Abstract
The title compound, C13H8INO, was prepared by a condensation reaction of 4-nitrobenzene with phenylacetonitrile in NaOH–ethanol solution. There are two independent molecules in the asymmetric unit, in which the dihedral angles between the benzene ring and the benzoisoxazole unit are 4.2 (3) and 4.1 (3)°. The crystal packing is governed by C—H⋯N, C—I⋯π and C—I⋯O interactions.
Related literature
For the biologial activity and applications of benzo[c]isoxazoles, see: McEvoy et al. (1968 ▶); Hester et al. (1989 ▶); Walsh et al. (1990 ▶); Angibaud et al. (2003 ▶). For a related structure, see: Teslenko et al. (2008 ▶). For a general synthetic procedure, see: Davis & Pizzini (1960 ▶).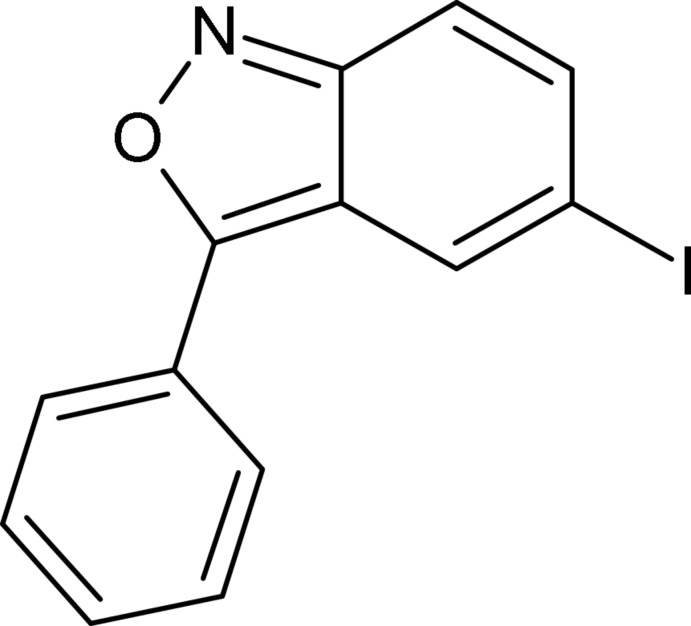
Experimental
Crystal data
C13H8INO
M r = 321.10
Monoclinic,
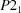
a = 5.381 (3) Å
b = 15.225 (7) Å
c = 13.749 (7) Å
β = 94.92 (3)°
V = 1122.2 (10) Å3
Z = 4
Mo Kα radiation
μ = 2.83 mm−1
T = 100 K
0.25 × 0.08 × 0.03 mm
Data collection
Kuma KM-4-CCD four-circle diffractometer
Absorption correction: analytical (CrysAlis RED; Oxford Diffraction, 2006 ▶) T min = 0.44, T max = 0.80
15060 measured reflections
6015 independent reflections
4621 reflections with I > 2σ(I)
R int = 0.053
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.090
S = 1.00
6015 reflections
289 parameters
1 restraint
H-atom parameters constrained
Δρmax = 1.98 e Å−3
Δρmin = −1.01 e Å−3
Absolute structure: Flack (1983 ▶), 1659 Friedel pairs
Flack parameter: 0.00 (3)
Data collection: CrysAlis CCD (Oxford Diffraction, 2006 ▶); cell refinement: CrysAlis RED (Oxford Diffraction, 2006 ▶); data reduction: CrysAlis RED; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813005862/gk2554sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813005862/gk2554Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813005862/gk2554Isup3.cdx
Supplementary material file. DOI: 10.1107/S1600536813005862/gk2554Isup4.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Intermolecular interactions (Å, °).
Cg is the centroid of the C1B–C6B ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3A—H3A⋯N1B i | 0.95 | 2.40 | 3.247 (7) | 149 |
| C11A—H11A⋯N1A ii | 0.95 | 2.47 | 3.339 (8) | 152 |
| C4A—I1A⋯Cg iii | 2.100 (5) | 3.618 (2) | 5.637 (6) | 160.0 (2) |
| C4B—I1B⋯O1A | 2.100 (5) | 3.335 (5) | 5.325 (7) | 156.3 (2) |
Symmetry codes: (i) 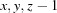 ; (ii)
; (ii) 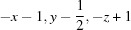 ; (iii)
; (iii) 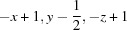 .
