Abstract
The neuropeptide PDF is crucial for Drosophila circadian behavior: it keeps circadian neurons synchronized. Here, we identify GW182 as a key regulator of PDF signaling. Indeed, GW182 downregulation results in phenotypes similar to those of Pdf and Pdf-receptor (Pdfr) mutants. gw182 genetically interacts with Pdfr and cAMP signaling, which is essential for PDFR function. GW182 mediates miRNA-dependent gene silencing through its interaction with AGO1. Consistently, GW182's AGO1 interaction domain is required for GW182's circadian function. Moreover, our results indicate that GW182 modulates PDFR signaling by silencing the expression of the cAMP phosphodiesterase DUNCE. Importantly, this repression is under photic control, and GW182 activity level - which is limiting in circadian neurons - influences the responses of the circadian neural network to light. We propose that GW182's gene silencing activity functions as a rheostat for PDFR signaling, and thus profoundly impacts the circadian neural network and its response to environmental inputs.
Introduction
Most animals have to cope with important environmental changes caused by the day/night cycle. Their physiology and behavior are therefore temporally controlled and optimized with their ever-changing environment. 24-hr (circadian) rhythms are generated by intracellular pacemakers called circadian clocks, which consist of interlocked transcriptional feedback loops that control the rhythmic expression of clock-controlled genes. In Drosophila, the PERIOD (PER) feedback loop generates transcriptional rhythms that peak in the early night, while the PAR Domain Protein1/VRILLE (PDP1/VRI) feedback loop generates rhythms with a peak in the early day (Hardin, 2006). These two interlocked feedback loops are connected by the dimeric transcription factor CLOCK/CYCLE (CLK/CYC), which transactivates both per and timeless (tim) in one loop, and pdp1 and vri in the other. PDP1 and VRI feed back positively and negatively on the Clk promoter, respectively. PER and TIM form a dimer that acts as a CLK/CYC transcriptional repressor to negatively regulate their own genes’ transcription.
The fly brain contains a mosaic of ca. 150 circadian neurons, which express various neuropeptides and classic neurotransmitters, and have different patterns of neuronal projections (Johard et al., 2009; Nitabach and Taghert, 2008). Studies in the past ten years have begun to shed light on the function of such complex neural organization. Specific neurons have specific roles in the control of circadian behavior. For example, the Pigment Dispersing Factor (PDF) positive small ventral lateral neurons (sLNvs) predominantly generate morning activity in a light: dark (LD) cycle, while the dorsal lateral neurons (LNds) and the PDF negative sLNv are important for evening activity (Grima et al., 2004; Stoleru et al., 2004). Some neurons are more sensitive to temperature cycles (lateral posterior neurons [LPNs], Dorsal Neurons [DN] 1 and 2) and can influence circadian behavior specifically when such environmental cycles are present (Busza et al., 2007; Miyasako et al., 2007; Picot et al., 2009; Yoshii et al., 2009a). Others (large LNvs, LNds, DN1s) appear to be particularly important for light responses (Murad et al., 2007; Picot et al., 2007; Shang et al., 2008; Stoleru et al., 2007; Tang et al., 2010). Finally, a subset of Dorsal Neurons (DN1s) integrates light and temperature inputs to influence circadian behavior (Zhang et al., 2010). Thus, in addition to having specific functions in the control of circadian behavior, different circadian neurons gather specific environmental cues to properly synchronize and organize circadian locomotor rhythms (Zhang and Emery, 2012).
Besides promoting morning activity, the sLNvs have an additional and crucial function. They keep brain pacemaker neurons coherently synchronized, and can thus maintain circadian behavioral rhythms even if flies are under constant conditions (Lin et al., 2004; Renn et al., 1999; Yoshii et al., 2009b). They perform this remarkable task by secreting the neuropeptide PDF (Renn et al., 1999). The Receptor for PDF (PDFR) is broadly expressed in circadian neurons (Hyun et al., 2005; Im and Taghert, 2010; Lear et al., 2005; Lear et al., 2009; Mertens et al., 2005). If PDF or its receptor is missing, flies become rapidly arrhythmic in constant darkness (DD), and in Pdf0 flies circadian neurons are desynchronized in DD (Hyun et al., 2005; Lear et al., 2005; Lin et al., 2004; Mertens et al., 2005; Renn et al., 1999; Yoshii et al., 2009b). These phenotypes are remarkably similar to those seen in mice lacking either the neuropeptide Vasoactive Intestinal Polypeptide (VIP) or its receptor (called either VIPR or VPAC2) (Aton et al., 2005), which are both expressed in the brain pacemaker structure of the mammalian brain: the Suprachiasmatic Nucleus (SCN). Interestingly, VPAC2 and PDFR are not just functional homologs, but actually share considerable sequence similarities (Helfrich-Forster, 2005). The neural mechanisms by which coherent circadian behavior is generated are thus well conserved in the Animal Kingdom. Beside arrhythmicity in constant darkness (DD), mutations in Drosophila PDF or its receptor have other characteristic consequences under LD conditions: the morning peak of activity is severely reduced, and the phase of the evening peak is advanced (Hyun et al., 2005; Lear et al., 2005; Mertens et al., 2005; Renn et al., 1999). This reflects the importance of the sLNvs in the control of morning activity, and their ability to determine the phase of circadian molecular rhythms in other circadian neurons. PDFR belongs to the Class II G-Protein Coupled Receptor (GPCR) family. Solid evidence indicates that it is positively coupled to cAMP signaling (Choi et al., 2012; Duvall and Taghert, 2012; Mertens et al., 2005; Shafer et al., 2008). However, the proteins participating in the PDFR signaling pathway only begins to be identified, with Gsα and the adenylate cyclase AC3 playing an important role in the sLNvs (Choi et al., 2012; Duvall and Taghert, 2012).
Gene expression can be modulated by small RNA molecules called miRNAs (Bartel, 2004). They are generated by an enzymatic cascade from precursor RNAs (Liu and Paroo, 2010). After being transcribed, pri-mRNAs are cleaved in Drosophila by PASHA and DROSHA into pre-miRNAs, which are processed into mature miRNAs by DICER1 (DCR1) and LOQUASCIOUS (LOQS). miRNAs are then loaded into the RISC complex with AGO1. miRNA-targeted RNAs can then be degraded or translationally silenced. This latter mechanism is dependent on GW182, which interacts with AGO1 (Chekulaeva et al., 2009; Eulalio et al., 2008; Eulalio et al., 2009b). Recent studies suggest that miRNA-mediated silencing might play an important role in the control of circadian behavior in both mammals and fruit flies. Two rhythmically expressed miRNAs were identified in mammals (Cheng et al., 2007). Evidence indicates that one of them (miR-132) modulates circadian light responses, while the other (miR-219) affects the pace of the circadian pacemaker. In flies, there are also rhythmically expressed miRNAs, but their function is not known (Yang et al., 2008). Knocking down DCR1 expression with double-stranded RNAs (dsRNA) appears to have surprisingly little effect on circadian rhythms, although this weak effect might be explained by residual DCR1 expression (Kadener et al., 2009). Interestingly however, binding sites for the miRNA bantam (Brennecke et al., 2003) in the 3’-untranslated region (UTR) of the Clk mRNA are important for the amplitude of circadian rhythms, and bantam overexpression alters the period of circadian behavioral rhythms (Kadener et al., 2009). Finally, miR-279 has recently been proposed to affect circadian behavioral output through regulation of the JAK/STAT pathway (Luo and Sehgal, 2012). Despite these recent studies, the role played by miRNA silencing in the control of circadian behavior in Drosophila remains poorly understood.
Results
GW182 downregulation phenocopies Pdfr or Pdf0 mutants
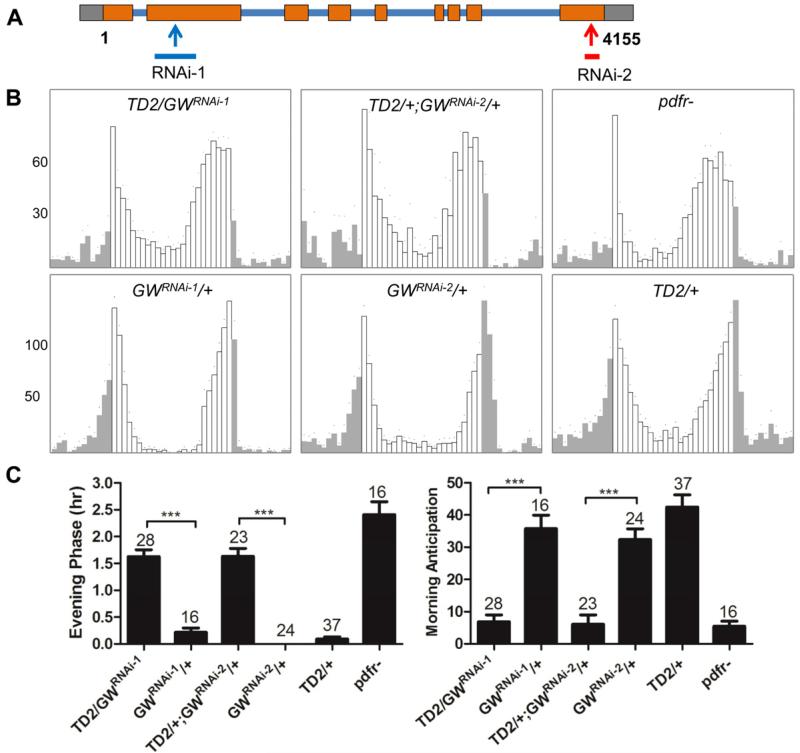
To try to understand better the role that miRNA silencing might play in the control of circadian behavior, we downregulated PASHA, DROSHA, LOQS, DCR1, AGO1 and GW182 with either long dsRNAs (Vienna Drosophila RNAi Center [VDRC] and Transgenic RNAi Project [TRiP] collections) or short hairpin RNAs (shRNA; TRiP collection) (Dietzl et al., 2007; Ni et al., 2011). Flies bearing these RNAi transgenes were crossed to tim-GAL4/UAS-dcr2 flies (TD2). tim-GAL4 is expressed in all circadian tissues. DICER2 (DCR2) was coexpressed with the dsRNAs to enhance RNAi effects (Dietzl et al., 2007). Only one RNAi line, directed against AGO1, was essentially lethal when combined with TD2 (only a few flies survived, see below). Most lines showed either no phenotypes under constant darkness (DD), or a minor period lengthening of about 0.5 hours (Table S1). The most striking phenotype was observed with one line directed against Dcr-1 and two independent lines targeting GW182 (Table 1, Table S1): flies were completely arrhythmic. The two gw182 RNAi lines target non-overlapping regions of the GW182 mRNAs (Figure 1A). Thus, RNAi off-target effects are very unlikely to explain the arrhythmic phenotype observed with these lines. Hence, the arrhythmicity observed with the two gw182 RNAi lines strongly suggests that GW182 is essential for circadian behavior. We therefore focused our work on this protein.
