Abstract
Peripheral nerve sheath tumors are common neoplasms, with classic identifiable features, but on occasion, they are diagnostically challenging. Although well defined subtypes of peripheral nerve sheath tumors were described early in the history of surgical pathology, controversies regarding the classification and grading of these tumors persist. Advances in molecular biology have provided new insights into the nature of the various peripheral nerve sheath tumors, and have begun to suggest novel targeted therapeutic approaches. In this review we discuss current concepts and problematic areas in the pathology of peripheral nerve sheath tumors. Diagnostic criteria and differential diagnosis for the major categories of nerve sheath tumors are proposed, including neurofibroma, schwannoma, and perineurioma. Diagnostically challenging variants, including plexiform, cellular and melanotic schwannomas are highlighted. A subset of these affects the childhood population, and has historically been interpreted as malignant, although current evidence and outcome data suggests they represent benign entities. The growing current literature and the authors experience with difficult to classify borderline or “hybrid tumors” are discussed and illustrated. Some of these classification gray zones occur with frequency in the gastrointestinal tract, an anatomical compartment that must always be entertained when examining these neoplasms. Other growing recent areas of interest include the heterogeneous group of pseudoneoplastic lesions involving peripheral nerve composed of mature adipose tissue and/or skeletal muscle, such as the enigmatic neuromuscular choristoma. Malignant peripheral nerve sheath tumors (MPNST) represent a diagnostically controversial group; difficulties in grading and guidelines to separate “atypical neurofibroma” from MPNST are provided. There is an increasing literature of MPNST mimics which neuropathologists must be aware of, including synovial sarcoma and ossifying fibromyxoid tumor. Finally, we discuss entities that are lacking from the section on cranial and paraspinal nerves in the current WHO classification, and that may warrant inclusion in future classifications. In summary, although the diagnosis and classification of most conventional peripheral nerve sheath tumors are relatively straightforward for the experienced observer, borderline and difficult to classify neoplasms continue to be problematic. In the current review, we attempt to provide some useful guidelines for the surgical neuropathologist to help navigate these persistent, challenging problems.
Keywords: peripheral nerve, neurofibroma, schwannoma, perineurioma, MPNST
Introduction
Peripheral nerve sheath tumors encompass a spectrum of well defined clinicopathologic entities [87], ranging from benign tumors, such as schwannoma, to high grade malignant neoplasms termed malignant peripheral nerve sheath tumors (MPNST)(Table 1), which are often resistant to conventional treatments[126].
Table 1.
Pathologic and immunophenotypic features useful in the differential diagnosis of Schwann cell neoplasms
| Neurofibroma | Schwannoma | MPNST | |
|---|---|---|---|
|
| |||
| Cytology | |||
| Nuclear size | + | ++ | ++/+++ |
| Nuclear hyperchromasia | + | ++ | +++ |
| Wavy nuclei | +++ | + | ++ |
|
| |||
| Histology | |||
| “Shredded carrot” type collagen | +++ | − | −/+ |
| Capsule | − | +++ | − |
| Hyalinized vessels | −/+ | +++ | − |
| Fascicular growth pattern | −/+ | ++ | +++ |
| Mitotic Activity | −/+ | −/+ | +++ |
| Necrosis | − | −/+ | +++ |
|
| |||
| IHC marker | |||
| S100 | ++/+++ | +++ | +/++ |
| Collagen IV | ++/+++ | +++ | +/++ |
| EMA | + | −(capsular) | −(except MPNST with perineurial diff) |
| CD34 | +++ | +++ | ++ |
| Neurofilament protein | ++ | + (capsular, rare intratumoral axons | +/+++ |
| Podoplanin | + | ++ | + |
| Calretinin | + | +++ | NA |
| Sox10 | +++ | +++ | +/++ |
Beyond the long-established problems in peripheral nerve sheath pathology, such as the distinction of atypical neurofibromas from malignant peripheral nerve sheath tumors, a number of newer issues have arisen in this area. For example, a subset of peripheral nerve tumors are difficult to classify, demonstrating hybrid morphologic features overlapping with previously discrete diagnostic categories, such as schwannoma and perineurioma [63, 69, 98, 124, 128]. Nerve sheath tumors arising in children represent another difficult area that has received recent attention [94, 95, 151]. In this review we will discuss current concepts relating to the diagnostic criteria for peripheral nerve sheath tumors, chiefly those with Schwann cell differentiation, as well as less common problems such as hybrid nerve sheath tumors, cellular nerve sheath tumors in the pediatric population, the distinction of atypical changes in neurofibroma from MPNST, and non-Schwann cell-derived mimics of MPNST in peripheral nerves and paraspinal regions. Molecular aspects of nerve sheath tumors will be discussed when pertinent. More comprehensive discussion of this rapidly evolving area can be found elsewhere, including the accompanying review by Carroll S. in this issue[15]. In addition, for more comprehensive coverage, the reader is referred to the upcoming AFIP fascicle of tumors of the peripheral nervous system[8].
