Abstract
Impact of activating (aKIR) and inhibitory (iKIR) on overall survival (OS), relapse-related mortality (RRM), and acute graft-vs.-host disease (aGVHD) were studied in 84 adults with high risk hematologic malignancies receiving reduced intensity conditioning (RIC) T-cell depleted hematopoietic stem cell transplantation (HSCT) from haploidentical related donors. In this clinical model, freedom from RRM was more dependent on graft-vs-leukemia (GVL) effect. Patients were divided into myeloid (n=49) and lymphoid (n=35) malignancy groups. KIR-ligand and ligand-ligand models were studied in both directions and statistically correlated with outcome measures. In the myeloid group, OS was higher (p=0.009) and RRM lower (p=0.036) in patients missing HLA-C group2 ligand to donor inhibitory KIR. OS was higher if patients had >1 missing ligand (p=0.018). In lymphoid malignancy, missing ligand to donor KIR had no impact on OS or RRM. However, OS was longer with donor activating KIR 2DS2 (p=0.028). There was a trend toward shorter OS in recipient with KIR 2DS1, 2DS5 and 3DS1, although sample sizes are too small to provide inferential statistics. These results suggest that absence of appropriate HLA ligands in the recipient to donor iKIR may induce GVL without aGVHD in myeloid malignancy patients undergoing TCD-RIC transplants.
Keywords: KIR, Activating KIR, Inhibitory KIR, Haploidentical, Reduced Intensity Conditioning (RIC), Hematopoietic Stem Cell Transplantation (HSCT)
INTRODUCTION
Benefit of natural killer (NK) cell mediated graft-versus-leukemia (GVL) effect in haploidentical T cell–depleted hematopoietic stem cell transplantation (HSCT) was initially reported after myeloablative conditioning regimen(1). However, many patients, particularly those of older age and with co-morbidities are unable to tolerate myeloablative conditioning. Use of reduced intensity conditioning (RIC) allows them access to HSCT. Additionally, RIC offers a very useful model for the study of factors like KIR that may influence GVL effect which is largely responsible for the anti-leukemic benefits of RIC transplants(2-5).
The KIR gene family currently consists of inhibitory KIRs (KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL4, KIR2DL5A, KIR2DL5B, KIR3DL1, KIR3DL2 and KIR3DL3) and activating KIRs (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5 and KIR3DS1) located on chromosome 19 and encode for corresponding receptors on NK cells(6). KIR2DL1 recognizes HLA-C alleles of “C2 group” identifiable by Lys at position 80 (HLA-CLys80), KIR2DL2/3 recognizes HLA-C alleles of “C1 group” with Asn at position 80 (HLA-CAsn80), KIR3DL1 recognizes “Bw4” alleles, and KIR3DL2 recognizes HLA-A3 and HLA-A11 alleles(7-9). It is important to remember that not all KIR genes from an individual’s genotype may be expressed nor all NK cells in that individual will carry corresponding KIR protein on the cell surface. Inhibitory receptors inhibit lysis after binding with the corresponding ligand while stimulation of activating KIRs leads to killing by the NK cells(10). In normal individuals, activating and inhibitory KIRs mediate killing of virally infected or tumor cells through corresponding signals after interactions with specific class I HLA molecules that act as their cognate ligands(11).
Following HSCT, donor-derived NK cells attack allogeneic cells in the patient if the recipient HLA class I ligands do not sufficiently engage their inhibitory receptors(12). Reduced intensity cytoreduction is expected to spare many host derived immune cells and may allow an anti-graft attack by host NK cells if alloreactivity is present in the reverse direction. HLA ligands for activating KIRs are not yet defined but KIR2DS1 recognizes the C2 group of alleles in Epstein-Barr virus–transformed B-lymphocytes(13;14) and leukemia cells(12). The current study examines the differences in overall survival (OS), relapse-related mortality (RRM) and incidence of Grade II-IV acute graft-versus-host disease (aGvHD) following TCD haploidentical RIC HSCT in patients with lymphoid and myeloid malignancies with respect to the impact of inhibitory and activating KIRs in the patients and their donors, ligand to ligand mismatch as well as inhibitory KIR to ligand alloreactivity.
METHODS
Patient, Donor, and Graft Characteristics
Between March 2000 and July 2008, 84 consecutive adult patients with relapsed or refractory hematologic malignancies treated at Duke University Medical Center with T-cell depleted peripheral blood stem cell transplantation (PBSC-T) from haploidentical related donors following RIC regimen were included in this study. Donor and patient DNA samples had to be available to be included in the study. These patients lacked suitable related or unrelated bone marrow donors as well as suitable single cord blood units or were not eligible for ablative therapy due to age or co-morbidities. All patients were enrolled in IRB approved protocols and signed informed consent. Diagnoses are shown in table 1. The patient and donor pairs were 3/6 (n=41), 4/6 (n=29), 5/6 (n=12), or 6/6 (n=2). HLA-C and – DQB1 revealed further mismatches. The distribution of HLA matching was similar between the myeloid and lymphoid malignancy patients by Yates Chi-square test. Patients were categorized as having lymphoid (n=35) or myeloid (n=49) malignancy (Table 1). The median age was 48.5 years (range, 18 – 72 years). Myeloid patients were older (51 vs. 43 years; p=0.032). In general, the patients were heavily pretreated with multiple regimens. In addition, a number of patients had undergone prior autologous (n=14; 16.5%) or allogeneic (n=10; 11.7%) transplants. Fifty-one percent (n=43) of patients had relapsed or refractory disease at the time of transplant. There was no statistical difference between the myeloid and lymphoid groups with respect to prior transplantation and advanced disease status. These characteristics were similar to those reported in an earlier paper from our center that focused on the clinical and immunological recovery in a subgroup of these patients(4). The median duration of follow-up of surviving patients was 30.4 months (range, 13 to 77 months). The patient-donor sex matching was similar between the lymphoid and myeloid groups. Donors received filgrastim 8 μg/kg twice daily beginning 5 days before pheresis and continuing until apheresis was completed. All purging was accomplished in vivo after stem cell infusion into the patient. In vitro estimates of the degree of T-cell purging were performed in a subset of patients. A 5-mL aliquot of patient serum and pheresed donor cells were mixed to a cell suspension containing 25% serum volume and incubated for 30 minutes at 37°C with 2 μg of alemtuzumab followed by a flow analysis for viability of the cellular suspension using the 7-aminoactinomycin-D (7AAD) method(15;16). Donor lymphocyte infusions (DLI) were planned for all patients who had persistence of disease after transplantation if they did not have severe GVHD. Details of cell collection, in-vivo T-cell depletion, estimates of CD34 and CD3+ cell counts, other graft characteristics, clinical protocol and outcomes on partial cohort is described previously(4;17).