Table 1.
Circadian locomotor behavior under constant darkness (DD)
| GW182 down-regulation | ||||
|---|---|---|---|---|
| Genotype | N | Percent rhythmic | Period (hr) ± S.D. | Power ± S.D. |
| tim-GAL4, UAS-dicer2(=TD2)/+ | 48 | 93.8 | 24.7±0.4 | 65.8±28.1 |
| Pdf-GAL4, UAS-dicer2 (=PD2)/+ | 32 | 96.9 | 24.6±0.4 | 60.5±20.3 |
| TD2/+;Pdf-GAL80/+ | 19 | 100.0 | 24.3±0.3 | 89.3±29.8 |
| GWRNAi-1/+ | 31 | 93.5 | 23.8±0.2 | 90.2±23.6 |
| GWRNAi-2/+ | 37 | 100.0 | 23.9±0.2 | 82.1±32.9 |
| PD2/GWRNAi-1 | 44 | 97.7 | 24.5±0.3 | 64.8±16.2 |
| PD2/+;GWRNAi-2/+ | 31 | 93.5 | 24.3±0.4 | 62.7±20.1 |
| TD2/GWRNAi-1 | 82 | 2.4 | 24.7±0.5 | 25.5±5.2 |
| TD2/+;GWRNAi-2/+ | 30 | 0 | - | - |
| TD2/GWRNAi-1;Pdf-GAL80/+ | 32 | 25.0 | 23.6±0.2 | 48.6±14.5 |
| Pdfr-han5304 | 24 | 4.2 (N=1) | 23.2 | 28.4 |
| Genetic interactions with PDFR/cAMP signaling | ||||
| TD2/ GWRNAi-1 ;t-PDF 58F1a/+ | 40 | 67.5 | 24.2±0.4 | 44.9±18.8 |
| TD2/ GWRNAi-1; t-PDF 58F4a/+ | 22 | 41.0 | 24.1±0.6 | 37.5 ±16.8 |
| TD2/ GWRRNAi-1 ; t-SCRB/+ | 30 | 0 | - | - |
| dnc1 | 22 | 59.1 | 24.7±0.4 | 57.5±14.8 |
| dnc1;TD2/+; GWRNAi-2 /+ | 32 | 41.0 | 25.2±1.3 | 44.2±21.4 |
| UAS-dnc/+ | 16 | 100 | 23.9±0.2 | 111.8±13.1 |
| TG4/UAS-dnc | 18 | 0 | - | - |
| TD2/Pka-C1RNAi | 32 | 0 | - | - |
| Pka-C1RNAi/+ | 31 | 100 | 24.2±0.3 | 103.3±35.8 |
| TD2/GsαRNAi | 50 | 4 | 24.9±0.4 | 35.2±5.4 |
| GsαRNAi /+ | 24 | 100 | 24.1±0.3 | 118.0±18.2 |
| GW182 knockdown rescues (Rescue lines showing significant changes of periods compared to control flies are shown in bold ) | ||||
| TG4/+ | 20 | 100.0 | 24.9±0.3 | 87.1±18.1 |
| GW_12AA#3/+ | 15 | 86.7 | 24.3±0.3 | 64.2±20.1 |
| GW_12AA#7/+ | 16 | 87.5 | 23.7±0.2 | 80.3±32.7 |
| GW#27/+ | 15 | 100.0 | 24.4±0.2 | 73.7±18.0 |
| GW#38b/+ | 16 | 100.0 | 24.3±0.2 | 63.7±27.5 |
| GW#64/+ | 15 | 100.0 | 23.9±0.4 | 50.5±36.4 |
| TD2/UAS-GFP;GWRNAi-2/+ | 27 | 0 | - | - |
| TD2/GW_12AA#3;GWRNAi-2/+ | 20 | 0 | - | - |
| TG4/GW_12AA #3 | 27 | 74.1 | 25.0±0.3 | 70.8±24.1 |
| TD2/GW_12AA#7;GWRNAi-2/+ | 27 | 59.2 | 23.2±0.7 | 49.1±24.3 |
| TG4/GW_12AA #7 | 32 | 90.6 | 24.7±0.4 | 72.9±36.3 |
| TD2/GW#27;GWRNAi-2/+ | 54 | 88.9 | 26.5±0.3 | 74.7±28.4 |
| TG4/GW #27 | 73 | 90.5 | 26.7±0.3 | 65.3±24.8 |
| TD2/+;GW#38b/GWRNAi-2 | 44 | 68.2 | 24.8±0.5 | 57.0±32.3 |
| TG4/+;GW#38b/+ | 22 | 100.0 | 25.4±0.3 | 59.6±31.4 |
| TD2/+;GW#53/GWRNAi-2 | 20 | 50.0 | 25.7±0.4 | 50.5±17.2 |
| TG4/+;GW#53/+ | 21 | 90.5 | 25.8±0.4 | 61.4±18.4 |
| TD2/GW#64;GWRNAi-2/+ | 62 | 93.5 | 25.4±0.4 | 87.1±89.9 |
| TG4/GW #64 | 37 | 81.1 | 26.0±0.3 | 52.8±26.9 |
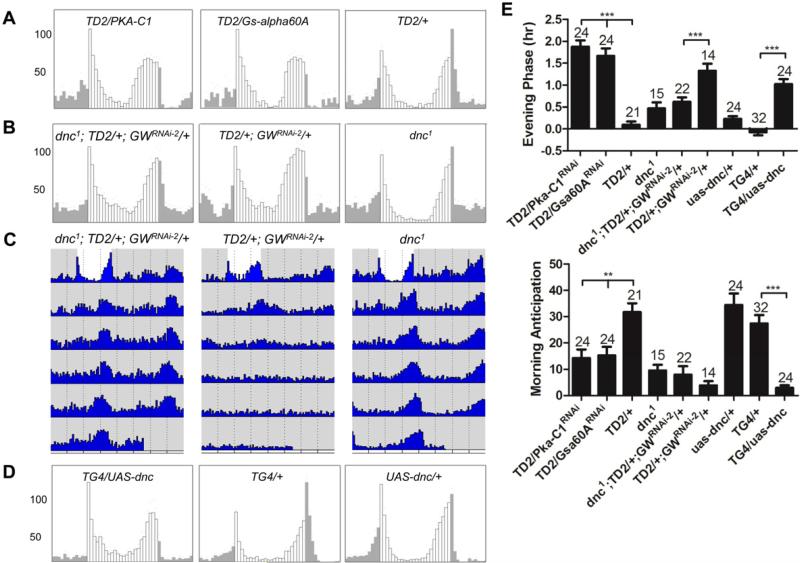
Figure 1. gw182 downregulation phenocopies Pdf/Pdfr mutants.
(A) Regions of the gw182 mRNA targeted by the two non-overlapping RNAi lines. RNAi-1 is a long double-stranded RNAi line from the VDRC stocks center (KK101472), while RNAi-2 is a short hairpin RNAi line from the TRiP stock center (HMS00105). (B) Average locomotor activity of flies with different genotypes under 3 days of 12hr: 12hr light:dark (LD) conditions. Grey activity bars represent the night, white bars the day. gw182 is downregulated with tim-GAL4, UAS-dcr2 (TD2). The evening peak of activity anticipating the light-off transition is advanced and the morning peak anticipating the light-on transition is severely disrupted, as in Pdfr mutants. Control genotypes are shown on the three bottom panels. (C) Quantification of morning anticipation and evening phase. Evening peak phase was determined in individual flies. Average phase is shown for each genotype, expressed in hours before lights-off transition. Morning anticipation in individual flies was calculated by subtracting average activity measured in 30 minute bin during the middle of the night (Zeitgeber Time [ZT]17-ZT19.5) from the average activity measured prior to light on (ZT21.5-ZT24). Individual fly anticipations were then averaged and the averages are shown on the bar graphs. Compared to control flies, knocking down gw182 significantly decreased the morning anticipatory activity and advanced the evening phase. The numbers of tested flies are shown above the error bar. *** = P< 0.001 as determined by Tukey's multiple comparison test after one -way ANOVA. See material and methods for additional details on quantification of behavior. Error bars correspond to S.E.M.
