Abstract
Cognitive changes related to cancer and its treatment have been intensely studied, and neuroimaging has begun to demonstrate brain correlates. In the first prospective longitudinal neuroimaging study of breast cancer (BC) patients we recently reported decreased gray matter density one month after chemotherapy completion, particularly in frontal regions. These findings helped confirm a neural basis for previously reported cognitive symptoms, which most commonly involve executive and memory processes in which the frontal lobes are a critical component of underlying neural circuitry. Here we present data from an independent, larger, more demographically diverse cohort that is more generalizable to the BC population. BC patients treated with (N = 27) and without (N = 28) chemotherapy and matched healthy controls (N = 24) were scanned at baseline (prior to systemic treatment) and one month following chemotherapy completion (or yoked intervals for non-chemotherapy and control groups) and APOE-genotyped. Voxel-based morphometry (VBM) showed decreased frontal gray matter density after chemotherapy, as observed in the prior cohort, which was accompanied by self-reported difficulties in executive functioning. Gray matter and executive symptom changes were not related to APOE ε4 status, though a somewhat greater percentage of BC patients who received chemotherapy were ε4 allele carriers than patients not treated with chemotherapy or healthy controls. These findings provide confirmatory evidence of frontal morphometric changes that may be a pathophysiological basis for cancer and treatment-related cognitive dysfunction. Further research into individual risk factors for such changes will be critical for development of treatment and prevention strategies.
Keywords: Adjuvant chemotherapy, APOE genotype, Brain, Breast cancer, BRIEF-A, Executive function, Frontal lobes, Magnetic resonance imaging, Neuroimaging, Voxel-based morphometry
Cognitive changes related to breast cancer and its treatment have been an area of increasing study, with numerous reports demonstrating cognitive impairment in patients relative to controls. These changes have been differentially attributed to chemotherapy, radiation, and anti-estrogen treatment (Agrawal et al., 2010; Ahles et al., 2010; Collins et al., 2009; Jim et al., 2009; Quesnel et al., 2009), and have been reported most prominently in executive functions (e.g., working memory) and processing speed, cognitive processes largely subserved by frontally mediated brain systems (impairment in other cognitive domains has also been noted; for review and meta-analysis see (Anderson-Hanley et al., 2003; Correa and Ahles, 2008; Stewart et al., 2006)). A higher than expected incidence of impaired cognitive performance has also been found in patients prior to systemic treatment (Ahles et al., 2008; Wagner et al., 2006; Wefel et al., 2004), suggesting that host factors and/or the cancer disease process itself may play a role. This prior work demonstrates the continued need for further investigation of the effects of cancer treatment and the disease process on cognition in vulnerable individuals (McDonald and Saykin, 2011; Vardy et al., 2008).
The neural mechanisms underlying these cognitive changes have likewise been the subject of increasing investigation. Several cross-sectional, retrospective structural MRI studies have utilized voxel-based morphometry (VBM) to assess gray matter changes after breast cancer treatment quantitatively, in an automated, unbiased manner (de Ruiter et al., in press; Hakamata et al., 2007; Inagaki et al., 2007; McDonald et al., 2008; Saykin et al., 2003; Yoshikawa et al., 2006). Those studies comparing gray matter between patients who did and did not receive chemotherapy have demonstrated residual gray matter deficits in the chemotherapy-treated group, even several years after treatment completion (de Ruiter et al., in press; Inagaki et al., 2007; McDonald et al., 2008; Saykin et al., 2003). We recently reported the first prospective VBM study examining such gray matter changes relative to pre-treatment baseline (McDonald et al., 2010). We predicted that these changes would be detectable in the short-term but would recover at least partially over time, given prior cognitive studies suggesting longitudinal improvement in brain function after chemotherapy (Ahles et al., 2010; Collins et al., 2009; Jansen et al., 2011; Jenkins et al., 2006; Schagen et al., 2002). Findings were consistent with study hypotheses, demonstrating reduced gray matter in chemotherapy-treated patients one month after chemotherapy completion in bilateral frontal, medial temporal, and cerebellar regions. One year later gray matter density had returned to baseline levels in some regions, though not all. No between-group differences were found at baseline, and changes were not seen in patients who did not receive chemotherapy or healthy controls.
The purpose of the current investigation was to assess gray matter alterations related to breast cancer and its treatment prospectively in an independent cohort of patients treated with and without standard-dose systemic chemotherapy and demographically matched healthy controls, in order to replicate our previous findings. Given the prominence of executive function changes among the cognitive domains affected in cancer patients after treatment (Anderson-Hanley et al., 2003), and the recent finding of a relationship between self-reported executive functioning and altered brain activation after breast cancer chemotherapy (Kesler et al., 2011), we also sought to examine the relationship of these gray matter changes to self-reported executive functioning. Finally, a large body of research has shown a significant relationship between the apolipoprotein E (APOE) ε4 allele and Alzheimer’s disease and its precursors, and has demonstrated a role for APOE in other neurocognitive disorders (for reviews see (Bookheimer and Burggren, 2009; Smith, 2000)). Given prior work demonstrating decreased cognitive functioning in cancer survivors treated with chemotherapy who carried the ε4 allele vs. those who did not (Ahles et al., 2003), we further evaluated possible risk factors for gray matter changes after chemotherapy by investigating their relationship to presence or absence of the APOE ε4 allele.
1. Participants
Written informed consent was obtained from all participants according to the Declaration of Helsinki under a protocol approved by the Indiana University Institutional Review Board. Participants were female breast cancer patients treated with (CTx+, N = 27) and without (CTx−, N = 28) systemic chemotherapy and healthy controls (N = 24). Patients had non-invasive (stage 0) or non-metastatic invasive (stages I, II, or III) disease, and were treated with common standard-dose chemotherapy regimens which all included a taxane (see Table 1 for demographic and treatment data). Exclusion criteria for all groups were: (1) prior treatment with cancer chemotherapy, CNS radiation, or intrathecal therapy; (2) current or past alcohol or drug dependence; (3) neurobehavioral risk factors including neurologic, medical, or psychiatric conditions known to affect brain structure or function, except history of depression or anxiety in breast cancer patients. Potential participants for all groups were excluded for current diagnosis of any DSM-IV Axis I disorder or a history of any psychiatric disorder requiring hospitalization. Anxiety and depression symptoms were assessed at each study visit with the Center for Epidemiologic Studies-Depression Scale (CES-D) (Radloff, 1977) and the State-Trait Anxiety Inventory-State subscale (STAI-S) (Spielberger, 1983).