Table 1.
Patient Characteristics
| Characteristic | All Patients (N=84) |
Myeloid Malignancy (N=49) |
Lymphoid Malignancy (N=35) |
||||
|---|---|---|---|---|---|---|---|
| Age, years | |||||||
| Median | 48.5 | 51 | 43 | p =0.032 | |||
| Range | 18 – 72 | 21 – 72 | 18 – 69 | ||||
| Patients ≥ 60 years old (n, %) | 15 | 17.9% | 11 | 22.5% | 4 | 11.4% | |
| Duration of follow-up, months | |||||||
| Median | 63 | 45.5 | 78 | p =0.008 | |||
| Range | 1 - 113 | 1 - 113 | 5 – 113 | ||||
| Diagnosis | |||||||
| Acute Myeloid Leukemia (AML) | 36 | 42.8% | 36 | 73% | |||
| Myelodysplastic syndrome (MDS) | 13 | 15.5% | 13 | 27% | |||
| Acute Lymphoid Leukemia (ALL) | 12 | 14.1% | 12 | 34.3% | |||
| Hodgkin’s Disease (HD) | 4 | 4.7% | 4 | 11.4% | |||
| Mantle Cell Lymphoma (MCL) | 2 | 2.4% | 2 | 5.7% | |||
| Non Hodgkin’s Lymphoma (NHL) | 17 | 20.0% | 17 | 48.6% | |||
| Pre-transplantation therapies | |||||||
| Prior autologous transplantation | 14 | 16.5% | 8 | 16.0% | 6 | 17.1% | p = 0.89 |
| Prior allogeneic transplantation | 10 | 11.7% | 6 | 12.0% | 4 | 11.4% | p = 0.94 |
| Relapse or Refractory Disease at Transplant |
43 | 50.6% | 23 | 46.0% | 20 | 57.1% | p = 0.43 |
Regimen
The preparative regimen included intravenous infusions of 5 days of Alemtuzumab 20 mg/day on days −4 to 0 and 4 days of fludarabine (Flu) 30 mg/m2/day and cyclophosphamide (Cy) 500 mg/m2/day on days −5 to −2. All patients with 3–5/6 HLA-matched grafts received mycophenolate 1 gm orally twice daily for 45-60 days after transplantation. Starting day +1, patients received Filgrastim 5 mcg/kg (rounded to nearest vial) till the absolute neutrophil count was >1 × 109/l for 2 days.
HLA and KIR Typing
HLA- A, B, C, DRB1 and DQB1 were performed on each recipient and donor by reversed Sequence-Specific Oligonucleotide Probe (rSSOP, Luminex), Sequence-Specific Primer (SSP), or Sequence-Based Typing (SBT). High resolution HLA-C typing was performed retrospectively by SBT when necessary to characterize alleles in the different HLA-C ligand groups. Low resolution KIR typing was performed by Luminex-rSSOP reagents (One Lambda, Canoga Park, CA) for the presence or absence of 16 KIR genes including inhibitory KIR genes (KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL4, KIR2DL5, KIR3DL1, KIR3DL2 and KIR3DL3), activating KIR genes (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5 and KIR3DS1) and pseudogenes (KIR2DP1 and KIR3DP1). KIR genotyping was validated by typing known controls (n=16) from IHIWS panel.
Interpretation of NK alloreactivity
Ligand-ligand Model (Ligand Incompatibility Model) The presence of an epitope of HLA ligand for KIR in the donor and its absence in the patient is assumed to represent potential for donor NK alloreactivity against the patient target cells resulting in alloreactivity in the GVH direction. Conversely, the presence of an epitope of HLA ligand for KIR in the patient and its absence in the donor will result in alloreactivity in the rejection direction. In this study, KIR alloreactivity was measured for HLA-C group 1 and 2, and Bw4 ligand incompatibilities. Each recipient/donor pair was evaluated for ligand-ligand incompatibility in both directions. A combined ligand incompatible score was also considered. A positive combined score was assigned when either one or both of HLA-C and Bw4 ligands were incompatible. Receptor-ligand Model (Missing Ligand Model) - We measured the KIR alloreactivity for missing HLA-C group 1 and 2, Bw4 and/or A3/11 ligands. Each pair was evaluated for the donor KIR genotype and lack of corresponding ligands in recipient or vice versa. A combined KIR score was also considered. The combined score was the sum of the number of the KIR genes (2DL1/2/3, 3DL1/2) without ligand. Activating KIR receptors: Genotyping was performed for the presence of each activating KIR receptors (2DS1, 2DS2, 2DS3, 2DS4, 2DS5, and 3DS1) in donors and patients.