To understand further the role that GW182 might be playing in the control of circadian behavior, we observed gw182 RNAi flies under light:dark (LD) cycles. Circadian behavior was also altered under these conditions, but not as severely as under DD (Figure 1B and 1C). The morning peak of activity was severely blunted, but a robust evening peak of activity was present, indicating that the molecular circadian pacemaker was still functional under LD, at least in the Evening oscillators. Interestingly though, the phase of the evening peak of activity was clearly advanced compared to control flies (Figure 1B and 1C). The trio of phenotypes observed when downregulating GW182 is not unprecedented. Pdf0 and Pdfr mutant flies are also mostly arrhythmic in DD, show severely reduced morning anticipation, and have an advanced evening peak of activity (Hyun et al., 2005; Lear et al., 2005; Mertens et al., 2005; Renn et al., 1999) (Figure 1B and 1C). Thus, our results strongly suggest that GW182 is implicated in the PDF/PDFR signaling pathway, which plays an essential role in the control of circadian behavior.
GW182 is expressed in circadian neurons and is required in adult flies for rhythmic behavior
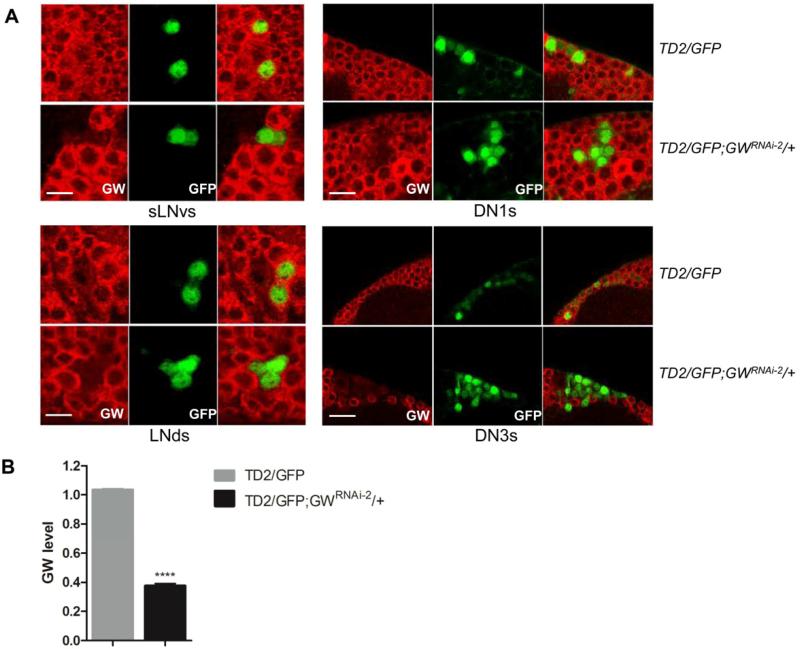
If GW182 were important for PDF/PDFR signaling, we would expect it to be expressed in circadian neurons. We stained fly brains with an anti-GW182 antibody and found GW182 to be widely expressed in the brain, which is expected since it plays a crucial role in miRNA silencing (Eulalio et al., 2009b). Notably, all circadian neurons that we could visualize with GFP expression driven by tim-GAL4 expressed GW182(Figure 2A). We also stained brains of flies expressing gw182 dsRNAs in clock neurons. We found GW182 levels to be severely reduced in these cells (Figure 2A). Quantifications in DN1s showed a reduction of ca. 60% (Figure 2B). This is probably an underestimation of the actual downregulation. Indeed, we could not subtract background signal since GW182 is expressed in all neurons. In summary, GW182 is expressed in both PDF-positive and PDF-negative circadian neurons, and downregulated in these cells in the presence of dsRNAs.
Figure 2. GW182 is downregulated in circadian neurons expressing gw182 shRNAs.
(A) Representative confocal images showing GW182 downregulation in different groups of circadian neurons of TD2/UAS-GFP; GWRNAi-2/+ flies (lower images of each panel) compared with TD2/UAS-GFP control flies (upper images of each panel). The brains were stained with anti-GW182 antibody (red) and anti-GFP antibody (green). Scale bars indicate 20 μm. (B) Quantifications of GW182 downregulation in DN1s. y-axis shows the relative GW182 level in DN1s compared with the average level in three neighboring non-circadian neurons. GW182 is reduced by over 60% in flies expressing gw182 shRNAs. Error bars correspond to S.E.M. **** = p<0.0001 was determined by t test.
No obvious anatomical defects were observed in the cell bodies of circadian neurons and in the projections of sLNvs when GW182 was downregulated (Figure 2 and S2A). However, more subtle developmental defects could be responsible for the circadian phenotype we observed when expressing gw182 dsRNAs. Thus, we restricted the expression of gw182 dsRNAs either to the developmental or to the adult stage using GAL80ts, which is a temperature sensitive repressor of GAL4 (McGuire et al 2004). When GW182 was downregulated only during development, no phenotypes were observed in LD or DD (Figure S2B, S2C). However, most flies were arrhythmic when the gw182 dsRNAs were expressed only during adulthood. In LD, morning activity was partially suppressed and the onset of evening activity advanced by about one hour. This slightly weaker phenotype compared to that observed with constitutive gw182 dsRNA expression is probably explained by a less extensive GW182 downregulation. We therefore conclude that GW182 is required in adult circadian neurons for normal behavioral rhythms.
GW182 is required in PDFR expressing circadian neurons
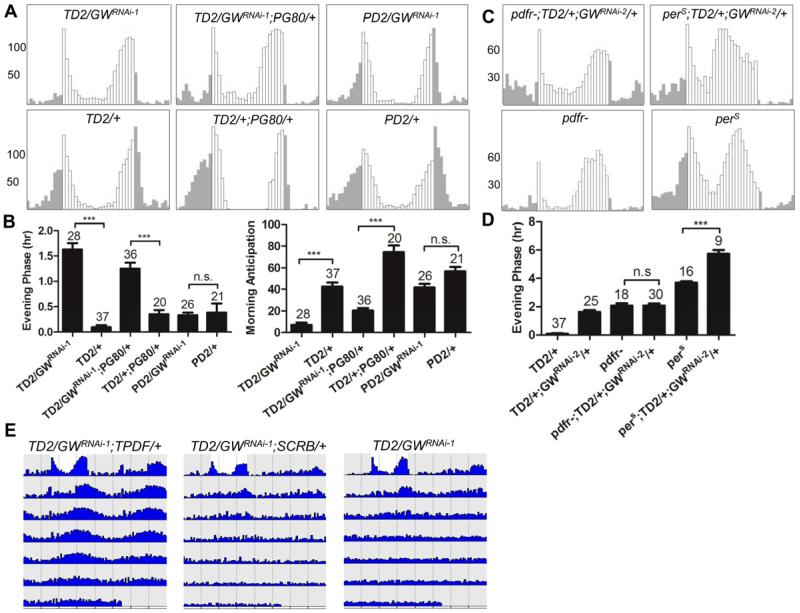
Since GW182 downregulation result in a phenotype reminiscent of those of flies with no PDF signaling, and since GW182 is expressed in both PDF positive and negative circadian neurons, it could affect either PDF expression/release or PDFR signaling. To distinguish between these two hypotheses, we determined in which circadian neurons GW182 is required. We first crossed gw182 RNAi transgenic flies to Pdf-GAL4/UAS-dcr2 (PD2) flies to downregulate GW182 only in PDF positive circadian neurons. This tissue-specific downregulation had no effect on circadian behavior in LD and DD (Figure 3A, 3B and Table 1), which strongly suggests that GW182 is primarily required in PDF-negative circadian neurons. Although we have previously observed that Pdf-GAL4 is as efficient as tim-GAL4 to downregulate genes in PDF-positive LNvs (Dubruille et al., 2009), we cannot entirely exclude the possibility that there is higher residual GW182 expression in these neurons when using Pdf-GAL4. We therefore also combined TD2 with Pdf-GAL80 (PG80), to block expression of the dsRNAs in PDF-positive LNvs. The phenotypes were comparable to those with TD2 alone, although slightly weaker (Figure 3A, 3B, Table 1). 75% of TD2/GWRNAi-1; PG80/+ flies were arrhythmic (98% without PG80), morning peak was blunted, and the evening peak phase advanced. We therefore conclude that GW182's primary role is in PDF-negative circadian neurons, which strongly suggest that it functions in the PDFR pathway (Lear et al., 2009).
Figure 3. gw182 and Pdfr are in the same signaling pathway.