Origin and ontogenesis of peripheral nerve sheath tumors
The main peripheral nerve sheath tumors are characterized by neoplastic proliferations with Schwann cell differentiation. For instance, the Schwann cell represents the primary neoplastic cell component of neurofibroma[111], characterized cytologically by wavy nuclear contours and S-100 protein expression[140] [19]. Neurofibromas also incorporate a mixture of non-neoplastic peripheral nerve components, including axons, perineurial cells, fibroblasts, and variable inflammatory elements, such as mast cells and lymphocytes. In addition, a population of CD34 positive cells of unclear histogenesis is present[15, 141].
Recent mouse models of neurofibroma have provided key insights into possible cells of origin of neurofibroma subtypes. In one model, non-myelinating p75+ Schwann cell progenitors are the candidate cell for Nf1 loss in plexiform neurofibroma[156], while in other models, the cell of origin has been temporally assigned to the Schwann cell precursor/immature Schwann cell boundary, in dessert hedgehog expressing cells[152]. Dermal neurofibromas may even have a non schwannian precursor altogether, such as a neural stem cell/progenitor[82]. MPNST may in theory arise from similar precursors, but in addition to NF1 loss, mutations in multiple tumor suppressor genes (TP53, CDKN2A) and receptor tyrosine kinase amplification (e.g. EGFR) are acquired[77, 97, 106, 112]. Compared to neurofibroma and MPNST, schwannoma represents a more homogeneous neoplastic proliferation of mature Schwann cells. Unlike neurofibroma, several genetic syndromes with different alterations are characterized by multiple schwannoma development, including NF2, schwannomatosis and Carney complex. However, homozygous Nf2 loss in the Schwann cell lineage leads to schwannoma formation in mice[50]. Conversely, in mouse models of neurofibromas, tumor formation is greatly facilitated by a NF1 hemizygous genetic background in non-neoplastic cells in the microenvironment (see below). Biological evidence about the cell of origin of perineurioma is lacking, as well as suitable model systems to study it.