Statistical Analysis
Outcome measures used in this study included overall survival (OS), relapse related mortality (RRM), and acute graft-versus-host disease (GvHD). Acute GVHD was scored in all patients by standard criteria(18) and only grades II – IV were measured for this analysis. The probabilities of acute GvHD were displayed using cumulative-incidence-function method(19). For acute GvHD, patients were evaluable after surviving to Day 14 and death without the event was the competing event while patients were censored on the last day of follow-up. Overall survival (OS) was measured from the first day of transplant to death and censored at the last follow-up. Relapse related mortality (RRM) was measured from the first day of transplantation to death following a relapse or was censored at time of death from non relapse cause or date of follow-up for living patients. Kaplan-Meier probability curves(20) were used to analyze OS, RRM, and acute grades II-IV GvHD by KIR alloreactivity for myeloid malignancy and lymphoid malignancy groups separately and then with the two groups combined. The differences between groups were compared using the log-rank statistics(21). Central tendencies of age and duration of follow-up are compared between myeloid and lymphoid patients using the Wilcoxon Rank-Sums test. Proportion with pre-transplant therapies and relapse or refractory disease at transplant in the myeloid and lymphoid groups are compared using the Chi-square test. All p-values are two-sided. Analyses were completed using the SAS system, version 9.2, and R, version 2.1.1.