Table 1.
Sample demographics.
| CTx+ (N = 27) |
CTx− (N = 28) |
Control (N = 24) |
|
|---|---|---|---|
| Age at baseline (yrs.) | 49.9 (7.6) | 52.4 (9.1) | 47.0 (9.2) |
| Education (yrs.) | 15.5 (2.8) | 15.4 (2.3) | 15.4 (2.4) |
| Estimated full scale IQ (Barona Index (Barona et al., 1984)) | 110.1 (6.5) | 111.3 (6.1) | 110.6 (6.5) |
| Handedness (R,L/Amb) | 26, 1 | 26, 2 | 22, 2 |
| Percent Caucasian, Non-hispanic | 78 | 89 | 83 |
| CES-D raw score: Baseline | 10.8 (9.5) | 8.6 (8.6) | 7.8 (7.6) |
| M1 | 14.6 (9.3) | 9.3 (9.5) | 7.4 (7.2) |
| STAI-S raw score: baseline | 35.3 (15.2) | 28.9 (7.7) | 31.6 (10.3) |
| M1 | 35.4 (12.4) | 32.3 (12.2) | 32.5 (11.9) |
| Inter-scan interval (days) | 158.7 (68.9) | 204.3 (151.7) | 160.8 (28.9) |
| Cancer stage: 0 (DCIS) | 0 | 7 | |
| I | 11 | 18 | |
| II | 12 | 3 | |
| III | 4 | 0 | |
| Received radiotherapya | 22 | 18 | |
| Number on anti-estrogen therapya,b baseline | 0 | 1 TAM | |
| M1 | 2 ANA | 12 TAM | |
| 1 TAM | 5 LET | ||
| 2 ANA | |||
| 1 EXE | |||
| 1 RAL | |||
| Chemotherapy regimena,c | |||
| Doxorubicin/cyclophosphamide/paclitaxel | 9 | ||
| Docetaxel/cyclophosphamide | 9 | ||
| Docetaxel/carboplatin | 5 | ||
| Docetaxel/doxorubicin/cyclophosphamide | 1 | ||
| Docetaxel/cisplatin | 1 | ||
| Paclitaxel | 1 |
Values are Mean (SD).
CES-D = Center for Epidemiologic Studies-Depression Scale.
STAI-S = State-Trait Anxiety Inventory-State subscale.
M1 = one month post chemotherapy completion (or yoked intervals).
Details regarding radiation, chemotherapy regimen, and anti-estrogen treatment were not available for one CTx+ patient.
ANA = anastrozole; TAM = tamoxifen; LET = letrozole; EXE = exemestane; RAL = raloxifene.
Nine CTx+ patients were also treated with trastuzumab; one was also treated with sunitinib; one was also treated with bevacizumab.
2. Methods
Study measures were completed at baseline (after surgery but before radiation, chemotherapy, and/or anti-estrogen treatment) and approximately one month following the completion of chemotherapy (M1), or yoked intervals for the CTx− and control groups, for all participants except nine CTx+ patients who received neoadjuvant chemotherapy prior to surgery and additional treatment. For these nine participants the baseline study visit was prior to both cancer surgery and systemic treatment, and the second study visit was approximately one month after chemotherapy completion. For CTx+ patients the baseline visit was conducted on average 9.9 days (SD 11.0) prior to the start of chemotherapy (range 1– 43 days). One CTx− participant began tamoxifen about three weeks prior to her baseline scan. Of note, data reported here are drawn from a larger study in which participants undergo a comprehensive assessment including structural and functional neuroimaging, objective and subjective cognitive evaluation, and genetic and other biomarkers at three time-points. Data collection is ongoing, particularly for the final study visit (not reported here, given our current partial sample), and the present findings therefore represent an interim analysis of a subset of the larger study.
2.1. Self-reported executive function
Self-report of executive functioning was obtained with the Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) (Roth et al., 2005), which includes an overall composite score (the Global Executive Composite, or GEC) and two major index scores: the Behavioral Regulation Index (BRI), composed of the Inhibit, Shift, Emotional Control and Self-Monitor scales, and the Metacognition Index (MI), which includes the Initiate, Working Memory, Plan/Organize, Task Monitor, and Organization of Materials scales. Between-group differences on BRIEF-A scale and index T-scores were compared using the general linear model in SPSS (SPSS Statistics 19, IBM Corporation, Somers, NY) to examine differences in self-reported executive function at M1 controlling for baseline levels. Of note, higher T-scores on this measure indicate greater levels of executive complaints.
2.2. APOE genotyping
APOE alleles were determined using standard assays for the two single nucleotide polymorphisms (SNPs) coding for the ε4 (rs429358) and ε2 (rs7412) vs. more common ε3 allele of APOE. Participants who were carriers of one or two copies of the ε4 allele were considered APOE ε4 positive. Within the CTx+ group, differences between APOE ε4 positive and negative patients for significant gray matter clusters and BRIEF-A scales were compared using the general linear model in SPSS (SPSS Statistics 19, IBM Corporation, Somers, NY) to examine differences at M1 controlling for baseline levels.
2.3. MRI scan acquisition
All scans were acquired on the same Siemens Tim Trio 3T scanner using a 12-channel head coil. A T1-weighted three-dimensional magnetization prepared rapid gradient echo (MPRAGE) volume was used for VBM, with the following parameters: TR = 2300 ms, TE = 2.98 ms, FOV = 256 mm, FA = 9 deg, 160 1.2 mm thick sagittal slices with no skip, 256 × 256 matrix, in-plane resolution of 1 mm2. This MPRAGE sequence has been extensively tested and validated via the multicenter, international Alzheimer’s Disease Neuroimaging Initiative (ADNI) study (see http://adni.loni.ucla.edu/ for additional information). T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences were also acquired to rule out incidental pathology.