Benign Nerve Sheath Tumors
Neurofibroma
Neurofibromas are benign nerve sheath tumors with a tan-white, glistening cut surface apparent grossly (Figure 1a). Their growth pattern is either well-demarcated intraneural (Figure 1b) or diffuse infiltration of soft tissue at extraneural sites (Figure 1c). They are relatively common, particularly at superficial cutaneous sites, where they present as localized, pedunculated growths.
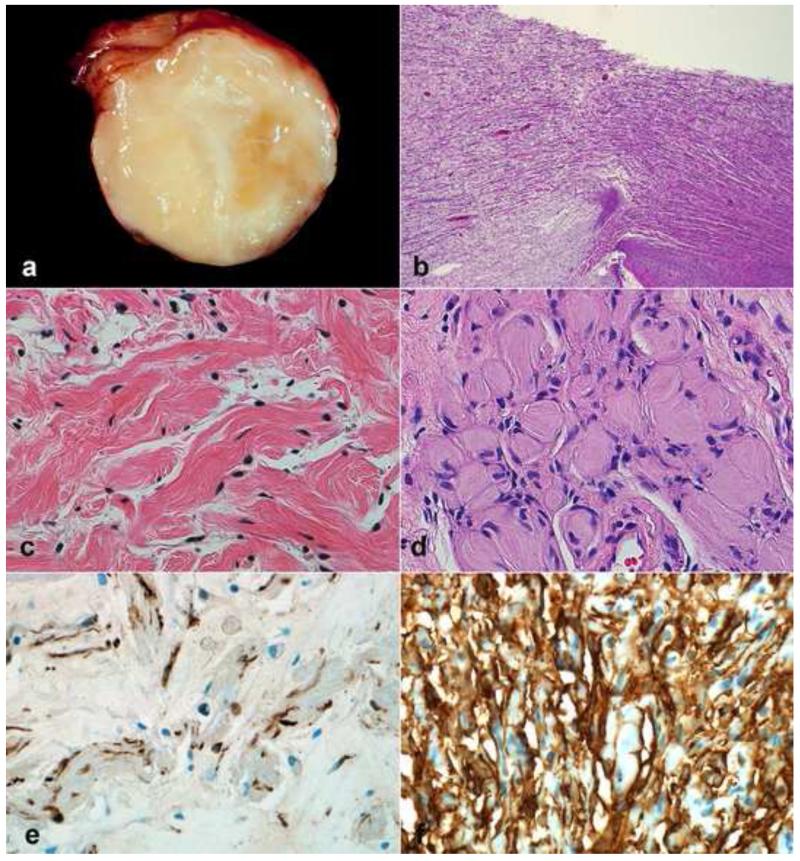
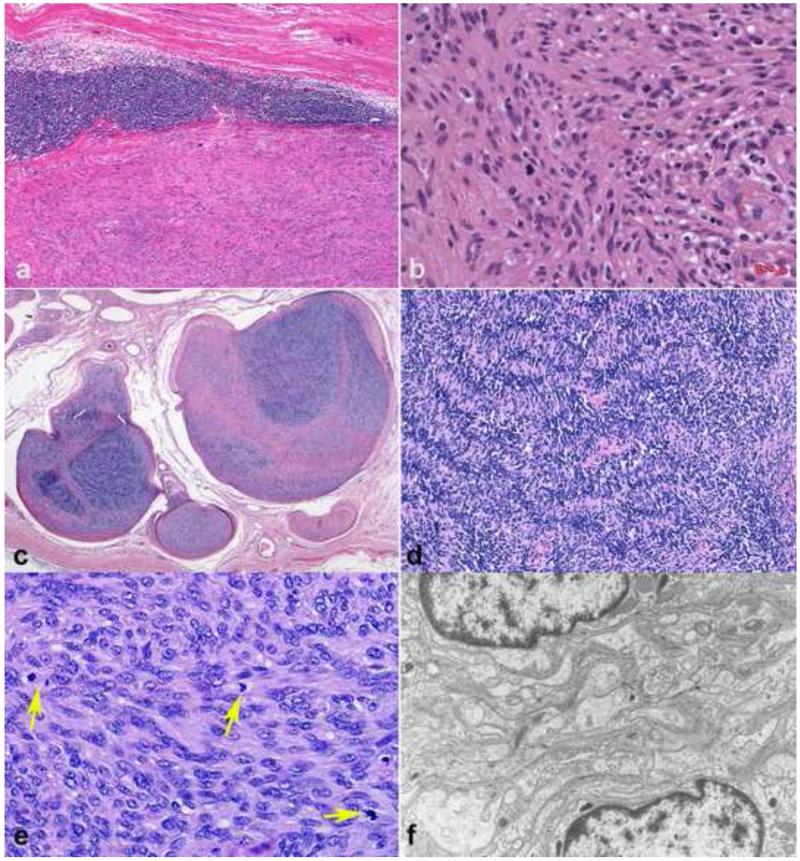
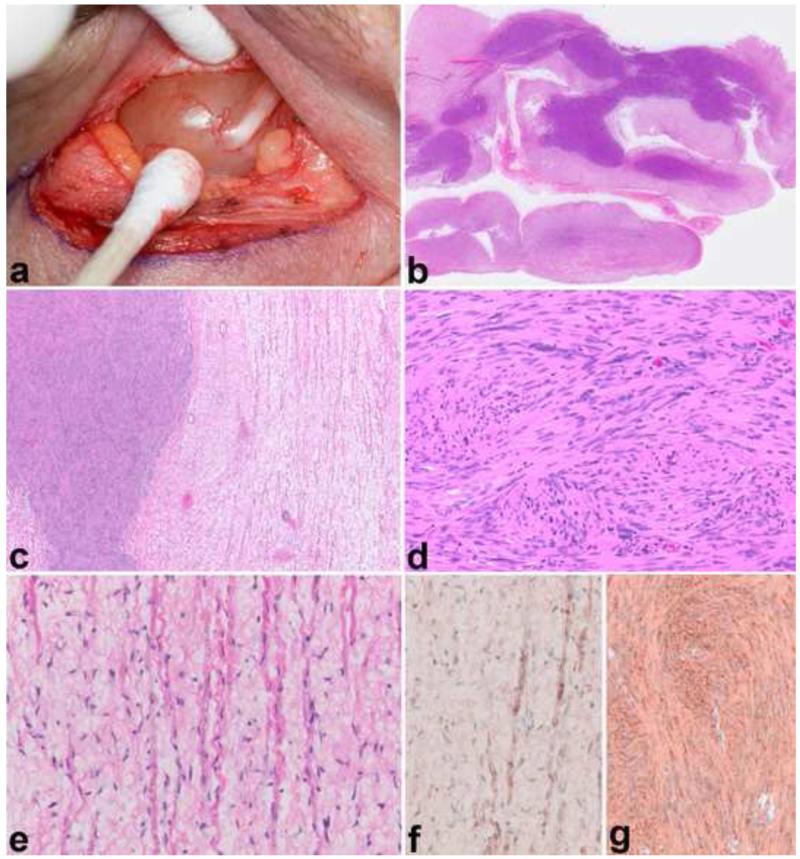
Figure 1. Pathologic Features of Neurofibroma.
Neurofibromas may form circumscribed masses in dermis or soft tissue with variable, white-tan appearance (a). When involving a main peripheral nerve trunk, neurofibromas cause fusiform expansion (b). Wavy nuclei and “shredded carrot” type collagen are typical (c). Diffuse neurofibroma, a less common subtype, may form large masses with infiltration of fat and numerous pseudomeissnerian corpuscles (d). S100 usually labels a subset of cells of probable schwannian origin in neurofibromas (e), while CD34 shows variable reactivity, highlighting cells of an uncertain nature (f).
Neurofibroma Variants
Specific clinicopathologic subtypes based on architectural growth patterns include localized, diffuse and plexiform neurofibromas. The localized cutaneous neurofibroma is the most common, and occurs sporadically in the majority of cases. Localized neurofibromas may also involve a major nerve, and result typically in fusiform expansion of the nerve trunk (intraneural subtype). Diffuse neurofibromas are characterized by a plaque-like enlargement usually in the head and neck region (Figure 1d). S100 positive pseudomeissnerian corpuscles may be abundant [125, 127, 138]. Most neurofibromas occur sporadically, although approximately 10% ultimately prove to be associated with neurofibromatosis type 1 (NF1).