RESULTS
Ligand-Ligand (L-L) Incompatibility: Impact on OS, RRM and acute GVHD
In the GVHD/GVL direction, L-L incompatibility for either HLA-C or HLA-Bw4 was present in 24% (n=12) of myeloid and 48.6% (n=17) of lymphoid malignancy patients. In the rejection direction, incompatibility for either HLA-C or HLA-Bw4 was seen in 24% (n=12) of myeloid and 37.1% (n=13) of lymphoid malignancy patients. Subgroup sample sizes were too small to perform statistical analysis for significant differences in L-L incompatibility on OS, RRM or acute GVHD.
Missing Recipient Ligand for Donor’s Inhibitory KIR (Receptor-Ligand alloreactivity in GVH/GVL Direction): Impact on OS, RRM and acute GVHD
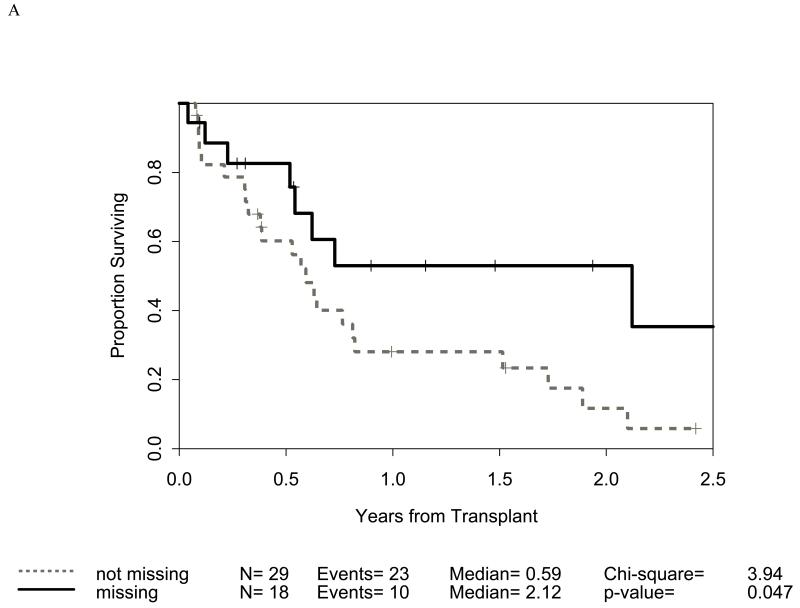
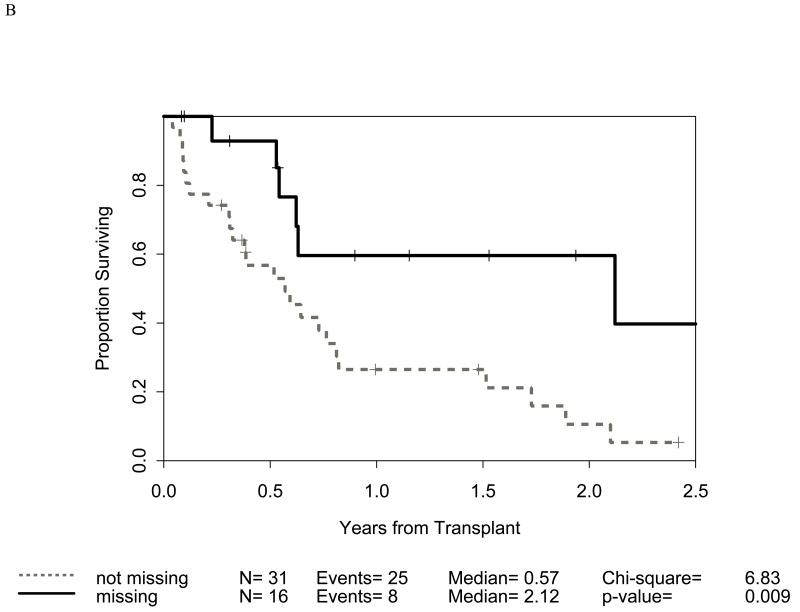
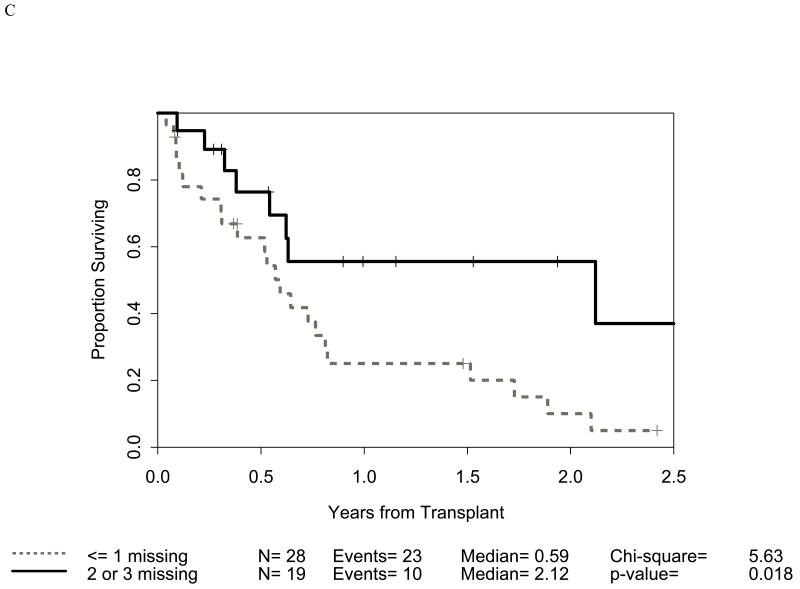
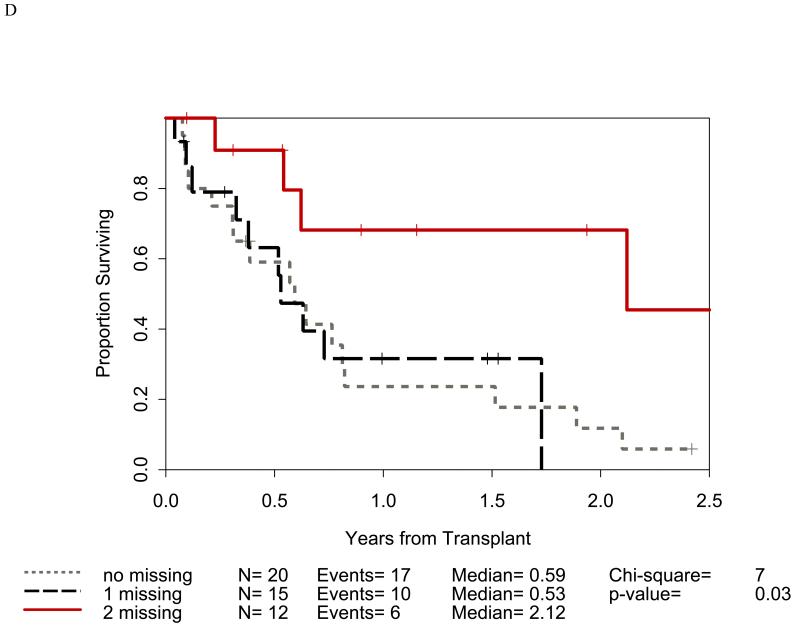
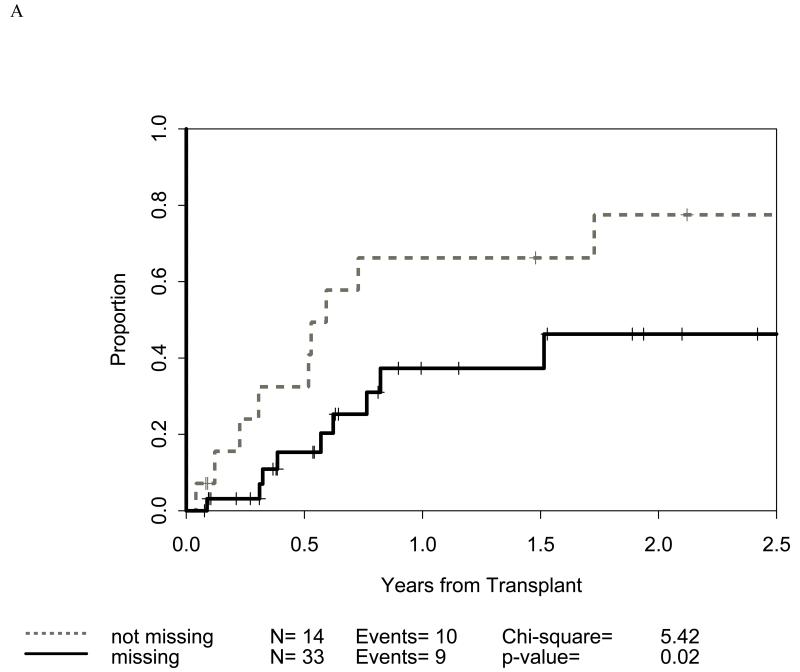
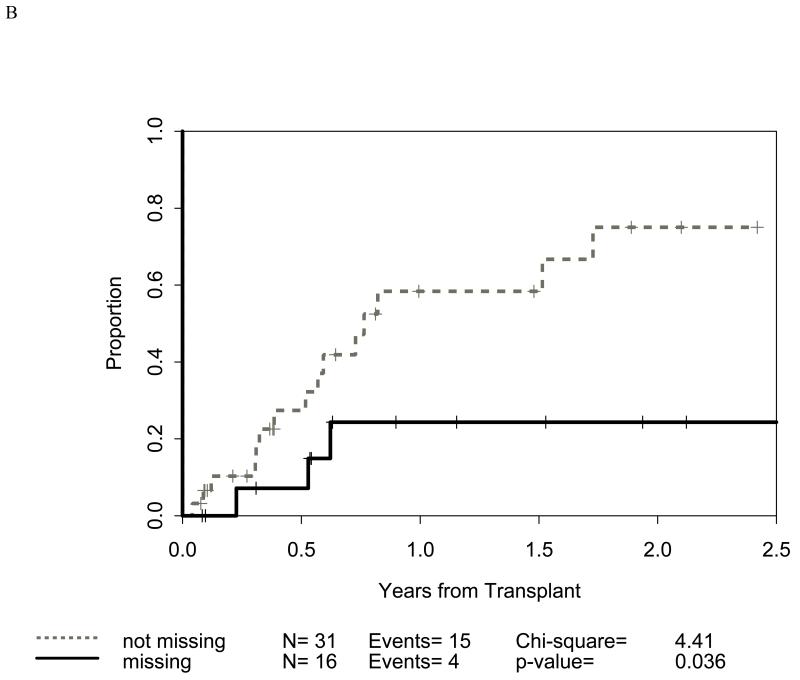
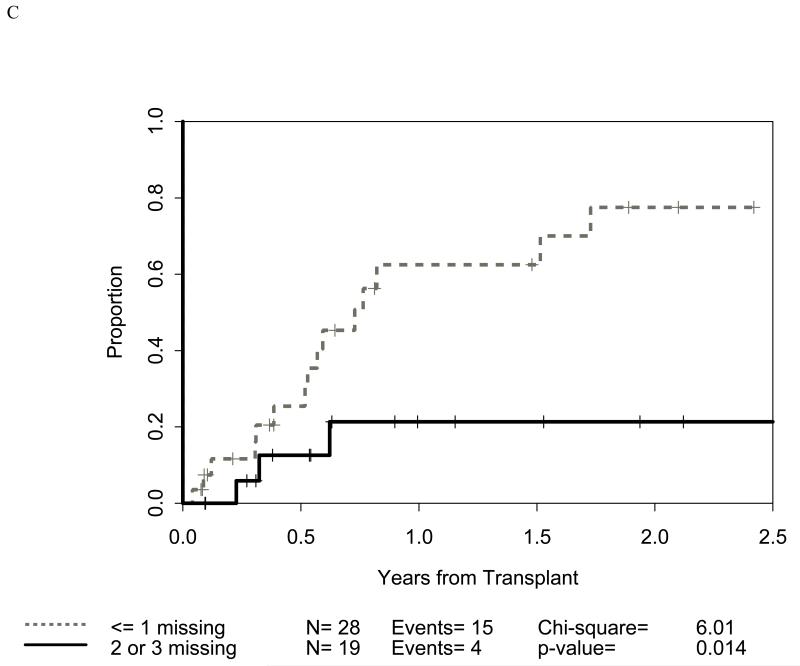
Distribution of incompatibilities for HLA-A3/A11, - Bw4, -C1, and -C2 were similar in the myeloid and lymphoid cohorts. In the myeloid cohort, alloreactivity for i) HLA-C2 was associated with higher OS (p=0.009: figure 1B) as well as lower RRM (p=0.036: figure 2B); ii) Bw4 was associated with higher OS (p=0.047: figure 1A); and iii) A3/A11 was associated with lower RRM (p=0.02; figure 2A). Furthermore, increasing number of alloreactivities led to