(A-B) GW182 is primarily required in PDF negative circadian neurons. (A) Average locomotor activity under LD conditions of flies expressing gw182 dsRNAs in all circadian neurons, or different groups of circadian neurons (PG80 = Pdf-GAL80, PD2 = Pdf-GAL4, UAS-dcr2). (B) Quantification of evening phase and morning anticipation. The advanced evening peak phase and decreased morning anticipation are due to downregulation of GW182 in PDF negative circadian neurons. TD2/+ control is the same as in Figure 1. *** = P< 0.001 as determined by Tukey's multiple comparison test after one -way ANOVA. n.s. = not significant at the 0.05 level. (C-D) gw182 genetically interacts with Pdfr. (C) Locomotor activity of Pdfr or perS mutants with or without GW182 downregulation, in LD conditions. (D) Quantification of evening phase. There are no additive effects on the advance of evening anticipation in Pdfr/gw182 double-mutant flies. However, there are clear additive effects when GW182 is downregulated in perS mutants. (E) Actograms showing the average activities on the last day of LD and during 6 days in DD. Light represents the day and grey darkness. From left to right: 1) gw182 RNAi flies expressing the membrane-tethered PDF. 2) gw182 RNAi flies expressing a membrane-tethered scrambled PDF (negative control). 3) gw182 RNAi flies. Note that rhythms in DD are restored with tethered PDF, but in LD, evening activity remains advanced. Error bars correspond to S.E.M.
gw182 and Pdfr genetically interact
The results presented so far strongly suggest that GW182 plays a positive role in the PDFR signaling pathway. If indeed this is the case, flies in which expression of gw182 dsRNAs is combined with a severely hypomorphic Pdfr mutation should behave similarly as single mutant flies. If on the contrary GW182 and PDFR affect two separate pathways, we would expect an additive effect. Since the morning peak of activity is almost entirely eliminated in both gw182 RNAi flies and Pdfr mutant flies, and both are almost completely arrhythmic in DD, the only phenotype that can show additive effects is the evening peak. We observed no additive effects when combining a Pdfr mutation with GW182 downregulation on the phase of evening activity (Figure 3C, and 3D). This absence of additive effect is not caused by a limitation in how early the evening peak can be advanced. Indeed, the evening peak in perS mutant flies (Konopka and Benzer, 1971) is more advanced than gw182 or Pdfr mutants, and could even be further advanced when perS was combined with gw182 downregulation (Figure 3C and 3D). The absence of additive effect is thus specific to the gw182-RNAi/Pdfr mutant combination, and therefore strongly suggests that GW182 and PDFR are in the same signaling pathway.
To strengthen further this notion, we determined whether hyperactivation of PDFR could at least partially rescue the phenotypes associated with decreased GW182 expression. To increase PDFR signaling, we overexpressed a membrane-tethered PDF peptide (t-PDF). Expression of this peptide in PDF negative neurons is known to result in phenotypes reminiscent to those of flies with high PDF levels (Choi et al., 2009). Strikingly, we could rescue rhythmicity in DD in 60% of flies with one of the t-PDF transgenic line (40% in the other, Figure 3E, Table 1). Importantly, a scrambled version of the t-PDF (t-SCRB) was totally unable to do so. LD behavior was not rescued with t-PDF, however. Thus, hyperactivation of PDFR can partially suppress the phenotypes associated with downregulation of GW182. This result, combined with all the results presented above, clearly demonstrate that GW182 is an essential element of the PDFR pathway.
GW182's AGO1 binding domain is required for its circadian function
GW182 plays a central role in miRNA-mediated translation silencing. It does so by interacting with AGO1, which binds directly to miRNAs (Eulalio et al., 2009b). Unfortunately, we could not determine directly whether AGO1 is important for GW182's circadian function. AGO1 null mutants are lethal, one of the two AGO1 RNAi line showed no phenotype, and the other RNAi line is as mentioned above almost completely lethal when combined with TD2 (or even in the absence of DCR2). A few unhealthy escapers were obtained. Not surprisingly, they were arrhythmic both in DD and LD, with very low activity levels (data not shown).
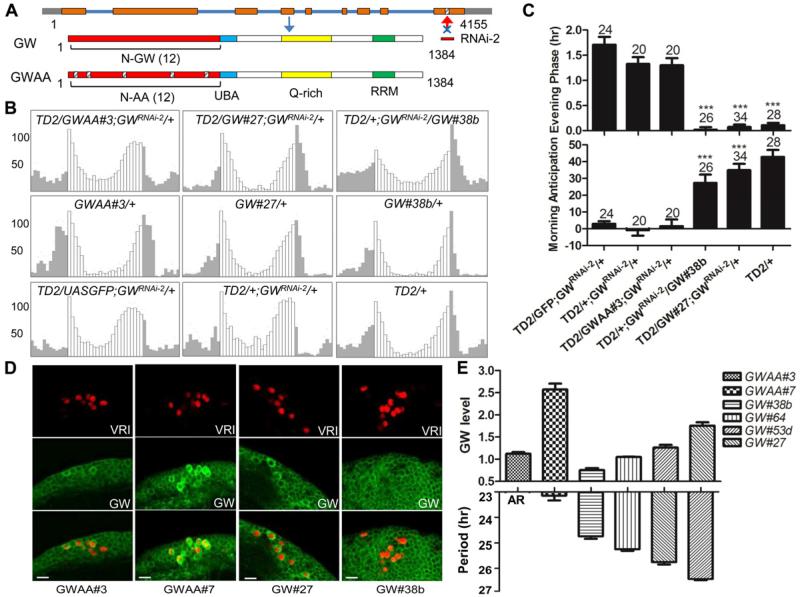
To determine whether GW182 works with AGO1 to regulate circadian behavior, we used a rescue strategy. We generated two transgenes resistant to the GW182 shRNA by mutagenizing extensively the binding site for this shRNA without affecting the amino acid sequence of the GW182 protein (Figure 4A). The first transgene encodes a wild-type GW182 (GW), while the other encodes a mutant protein (GWAA) in which the 12 N-terminal Glycine-Tryptophane (GW) motifs critical for AGO1 binding were changed to Alanines (AA) (Eulalio et al., 2009a).
Figure 4. Wild-type GW182, but not the GW-repeat mutant (GWAA) rescues gw182 RNAi phenotypes.
(A) Design of the GW182 rescue experiments. The binding site for the shRNA was extensively mutagenized with synonymous codons to make the transgenic mRNAs resistant to the gw182 shRNA. Two constructs were generated: A wild-type construct (GW) and a mutant construct (GWAA) in which the 12 GW amino acid residues critical for AGO1 binding were mutagenized. (B) Average activity of flies of different genotypes under LD conditions. Representative lines expressing either the GW-repeat mutant (GWAA) or the wild-type GW182 are shown. TD2/UASGFP; GWRNAi-2/+ is used as an additional control. Note the normal phase of the evening peak and rescued morning peak with the wild-type transgenes (#27 and 38b). The evening peak remains advanced with the mutant protein, and the morning activity is still blunted (GWAA#3). (C) Quantification of evening phase and morning anticipation. Wild-type GW182 transgenes significantly rescued the LD behavior defects for GW182 knock down flies. Statistics are the same as Figure 3. (D-E) The level of GW182 activity correlates with circadian period length. (D) Rescued GW182 expression in different transgenic lines in the presence of GW182 dsRNAs. The brains were stained with anti-GW182 antibody (green) and anti-VRI antibody (red). (E) Circadian behavior period in DD is correlated with GW182 protein and activity levels. The upper half of the bar graph shows the quantification of GW182 protein levels in DN1s with GWAA mutant or wild-type GW transgenes. Y-axis is the ratio of GW182 levels in DN1s compared with those of neighboring non-circadian neurons (in wild-type flies, this ratio is about 1). The lower half of the bar graph shows the circadian periods (tau) of different lines in DD, plotted on the Y-axis (hr). X-axis is the genotypes in both graphs. Error bars correspond to S.E.M. Note that with the severely hypomorphic GWAA mutant protein, flies are either arrhythmic, or rhythmic with a short period reminiscent of the short period rhythms of the rare Pdf/Pdfr mutant flies that remain rhythmic in DD. On the contrary, period lengthen with increased GW182 activity and protein levels, as in flies with excessive PDF signaling. This indicates that GW182 functions as a rheostat of PDFR signaling and regulates the circadian period. Error bars correspond to S.E.M.
We then coexpressed the shRNA and the resistant constructs with the TD2 combination. As expected, rhythmicity was restored in DD with the wild-type gw182 transgene (although frequently with a long period phenotype, see below), and under a LD cycle both the morning peak and the evening were entirely normal in phase and amplitude (Figure 4B, 4C and Table 1, Table S2). This definitely establishes that all the phenotypes we observed with the gw182 dsRNAs are caused by GW182 downregulation. Importantly, the GWAA mutant had a very limited ability to rescue the GW182 downregulation phenotype. None of the 5 mutant lines we obtained could rescue behavior under LD (Figure 4B, 4C and data not shown). 3/5 lines did not rescue behavior in DD (Table 1, Table S2). One line showed very weak rescue in DD. The strongest rescue was observed with GWAA line #7, with about 50% of flies being rhythmic in constant conditions. Amplitude of these rhythms was weaker than in control flies.