The plexiform neurofibroma localized to a major nerve trunk and the rarest form, the massive soft tissue neurofibroma are almost always associated with NF1. Plexiform neurofibroma is defined by the involvement of numerous adjacent nerve fascicles or multiple components of a nerve plexus (Figure 2 a,b). Microscopically, plexiform neurofibromas often show an admixture of areas resembling localized and diffuse-type neurofibromas. Plexiform neurofibroma has a potential for malignant degeneration, and is a recognized precursor for MPNST in NF1 patients[92].
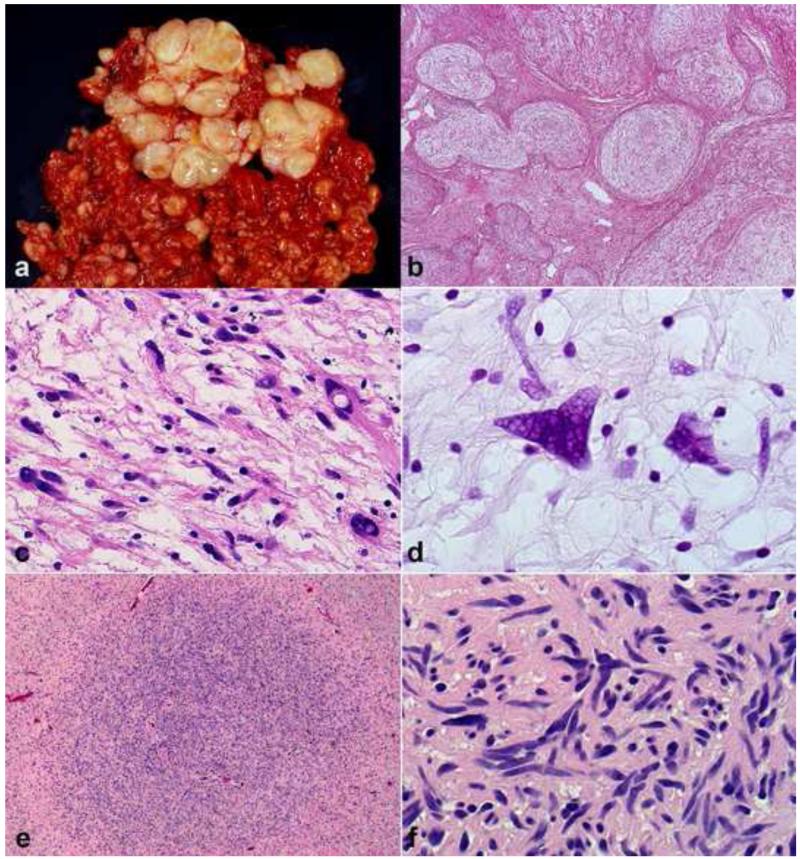
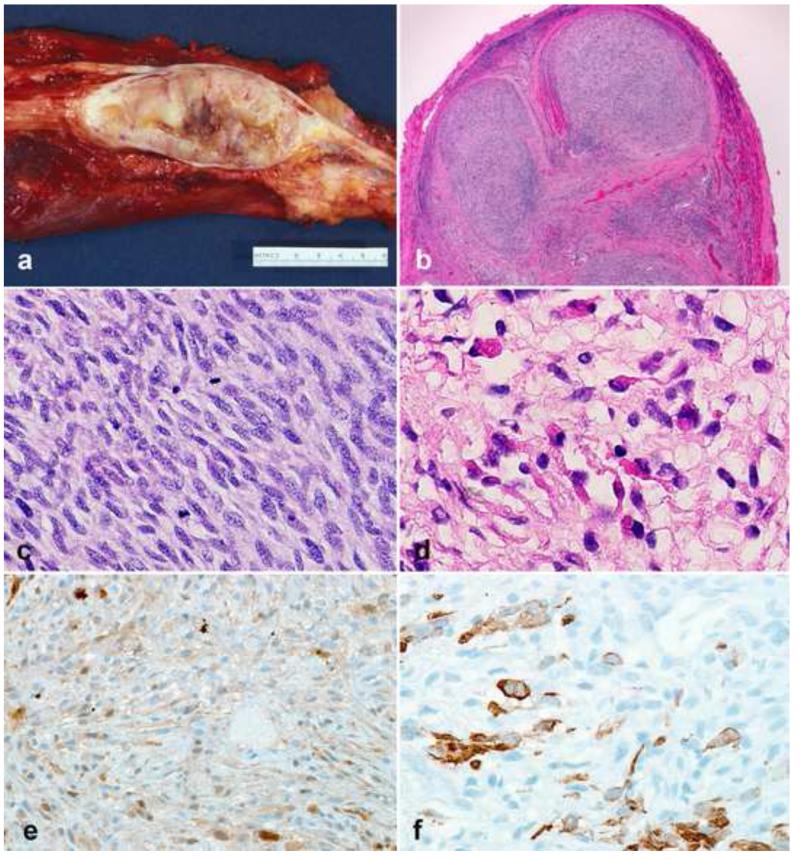
Figure 2. Diagnostically relevant neurofibroma variants.