higher OS (p=0.018 for 2 or 3; p=0.03 for 3 categories) as shown in figures 1C and 1D. RRM was lower in the presence of incompatibility involving A3/A11 (p=0.02), C2 (p=0.036), or combined (p=0.014) as shown in figures 2A-C. In contrast, in the lymphoid cohort, there was no impact of either individual incompatibility for HLA-A3/A11, - Bw4, -C1, and -C2 or multiple incompatibilities on OS or RRM but grades II-IV acute GVHD trended lower when donor-recipient pairs with C1 or C2 incompatibility (n=24; p value = 0.011) were analyzed together.
Figure 1.
Impact of receptor-ligand incompatibility in the GVH/GVL direction on overall survival (OS) in patients with myeloid malignancy undergoing T cell depleted reduced intensity conditioning related donor haploidentical transplantation. In this model, incompatibility is defined as absence of corresponding ligand in the recipient to the inhibitory KIR present in the donor. The combinations used were KIR3DL1 for HLA Bw4 alleles (figure 1A), KIR2DL1 for HLA C2 alleles (figure 1B) that are characterized by lysine at position 80 (HLA-CLys80), KIR2DL2 and KIR2DL3 for HLA C1 alleles (graph not shown because of lack of statistical significance) characterized by Asparagine at position 80 (HLA-CAsn80), and KIR3DL2 for HLA-A3 and HLA-A11 alleles (graph not shown because of lack of statistical significance). Figure 1C depicts the impact of incompatibility for any of the above locus and figure 1D depicts the impact of multiple incompatibilities.
Figure 2.