To correctly interpret the partial rescue observed with GWAA mutant line #7, and the absence of rescue with other mutant lines such as #3, we measured GW182 protein levels in the presence of the shRNA. With line #3, protein levels were slightly higher than those of wild-type flies (Figure 4D and 4E). Thus, the complete failure of this transgene to rescue the GW182 knockdown phenotype is not the result of low protein level. This clearly shows that the AGO1 binding residues of GW182 are critical for its circadian function. However, the GWAA mutant protein must retain a very weak ability to bind to AGO1, because we could detect a partial rescue of rhythmicity in DD with line #7, which has much higher GW182 levels than wild-type flies or mutant line #3.
The level of GW182 activity correlates with circadian period length
The period obtained in DD with GWAA mutant line #7 is short (Table 1, Figure 4E). Interestingly, this is what is observed in the rare Pdf0 or Pdfr mutant flies that remain rhythmic in DD. When we rescued GW182 knockdown phenotypes with wild-type rescue transgenes, we observed various period lengths in DD. With most lines, period was long. Line #27, for example had a 26.5hr period phenotype in the presence of the gw182 dsRNAs (Table 1, Figure 4E). With line #38b however, a similar period length as control flies was observed (Table 1). Again, we measured protein levels in these rescued flies to understand these phenotypes. With wild-type line #38b, GW182 levels in clock neurons were slightly below those of wild-type flies (Figure 4D, 4E). However, with line #27, protein levels were about 2 fold higher than wild-type (Figure 4D, 4E). Two additional lines were tested and confirmed a correlation between period length and GW182 expression (Figure 4E). Thus, period length in DD is exquisitely sensitive to GW182 levels. This is also supported by the fact that period is always slightly longer when the wild-type transgenes are expressed in a wild-type background (in the absence of shRNAs), and thus in the presence of genomically encoded GW182 (Table 1, Table S2). Behavior with long period has been observed when PDF is overexpressed or when PDFR is hyperstimulated (Choi et al., 2009; Wulbeck et al., 2008; Yoshii et al., 2009b). Thus, we conclude that the level of GW182 activity is directly correlated with period length and the level of PDFR signaling (Figure 4E). GW182 is therefore a critical regulator of circadian behavior and communication between circadian neurons, and its activity is limiting in clock neurons. Interestingly, the long period phenotype observed with GW182 overexpression was partially suppressed by lowering AGO1 levels, but not AGO2 (Figure S3). This genetic interaction further demonstrates that GW182 regulates circadian behavior through miRNA-mediated gene regulation, and that period length is exquisitely sensitive to RISC complex activity.
GW182 modulates DNC expression in the PDFR signaling cascade
PDFR has been shown in vivo and in cell culture to promote the production of cAMP, suggesting that cAMP is an important secondary messenger in the PDFR signaling cascade (Mertens et al., 2005; Shafer et al., 2008). Moreover, in PDF-positive sLNvs, PDFR signaling is dependent on Gsα and the adenylyl cyclase AC3 (Choi et al., 2012; Duvall and Taghert, 2012). To determine whether cAMP signaling is also essential in PDF-negative circadian neurons, we downregulated Gsα and the three Drosophila PKA catalytic subunits with tim-GAL4. We observed the typical trio of phenotypes characteristic of PDFR signaling disruption when Gsα and PKA-C1 were downregulated, but not when PKA-C2 and -C3 were targeted (Figure 5A, 5E, Table 1, Table S3, data not shown). PKA-C1 downregulation combined with a Pdfr mutation confirmed that PKA-C1 is indeed in the PDFR pathway (no additive effect on the evening peak, Figure S3). Thus, PDFR is dependent on cAMP for its signaling in both PDF-positive and negative circadian neurons.
Figure 5. GW182 interacts with the cAMP signaling cascade, which is required for PDFR function.
(A) Average locomotor activities of flies under LD cycles. Knocking down PKA-C1 or Gαs with dsRNAs in circadian neurons phenocopy Pdf/Pdfr mutants. Four independent RNAi lines for PKA-C1and two lines for Gsα were tested and they all showed similar phenotypes. See (E) for quantification. (B-D) dnc is in the PDFR signaling pathway. (B) Average locomotor activities of flies under LD cycles. From left to right: 1) gw182 RNAi flies carrying the dnc1 mutation. The phase of the Evening peak is almost perfectly restored. 2) gw182 RNAi flies. 3) dnc1 mutant flies. (C) Actograms showing average activities on the last day of LD and 5 days in DD. Light represents the day and grey darkness. DD rhythms are restored in gw182 RNAi flies carrying the dnc1 mutation. (D) Average locomotor activities of flies under LD cycles. Overexpression of DNC in circadian neurons (left panel) phenocopies Pdfr mutants, with severely disrupted morning anticipation and advanced evening peak phase. All flies were arrhythmic in DD (Table 1). Middle and right panels are control flies. (E) Quantification of evening phase and morning anticipation. Statistics are the same as Figure 3. Error bars correspond to S.E.M.
Since GW182 silences gene expression but plays a positive role in PDFR signaling, it is unlikely to target directly PDFR, Gsα or PKA-C1. A more likely candidate would be a negative regulator of cAMP signaling, such as a phosphodiesterase. The suppression of the gw182 downregulation phenotype observed with t-PDF shows that PDFR signaling is not entirely abolished in flies with downregulated GW182. We therefore decided to combine gw182 dsRNAs with dnc1, a hypomorphic mutation in the gene coding for the cAMP phosphodiesterase DUNCE (DNC). Indeed, it has been previously proposed that DNC might affect circadian behavior and photoreception (Dahdal et al., 2010; Levine et al., 1994). Interestingly, we found that gw182 and dnc genetically interact. LD behavior was partially rescued in dnc1/gw182-RNAi flies. The evening peak phase was much closer to that of wild-type flies than to GW182 knockdown flies (Figure 5B, 5E). The morning peak was however not restored, but was for unclear reasons weak even in dnc1 single mutants. The dnc1/gw182-RNAi flies also showed much greater rhythmicity in DD than gw182-RNAi flies (41% vs. 0, note that only 60 % of dnc1 flies are rhythmic in our hands, Figure 5C, Table 1). We did not observe any rescue with the rut1 mutation, which affects an adenylate cyclase involved in learning and memory, like dnc (data not shown) (Waddell and Quinn, 2001). The suppression of the GW182 knockdown phenotype is thus specific to dnc. Interestingly, DNC overexpression using tim-GAL4 resulted in a phenotype similar to that of Pdf/Pdfr mutants in LD (Figure 5D, 5E), and all DNC overexpressing flies were arrhythmic in DD (Table 1). Combined, these results show that DNC is a negative modulator of PDFR, as expected for a phosphodiesterase. They also reinforce the notion that cAMP is a key secondary messenger in the PDFR pathway. Finally it strongly suggests that GW182 negatively regulates DNC expression.
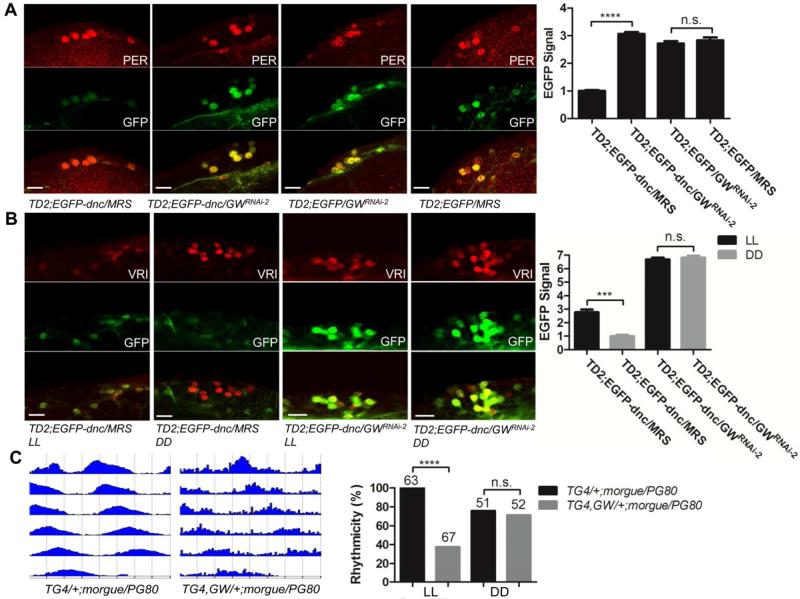
Since there are to our knowledge no antibody available for DNC, we tested whether GW182 indeed regulates DNC levels by expressing an EGFP reporter fused (or not) to the 3’-untranslated region (3’-UTR) of dnc, which contains a predicted conserved miRNA binding site. Strikingly, we found that expression of EGFP under the control of the dnc 3’UTR is highly sensitive to GW182 downregulation (Figure 6A). EGFP signal was dramatically increased in gw182 RNAi flies, as expected since GW182 silences gene expression. On the contrary, the control construct missing the 3’UTR of dnc was insensitive to GW182 downregulation. Thus, our genetic and imaging results converge in identifying DNC as a critical target of GW182 in the PDFR signaling cascade.
Figure 6. dnc 3’UTR is under both GW182 and photic control.