Plexiform neurofibromas typically form large, multinodular masses (a). Involvement and expansion of multiple peripheral nerve fascicles is definitional (b). Degenerative atypia, in the absence of hypercellularity has been interpreted by some authors as “atypical neurofibroma”, has no prognostic significance, but may create diagnostic difficulties (c). The atypia in some neurofibromas is reflected in hyperchromatic nuclei with “smudgy” chromatin, which is probably degenerative (d). Foci of hypercellularity may be present in some neurofibromas (e). Areas of hypercellularity (“cellular” neurofibromas) in the absence of other atypical features is still compatible with a benign course (f).
Some neurofibromas show unusual features such as degenerative cytological atypia (neurofibroma with ancient change, atypical neurofibroma) (Figure 2c,d) and/or increased cellularity (cellular neurofibroma)(Figure 2e,f), often raising the differential diagnosis with MPNST. Cellular neurofibromas may show moderate cellularity and a more pronounced fascicular growth pattern, but lack the “monotonous” cytological atypia, chromatin abnormalities and mitotic activity seen in MPNST. Neurofibromas with ancient change feature degenerative nuclear atypia, containing scattered cells with markedly enlarged, hyperchromatic nuclei, often with “smudgy” chromatin; however, they lack increased cellularity, fascicular growth, or mitotic activity. Similar changes may be seen in so-called “ancient schwannomas”. Other less common morphological findings in neurofibroma include the presence of melanin pigment (Figure 3a) [40], metaplastic bone (Figure 3b) and glandular differentiation[120]. Massive soft tissue neurofibroma, a very rare subtype, is characterized by large size, infiltration of soft tissue and skeletal muscle, often involving large anatomical regions, and histologically demonstrating the presence of a cellular component (Figure 3c)[150]. They may contain plexiform components, but usually do not undergo malignant degeneration.
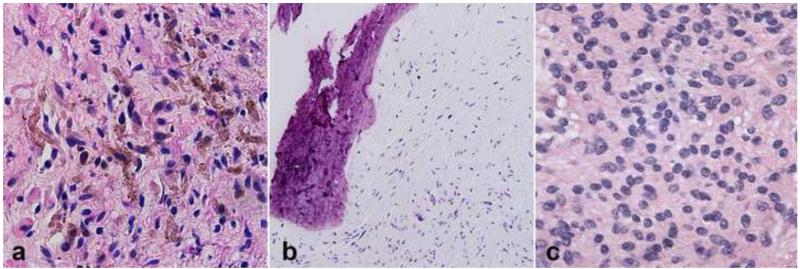
Figure 3. Rare neurofibroma changes and variants.
Pigmented or melanotic change in neurofibroma is a very rare finding, usually encountered in the diffuse variant (a). Ossification may also be an occasional finding in neurofibromas (b). Massive soft tissue neurofibroma is a very rare neurofibroma variant essentially limited to NF1, and which may contain alarming, cellular areas (c).
Differential diagnosis of neurofibroma
The possible differential diagnosis for neurofibromas is ample and influenced by the site of occurrence (intra vs. extraneural), including a number of neoplastic and non-neoplastic nerve lesions, such as schwannoma, nerve sheath myxoma, neurothekeoma, ganglioneuroma and traumatic neuroma, as well as a variety of non-nerve sheath tumors, in particular dermatofibrosarcoma protuberans (DFSP) and desmoplastic malignant melanoma. Among benign lesions, traumatic neuroma, a non-neoplastic proliferation at a site of nerve transection and ganglioneuroma, since neurofibroma may infiltrate dorsal root or sympathetic ganglia, represent the main entities in the differential diagnosis. The abnormal appearance of ganglion cells, which vary greatly in size and are frequently binucleated and their haphazard distribution, single or in clusters, makes the distinction of ganglioneuroma from infiltrated normal ganglia quite simple in most cases. Desmoplastic malignant melanoma may be composed of deceptively bland cells with wavy nuclei, closely mimicking neurofibroma. Important clues to the diagnosis of melanoma include the presence of significant sun damage, atypical junctional melanocytic hyperplasia or melanoma in-situ, the presence of very long, hyperchromatic cells, a “packeted” pattern of growth, dense fibrosis, and deep nodular lymphoid aggregates. Immunohistochemistry is of limited value in this differential diagnosis, as both tumors express S100 protein, and more specific melanocytic markers (e.g., HMB45, Melan-A, tyrosinase) are essentially never positive in desmoplastic melanoma. A recent report suggests that CD34 immunoreactivity in a “fingerprint” pattern is more typical of neurofibroma than desmoplastic melanoma[153]. Although desmoplastic melanoma represents a true malignancy, recent reports suggest that desmoplastic melanoma may not be as aggressive as previously thought[20].