.
Acknowledgments
The authors are grateful to the State fund for fundamental research of Ukraine for the financial support (Project F54.3/004).
supplementary crystallographic information
Comment
Our interest in benzo[c]isoxazoles is concerned with their application as precursors of a variety of bioactive compounds (Angibaud et al., 2003; Walsh et al., 1990; Hester et al., 1989; McEvoy et al., 1968). The title compound will be used in our further investigations as arylation agent in palladium-catalyzed reactions with alkenes and alkynes.
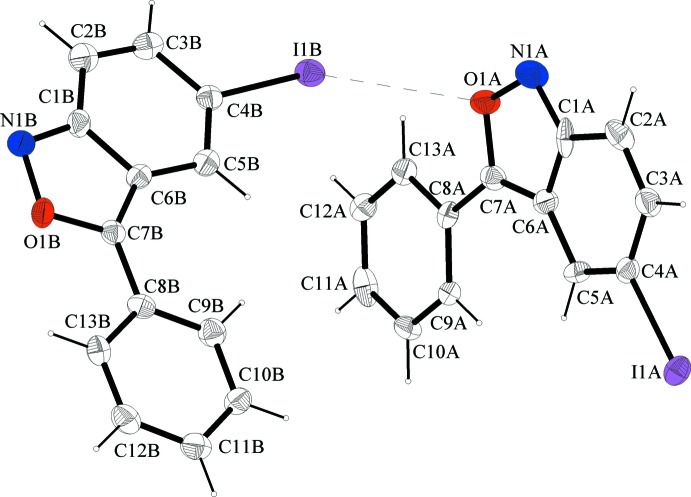
The title compound crystalizes in the noncentrosymmetric monoclinic P21 space group with two independent molecules in the asymmetric part (A and B), see Fig. 1. The molecules are almost planar, the dihedral angles between the mean planes of benzoisoxazole and benzene rings being 4.2 (3)° and 4.1 (3)° for A and B, respectively. The geometrical parameters of the molecules are similar and consistent with the previously studied 2,1-benzoxazole derivatives (Teslenko et al., 2008).
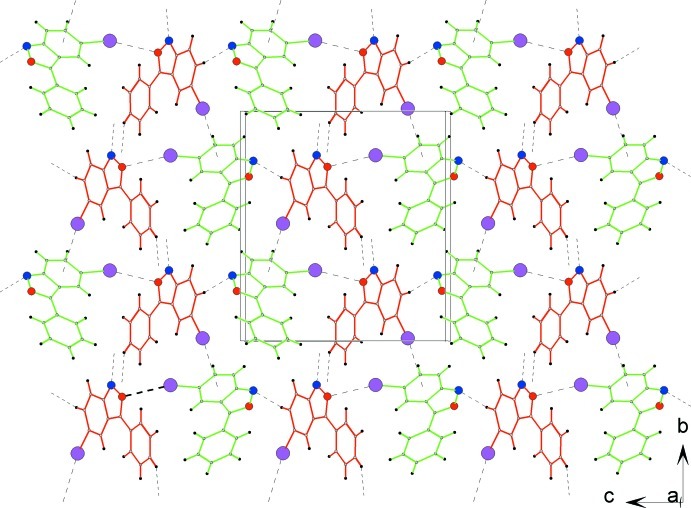
Crystal packing is governed by hydrogen bonds of C–H···N type and other intermolecular interactions including C–I···π and C–I···O. Intermolecular interactions C4A–I1A···Cgiii (Cg is a centroid of C1B/C6B aromatic ring) and C4B–I1B···O1A connect the molecules into chains propagating in b-axis direction along 21 screw axis (see Fig. 2). Hydrogen bond C3A–H3A···N1Bi connects the chains into corrugated layer parallel to the bc-plane. Hydrogen bond C11A–H11A···N1Aii binds successive layers.