2.4. Image analysis
Locally developed MATLAB (R2009b, Mathworks, Inc., Natick, MA) scripts were used to implement optimized VBM methods (Ashburner and Friston, 2000; Ashburner and Friston, 2001; Good et al., 2001) using SPM (Version 8, Wellcome Department of Imaging Neuroscience, London, UK), similar to our prior longitudinal study (McDonald et al., 2010). Briefly, after reconstruction MPRAGE follow-up scans were registered to the baseline scan for each subject. Scans were then registered to the Montreal Neurological Institute (MNI) T1-weighted template and segmented into gray matter, white matter, and cerebrospinal fluid compartments using the MNI T1-weighted template and corresponding tissue probability maps. Gray matter maps were then spatially normalized to MNI space, resampled to 1 mm isotropic voxels, and smoothed using an isotropic Gaussian spatial filter (FHWM = 10 mm) to reduce residual inter-individual variability. The smoothed, normalized gray matter maps were subjected to statistical parametric mapping on a voxel-by-voxel basis using the general linear model as implemented in SPM8. The SPM8 prior probability gray matter template was used to restrict the statistical comparisons to the gray matter compartment. As multiple prior structural and functional MRI studies in breast cancer patients have consistently shown alterations in frontal brain regions (Cimprich et al., 2010; de Ruiter et al., 2011; Inagaki et al., 2007; Kesler et al., 2009; Kesler et al., 2011; McDonald et al., 2010; Scherling et al., 2011; Silverman et al., 2007), all imaging analyses were restricted to the frontal lobes using a mask composed of frontal lobe subregions from the WFU PickAtlas toolbox in SPM8, which was included as an explicit mask in the SPM8 design matrix.
Random effects analyses were conducted using analysis of variance (ANOVA) to construct contrast maps of voxels in which local gray matter density differed between groups and over time. Comparisons were conducted within an omnibus group (three independent levels: CTx+, CTx−, control) by time (two non-independent levels: baseline, M1) ANOVA. The critical significance threshold (Pcrit) was set to 0.001. Cluster extent (k) for all analyses was set to limit results only to regions that survived an unbiased search of the entire frontal region of interest at a cluster-level threshold of PFWE-corrected < 0.05. Within the omnibus SPM8 ANOVA design matrix between-group comparisons were conducted using weighted contrast vectors. For example, pair-wise comparisons of gray matter density at baseline (CTx+ vs. CTx−, CTx+ vs. control, CTx− vs. control) were conducted by entering values of 1 and −1 in the appropriate columns in the matrix. In this manner examination of regions where controls showed greater gray matter density than CTx+ at baseline would be conducted by entering 1 in the control baseline column and −1 in the CTx+ baseline column. Group-by-time interactions were conducted in a similar fashion. For example, to evaluate regions in which the control and CTx+ groups showed significant differences from baseline to M1, values of 1 would be entered in the CTx+ baseline and control M1 columns, and values of −1 would be entered in the CTx+ M1 and control baseline columns (and vice versa for the inverse interaction).
We hypothesized that gray matter decreases would be seen from baseline to M1 in the CTx+ group, consistent with our prior findings in an independent cohort (McDonald et al., 2010). Mean values for significant clusters in the analysis of regions showing decreasing gray matter density from baseline to M1 in the CTx+ group were extracted using MarsBaR v0.42 (http://marsbar.sourceforge.net/). The general linear model in SPSS was used to investigate the relationship of these mean gray matter change values in the CTx+ group to APOE status, examining between-group differences in M1 gray matter density accounting for baseline levels. To evaluate relationships between self-reported executive function symptoms and gray matter linear regression in SPSS was used to calculate adjusted T-scores for M1 accounting for baseline score. These were then entered as a covariate into the SPM8 design matrix separately for each group (CTx+, CTx−, control) to assess positive and negative correlations with gray matter density at M1.
3. Results
As expected and consistent with conventional treatment patterns, CTx+ patients had significantly higher stage disease than CTx− patients (χ2 = 18.08, df = 3, P < 0.001). There were no other between-group demographic differences, and no group-by-time interactions were observed for depression or anxiety symptoms (CES-D, STAI-S; P > 0.05, Table 1). The second scan session was about six months after the baseline visit on average, and interscan intervals did not differ between groups (P > 0.05, Table 1). MNI coordinates, cluster extents, P values, T and Z scores, and region descriptions are presented in Table 2. Imaging analyses described below were repeated including age as a covariate to control for possible gray matter density decline with aging, without a significant change in the pattern of findings.
Table 2.
Regional gray matter changes (Pcrit < 0.001, cluster-level PFWE-corr < 0.05).
| MNI coordinates (x y z) | Cluster extent (k) | Cluster-level PFWE-corrected | T | Z | Region description (for cluster peak) |
|---|---|---|---|---|---|
| Between-Group Analyses | |||||
| Control > CTx− at baseline | |||||
| −22 −14 43 | 1566 | 0.001 | 5.78 | 5.49 | L cingulate gyrus (BA24) |
| Interaction Control > CTx+ from baseline to M1 | |||||
| −49 41 30 | 1357 | 0.002 | 4.18 | 4.06 | L middle frontal gyrus (BA46) |
| Within-Group (CTx+) Analyses | |||||
| Gray matter decline from baseline to M1 | |||||
| −12 36 50 | 717 | 0.04 | 4.41 | 4.27 | L superior frontal gyrus (BA8) |
| −45 37 29 | 4320 | <0.001 | 4.36 | 4.22 | L middle frontal gyrus (BA46) |
| Correlation with BRIEF-A initiate scale adjusted T-score | |||||
| −27 52 −7 | 1410 | 0.035 | 7.19 | 5.24 | L middle frontal gyrus (BA10) |
BA = Brodmann area.