Schwannoma
Schwannomas are benign neoplasms of Schwann cell origin. The gross appearance is characteristic, in the form of well circumscribed masses (Figure 4a) with degenerative changes and variable admixture of compact spindled (Antoni A) areas and hypocellular, microcystic (Antoni B) areas rich in macrophages and collagen fibers (Figure 4b). A well formed collagenous capsule is a consistent finding (Figure 4c), as well as hyalinized vessels (Figure 4d). By immunohistochemistry, schwannomas typically show diffuse, strong expression of S100 protein [140] (Figure 4e) and abundant pericellular collagen type IV (Figure 4f), consistent with the presence of a continuous pericellular basal lamina [108]. Glial fibrillary acid protein (GFAP) is expressed in a subset of schwannomas [68]. Recent markers frequently positive in schwannomas include podoplanin[67, 104], calretinin[41], and SOX10[107]. Very rarely, otherwise typical schwannomas may show anomalous expression of cytokeratins[35]. In our experience such tumors are always strongly GFAP-positive, suggesting cross reactivity of cytokeratin antibodies with GFAP, rather than true protein expression. Unlike neurofibroma, neurofilament protein staining is usually limited to entrapped axons at the periphery of the tumor, although some recent studies suggest that the presence of intralesional axons is actually more frequent than previously reported[105].
Figure 4. Pathologic Features of Schwannoma.
Schwannomas form well circumscribed, variegated masses with a tan appearance and macrocysts (a). Verocay bodies, characterized by distinct palisades with a fibrillary core are important diagnostic features (b). A well formed capsule is seen in almost all cases (c). Hyalinized vessels (d) are frequent findings. Diffuse immunoreactivity for S100 (e) and pericellular collagen IV (f) is almost universal in all schwannomas subtypes.
Schwannoma variants
Cellular schwannoma, although relatively uncommon, is an important variant of schwannoma to recognize, because its high cellularity, fascicular growth pattern, increased mitotic activity, and occasional locally destructive behavior, including bone erosion, often prompt consideration of malignancy. Cellular schwannoma is defined as a schwannoma composed almost entirely of a compact, fascicular proliferation of well-differentiated, cytologically bland Schwann cells, lacking Verocay bodies[17, 43, 144] (Figure 5a,b), and showing no more than very focal Antoni B pattern growth (<10% of the tumor area). Important clues to this diagnosis include the presence of foamy histiocyte aggregates, a well formed capsule containing lymphoid aggregates, and diffuse strong S100 protein and pericellular collagen IV expression. Diffuse S100 protein expression is exceedingly uncommon in spindled MPNST, and this finding should always raise the possibility of cellular schwannoma. Cytokeratin immunoreactivity may be seen in some cellular schwannomas, and may represent cross reactivity with GFAP as mentioned above. Importantly, cellular schwannomas lack expression of smooth muscle actin, desmin, CD117 and DOG1, allowing exclusion of other important tumors in its differential diagnosis, leiomyosarcoma and GIST, respectively. DOG1 (“discovered on GIST 1/Anoctamin-1”) is a recently described membrane protein, a highly sensitive and specific marker for GIST, expressed even in tumors lacking KIT or PDGFRA mutations[52, 143].
Figure 5. Important schwannoma variants.
Cellular schwannomas, despite their alarming cellularity usually have a well formed capsule, often with multiple subcapsular lymphoid aggregates (a). Verocay bodies are usually lacking (b). Plexiform schwannomas involving major peripheral nerves are rare, characteristically involving multiple fascicles (c). Plexiform schwannomas are composed primarily of Antoni A areas (d). Mitotic activity in plexiform/cellular schwannomas may be brisk (arrows), a finding still compatible with a benign diagnosis(e). Electron microscopy demonstrates extensive basal lamina in all schwannoma subtypes, a diagnostically useful finding (f).
Cellular schwannomas, despite their occasional alarming cellularity, lack malignant potential for practical purposes and never metastasize. Local recurrence is variable (5-40%)[17, 144] and may be higher than in conventional schwannomas. This may be related in part to location given the propensity for deep anatomic regions that are not always amenable to gross total resection. However, even recurrent lesions grow slowly and do not result in death. Mitotic activity is usually less than 5 per 10 high power fields. However, brisk mitotic activity, even in excess of 10 per 10 high power fields, may be present in rare instances, and if other features diagnostic of cellular schwannoma are present, this proliferative activity is still compatible with a benign diagnosis.
Plexiform schwannoma is a distinctive subtype of schwannoma that usually occurs in superficial (cutaneous or subcutaneous) locations and is defined by a plexiform (intraneural-nodular) pattern of growth [42, 146] Although they may be associated with schwannoma predisposition syndromes such as NF2 and schwannomatosis, the association is weak (approximately 5% of cases)[11]. These tumors may be less circumscribed than conventional schwannoma, or even lack a capsule. The tumors are usually composed of Antoni A patterns. Neurofilament protein immunoreactive axons are usually identified within the lesion.
More problematic are the rare plexiform schwannomas that arise in deep anatomic locations, in soft tissue[4] or major peripheral nerves[56] (Figure 5c-e), since they may demonstrate increased cellularity and mitotic activity and thus, may be difficult to distinguish from MPNST. Although these tumors have a negligible malignant potential, local recurrence may be relatively high, occurring in approximately half of the cases[4]. Again, the presence of widespread S100, collagen IV immunoreactivity or basal lamina by electron microscopy is reassuring (Figure 5f).