Experimental
Phenylacetonitrile (1.4 g, 12 mmol) and 5 ml of benzene solution of 4-iodonitrobenene (2.49 g, 10 mmol) were added with stirring to 40 ml of ethanol solution of potassium hydroxide (4 g, 0.1 mole). The mixture was stirred for 4 h at 323 K, then poured into 150 ml of water and acidified with hydrochloric acid. The precipitate was isolated by filtration, washed with water and dried. Recrystallization of crude product from ethanol gave 2.57 g (80% yield) of 5-iodo-3-phenyl-2,1-benzoxazole as pale yellow needles suitable for X-ray analysis, m.p. 390–391 K.
Refinement
All H atoms were found in difference Fourier maps. All H atoms were positioned geometrically and treated as riding on their carriers, with C–H = 0.95 Å and Uiso(H) = values of 1.2Ueq(C).
Figures
Fig. 1.
The asymmetric unit of the title compound with atom labeling scheme. The displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
The crystal packing of the title compound showing intermolecular interactions as dashed lines (molecule A - red, molecule B - green).
Crystal data
| C13H8INO | F(000) = 616 |
| Mr = 321.10 | Dx = 1.901 Mg m−3 |
| Monoclinic, P21 | Melting point = 390–391 K |
| Hall symbol: P 2yb | Mo Kα radiation, λ = 0.71073 Å |
| a = 5.381 (3) Å | Cell parameters from 15060 reflections |
| b = 15.225 (7) Å | θ = 3.0–34.7° |
| c = 13.749 (7) Å | µ = 2.83 mm−1 |
| β = 94.92 (3)° | T = 100 K |
| V = 1122.2 (10) Å3 | Needle, pale yellow |
| Z = 4 | 0.25 × 0.08 × 0.03 mm |
Data collection
| Kuma KM-4-CCD four-circle diffractometer | 6015 independent reflections |
| Radiation source: fine-focus sealed tube | 4621 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.053 |
| ω scans | θmax = 34.7°, θmin = 3.0° |
| Absorption correction: analytical (CrysAlis RED; Oxford Diffraction, 2006) | h = −8→7 |
| Tmin = 0.44, Tmax = 0.80 | k = −17→23 |
| 15060 measured reflections | l = −20→21 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.041 | H-atom parameters constrained |
| wR(F2) = 0.090 | w = 1/[σ2(Fo2) + (0.046P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.00 | (Δ/σ)max = 0.001 |
| 6015 reflections | Δρmax = 1.98 e Å−3 |
| 289 parameters | Δρmin = −1.01 e Å−3 |
| 1 restraint | Absolute structure: Flack (1983), 1659 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.00 (3) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| I1A | 0.51472 (6) | 0.01154 (2) | 0.19512 (2) | 0.02514 (9) | |
| O1A | −0.2353 (8) | 0.2574 (3) | 0.4305 (3) | 0.0262 (9) | |
| N1A | −0.0803 (9) | 0.3076 (4) | 0.3739 (4) | 0.0316 (10) | |
| C1A | 0.0530 (10) | 0.2453 (4) | 0.3322 (4) | 0.0256 (12) | |