3.1. Between-group analyses
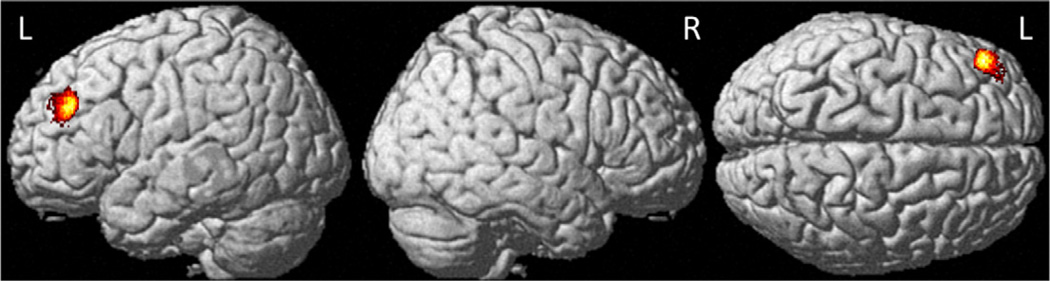
At baseline the only significant between-group difference was a single cluster in the left cingulate gyrus in which controls showed greater gray matter than CTx− patients (Table 2). Group-by-time interaction analyses showed reduced gray matter density in CTx+ patients relative to controls at M1 relative to baseline in the left middle frontal gyrus (Fig. 1). This pattern of change over time was not apparent for the CTx− group. There were no regions where the control group showed lower gray matter than either cancer group at M1 relative to baseline, nor were there any regions where a significant group-by-time interaction was found between the two cancer groups from baseline to M1.
Fig. 1.
Between-group interaction analyses of regional gray matter density declines in chemotherapy-treated breast cancer patients relative to healthy controls from baseline to one month after chemotherapy (Pcrit < 0.001, cluster-level PFWE-corr < 0.05, see Table 2 for region descriptions).
3.2. Within-group analyses
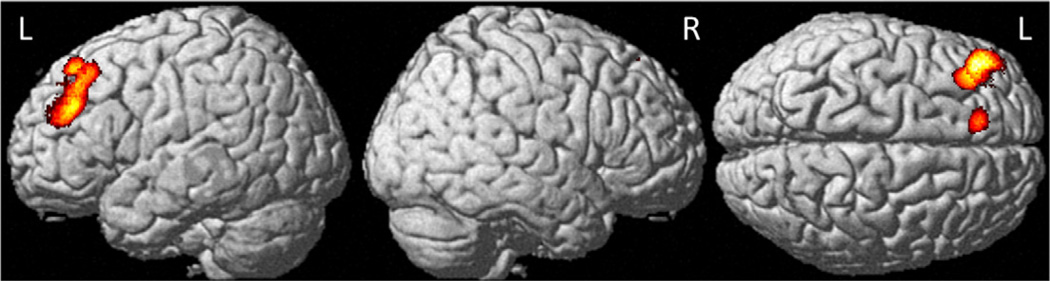
At M1 relative to baseline the CTx+ group showed decreased gray matter density in the left middle and superior frontal gyri (Fig. 2), including in the same middle frontal gyrus regions shown to be significant in the interaction analyses above. Within the control and CTx− groups there were no gray matter regions which showed significant decline from baseline to M1. There were also no regions showing increased gray matter from baseline to M1 for any group.
Fig. 2.
Regional gray matter density declines in chemotherapy-treated breast cancer patients from baseline to one month after chemotherapy (Pcrit < 0.001, cluster-level PFWE-corr < 0.05, see Table 2 for region descriptions).
3.3. Self-reported executive function changes and relationship to gray matter density
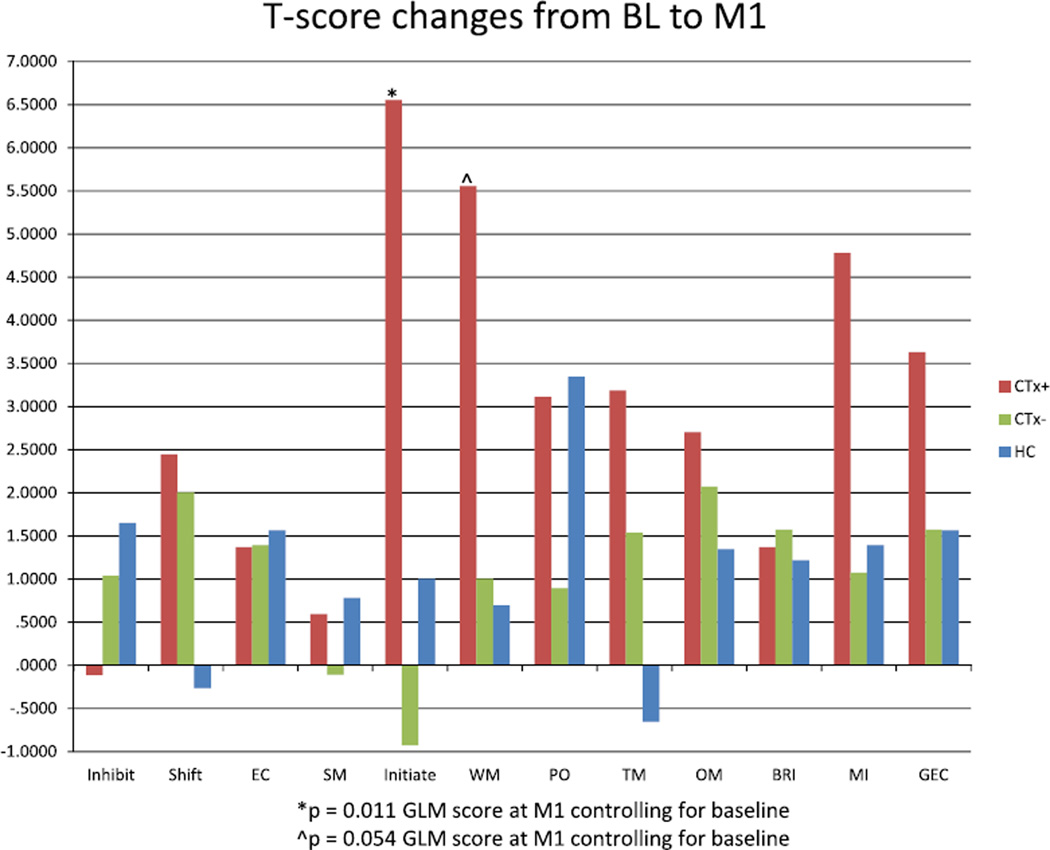
There were no between-group differences in BRIEF-A T-scores at baseline for any scale or index (Table 3). Longitudinal analysis of BRIEF-A T-scores revealed a significant difference in the Initiate scale (P = 0.011), with CTx+ patients showing increased scores over time, indicating more self-perceived symptoms in the area of ability to initiate problem-solving or activity. A trend in the same direction was also evident on the BRIEF-A Working Memory scale (P = 0.054). No significant differences were evident on other scales. Graphical representation of raw T-score changes (Fig. 3) demonstrates that for many BRIEF-A scales, particularly those which make up the Metacognition Index, CTx+ patients showed the greatest score increase at M1 relative to baseline, indicative of greater increase in self-perceived executive difficulties.