Melanotic schwannoma is a rare, distinctive, potentially malignant neoplasm characterized by epithelioid cells with variably sized nuclei and marked accumulation of melanin in neoplastic cells and associated melanophages[96] (Figure 6a). The main differential diagnosis is with other melanin-producing neoplasms, in particular melanoma. The presence of psammoma bodies in these tumors (i.e., psammomatous melanotic schwannoma) is associated in approximately half the cases with Carney complex[14], and therefore this neoplasm is discussed in more detail in an accompanying review of peripheral nerve sheath tumors in inherited tumor syndromes[117].
Figure 6. Rare schwannoma patterns and variants.
Melanotic schwannoma is a unique variant that is difficult to separate from other melanocytic tumors (a). “Neuroblastoma-like” schwannoma is a rare variant characterized by collagenous rosettes surrounded by small cells with high nuclear cytoplasmic ratios (b). Pseudoglandular spaces mimicking glands may be rarely encountered (c). Epithelioid change in a schwannoma demonstrating otherwise reassuring features, including negligible proliferation and hyalinized vasculature (d).
Although most schwannomas demonstrate classic histology, curious morphologic variations are occasionally encountered. The recently described “reticular” schwannoma is characterized by abundant myxoid change, microcysts, and a tendency to arise in viscera [84]. Rare findings in schwannomas include large cellular palisades resembling neuroblastic rosettes (i.e. “Neuroblastoma-like schwannoma”)[51](Figure 6b), pseudoglandular structures[38](Figure 6c), benign epithelioid change[73](Figure 6d), and lipoblastic differentiation[113].
Perineurioma
Perineurioma is currently considered a benign neoplasm with advanced perineurial differentiation. Two distinct types are recognized: intraneural and soft tissue. Although historically the intraneural variety was interpreted as a reactive hypertrophic process[135] the presence of 22q deletions in both intraneural and soft tissue perineurioma supports a neoplastic origin for both types [33, 48].
Intraneural perineuriomas are characterized by localized, solitary expansion of peripheral nerves, due to involvement of one or more nerve fascicles. These tumors remain stable over time or progress very slowly [90]. On gross examination, multinodularity secondary to firm, enlarged individual fascicles when exposed is the main finding (Figure 7a). Histologically, it is characterized by a complex perineurial cell proliferation extending into the endoneurium and concentrically surrounding individual nerve fibers and endoneurial capillaries producing characteristic “pseudo-onion bulbs”, which are best appreciated on cross sections of nerve fascicles (Figure 7b-f).
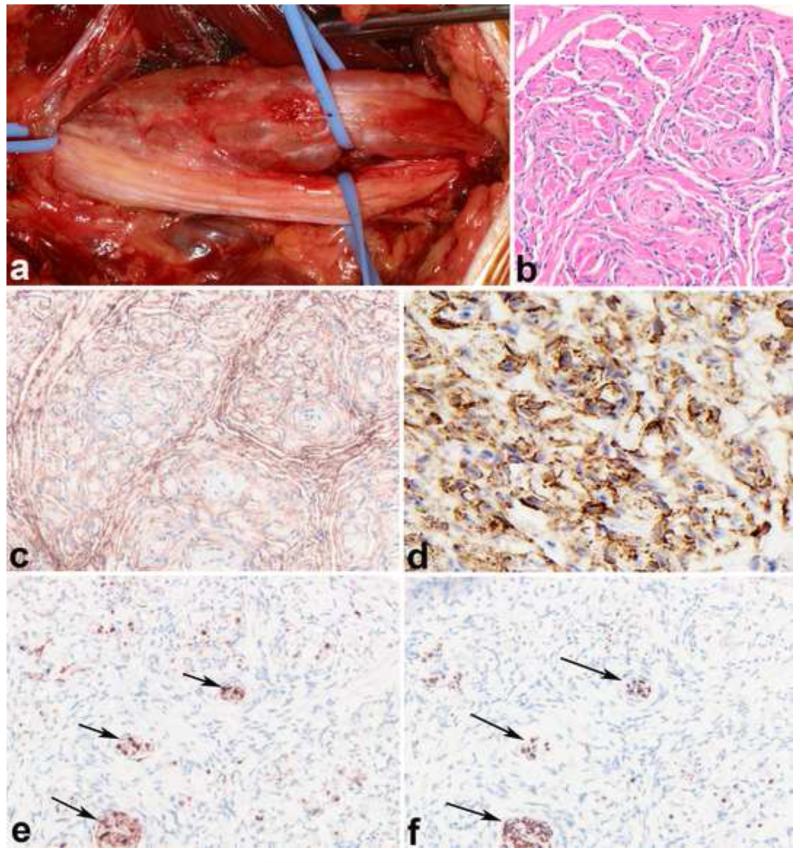
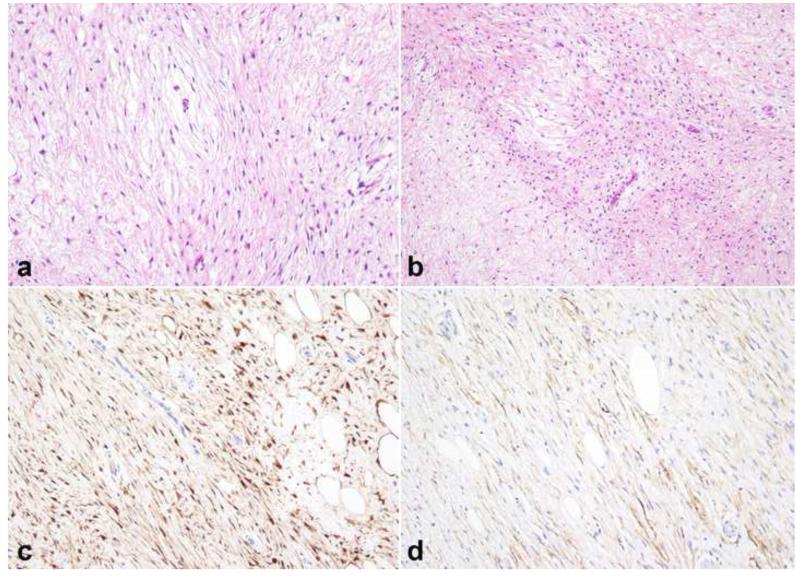
Figure 7. Pathologic features of intraneural perineurioma.