| C2A | 0.2425 (11) | 0.2686 (4) | 0.2666 (5) | 0.0303 (13) | |
| H2A | 0.2765 | 0.3276 | 0.2499 | 0.036* | |
| C3A | 0.3651 (11) | 0.2002 (4) | 0.2316 (4) | 0.0261 (11) | |
| H3A | 0.4926 | 0.2110 | 0.1894 | 0.031* | |
| C4A | 0.3088 (10) | 0.1115 (4) | 0.2561 (4) | 0.0219 (10) | |
| C5A | 0.1314 (10) | 0.0903 (4) | 0.3171 (4) | 0.0204 (10) | |
| H5A | 0.0981 | 0.0309 | 0.3328 | 0.024* | |
| C6A | −0.0029 (10) | 0.1613 (3) | 0.3564 (4) | 0.0201 (10) | |
| C7A | −0.1871 (10) | 0.1707 (4) | 0.4203 (4) | 0.0213 (10) | |
| C8A | −0.3335 (10) | 0.1101 (4) | 0.4753 (4) | 0.0204 (10) | |
| C9A | −0.2895 (11) | 0.0191 (4) | 0.4735 (4) | 0.0260 (11) | |
| H9A | −0.1608 | −0.0034 | 0.4373 | 0.031* | |
| C10A | −0.4324 (12) | −0.0380 (4) | 0.5240 (4) | 0.0267 (12) | |
| H10A | −0.3982 | −0.0992 | 0.5230 | 0.032* | |
| C11A | −0.6211 (10) | −0.0080 (4) | 0.5750 (4) | 0.0296 (14) | |
| H11A | −0.7201 | −0.0478 | 0.6084 | 0.036* | |
| C12A | −0.6676 (11) | 0.0838 (4) | 0.5776 (4) | 0.0229 (11) | |
| H12A | −0.7973 | 0.1054 | 0.6138 | 0.027* | |
| C13A | −0.5264 (10) | 0.1421 (4) | 0.5282 (4) | 0.0220 (11) | |
| H13A | −0.5597 | 0.2033 | 0.5300 | 0.026* | |
| I1B | −0.05111 (7) | 0.30782 (2) | 0.66158 (3) | 0.02711 (9) | |
| O1B | 0.7816 (8) | 0.2167 (3) | 1.0229 (3) | 0.0258 (8) | |
| N1B | 0.6242 (9) | 0.2844 (3) | 1.0482 (4) | 0.0270 (10) | |
| C1B | 0.4622 (10) | 0.2937 (3) | 0.9704 (4) | 0.0232 (11) | |
| C2B | 0.2531 (11) | 0.3519 (4) | 0.9611 (5) | 0.0272 (12) | |
| H2B | 0.2166 | 0.3890 | 1.0136 | 0.033* | |
| C3B | 0.1074 (11) | 0.3530 (4) | 0.8749 (4) | 0.0249 (11) | |
| H3B | −0.0335 | 0.3907 | 0.8673 | 0.030* | |
| C4B | 0.1657 (9) | 0.2974 (3) | 0.7956 (4) | 0.0206 (10) | |
| C5B | 0.3589 (10) | 0.2384 (4) | 0.8030 (4) | 0.0220 (11) | |
| H5B | 0.3902 | 0.2010 | 0.7501 | 0.026* | |
| C6B | 0.5112 (10) | 0.2353 (3) | 0.8931 (4) | 0.0199 (10) | |
| C7B | 0.7150 (10) | 0.1873 (3) | 0.9304 (4) | 0.0196 (10) | |
| C8B | 0.8618 (10) | 0.1135 (4) | 0.8959 (4) | 0.0216 (10) | |
| C9B | 0.8005 (11) | 0.0769 (4) | 0.8031 (4) | 0.0258 (12) | |
| H9B | 0.6644 | 0.0996 | 0.7621 | 0.031* | |
| C10B | 0.9389 (10) | 0.0082 (4) | 0.7720 (4) | 0.0255 (10) | |
| H10B | 0.8969 | −0.0158 | 0.7089 | 0.031* | |
| C11B | 1.1383 (12) | −0.0274 (4) | 0.8297 (4) | 0.0285 (12) | |
| H11B | 1.2315 | −0.0753 | 0.8076 | 0.034* | |
| C12B | 1.1968 (11) | 0.0099 (5) | 0.9218 (4) | 0.0301 (11) | |
| H12B | 1.3328 | −0.0131 | 0.9626 | 0.036* | |
| C13B | 1.0630 (11) | 0.0790 (4) | 0.9549 (4) | 0.0253 (11) | |
| H13B | 1.1071 | 0.1032 | 1.0177 | 0.030* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| I1A | 0.02109 (16) | 0.03002 (19) | 0.02445 (17) | 0.00079 (15) | 0.00275 (13) | −0.00571 (15) |