Table 3.
Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) T-scores.
| CTx+ (N = 27) |
CTx− (N = 28) |
Control (N = 24)a |
||||
|---|---|---|---|---|---|---|
| Baseline | M1 | Baseline | M1 | Baseline | M1 | |
| Inhibit | 51.2 (7.4) | 51.0 (9.0) | 49.8 (10.6) | 50.8 (8.4) | 49.0 (8.6) | 50.0 (7.6) |
| Shift | 51.2 (10.0) | 53.7 (9.2) | 51.6 (12.3) | 53.6 (11.1) | 52.8 (11.1) | 53.0 (11.1) |
| Emotional control | 51.2 (10.3) | 52.6 (10.4) | 51.4 (11.2) | 52.8 (11.1) | 50.2 (10.9) | 51.8 (11.5) |
| Self-monitor | 47.9 (8.1) | 48.4 (8.5) | 48.0 (11.7) | 47.9 (8.4) | 48.8 (7.4) | 48.4 (7.8) |
| Behavioral regulation index | 50.6 (9.3) | 52.0 (10.7) | 50.3 (12.2) | 51.9 (10.1) | 50.0 (8.8) | 51.0 (9.7) |
| Initiate* | 49.8 (11.0) | 56.3 (11.1) | 51.6 (12.6) | 50.6 (11.0) | 47.5 (7.8) | 48.3 (7.1) |
| Working memory^ | 54.0 (9.4) | 59.5 (10.3) | 53.0 (12.4) | 54.0 (11.5) | 52.1 (10.7) | 52.4 (10.4) |
| Plan/organize | 51.2 (9.2) | 54.3 (11.1) | 51.0 (11.6) | 51.9 (10.4) | 50.5 (9.3) | 53.3 (11.4) |
| Task MOnitor | 52.9 (9.2) | 56.0 (9.7) | 52.2 (11.2) | 53.8 (9.5) | 53.0 (9.4) | 52.0 (9.3) |
| Organization of materials | 50.3 (9.5) | 53.0 (11.7) | 51.1 (11.0) | 53.2 (9.7) | 52.8 (12.9) | 53.1 (11.9) |
| Metacognition index | 51.7 (9.3) | 56.4 (11.1) | 51.9 (12.4) | 53.0 (10.5) | 51.4 (9.9) | 52.1 (10.2) |
| Global executive composite | 51.2 (9.5) | 54.9 (10.6) | 51.2 (12.7) | 52.8 (10.5) | 50.7 (9.5) | 51.8 (10.0) |
Values are mean (sd) of scale/index T-scores, higher score reflects greater complaints.
One control participant did not complete the BRIEF-A at M1.
Significant between-group difference at M1 controlling for baseline (P = 0.011), CTx+ group showing greatest change.
Trend for between-group difference at M1 controlling for baseline (P = 0.054), CTx+ group showing greatest change.
Fig. 3.
T-score changes for BRIEF-A scales and indexes from baseline to M1. Note relatively greater increases for CTx+ patients on scales which make up the Metacognition Index (Initiate, WM, PO, TM, OM). (BRIEF-A = Behavior Rating Inventory of Executive Function-Adult Version, BL = baseline, M1 = one month after chemotherapy completion, CTx+ = chemotherapy-treated, CTx− = nonchemotherapy-treated, HC = healthy control, EC = Emotional Control, SM = Self-Monitor, WM= Working Memory, PO = Plan/Organize, TM = Task Monitor, OM = Organization of Materials, BRI = Behavioral Regulation Index, MI = Metacognition Index, GEC = Global Executive Composite).
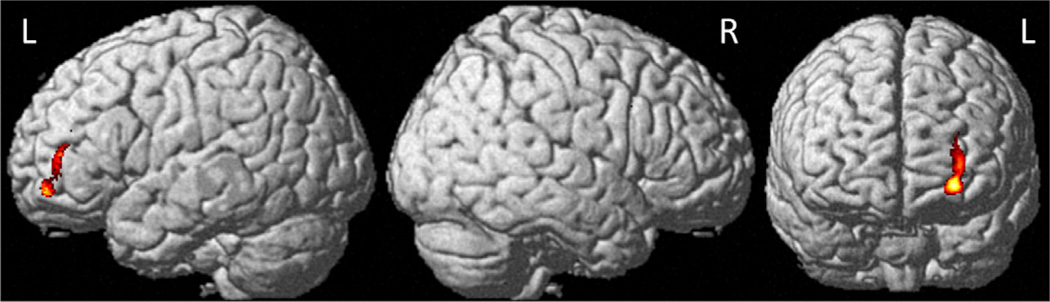
When BRIEF-A Initiate scale adjusted T-score was entered as a covariate into the SPM8 design matrix, a significant negative correlation with M1 gray matter density was seen in the left middle frontal gyrus for the CTx+ group (Table 2, Fig. 4), indicating that reduced gray matter density was associated with higher levels of executive complaints in this domain. There were no positive correlations between Initiate scale adjusted T-score and gray matter density at M1 in the CTx+ group, and no significant relationships in either direction were apparent at this threshold for the CTx− or control groups.
Fig. 4.
Negative correlation of BRIEF-A Initiate scale adjusted T-score with gray matter density one month after chemotherapy completion (Pcrit < 0.001, cluster-level PFWE-corr < 0.05, see Table 2 for region descriptions, BRIEF-A = Behavior Rating Inventory of Executive Function-Adult Version).
3.4. Relationship of gray matter and BRIEF-A changes to APOE status
Regions of gray matter which showed significant decline from baseline to M1 and BRIEF-A initiate scale T-scores (where a significant difference over time was seen in the CTx+ group, as noted above) were compared between APOE ε4 positive and negative CTx+ patients, but no significant between-group differences were observed (all P > 0.05). Of note, a higher percentage of patients in the CTx+ group were ε4 positive than in the other two groups (CTx+ 42%, CTx− 21%, control 25%; APOE status was not available for one CTx+ patient), though this difference was not statistically significant.