(A) Representative confocal images showing EGFP reporter expression in DN1s. Knocking down GW182 increased the level of EGFP-dnc 3’UTR expression but had no effects in the absence of dnc 3’UTR. Flies were entrained for 3 days in LD and brains were dissected at ZT1 for anti-PER (red) and anti-GFP staining. MRS is a “balancer” chromosome that does not carry the GWRNAi-2 transgene. Right panel is the quantification of EGFP levels. (B) Representative confocal images showing EGFP-dnc 3’ UTR expression in DN1s during the 3rd day of constant light (LL) or constant dark (DD). Brains were dissected at Circadian TIme (CT) 16 for anti-VRI (red) and anti-GFP staining. Right panel is the quantification of EGFP levels. (C) GW182 influences light-dependent responses of the circadian neural network. Actograms showing average activities of 16 flies under constant light (LL) for 5 days, and quantification of rhythmic behavior. Overexpression of morgue only in PDF negative circadian neurons render flies rhythmic in LL, but co-overexpression of gw182 severely decreased this rhythmicity. Circadian behavior in DD is unaltered by GW182 overexpression. Four independent experiments were performed for LL and three for DD. Quantification is based on all these independent experiments. The number of tested flies is shown above each column. ****= p<0.0001, n.s. not significant, determined by z test. Error bars correspond to S.E.M.
Light modulates DNC expression and circadian behavior in a GW182-dependent manner
Several studies have demonstrated that the organization of the circadian neural network responds to environmental light. While the sLNvs drive circadian behavior in the dark or under short photoperiod, PDF negative circadian neurons can take control of circadian behavior under constant light (LL) or long photoperiod (Murad et al., 2007; Picot et al., 2007; Stoleru et al., 2007). This plasticity in neural hierarchy - thought to contribute to seasonal adaptation of circadian behavior (Stoleru et al., 2007) - results from photic inhibition of sLNv output and activation of PDF negative circadian neuron output (Picot et al., 2007).
Since PDF is a major sLNv output and since our data indicate that GW182 modulates PDFR signaling through the 3’UTR of dnc, we decided to test whether dnc expression is controlled by light. We measured EGFP-dnc 3’UTR level of expression in control and gw182 dsRNA flies under two conditions: LL and DD (Figure 6B). The results were striking: EGFP expression was ca. three times higher in LL than in DD in control flies, but it was not affected at all by light when GW182 was downregulated. dnc 3’UTR activity is not under circadian control (Figure S5), which means that its derepression in LL is not a secondary effect of LL-induced disruption of the molecular circadian pacemaker. Our results therefore indicate that DNC expression is derepressed by prolonged light exposure, which is predicted to result in decreased PDFR signaling and therefore a weakening of the connection between the sLNvs and its neuronal targets. Since this is GW182-dependent, and that GW182 activity is in a dynamic range in circadian neurons (Figure 4E), it also suggests that GW182 activity is repressed in the dark (see discussion).
Does GW182 indeed impact the light-dependent reorganization of the circadian network? A method to reveal this neural plasticity is to inhibit the circadian photoreceptor Cryptochrome (CRY) or its signaling pathway to allow flies to remain rhythmic under constant light (Murad et al., 2007; Picot et al., 2007; Stoleru et al., 2007). We have previously shown that we can achieve this by overexpressing MORGUE only in PDF negative circadian neurons, leaving the PDF positive circadian neurons unprotected from LL and thus arrhythmic (Murad et al., 2007). Hence, we compared the behavior of flies overexpressing MORGUE or both MORGUE and GW182 in PDF-negative circadian neurons. As previously described, MORGUE overexpressing flies are very strongly rhythmic in LL (100% rhythmic, Figure 6C, Table S4). When PDFR signaling is increased by overexpressing GW182, only ca. 40% of flies are rhythmic, and the rhythmic flies have reduced amplitude of circadian behavior (Figure 6C, Table S4). We conclude that the arrhythmic signal the unprotected PDF positive sLNvs send to downstream PDFR positive circadian neurons is amplified by increased PDFR signaling, and thus partially disrupt LL rhythms. Importantly, GW182 overexpression does not increase arrhythmicity in DD. GW182 levels thus modulate light-dependent changes in circadian neurons hierarchy.
Discussion
We have demonstrated that circadian behavior and PDFR signaling are under the control of GW182, a protein critical for miRNA silencing. Our results strongly suggest that GW182 functions as a rheostat determining the intensity of PDFR signaling through repression of the cAMP phosphodiesterase DNC (Figure 7). Indeed, we find interesting correlations between GW182 levels of activity and the phenotype we observed.
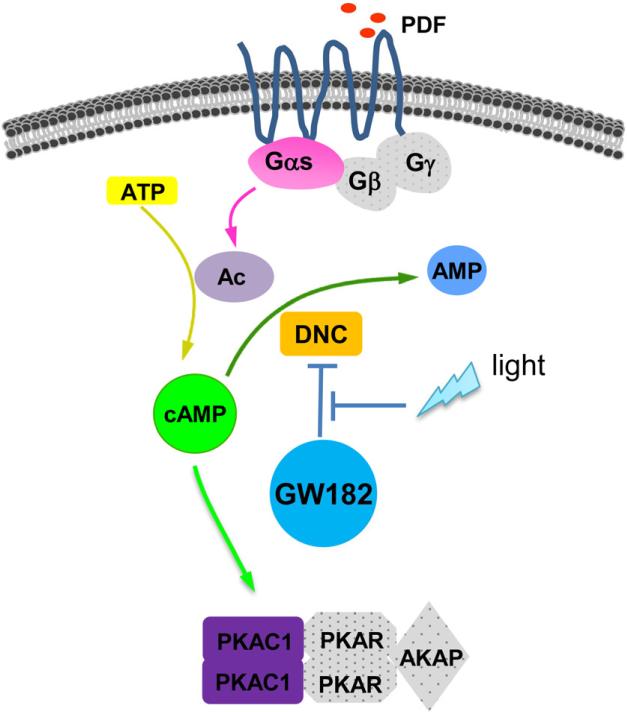
Figure 7. GW182 regulates PDFR signaling pathway through modulation of DNC.
Gαs (Choi et al., 2012, this study), PKA-C1 (this study) and adenylate cyclase AC3 (at least in PDF-positive sLNvs) (Duvall and Taghert, 2012) are positive components of PDFR signaling. Our work reveals that GW182 also promotes PDFR signaling by repressing DNC, which degrades cAMP and thus represses PDFR signaling. GW182-mediated DNC repression is controlled by light. Grey shapes are as yet unidentified components of the PDFR signaling pathway.
Very severe reduction in GW182 levels results in behaviors that are reminiscent of those found in flies lacking PDF or PDFR. The only difference is that GW182 knockdown is slightly more severe as we see virtually complete arrhythmicity in DD, while a small percentage of Pdf0 or Pdfr mutant flies remain rhythmic, with a short period phenotype (Hyun et al., 2005; Lear et al., 2005; Mertens et al., 2005; Renn et al., 1999). This might indicate that GW182 affects secondary signaling pathways that work in parallel of PDFR. Interestingly, as in Pdf/Pdfr mutants, short period rhythms are observed in ca. 50% of flies expressing very high levels of the GWAA mutant, which must thus retain a very weak ability to interact with AGO1 and thus repress translation. As GW182 activity increases, longer period phenotypes are observed. It is striking that only a ca. 2 fold overexpression of GW182 can lengthen period by about 2 hours. This period lengthening parallels again what is observed with various manipulations of PDF signaling: PDF overexpression or hyperexcitation of PDFR results in long period phenotypes as well (Choi et al., 2009; Wulbeck et al., 2008; Yoshii et al., 2009b). However, these manipulations also frequently result in internal desynchronization, with some groups of circadian neurons running fast and some slow. Thus, in many flies, behavior is not simply long, but complex. Both a long period rhythm and a short period rhythm are observed in single flies. We did not observe such behavioral complexity with GW182 overexpression, but the period length we obtained with GW182 overexpression (up to 2 hours) is in the range of the long period component obtained when PDFR was hyperexcited with t-PDF (Choi et al., 2009). As discussed above, GW182 might not just affect PDFR signaling, and its overexpression might have slightly different consequences for circadian behavior than increases in PDFR signaling. There is however a more likely and interesting possibility. GW182 overexpression may preferentially affect circadian neurons that lengthen their period when stimulated by PDF, because GW182 is limiting only in these neurons. Interestingly, neurons that lengthen their period length in response to PDF overlap with those that can drive circadian behavior under constant light (LL) conditions: the CRY positive LNds and the DN1s (Murad et al., 2007; Picot et al., 2007; Stoleru et al., 2007; Yoshii et al., 2009b). The disruption of LL behavior when GW182 is overexpressed (Figure 6C) thus fits nicely with the notion that these neurons are particularly sensitive to GW182 and PDFR signaling. Strikingly, these neurons also express high PDFR levels (Im and Taghert, 2010).