| O1A | 0.028 (2) | 0.021 (2) | 0.031 (2) | 0.0056 (16) | 0.0085 (17) | 0.0011 (16) |
| N1A | 0.036 (3) | 0.024 (2) | 0.037 (3) | −0.001 (3) | 0.014 (2) | 0.007 (2) |
| C1A | 0.010 (2) | 0.055 (4) | 0.011 (2) | 0.000 (2) | −0.0008 (18) | 0.000 (2) |
| C2A | 0.029 (3) | 0.033 (3) | 0.030 (3) | −0.001 (3) | 0.006 (2) | 0.011 (2) |
| C3A | 0.029 (3) | 0.028 (3) | 0.022 (3) | 0.001 (2) | 0.006 (2) | 0.004 (2) |
| C4A | 0.021 (3) | 0.025 (3) | 0.020 (2) | 0.006 (2) | −0.0003 (19) | −0.002 (2) |
| C5A | 0.021 (3) | 0.015 (2) | 0.025 (3) | 0.000 (2) | 0.001 (2) | −0.0027 (19) |
| C6A | 0.021 (3) | 0.018 (3) | 0.021 (3) | 0.000 (2) | 0.001 (2) | 0.0002 (19) |
| C7A | 0.020 (3) | 0.021 (3) | 0.022 (3) | 0.001 (2) | −0.003 (2) | 0.0008 (19) |
| C8A | 0.021 (2) | 0.023 (3) | 0.017 (2) | 0.001 (2) | 0.0032 (19) | −0.0026 (19) |
| C9A | 0.042 (3) | 0.018 (3) | 0.019 (2) | 0.005 (3) | 0.005 (2) | −0.001 (2) |
| C10A | 0.039 (3) | 0.017 (3) | 0.024 (3) | −0.001 (2) | 0.000 (2) | 0.002 (2) |
| C11A | 0.021 (3) | 0.048 (4) | 0.020 (3) | −0.012 (2) | 0.001 (2) | 0.008 (2) |
| C12A | 0.020 (3) | 0.026 (3) | 0.023 (3) | 0.000 (2) | 0.000 (2) | −0.003 (2) |
| C13A | 0.020 (3) | 0.025 (3) | 0.021 (3) | 0.003 (2) | 0.001 (2) | −0.002 (2) |
| I1B | 0.02550 (18) | 0.02499 (18) | 0.03013 (19) | 0.00099 (17) | −0.00173 (14) | 0.00057 (16) |
| O1B | 0.032 (2) | 0.028 (2) | 0.0171 (19) | −0.0014 (18) | 0.0017 (15) | −0.0014 (16) |
| N1B | 0.035 (3) | 0.021 (2) | 0.025 (2) | −0.0026 (19) | 0.004 (2) | 0.0006 (17) |
| C1B | 0.030 (3) | 0.022 (3) | 0.019 (2) | −0.005 (2) | 0.007 (2) | 0.0006 (18) |
| C2B | 0.027 (3) | 0.026 (3) | 0.030 (3) | −0.003 (2) | 0.012 (2) | 0.001 (2) |
| C3B | 0.021 (3) | 0.024 (3) | 0.032 (3) | 0.000 (2) | 0.009 (2) | −0.001 (2) |
| C4B | 0.022 (2) | 0.018 (3) | 0.022 (2) | −0.004 (2) | 0.0011 (19) | 0.0015 (18) |
| C5B | 0.023 (3) | 0.021 (3) | 0.022 (3) | −0.004 (2) | 0.003 (2) | −0.0012 (19) |
| C6B | 0.021 (3) | 0.018 (2) | 0.021 (3) | −0.003 (2) | 0.005 (2) | −0.0020 (18) |
| C7B | 0.023 (3) | 0.017 (2) | 0.019 (2) | −0.005 (2) | 0.002 (2) | 0.0018 (18) |
| C8B | 0.020 (3) | 0.019 (2) | 0.026 (3) | −0.003 (2) | 0.003 (2) | 0.003 (2) |
| C9B | 0.024 (3) | 0.028 (3) | 0.025 (3) | 0.000 (2) | 0.000 (2) | 0.001 (2) |
| C10B | 0.026 (3) | 0.025 (3) | 0.026 (2) | 0.002 (3) | 0.0045 (19) | −0.004 (2) |
| C11B | 0.029 (3) | 0.026 (3) | 0.031 (3) | 0.003 (2) | 0.009 (2) | 0.004 (2) |
| C12B | 0.029 (3) | 0.031 (3) | 0.029 (3) | 0.005 (3) | −0.003 (2) | 0.006 (3) |
| C13B | 0.027 (3) | 0.028 (3) | 0.020 (3) | 0.001 (2) | −0.002 (2) | 0.002 (2) |