4. Discussion
These findings replicate our previous work showing decreased frontal gray matter shortly after chemotherapy completion in breast cancer patients. Relative to our prior study (McDonald et al., 2010), the current cohort is larger, more racially and ethnically diverse, includes patients receiving neoadjuvant chemotherapy, and was conducted on a new generation 3T vs. older 1.5T magnet. Demonstration of reduced frontal gray matter in this cohort provides independent confirmation of the prior results, strengthening the evidence that breast cancer chemotherapy is associated with frontal gray matter changes. Also consistent with our prior work, such changes were not evident in controls or patients who received anti-estrogen treatment but not chemotherapy, suggesting that these frontal gray matter decreases are specific to chemotherapy treatment, rather than solely reflecting host factors, the cancer disease process, or effects of other cancer treatments. Gray matter changes in the current study were also consistent with frontal regions in which prior work has demonstrated structural and functional abnormalities in breast cancer patients prior to adjuvant treatment (Scherling et al., 2011, 2012), post-treatment (de Ruiter et al., 2011; Kesler et al., 2009; Kesler et al., 2011; McDonald et al., 2010; Silverman et al., 2007), and longitudinally (McDonald et al., in press), further supporting the importance of frontal abnormalities in the observed subjective and objective cognitive changes.
These findings extend our prior prospective work by demonstrating self-reported executive complaints that follow the same pattern as gray matter changes. Across BRIEF-A subscales, and particularly in the area of metacognitive functioning, chemotherapy-treated patients were more likely to show increased T-scores from baseline to one month post-treatment, indicative of greater perceived executive dysfunction. The CTx+ group also showed the only significant increase in symptoms over time (on the Initiate scale), and a trend in the same direction on the Working Memory scale. Change in Initiate scale adjusted T-score showed a negative correlation with gray matter density one month after chemotherapy completion (reduced gray matter density at M1 was correlated with greater executive complaints). No such correlation was seen in patients who did not receive chemotherapy or controls. These findings are consistent with prior cognitive studies showing greater symptoms in the short-term following chemotherapy treatment. In addition, the frontal regions in which we found significant gray matter changes over time and correlations with executive complaints (Brodmann areas 8, 10, and 46) are the same regions where a recent study demonstrated functional abnormalities in breast cancer patients post-treatment and found relationships between brain activation and self-perceived executive functioning as measured by the BRIEF-A (Kesler et al., 2011). Our findings therefore provide independent support for Kesler et al.’s previous results. In addition, previous work has demonstrated that BRIEF-A measures correlate with frontal lobe volume in schizophrenia (Garlinghouse et al., 2010). Our findings therefore offer additional support for the BRIEF-A as a measure sensitive to functionally meaningful brain changes in neuropsychiatric populations.
In our prior cohort there were no between-group differences apparent at baseline. In the current study, the only baseline difference was a single cluster in the left cingulate gyrus in which CTx− patients showed lower gray matter density than controls. This finding seems unlikely to be related to cancer per se, as no such group difference was seen in the CTx+ group. In addition, our primary interest was in examination of changes over time. As this region showed no significant change over time in within-group or interaction analyses, it remains of uncertain clinical significance. We also examined self-reported symptoms of depression and anxiety (CES-D, STAI-S). As in our prior cohort, there were no significant group-by-time interactions on these factors, and group means were below levels typically considered to be clinically significant, suggesting that these psychosocial factors do not account for the observed differences in gray matter density or self-reported executive dysfunction.
While at present the systemic effects of chemotherapy and other cancer treatments remain poorly understood, we and others have proposed possible mechanisms for chemotherapy-induced cognitive and brain changes, including chemotherapy-induced DNA damage (directly or through increases in oxidative stress), individual variation in genes related to neural repair and/or plasticity, and chemotherapy-induced hormonal changes (Ahles and Saykin, 2007). The question of whether chemotherapy-related cognitive and brain changes are related to direct cytotoxic effects of chemotherapeutic agents crossing the blood–brain barrier has not been conclusively addressed. All but one of the CTx+ participants in the current study received cyclophosphamide, carboplatin, or cisplatin, and all CTx+ patients in the prior cohort received cyclophosphamide. As these agents are believed to cross the blood–brain barrier to some degree, this remains a possible explanation for the observed decreases in gray matter density.
Prior work has also suggested that such cancer and treatment-related changes are likely to affect only a subgroup of cancer patients, who may be more vulnerable to these effects for as yet undetermined reasons. Previous studies have suggested that patients who have more advanced stage disease, are older at the time of breast cancer diagnosis, have lower baseline cognitive reserve, or are APOE ε4 positive may be at increased risk for cognitive changes related to cancer and its treatment (Ahles et al., 2008; Ahles et al., 2010; Ahles et al., 2003). To examine a potential biological mechanism for the observed gray matter and executive symptom changes we investigated their relationship to APOE status, but did not find a significant effect of APOE ε4 carrier status on either gray matter change or increase in self-reported executive symptoms. This may reflect relatively low power to detect genetic influences in the present study. It was noteworthy that a relatively greater percentage of chemotherapy-treated patients were ε4 positive than in the other two groups. This is consistent with prior work showing an association between breast cancer and ε4 status (Chang et al., 2005; Moysich et al., 2000; Porrata-Doria et al., 2010), though other studies have failed to find such an effect (Chang et al., 2006; Niemi et al., 2000; Yaylim et al., 2003). By comparison, in a meta-analysis Farrer et al. (1997) found that 25.7% of a group of 6262 Caucasian healthy older adults were APOE ε4 positive, in contrast to patients with Alzheimer’s disease, of whom 58.5% were APOE ε4 positive. While these figures were drawn from an older population, recruited for comparison to individuals with Alzheimer’s disease, we note that the percentages of APOE ε4 positive participants in our CTx− and control groups (21% and 25%, respectively) are similar to the control group of Farrer et al., while our CTx+ group had a notably higher percentage of APOE ε4 positive individuals (42%).
