytochemistry, 86, 195–200. [DOI] [PubMed]
- Andersson, P. F., Bengtsson, S., Stenlid, J. & Broberg, A. (2012). Molecules, 17, 7769–7781. [DOI] [PMC free article] [PubMed]
- Andersson, P. F., Johansson, S. B. K., Stenlid, J. & Broberg, A. (2010). For. Pathol. 40, 43–46.
- Blight, M. M. & Grove, J. F. (1986). J. Chem. Soc. Perkin Trans. 1, pp. 1317–1322.
- Brandenburg, K. (2001). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Brian, P. W., Curtis, P. J., Hemming, H. G. & Norris, G. L. F. (1957). Trans. Br. Mycol. Soc. 40, 365–368.
- Brian, P. W. & McGowan, J. C. (1945). Nature, 156, 144–145.
- Cross, M. J., Stewart, A., Hodgkin, M. N., Kerr, D. J. & Wakelam, M. J. O. (1995). J. Biol. Chem. 270, 25352–25355. [DOI] [PubMed]
- Dewick, P. M. (2002). Nat. Prod. Rep. 19, 181–222. [DOI] [PubMed]
- Flack, H. D. & Bernardinelli, G. (2000). J. Appl. Cryst. 33, 1143–1148.
- Hanson, J. R. (1995). Nat. Prod. Rep. 12, 381–384. [DOI] [PubMed]
- Harrison, D. M. (1990). Nat. Prod. Rep. 7, 459–484. [DOI] [PubMed]
- Jones, R. W. & Hancock, J. G. (1987). Can. J. Microbiol. 33, 963–966. [DOI] [PubMed]
- Lang, Y., Souza, F. E. S., Xu, X., Taylor, N. J., Assoud, A. & Rodrigo, R. (2009). J. Org. Chem. 74, 5429–5439. [DOI] [PubMed]
- MacMillan, J., Simpson, T. J., Vanstone, A. E. & Yeboah, S. K. (1972). J. Chem. Soc. Perkin Trans. 1, pp. 2892–2898.
- Moffatt, J. S., Bu’Lock, J. D. & Yuen, T. H. (1969). J. Chem. Soc. D, p. 839a.
- Neidle, S., Rogers, D. & Hursthouse, M. B. (1972). J. Chem. Soc. Perkin Trans. 2, pp. 760–766.
- Oxford Diffraction (2007). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Abingdon, England.
- Przybyl, K. (2002). For. Pathol. 32, 387–394.
- Queloz, V., Grunig, C. R., Berndt, R., Kowalski, T., Sieber, T. N. & Holdenrieder, O. (2011). For. Pathol. 41, 133–142.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Smith, A., Blois, J., Yuan, H., Aikawa, E., Ellson, C., Figueiredo, J. L., Weissleder, R., Kohler, R., Yaffe, M. B., Cantley, L. C. & Josephson, L. (2009). Mol. Cancer Ther. 8, 1666–1675. [DOI] [PMC free article] [PubMed]
- Wipf, P. & Kerekes, A. D. (2003). J. Nat. Prod. 66, 716–718. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813005606/hb7028sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813005606/hb7028Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813005606/hb7028Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report