By which mechanisms does GW182 regulate PDFR and cAMP signaling? GW182 interacts with AGO1 and is essential for miRNA-mediated translation. We actually identified GW182 as a regulator of circadian behavior in a mini-screen in which we downregulated miRNA-related genes, but most dsRNAs targeting these genes had little effects on circadian behavior. Only subtle period changes were observed. This however might be simply explained by insufficient downregulation of the enzymes responsible for miRNA synthesis, as proposed in a previous study in which DCR1 knockdown had very little effect on circadian behavior (Kadener et al., 2009). Surprisingly, one of the Dcr-1 lines we tested was arrhythmic, but unlike what was observed with GW182 downregulation, LD behavior was only very mildly affected (Figure S1), with possibly a slightly advanced evening peak. This could be indicative of a mild Pdf0-like phenotype, but we have to take these results very cautiously. First, they were observed with one dsRNA line only, and there is therefore the possibility of off-target effects. Second, it would actually be surprising that DD rhythms would be so profoundly disrupted, while LD behavior is almost unaffected. Indeed, in our rescues with GWAA mutants or with tethered PDF, DD behavior was partially restored, but LD behavior was not. With AGO1 downregulation, we could not get any informative results. One of the RNAi line showed no phenotypes, while the other one was semi-lethal, with a few unhealthy survivors. However, we found AGO1 levels to be limiting when GW182 is overexpressed (Figure S3). Moreover, the GW182 amino acid residues necessary for AGO1 binding (the N-terminus GW motifs) are essential to GW182's circadian function. We therefore conclude that GW182's role in the control of circadian behavior is dependent on AGO1 and thus miRNA silencing. Our identification of the 3’UTR of dnc as a target of GW182 fits perfectly with this notion. In the absence of GW182, we observed in circadian neurons a strong derepression of an EGFP reporter gene controlled by dnc 3’UTR, which contains a highly conserved miRNA binding site according to Pictar and Targetscan predictions (Krek et al., 2005; Ruby et al., 2007).
The evidence that dnc is a key target for GW182 in the PDFR pathway is particularly strong. In addition to showing that GW182 represses dnc 3’UTR, we have found that decreasing dnc activity can partially correct the loss of gw182 in clock neurons, and overexpressing DNC is sufficient to mimic closely the loss of GW182, or of PDFR signaling. Moreover, the idea that GW182 regulates DNC level would explain how hyperexciting the PDFR receptor partially corrects the loss of gw182. Increased PDFR signaling would compensate for increased cAMP catabolism. This said, other genes in the PDFR cascade might also be directly or indirectly regulated by GW182. Indeed, in S2 cells, several positive and negative elements of the cAMP cascade are misregulated when GW182 is depleted (Behm-Ansmant et al., 2006). Interestingly, two adenylate cyclases are downregulated, while PDE11 is upregulated. This again fits perfectly with a positive role of GW182 in promoting PDFR/cAMP signaling. Finally, misregulation of UPD and the JAK/STAT pathway might also contribute to the GW182 arrhythmic phenotype in DD, since it is regulated by miR279, and miR279 knockout decreases rhythm amplitude under these conditions (Luo and Sehgal, 2012).
GW182 activity is limiting in circadian neurons, since, as discussed above, decrease and even modest increase in GW182 activity result in phenotypes reminiscent of those observed with loss or gain of function in PDFR signaling, respectively. The fact that GW182 activity is set to such a dynamic range and is thus able to modulate the PDFR pathway is intriguing. This makes GW182 an ideal target for pathways that would impact the hierarchy between circadian neurons. For example, under constant light or long photoperiod, the role of PDF positive circadian neurons is decreased, while the role of PDF negative neurons is promoted (Murad et al., 2007; Picot et al., 2007; Stoleru et al., 2007). The inhibition of the PDF positive LNvs’ contribution to circadian behavior is dependent on visual inputs, and affect output mechanisms, not the molecular pacemaker (Picot et al., 2007). GW182 could thus be targeted by visual inputs to modulate PDF signaling downstream of PDFR in the presence of light. Our finding that GW182 overexpression severely reduces rhythmicity in LL, but not in DD, strengthens the idea that GW182 level of activity might be a target for photic regulation. Strikingly, we found that the 3’UTR of dnc is derepressed by light and that this derepression is dependent on GW182. DNC derepression in LL is predicted to decrease PDFR signaling, and thus to weaken the influence of the sLNvs on downstream neurons, which is what Picot et al. (2007) observed. Although we strongly favor the idea that GW182 is the target of visual inputs and thus mediates photic modulation of the circadian network, we cannot at the present time exclude that another element of the RISC complex (AGO1, or a miRNA targeting dnc 3’UTR) is controlled by light. Indeed, GW182 level is not obviously altered by the presence of light (data not shown). GW182 activity might thus be mostly regulated by a posttranslational mechanism such as phosphorylation. Indeed, in mammals, GW182 is a phosphoprotein, (Eystathioy et al., 2002). In any case, our result reveals a novel mechanism by which light might modulate circadian behavior and the hierarchy between circadian neurons: the modulation of DNC expression. This could be an important mechanism for seasonal adaptation to different photoperiods, which is thought to depend on changes in circadian neuron hierarchy (Stoleru et al., 2007)
In summary, our work demonstrates that GW182 is a critical regulator of PDFR signaling. Since VIP/VIPR play a very similar function as PDF/PDFR in the SCN (Aton et al., 2005), which control circadian rhythms in mammals, and since PDFR and VIPR share extensive sequence homologies and signaling mechanisms, it will be interesting to determine whether the three human homologs of GW182 also modulate VIPR signaling and circadian behavior. Our results also reveal a novel mechanism by which GPCR signaling as well as neural networks and their organization can be modulated by miRNA silencing mechanisms.
Methods
Fly stocks
All the flies were raised on cornmeal/agar medium at 25°C under a light:dark (LD) cycle. The following strains were used: w1118, y w; tim-GAL4/CyO (Kaneko et al., 2000), y w; Pdf-GAL4/CyO (Renn et al., 1999); y w; tim-GAL4/CyO; Pdf-GAL80/TM6B (Murad et al., 2007), y w; tim-GAL4 UAS-dcr2/CyO, y w; Pdf-GAL4 UAS-dcr2/CyO (Dubruille et al., 2009), perS (Konopka and Benzer, 1971), dnc1 , rut1 (Duerr and Quinn, 1982), ago1k08121 (Kataoka et al., 2001), ago2414 (Okamura et al., 2004). The Pdfr mutant flies contain the han5304 allele (Hyun et al., 2005). RNAi stocks were obtained from VDRC and TRiP stock centers. Wild-type gw182 and GW-repeat mutant cDNA were cloned from pAc5.1(Eulalio et al., 2009a) and the binding sites for the shRNA were mutagenized with synonymous codons to make it resistant to the gw182 shRNA. The cDNAs were cloned into pUAST to make transgenic flies. For EGFP reporter flies, EGFP with or without dnc 3’UTR cDNA were cloned into pUAST-attB1 constructs and injected for site directed transgenes.
Behavioral Experiments and Analysis
For almost all experiments, adult male flies (2-5 days old) were used for testing locomotor activity rhythms. Only when using the Pdfr-GAL4 driver did we use females, because this driver is an enhancer trap located into the proximal promoter of the Pdfr gene, which is located on the X chromosome. Flies were entrained for 3 full days LD cycle at 25°C, using about 500 lux light intensities, and then released into DD at 25°C for at least 5 days. Locomotor activity was measured with TriKinetics Activity Monitors (Waltham, MA) in I36-LL Percival Incubators. Locomotor activity was averaged over the 3 days entrainment for LD and 5 days for DD. Data analysis was performed with the FAAS-X software(Grima et al., 2002). Actograms were generated using a signal-processing toolbox implemented in MATLAB (MathWorks)(Levine et al., 2002). For GAL80ts experiments, flies were raised at 18°C and tested at 30°C, or raised at 30°C and tested at 18°C. They were entrained for 5 days and then released in DD for at least 5 days.
For each fly, morning anticipation amplitude was measured by averaging the activity count obtained in five 30-minute bins between ZT17-19.5 (middle of the night) and between ZT21.5 and ZT24 (just before lights on). The first value was subtracted to the second to obtain the amplitude of the morning peak. Morning anticipations of Individual flies were then averaged and plotted on the graphs. Evening peak phase was also measured in individual flies. The highest 30-minute bin count in the evening (or midday in extremely advanced flies) was defined as the evening peak. Its value was set relative to the light-off transition. For example, if the peak occurred two hours before lights-off, than its phase was equal to 2. If activity had not reached a peak before the startle response caused by the light-off transition (as in most control flies), evening phase was equal to 0, or even to negative values if activity kept increasing after lights-off. Individual fly's evening peaks were then averaged and plotted on the bar graph.
Whole-mount Immunohistochemistry
Whole-mount immunohistochemistry for fly brains were done as previously described (Zhang et al., 2010). Adult fly (3-6 days old) were dissected in chilled PBT (PBS with 0.1% Triton X-100), and fixed in 4% formaldehyde diluted in PBS for 30 min at room temperature. The brains were rinsed and washed with PBT three times (10 min each). Then brains were incubated with 10% normal donkey serum diluted in PBT to block for 40 min at room temperature and incubated with primary antibodies at 4 °C overnight. For VRI staining we used 1:10,000 guinea pig anti-VRI (generous gift from Dr. Hardin). We used a 1:2,000 dilutions for rabbit anti-GW182 (generous gift from Dr. Izaurralde) and 1:200 for mouse anti-GFP. After six times washes with PBT (20 min each), brains were incubated with relative secondary antibody at 4°C overnight followed by another six washes with PBT. All samples were imaged on a Zeiss LSM5 Pascal confocal microscope, with laser settings kept constant within each experiment. Eight to ten fly brains for each genotype were dissected for imaging. Representative images are shown. ImageJ software (NIH) was used for GW182 quantification in 15-20 DN1s from at least five brains. For quantification, signal intensity in each DN1 and average signals in three neighboring non-circadian neuron was measured and the ratio between signals in DN1s and non-circadian neurons were calculated.