Some limitations of the current work should be considered. First, while group sizes were larger than in our prior cohort, they remain relatively small, particularly for exploration of genetic or other risk factors for cognitive changes related to cancer and treatment. This is also a highly educated cohort. As noted above, previous work has shown that patients with lower baseline cognitive reserve (for which level of education is sometimes used as a proxy) appear to be at greater risk for cancer- and treatment-related cognitive changes. It may therefore be that even greater treatment-related changes in gray matter density would be apparent in a less educated cohort. Alternatively, it may be that individuals with greater education are more likely to be aware of literature regarding cognitive effects of cancer treatment, and may therefore report subjective changes more frequently (see (Schagen et al., 2009) for examination of priming effects in cancer patients). Also, as noted above, CTx+ patients had higher disease stage, on average, than CTx− patients. This is consistent with standard clinical care, as patients with more advanced disease are more likely to receive chemotherapy; however, it does also prevent separation of potential effects of treatment from disease stage. While there was significant commonality in treatment regimen for CTx+ patients (all received a taxane, most also received cyclophosphamide) and anti-estrogen treatment for CTx− patients (over half received tamoxifen), variation in treatment may potentially contribute to data variability (e.g., more patients in the CTx+ than CTx− group received local radiation). The inclusion of patients who received neoadjuvant treatment (one third of the CTx+ group) also potentially increases data variability; this group has not yet been exposed to some surgery-related variables at the M1 visit, but also may have somewhat more advanced disease than patients receiving standard adjuvant treatment.
It is also possible that changes in hormonal status (e.g., chemotherapy-induced ovarian failure) might play a role in the functional and structural brain changes noted in this population. In the CTx+ group 44% (12 of 27 patients) reported that periods were regular at the baseline visit, but had stopped or begun to stop at M1, a change they most commonly attributed to chemotherapy. However, as might be expected, patients who were menstruating at study entry were on average 10 years younger than those who were postmenopausal at study entry (mean age (SD) 44.6 (4.7) and 54.1 (6.9), respectively, P < 0.001), such that change in menstrual status is confounded with age. Comparison of values for the regions where significant gray matter changes were found from baseline to M1 between patients with and without changes in menstrual status showed no group-by-time interaction (P > 0.05), suggesting that chemotherapy-induced ovarian failure did not account for the observed structural changes. However, it will be important to continue to examine the effects of changes in hormonal (estrogen) status on brain functioning in future studies while also considering potential confounds.
Regarding these limitations, it will be advantageous in future work to pool samples when possible, to allow further investigation of APOE and/or other genetic or other biological factors thought likely to convey risk for these changes, as well as examination of individual contributions of specific cancer treatments and demographic factors (e.g., education, cognitive reserve). It will also be beneficial in the future to examine the relationship of these gray matter changes to objective psychometrically defined cognitive functioning. While prior work has consistently shown increases in both objective and subjective cognitive impairment after cancer chemotherapy, objective cognitive performance and subjective complaints are often not directly correlated, highlighting the need to examine both factors. It will also be helpful to examine other potential genetic factors which may be contributory (e.g., COMT (Small et al., 2011)). In our prior cohort (McDonald et al., 2010), reductions in gray matter density in the CTx+ group showed partial but not complete recovery to baseline levels at a follow-up scan conducted one year after the M1 visit. Other recent work (Koppelmans et al., 2012) has shown persistent decreases in total brain and gray matter volume in breast cancer survivors on average 21 years post-treatment. These findings suggest that while some improvement may be expected over time, persistent brain changes may be apparent, at least for a subgroup of patients. We anticipate being able to investigate these and other factors in this cohort in the future, as well as to examine longer-term outcome in terms of gray matter density, when members of this cohort complete additional follow-up visits.
In summary, the current findings replicate and extend our prior work and that of others demonstrating structural brain changes related to breast cancer chemotherapy and concurrent changes in perceived cognitive functioning. This pattern of gray matter change was not observed in breast cancer patients who did not receive chemotherapy or healthy controls, and was found in frontal regions important for attentional and executive functioning, domains commonly found to be affected by cancer and its treatment. These findings therefore provide additional supportive data for a structural neuroanatomic basis for the cognitive problems most commonly reported during and after chemotherapy.
Acknowledgments
The authors thank Kim Wagler-Ziner, PhD, the Indiana University-Melvin and Bren Simon Cancer Center recruitment core, and our oncologist colleagues for their invaluable assistance with patient recruitment. We also thank Bryan P. Schneider, MD, Darren P. O’Neill, MD, Michele A. Beal, RT, Courtney R. Robbins, RT, Sungeun Kim, PhD, and Kelly Holohan, BS for their assistance with aspects of this work, as well as the NIA National Cell Repository for Alzheimer’s Disease (NCRAD) for assistance with DNA preparation for genotyping. Finally, we are very grateful to our participants for their time and effort; this research would not have been possible without their willingness to participate during a challenging time in their lives.
Funding
This work was supported, in part, by the National Institutes of Health, including National Cancer Institute Grant Nos. R01 CA101318, P30 CA082709, and R25 CA117865, National Center for Research Resources Grant Nos. U54 RR025761, C06 RR020128, and S10 RR027710, and National Institute on Aging F30 AG039959, R01 AG019771, P30 AG010133, and U24 AG021886, as well as the Indiana Economic Development Corporation (Grant No. 87884).