Geometric parameters (Å, º)
| I1A—C4A | 2.100 (5) | I1B—C4B | 2.100 (5) |
| O1A—C7A | 1.354 (6) | O1B—C7B | 1.366 (6) |
| O1A—N1A | 1.413 (6) | O1B—N1B | 1.397 (6) |
| N1A—C1A | 1.346 (8) | N1B—C1B | 1.329 (8) |
| C1A—C6A | 1.361 (8) | C1B—C6B | 1.427 (7) |
| C1A—C2A | 1.462 (8) | C1B—C2B | 1.430 (8) |
| C2A—C3A | 1.344 (9) | C2B—C3B | 1.363 (9) |
| C2A—H2A | 0.9500 | C2B—H2B | 0.9500 |
| C3A—C4A | 1.430 (8) | C3B—C4B | 1.437 (8) |
| C3A—H3A | 0.9500 | C3B—H3B | 0.9500 |
| C4A—C5A | 1.362 (7) | C4B—C5B | 1.371 (8) |
| C5A—C6A | 1.432 (7) | C5B—C6B | 1.426 (8) |
| C5A—H5A | 0.9500 | C5B—H5B | 0.9500 |
| C6A—C7A | 1.387 (7) | C6B—C7B | 1.379 (7) |
| C7A—C8A | 1.466 (7) | C7B—C8B | 1.475 (8) |
| C8A—C9A | 1.405 (8) | C8B—C13B | 1.398 (8) |
| C8A—C13A | 1.405 (7) | C8B—C9B | 1.406 (8) |
| C9A—C10A | 1.387 (8) | C9B—C10B | 1.374 (8) |
| C9A—H9A | 0.9500 | C9B—H9B | 0.9500 |
| C10A—C11A | 1.362 (8) | C10B—C11B | 1.389 (8) |
| C10A—H10A | 0.9500 | C10B—H10B | 0.9500 |
| C11A—C12A | 1.420 (9) | C11B—C12B | 1.399 (9) |
| C11A—H11A | 0.9500 | C11B—H11B | 0.9500 |
| C12A—C13A | 1.384 (8) | C12B—C13B | 1.374 (9) |
| C12A—H12A | 0.9500 | C12B—H12B | 0.9500 |
| C13A—H13A | 0.9500 | C13B—H13B | 0.9500 |
| C7A—O1A—N1A | 110.0 (4) | C7B—O1B—N1B | 110.9 (4) |
| C1A—N1A—O1A | 102.4 (5) | C1B—N1B—O1B | 104.3 (4) |
| N1A—C1A—C6A | 114.9 (5) | N1B—C1B—C6B | 112.5 (5) |
| N1A—C1A—C2A | 121.2 (6) | N1B—C1B—C2B | 126.5 (5) |
| C6A—C1A—C2A | 123.9 (6) | C6B—C1B—C2B | 121.0 (5) |
| C3A—C2A—C1A | 115.1 (6) | C3B—C2B—C1B | 118.3 (5) |
| C3A—C2A—H2A | 122.5 | C3B—C2B—H2B | 120.8 |
| C1A—C2A—H2A | 122.5 | C1B—C2B—H2B | 120.8 |
| C2A—C3A—C4A | 121.7 (5) | C2B—C3B—C4B | 120.4 (5) |
| C2A—C3A—H3A | 119.1 | C2B—C3B—H3B | 119.8 |
| C4A—C3A—H3A | 119.1 | C4B—C3B—H3B | 119.8 |
| C5A—C4A—C3A | 122.9 (5) | C5B—C4B—C3B | 122.9 (5) |
| C5A—C4A—I1A | 119.7 (4) | C5B—C4B—I1B | 118.4 (4) |
| C3A—C4A—I1A | 117.4 (4) | C3B—C4B—I1B | 118.7 (4) |
| C4A—C5A—C6A | 117.1 (5) | C4B—C5B—C6B | 117.5 (5) |
| C4A—C5A—H5A | 121.4 | C4B—C5B—H5B | 121.2 |
| C6A—C5A—H5A | 121.4 | C6B—C5B—H5B | 121.2 |
| C1A—C6A—C7A | 104.0 (5) | C7B—C6B—C5B | 136.0 (5) |
| C1A—C6A—C5A | 119.2 (5) | C7B—C6B—C1B | 104.2 (5) |
| C7A—C6A—C5A | 136.8 (5) | C5B—C6B—C1B | 119.8 (5) |
| O1A—C7A—C6A | 108.7 (5) | O1B—C7B—C6B | 108.0 (4) |
| O1A—C7A—C8A | 116.4 (5) | O1B—C7B—C8B | 116.3 (5) |
| C6A—C7A—C8A | 134.9 (5) | C6B—C7B—C8B | 135.6 (5) |
| C9A—C8A—C13A | 119.0 (5) | C13B—C8B—C9B | 119.2 (5) |
| C9A—C8A—C7A | 120.9 (5) | C13B—C8B—C7B | 120.6 (5) |
| C13A—C8A—C7A | 120.1 (5) | C9B—C8B—C7B | 120.2 (5) |
| C10A—C9A—C8A | 120.5 (5) | C10B—C9B—C8B | 119.6 (6) |