Impact of receptor-ligand incompatibility in the GVH/GVL direction on relapse related mortality (RRM) in patients with myeloid malignancy undergoing T cell depleted reduced intensity conditioning related donor haploidentical transplantation. Similar to figure 1, incompatibility is defined as absence of corresponding ligand in the recipient to the inhibitory KIR present in the donor. The combinations used were KIR3DL2 for HLA-A3 and HLA-A11 alleles (figure 2A), KIR2DL1 for HLA C2 alleles (figure 2B) that are characterized by lysine at position 80 (HLA-CLys80), KIR3DL1 for HLA Bw4 alleles (graph not shown because of lack of statistical significance), and KIR2DL2 and KIR2DL3 for HLA C1 alleles (graph not shown because of lack of statistical significance) characterized by Asparagine at position 80 (HLA-CAsn80). Figure 2C depicts the combined impact of incompatibility for any of the above loci.
Missing Donor Ligand for Recipient’s Inhibitory KIR (Receptor-Ligand Alloreactivity in Rejection Direction): Impact on OS, RRM and acute GVHD
Distribution of alloreactivities for HLA-A3/A11, -Bw4, -C1, and -C2 were similar in the myeloid and lymphoid cohorts. In the myeloid cohort, alloreactivity for -Bw4 was associated with higher OS (p=0.014). There was no impact of individual or combined alloreactivities on OS or RRM in the lymphoid malignancy cohort.
Activating KIR in patients and donors
Genotyping was performed for activating KIR receptor (2DS1, 2DS2, 2DS3, 2DS4, 2DS5, and 3DS1) in donors and patients. However, for the outcomes analysis, KIR 2DS4 was excluded because almost all samples were positive for it. The presence or absence of any of the activating KIR in either recipient or donor has no impact on OS, RRM or GVHD in myeloid patients. However, in lymphoid patients, a trend toward higher GVHD probability was observed if donor was positive for activating KIR2DS1 (n=9; p=0.031). OS in lymphoid patients tended to be higher if the donor carried activating KIR2DS2 (p=0.028; n=16) and lower if the recipient carried activating KIR2DS1 (n=10; p=0.011), KIR2DS5 (n=9; p=0.035) or KIR3DS1 (n=10; p=0.011).
DISCUSSION
Despite numerous studies in the last decade investigating the impact of KIR on the outcomes of HSCT, a cohesive, all encompassing, and clear theory with supportive clinical evidence regarding the relevant associations is yet to emerge. These associations have been studied separately in various donor sources including matched sibling and unrelated donors, graft sources including bone marrow, peripheral blood stem cells, and umbilical cord blood, and following various cytoreductive regimens with or without T cell depletion(22-32). More recently, cellular and molecular studies have elucidated the complexity of the KIR genes, its ligand interactions, and effector responses and allowed the more recent outcome analyses to incorporate a wider range of variables and models of donor-host NK cell alloreactivities(10;27;33-42). Our study includes patients transplanted at a single center using uniform conditioning, graft source, GVHD prophylaxis, supportive care and follow-up on a prospective basis. In this cohort, high degree of T-cell depletion created a non-competing environment with low GVHD and minimal need for additional immunosuppression and thus allowed for a clearer evaluation of donor and recipient NK interactions and effector responses. Post-transplantation lymphocytic recovery patterns highlighting relative maintenance of the CD56+ compartment (including NK cells) relative to the CD4 and CD8 compartments with this type of conditioning regimen has been documented in our previous work (4). To our knowledge, this is the first single center analysis of inhibitory and activating KIR genotype and ligand in RIC transplants, a group that is arguably more dependent on GVL for overall survival and freedom from relapse related mortality.
Receptor-Ligand alloreactivity in GVL/GVH direction in our cohort revealed the greatest degree of impact on the outcome measures. Absence of recipient ligand for donor inhibitory KIR (GVH/GVL direction) impacted OS and RRM in the myeloid patients (figures 1 and 2). Alloreactivity involving HLA-C2 was most significant and led to higher overall survival as well as lower RRM. Functional study by Pende et al provides evidence that KIR2DS1, similar to KIR2DL1, can discriminate between C1 and C2 groups leading to differential lysis of C1 versus C2 allele expressing leukemia blasts(12). However, binding of KIR2DS1 was weaker than that of KIR2DL1. Additional impact of receptor-ligand alloreactivity in our myeloid patients was reflected in a higher OS in relation to Bw4 and lower RRM in relation to A3/A11. The presence of multiple alloreactivities led to the highest OS and lowest RRM. We also noticed a difference between HLA-C1 and HLA-C2 alleles. Myeloid patients with alloreactivity involving HLA-C1 did not have advantage in OS and RRM. In fact, C1 alloreactivity resulted in higher grades II-IV acute GVHD. Cook et al showed similar finding in matched sibling transplant if the donor carried activating KIR2DS2(43). KIR “ligand-ligand” (L-L) incompatibility did not predict OS, RRM, or acute GVHD in our study. It has been shown that persistence of T-cells inhibits NK cell alloreactivity(35). It is unclear if that was a factor in this cohort. Similar to our observations, Gagne et al did not find ligand-ligand analysis to be very useful in predicting survival and relapse following unrelated donor transplants(27). Overall, our findings support the view that receptor-ligand model alloreactivity (with the exception of C1) in GVH/GVL direction in myeloid patients induces GVL effect without causing GVHD and thus leading to a lower RRM and higher overall survival.