References
- Agrawal K, Onami S, Mortimer JE, Pal SK. Cognitive changes associated with endocrine therapy for breast cancer. Maturitas. 2010;67:209–214. doi: 10.1016/j.maturitas.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res. Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J. Clin. Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J. Int. Neuropsychol. Soc. 2003;9:967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KF. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Barona A, Reynolds C, Chastain R. A demographically based index of pre-morbid intelligence for the WAIS-R. J. Consult. Clin. Psychol. 1984;52:885–887. [Google Scholar]
- Bookheimer S, Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer’s and cognitive aging. Annu. 2009;5:343–362. doi: 10.1146/annurev.clinpsy.032408.153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N-W, Chen D-R, Wu C-T, Aouizerat BE, Chen F-N, Hung S-J, Wang S-H, Wei M-F, Chang C-S. Influences of apolipoprotein E polymorphism on the risk for breast cancer and HER2/neu status in Taiwan. Breast Cancer Res. Treat. 2005;90:257–261. doi: 10.1007/s10549-004-4656-7. [DOI] [PubMed] [Google Scholar]
- Chang S-J, Hou M-F, Tsai S-M, Kao J-T, Wu S-H, Hou LA, Tsai L-Y. Association between the apolipoprotein E genotypes and breast cancer patients in Taiwanese. Breast Cancer Res. Treat. 2006;98:109–113. doi: 10.1007/s10549-005-9137-0. [DOI] [PubMed] [Google Scholar]
- Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, Berman MG, Hayes DF, Noll DC, Peltier S, Welsh RC. Prechemotherapy alterations in brain function in women with breast cancer. J. Clin. Exp. Neuropsychol. 2010;32:324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S, Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18:134–143. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14:396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FSAM, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. doi: 10.1002/hbm.21422. in press, epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FSAM, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum. Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Garlinghouse MA, Roth RM, Isquith PK, Flashman LA, Saykin AJ. Subjective rating of working memory is associated with frontal lobe volume in schizophrenia. Schizophr. Res. 2010;120:71–75. doi: 10.1016/j.schres.2010.02.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Matsuoka Y, Inagaki M, Nagamine M, Hara E, Imoto S, Murakami K, Kim Y, Uchitomi Y. Structure of orbitofrontal cortex and its longitudinal course in cancer-related post-traumatic stress disorder. Neurosci. Res. 2007;59:383–389. doi: 10.1016/j.neures.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support. Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, Shah E, Stein R, Whitehead S, Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Brit J Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim HSL, Donovan KA, Small BJ, Andrykowski MA, Munster PN, Jacobsen PB. Cognitive functioning in breast cancer survivors: a controlled comparison. Cancer. 2009;115:1776–1183. doi: 10.1002/cncr.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Bennett FC, Mahaffey ML, Spiegel D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin. Cancer Res. 2009;15:6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch. Neurol. 2011;68:1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, Vrooman H, Niessen WJ, Breteler MMB, Schagen SB. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res. Treat. 2012;132:1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res. Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional MRI study. J. Clin. Oncol. doi: 10.1200/JCO.2011.38.5674. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Saykin AJ. Neurocognitive dimensions of breast cancer and its treatment. Neuropsychopharmacology. 2011;36:355–356. doi: 10.1038/npp.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Saykin AJ, Ahles TA. Brain imaging investigation of chemotherapy-induced neurocognitive changes. In: Meyers CA, Perry JR, editors. Cognition and Cancer. Cambridge, MA: Cambridge University Press; 2008. pp. 19–32. [Google Scholar]
- Moysich KB, Freudenheim JL, Baker JA, Ambrosone CB, Bowman ED, Schisterman EF, Vena JE, Shields PG. Apolipoprotein E genetic polymorphism, serum lipoproteins, and breast cancer risk. Mol. Carcinog. 2000;27:2–9. doi: 10.1002/(sici)1098-2744(200001)27:1<2::aid-mc2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Niemi M, Kervinen K, Kiviniemi H, Lukkarinen O, Kyllonen AP, Apaja-Sarkkinen M, Savolainen MJ, Kairaluoma MI, Kesaniemi YA. Apolipoprotein E phenotype, cholesterol and breast and prostate cancer. J. Epidemiol. Community Health. 2000;54:938–939. doi: 10.1136/jech.54.12.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrata-Doria T, Matta JL, Acevedo SF. Apolipoprotein E allelic frequency altered in women with early-onset breast cancer. Breast cancer. 2010;4:43–48. [PMC free article] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Ivers H, Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res. Treat. 2009;116:113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Roth RM, Isquith PK, Gioia GA. BRIEF-A Behavior Rating Inventory of Executive Function-Adult Version Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc.; 2005. [Google Scholar]
- Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8:201–216. [PubMed] [Google Scholar]
- Schagen SB, Das E, van Dam FSAM. The influence of priming and pre-existing knowledge of chemotherapy-associated cognitive complaints on the reporting of such complaints in breast cancer patients. Psychooncology. 2009;18:674–678. doi: 10.1002/pon.1454. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, van Rhijn D, Rodenhuis S, van Dam FS. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann. Oncol. 2002;13:1387–1397. doi: 10.1093/annonc/mdf241. [DOI] [PubMed] [Google Scholar]
- Scherling C, Collins B, Mackenzie J, Bielajew C, Smith A. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an FMRI study. Front. Hum. Neurosci. 2011;5:122. doi: 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherling C, Collins B, Mackenzie J, Bielajew C, Smith A. Prechemotherapy differences in response inhibition in breast cancer patients compared to controls: a functional magnetic resonance imaging study. J. Clin. Exp. Neuropsychol. 2012;34:543–560. doi: 10.1080/13803395.2012.666227. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res. Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rawson KS, Walsh E, Jim HSL, Hughes TF, Iser L, Andrykowski MA, Jacobsen PB. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- Smith JD. Apolipoprotein E4: an allele associated with many diseases. Ann. Med. 2000;32:118–127. doi: 10.3109/07853890009011761. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin. Neuropsychol. 2006;20:76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann. Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- Wagner L, Sweet J, Butt Z, Beaumont J, Havlin K, Sabatino T, Cella D. Trajectory of cognitive impairment during breast cancer treatment: a prospective analysis. J. Clin. Oncol. 2006;24:S8500. [Google Scholar]
- Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. ‘Chemobrain’ in breast carcinoma? A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- Yaylim I, Bozkurt N, Yilmaz H, Isbir T, Isik N, Arikan S. The apolipoprotein E epsilon 4 allele is not a risk factor for Turkish breast cancer patients. Cancer Genet. Cytogenet. 2003;146:86–87. doi: 10.1016/s0165-4608(03)00124-9. [DOI] [PubMed] [Google Scholar]
- Yoshikawa E, Matsuoka Y, Yamasue H, Inagaki M, Nakano T, Akechi T, Kobayakawa M, Fujimori M, Nakaya N, Akizuki N, Imoto S, Murakami K, Kasai K, Uchitomi Y. Prefrontal cortex and amygdala volume in first minor or major depressive episode after cancer diagnosis. Biol. Psychiatry. 2006;59:707–712. doi: 10.1016/j.biopsych.2005.08.018. [DOI] [PubMed] [Google Scholar]