Supplementary Material
Highlights.
- GW182 promotes PDFR signaling and is thus critical for circadian behavior
- GW182 gene silencing activity is limiting and determines PDFR signaling levels
- GW182 inhibits the expression of DNC, a negative regulator of PDFR signaling
- GW182 influences the response of the circadian neural network to light input
Acknowledgments
We would like to thank Diana Wentworth and Diane Szydlik for technical support, and the Emery, Weaver and Reppert lab, as well as V. Ambros, E. Izaurralde and M. Ramaswami for helpful discussions. We also thank the VDRC and TRiP stock centers for RNAi transgenic flies, as well as M. Nitabach, R. Allada, P. Zamore, S. Waddell and V. Budnik for various fly strains. We are very grateful to E. Izaurralde for the pAc5-GW182 and pAc5-GW182AA plasmids, as well as the anti-GW182 antibodies, and to P. Hardin for anti-VRI antibodies. This work was supported by NIH grant GM066777, GM079182 and GM100091 to P.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
P.E supervised the project. Y.Z. and P.E, designed the experiments. Y.Z performed the experiments and analysis. Y.Z. and P.E, wrote the manuscript.
References
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Busza A, Murad A, Emery P. Interactions between circadian neurons control temperature synchronization of Drosophila behavior. J Neurosci. 2007;27:10722–10733. doi: 10.1523/JNEUROSCI.2479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekulaeva M, Filipowicz W, Parker R. Multiple independent domains of dGW182 function in miRNA-mediated repression in Drosophila. RNA. 2009;15:794–803. doi: 10.1261/rna.1364909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Cao G, Tanenhaus AK, McCarthy EV, Jung M, Schleyer W, Shang Y, Rosbash M, Yin JC, Nitabach MN. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in Drosophila. Cell Rep. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Fortin JP, McCarthy E, Oksman L, Kopin AS, Nitabach MN. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Curr Biol. 2009;19:1167–1175. doi: 10.1016/j.cub.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahdal D, Reeves DC, Ruben M, Akabas MH, Blau J. Drosophila pacemaker neurons require g protein signaling and GABAergic inputs to generate twenty-four hour behavioral rhythms. Neuron. 2010;68:964–977. doi: 10.1016/j.neuron.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dubruille R, Murad A, Rosbash M, Emery P. A constant light-genetic screen identifies KISMET as a regulator of circadian photoresponses. PLoS Genet. 2009;5:e1000787. doi: 10.1371/journal.pgen.1000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr JS, Quinn WG. Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proc Natl Acad Sci U S A. 1982;79:3646–3650. doi: 10.1073/pnas.79.11.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall LB, Taghert PH. The circadian neuropeptide PDF signals preferentially through a specific adenylate cyclase isoform AC3 in M pacemakers of Drosophila. PLoS Biol. 2012;10:e1001337. doi: 10.1371/journal.pbio.1001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Helms S, Fritzsch C, Fauser M, Izaurralde E. A C-terminal silencing domain in GW182 is essential for miRNA function. RNA. 2009a;15:1067–1077. doi: 10.1261/rna.1605509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Huntzinger E, Izaurralde E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat Struct Mol Biol. 2008;15:346–353. doi: 10.1038/nsmb.1405. [DOI] [PubMed] [Google Scholar]
- Eulalio A, Tritschler F, Izaurralde E. The GW182 protein family in animal cells: new insights into domains required for miRNA-mediated gene silencing. RNA. 2009b;15:1433–1442. doi: 10.1261/rna.1703809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eystathioy T, Chan EK, Tenenbaum SA, Keene JD, Griffith K, Fritzler MJ. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol Biol Cell. 2002;13:1338–1351. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Grima B, Lamouroux A, Chelot E, Papin C, Limbourg-Bouchon B, Rouyer F. The F-box protein SLIMB controls the levels of clock proteins PERIOD and TIMELESS. Nature. 2002;420:178–182. doi: 10.1038/nature01122. [DOI] [PubMed] [Google Scholar]
- Hardin PE. Essential and expendable features of the circadian timekeeping mechanism. Curr Opin Neurobiol. 2006;16:686–692. doi: 10.1016/j.conb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C. PDF has found its receptor. Neuron. 2005;48:161–163. doi: 10.1016/j.neuron.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, et al. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J Comp Neurol. 2010;518:1925–1945. doi: 10.1002/cne.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johard HA, Yoishii T, Dircksen H, Cusumano P, Rouyer F, Helfrich-Forster C, Nassel DR. Peptidergic clock neurons in Drosophila: ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J Comp Neurol. 2009;516:59–73. doi: 10.1002/cne.22099. [DOI] [PubMed] [Google Scholar]
- Kadener S, Menet JS, Sugino K, Horwich MD, Weissbein U, Nawathean P, Vagin VV, Zamore PD, Nelson SB, Rosbash M. A role for microRNAs in the Drosophila circadian clock. Genes Dev. 2009;23:2179–2191. doi: 10.1101/gad.1819509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Park JH, Cheng Y, Hardin PE, Hall JC. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J Neurobiol. 2000;43:207–233. doi: 10.1002/(sici)1097-4695(20000605)43:3<207::aid-neu1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Takeichi M, Uemura T. Developmental roles and molecular characterization of a Drosophila homologue of Arabidopsis Argonaute1, the founder of a novel gene superfamily. Genes Cells. 2001;6:313–325. doi: 10.1046/j.1365-2443.2001.00427.x. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lear BC, Zhang L, Allada R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol. 2009;7:e1000154. doi: 10.1371/journal.pbio.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Casey CI, Kalderon DD, Jackson FR. Altered circadian pacemaker functions and cyclic AMP rhythms in the Drosophila learning mutant dunce. Neuron. 1994;13:967–974. doi: 10.1016/0896-6273(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Signal analysis of behavioral and molecular cycles. BMC Neurosci. 2002;3:1–25. doi: 10.1186/1471-2202-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Paroo Z. Biochemical principles of small RNA pathways. Annu Rev Biochem. 2010;79:295–319. doi: 10.1146/annurev.biochem.052208.151733. [DOI] [PubMed] [Google Scholar]
- Luo W, Sehgal A. Regulation of Circadian Behavioral Output via a MicroRNA-JAK/STAT Circuit. Cell. 2012;148:765–779. doi: 10.1016/j.cell.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Miyasako Y, Umezaki Y, Tomioka K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J Biol Rhythms. 2007;22:115–126. doi: 10.1177/0748730407299344. [DOI] [PubMed] [Google Scholar]
- Murad A, Emery-Le M, Emery P. A subset of dorsal neurons modulates circadian behavior and light responses in Drosophila. Neuron. 2007;53:689–701. doi: 10.1016/j.neuron.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire RE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE. 2004;220:pI6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, Yang-Zhou D, Shim HS, Tao R, Handler D, Karpowicz P, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods. 2011;8:405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Okamura K, Ishizuka A, Siomi H, Siomi MC. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol. 2007;5:e315. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Klarsfeld A, Chelot E, Malpel S, Rouyer F. A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain. J Neurosci. 2009;29:8312–8320. doi: 10.1523/JNEUROSCI.0279-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf Neuropeptide Gene Mutation and Ablation of PDF Neurons Each Cause Severe Abnormalities of Behavioral Circadian Rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Ruby JG, Stark A, Johnston WK, Kellis M, Bartel DP, Lai EC. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res. 2007;17:1850–1864. doi: 10.1101/gr.6597907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Griffith LC, Rosbash M. Feature Article: Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Nawathean P, Fernandez MP, Menet JS, Ceriani MF, Rosbash M. The Drosophila circadian network is a seasonal timer. Cell. 2007;129:207–219. doi: 10.1016/j.cell.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Tang CH, Hinteregger E, Shang Y, Rosbash M. Light-mediated TIM degradation within Drosophila pacemaker neurons (s-LNvs) is neither necessary nor sufficient for delay zone phase shifts. Neuron. 2010;66:378–385. doi: 10.1016/j.neuron.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S, Quinn WG. What can we teach Drosophila? What can they teach us? Trends Genet. 2001;17:719–726. doi: 10.1016/s0168-9525(01)02526-4. [DOI] [PubMed] [Google Scholar]
- Wulbeck C, Grieshaber E, Helfrich-Forster C. Pigment-dispersing factor (PDF) has different effects on Drosophila's circadian clocks in the accessory medulla and in the dorsal brain. J Biol Rhythms. 2008;23:409–424. doi: 10.1177/0748730408322699. [DOI] [PubMed] [Google Scholar]
- Yang M, Lee JE, Padgett RW, Edery I. Circadian regulation of a limited set of conserved microRNAs in Drosophila. BMC Genomics. 2008;9:83. doi: 10.1186/1471-2164-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Vanin S, Costa R, Helfrich-Forster C. Synergic entrainment of Drosophila's circadian clock by light and temperature. J Biol Rhythms. 2009a;24:452–464. doi: 10.1177/0748730409348551. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Wulbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Forster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J Neurosci. 2009b;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Emery P. Molecular and Neural Control of Insects Circadian Rhythms. In: Gilbert LI, editor. Insect Molecular Biology and Biochemistry. Academic Press; 2012. pp. 513–551. [Google Scholar]
- Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.