The lower probability of grades II-IV acute GHVD in lymphoid malignancy (n=24) in the presence of mismatch in the receptor-ligand model is previously unreported and merits further scrutiny. Additionally, conditioning regimen, GVHD prophylaxis, T cell dose, and infused NK cells may differ across centers, graft sizes, and T cell depletion protocols and may be another variable impacting NK mediated effects. Indeed, higher GVHD was reported in the presence of receptor-ligand mismatch in two studies done in myeloablative transplant recipients; one from related haploidentical donors(44) and another using T cell replete grafts from unrelated donors (23). In RIC cord blood transplant study, KIR-ligand alloreactivity resulted in significantly higher rates of grade III-IV acute GVHD (42% vs. 13%) and TRM (27% vs. 12%) with inferior survival (32% vs. 52%)(24). A recent study provides compelling evidence that the expression of mature KIR repertoires is affected by T cells in the graft which in turn impacts clinical outcomes after unrelated transplantation(35). In that context, it is noteworthy that we observed some impact of activating KIR in this study. In lymphoid patients, a higher GVHD probability was seen if donor was positive for activating KIR2DS1. OS in lymphoid patients was higher if donor carried activating KIR2DS2 and lower if the recipient carried activating KIR2DS1, KIR2DS5 or KIR2DS1. These findings must be interpreted cautiously due to small numbers and further explored in larger patient populations. Interestingly, Pende et al showed that leukemic blasts expressing C2/C2 ligands were killed by KIR2DL2/3+ NK cells that co-expressed KIR2DS1 because activating KIR2DS1 could directly recognize C2 on leukemia cells(12). In the large study of 1087 patients transplanted using unrelated donors through NMDP, recipients of KIR3DS1 positive donors had lower grade II-IV acute GVHD but no impact on relapse(29).
In conclusion, absence of appropriate HLA ligands in the recipient to donor inhibitory KIR may induce graft-vs.-leukemia (GVL) without aGVHD in myeloid malignancy patients undergoing haploidentical T cell depleted RIC transplant from haploidentical related donor. Similar alloreactivity in lymphoid malignancy patients did not have GVL effect although impact on acute GVHD was noted. Cumulative evidence from all previous studies and the current study points to the a level of uniqueness of NK mediated effects in specific contexts and suggests that clinical applicability of NK incompatibility must be determined and utilized separately for specific donor sources, conditioning regimen, level of HLA matching, graft manipulation and disease characteristics instead of extrapolating across these variables.
ACKNOWLEDGEMENT
NJC is supported by PO1 (NJC) 2PO1-CA047741-16A1 from NIH; DAR is a Leukemia Lymphoma Society Scholar in Clinical Research
Footnotes
AUTHORSHIP:
VKP conceptualized and designed the study, collected clinical data, analyzed the data, wrote the manuscript, and led all the coordination efforts; DAR and NJC were involved with the conceptualizing, designing, and overseeing the study, development of the transplant protocol, collection of clinical data, and manuscript preparation; DFC was involved in conceptualizing the study, generation of HLA and KIR typing data in the laboratory, data analysis, and editing the manuscript; GB was involved in statistical analysis, review of the data and preparation of the manuscript; NLR was involved with conceptualizing the study, laboratory support, and manuscript preparation; ADO and AC contributed to laboratory studies; KMS, JPC, MEH, CG, and GDL were involved with collection of clinical data and preparation of the manuscript; YY contributed in manuscript preparation.
Reference List
- (1).Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002 Mar 15;295(5562):2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- (2).Hale G, Zhang MJ, Bunjes D, Prentice HG, Spence D, Horowitz MM, et al. Improving the Outcome of Bone Marrow Transplantation by Using CD52 Monoclonal Antibodies to Prevent Graft-Versus-Host Disease and Graft Rejection. Blood. 1998 Dec 15;92(12):4581–90. [PubMed] [Google Scholar]
- (3).Morris E, Thomson K, Craddock C, Mahendra P, Milligan D, Cook G, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004 Dec 15;104(13):3865–71. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- (4).Rizzieri DA, Koh LP, Long GD, Gasparetto C, Sullivan KM, Horwitz M, et al. Partially Matched, Nonmyeloablative Allogeneic Transplantation: Clinical Outcomes and Immune Reconstitution. J Clin Oncol. 2007 Feb 20;25(6):690–7. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- (5).Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol. 2008 Sep;142(6):877–88. doi: 10.1111/j.1365-2141.2008.07260.x. [DOI] [PubMed] [Google Scholar]
- (6).Trowsdale J. Genetic and functional relationships between MHC and NK receptor genes. Immunity. 2001 Sep;15(3):363–74. doi: 10.1016/s1074-7613(01)00197-2. [DOI] [PubMed] [Google Scholar]
- (7).Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, et al. Receptors for HLA class-I molecules in human natural killer cells. Annual Review of Immunology. 1996 Apr 1;14(1):619–48. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- (8).Lanier LL. NK Cell receptors. Annual Review of Immunology. 1998 Apr 1;16(1):359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- (9).Long EO. Regulation of immune responses through inhibitory receptors. Annual Review of Immunology. 1999 Apr 1;17(1):875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- (10).Long EO. Immunology Signal sequences stop killer cells. Nature. 1998 Feb 19;391(6669):740–3. doi: 10.1038/35739. [DOI] [PubMed] [Google Scholar]