| C10A—C9A—H9A | 119.8 | C10B—C9B—H9B | 120.2 |
| C8A—C9A—H9A | 119.8 | C8B—C9B—H9B | 120.2 |
| C11A—C10A—C9A | 121.2 (5) | C9B—C10B—C11B | 122.1 (6) |
| C11A—C10A—H10A | 119.4 | C9B—C10B—H10B | 118.9 |
| C9A—C10A—H10A | 119.4 | C11B—C10B—H10B | 118.9 |
| C10A—C11A—C12A | 118.9 (5) | C10B—C11B—C12B | 117.5 (6) |
| C10A—C11A—H11A | 120.5 | C10B—C11B—H11B | 121.3 |
| C12A—C11A—H11A | 120.5 | C12B—C11B—H11B | 121.3 |
| C13A—C12A—C11A | 120.9 (5) | C13B—C12B—C11B | 121.8 (5) |
| C13A—C12A—H12A | 119.6 | C13B—C12B—H12B | 119.1 |
| C11A—C12A—H12A | 119.6 | C11B—C12B—H12B | 119.1 |
| C12A—C13A—C8A | 119.5 (5) | C12B—C13B—C8B | 119.8 (5) |
| C12A—C13A—H13A | 120.3 | C12B—C13B—H13B | 120.1 |
| C8A—C13A—H13A | 120.3 | C8B—C13B—H13B | 120.1 |
Hydrogen-bond geometry (Å, º)
Cg is the centroid of the C1B–C6B ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3A—H3A···N1Bi | 0.95 | 2.40 | 3.247 (7) | 149 |
| C11A—H11A···N1Aii | 0.95 | 2.47 | 3.339 (8) | 152 |
| C4A—I1A···Cgiii | 2.10 (1) | 3.62 (1) | 5.637 (6) | 160 (1) |
| C4B—I1B···O1A | 2.10 (1) | 3.34 (1) | 5.325 (7) | 156 (1) |
Symmetry codes: (i) x, y, z−1; (ii) −x−1, y−1/2, −z+1; (iii) −x+1, y−1/2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: GK2554).
References
- Angibaud, P., Bourdrez, X., Devine, A., End, D. W., Freyne, E., Ligny, Y., Muller, P., Mannens, G., Pilatte, I., Poncelet, V., Skrzat, S., Smets, G., Van Dun, J., Van Remoortere, P., Venet, M. & Wouters, W. (2003). Bioorg. Med. Chem. Lett. 13, 1543–1548. [DOI] [PubMed]
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Davis, R. B. & Pizzini, L. C. (1960). J. Org. Chem. 25, 1884–1888.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Hester, J. B., Ludens, J. H., Emmert, D. E. & West, B. E. (1989). J. Med. Chem. 32, 1157–1163. [DOI] [PubMed]
- McEvoy, F. J., Greenblatt, E. N., Osterrerg, A. C. & Allen, G. R. Jr (1968). J. Med. Chem. 11, 1248–1250. [DOI] [PubMed]
- Oxford Diffraction (2006). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Wrocław, Poland.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Teslenko, Y., Matiychuk, V., Obushak, M., Kinzhybalo, V. & Ślepokura, K. (2008). Acta Cryst. E64, o2420. [DOI] [PMC free article] [PubMed]
- Walsh, D. A., Moran, H. W., Shamblee, D. A. & Welstead, W. J. (1990). J. Med. Chem. 33, 2296–2304. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813005862/gk2554sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813005862/gk2554Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813005862/gk2554Isup3.cdx
Supplementary material file. DOI: 10.1107/S1600536813005862/gk2554Isup4.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report