- (11).Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008 May;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- (12).Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009 Mar 26;113(13):3119–29. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- (13).Moretta A, Sivori S, Vitale M, Pende D, Morelli L, Augugliaro R, et al. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. The Journal of Experimental Medicine. 1995 Sep 1;182(3):875–84. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Stewart CA, Laugier-Anfossi F, Vely F, Saulquin X, Riedmuller J, Tisserant A, et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13224–9. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gratama JW, Sutherland DR, Keeney M. Flow cytometric enumeration and immunophenotyping of hematopoietic stem and progenitor cells. Semin Hematol. 2001 Apr;38(2):139–47. doi: 10.1016/s0037-1963(01)90047-2. [DOI] [PubMed] [Google Scholar]
- (16).Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996 Jun;5(3):213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- (17).Rizzieri DA, Dev P, Long GD, Gasparetto C, Sullivan KM, Horwitz M, et al. Response and toxicity of donor lymphocyte infusions following T-cell depleted non-myeloablative allogeneic hematopoietic SCT from 3-6/6 HLA matched donors. Bone Marrow Transplant. 2009 Feb;43(4):327–33. doi: 10.1038/bmt.2008.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995 Jun;15(6):825–8. [PubMed] [Google Scholar]
- (19).Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999 Mar 30;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- (20).Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958 Jun;53(282):457–81. [Google Scholar]
- (21).Mantel C, Luo Z, Hendrie P, Broxmeyer HE. Steel factor and granulocyte-macrophage colony stimulating factor act together to enhance choline-lipid turnover during synergistically stimulated proliferation of the human factor dependent cell line, M07E. Biochem Biophys Res Commun. 1993 Dec 15;197(2):978–84. doi: 10.1006/bbrc.1993.2575. [DOI] [PubMed] [Google Scholar]
- (22).Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003 Aug 1;102(3):814–9. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- (23).Davies SM, Ruggieri L, DeFor T, Wagner JE, Weisdorf DJ, Miller JS, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Blood. 2002 Nov 15;100(10):3825–7. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- (24).Brunstein CG, Wagner JE, Weisdorf DJ, Cooley S, Noreen H, Barker JN, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009 May 28;113(22):5628–34. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009 Jan 15;113(3):726–32. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005 Jun 15;105(12):4878–84. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gagne K, Busson M, Bignon JD, Balere-Appert ML, Loiseau P, Dormoy A, et al. Donor KIR3DL1/3DS1 gene and recipient Bw4 KIR ligand as prognostic markers for outcome in unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009 Nov;15(11):1366–75. doi: 10.1016/j.bbmt.2009.06.015. [DOI] [PubMed] [Google Scholar]
- (28).Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006 Aug;12(8):876–84. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- (29).Venstrom JM, Gooley TA, Spellman S, Pring J, Malkki M, Dupont B, et al. Donor activating KIR3DS1 is associated with decreased acute GVHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2010 Apr 15;115(15):3162–5. doi: 10.1182/blood-2009-08-236943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Giebel S, Nowak I, Dziaczkowska J, Czerw T, Wojnar J, Krawczyk-Kulis M, et al. Activating killer immunoglobulin-like receptor incompatibilities enhance graft-versus-host disease and affect survival after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2009 Oct;83(4):343–56. doi: 10.1111/j.1600-0609.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- (31).Locatelli F, Pende D, Maccario R, Mingari MC, Moretta A, Moretta L. Haploidentical hemopoietic stem cell transplantation for the treatment of high-risk leukemias: how NK cells make the difference. Clin Immunol. 2009 Nov;133(2):171–8. doi: 10.1016/j.clim.2009.04.009. [DOI] [PubMed] [Google Scholar]
- (32).Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socie G, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia.[Erratum appears in Leukemia. 2009 Mar;23(3):630] Leukemia. 2009 Mar;23(3):492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Caligiuri MA. Human natural killer cells. Blood. 2008 Aug 1;112(3):461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Cambiaggi A, Darche S, Guia S, Kourilsky P, Abastado JP, Vivier E. Modulation of T-Cell Functions in KIR2DL3 (CD158b) Transgenic Mice. Blood. 1999 Oct 1;94(7):2396–402. [PubMed] [Google Scholar]
- (35).Cooley S, McCullar V, Wangen R, Bergemann TL, Spellman S, Weisdorf DJ, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005 Dec 15;106(13):4370–6. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Fehniger TA, Carson WE, Caligiuri MA. Costimulation of human natural killer cells is required for interferon gamma production. Transplantation Proceedings. 1999 May;31(3):1476–8. doi: 10.1016/s0041-1345(99)00011-1. [DOI] [PubMed] [Google Scholar]
- (37).Middleton D, Gonzelez F. The extensive polymorphism of KIR genes. Immunology. 2010 Jan;129(1):8–19. doi: 10.1111/j.1365-2567.2009.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Miller JS. How killers kill. Blood. 2008 Jul 15;112(2):213. doi: 10.1182/blood-2008-05-152405. [DOI] [PubMed] [Google Scholar]
- (39).Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007 Jul 1;110(1):433–40. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003 May 1;101(9):3730–40. doi: 10.1182/blood-2002-08-2568. [DOI] [PubMed] [Google Scholar]
- (41).Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol. 2009 Oct;21(5):525–30. doi: 10.1016/j.coi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- (42).Yu J, Venstrom JM, Liu XR, O’Reilly R, Pring J, Hasan RS, et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function following T-cell depleted allogeneic hematopoietic cell transplantation. Blood. 2009 Jan 28; doi: 10.1182/blood-2008-09-177055. blood-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Cook MA, Milligan DW, Fegan CD, Darbyshire PJ, Mahendra P, Craddock CF, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004 Feb 15;103(4):1521–6. doi: 10.1182/blood-2003-02-0438. [DOI] [PubMed] [Google Scholar]
- (44).Bishara A, De SD, Witt CC, Brautbar C, Christiansen FT, Or R, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004 Mar;63(3):204–11. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]