Intraneural perineurioma expands multiple nerve fascicles and may exhibit a multinodular gross appearance (photo courtesy of Dr. Robert Spinner)(a). An increase in perineurial and endoneurial cellularity is an important histologic clue (b). Immunohistochemical properties include EMA (c) and claudin (d) reactivity. S100 labels only residual Schwann cells (e), while neurofilament protein labels central axons within “pseudo-onion” bulbs (f)(arrows).
Soft tissue perineurioma almost always lacks an associated nerve, is usually well circumscribed and may have a capsule. Slender cells with very delicate, overlapping elongated cellular processes, arranged in loose fascicles or whorls is the typical pattern. Atypical histologic features, such as pleomorphic cells and limited infiltration, do not seem to have prognostic significance[61]. Abundant myxoid change, creating a microcystic or “reticular” pattern is present in a subset of cases (so-called “reticular perineurioma”) [53]. Sclerosing perineurioma is a distinctive variant most often seen in the hand of young men, and is characterized histologically by extensive collagen deposition and epithelioid cytomorphology [39].
Ancillary studies are required for the diagnosis of perineurioma. Among immunohistochemical markers, epithelial membrane antigen (EMA) is the most widely used and stains the majority of perineuriomas[9, 110], typically in a membranous fashion. Additional immunohistochemical markers of perineurioma include claudin 1[45], a marker of tight junctions, and GLUT1, a glucose transport protein involved in formation of the blood-nerve barrier. Neither of these markers is entirely specific for perineurial differentiation, and they are best used as part of a multi-antibody panel [5]. The differential diagnosis of intraneural perineurioma mainly includes localized reactive Schwann cell proliferations, while that of soft tissue perineurioma includes a variety of soft tissue tumors with fibrous and epithelioid morphologies, the most important of which is low-grade fibromyxoid sarcoma. Unlike perineurioma, low-grade fibromyxoid sarcoma shows prominent stromal collagen deposition and an “abrupt” transition into myxoid nodules, displaying a curvilinear vascular pattern. EMA expression may be present in up to 40% of low-grade fibromyxoid sarcomas, a potential pitfall. Demonstration of MUC4 expression and a FUS rearrangement may be required on occasion for the definitive distinction of low-grade fibromyxoid sarcoma (positive for both) from perineurioma (negative for both)[27, 28, 89, 109].
Hybrid Benign Nerve Sheath Tumors
Most peripheral nerve sheath tumors exhibit distinctive morphologic and immunophenotypic features that allow clear cut placement into a specific diagnostic category, predominantly neurofibroma, schwannoma or perineurioma. We have encountered on occasion tumors that are remarkably difficult to fit into one specific category. Some may arise in the setting of inherited syndromes. For example, occasional well circumscribed tumors may involve the soft tissues in patients with NF1 with focal areas reminiscent of neurofibroma, schwannoma and even perineurioma (Figure 8). This phenomenon is of the utmost importance, given that it may be incorporated into the overall clinical diagnostic criteria for a specific syndrome, with strong implications for future individual and familial screening.
Figure 8. Benign nerve sheath tumor in NF1.
Some benign nerve sheath tumors may be difficult to classify, even in clear cut genetic syndromes, containing areas typical of neurofibroma (a), or pattern variations reminiscent of schwannoma (b) and perineurioma (c).
The co-existence of benign nerve sheath tumors with distinct schwannoma and neurofibroma components was explored by Feany and colleagues in a series of 9 cases[36](Figure 9). Of note was the presence of a plexiform architecture in the majority of cases (5 of 9). Alternatively, some authors may interpret these tumors as neurofibromas with schwannian nodules[120]. An example of coexisting cellular schwannoma and plexiform neurofibroma involving the brachial plexus has also been reported[128].
Figure 9. Hybrid schwannoma-neurofibroma or “neurofibroma with Schwann cell nodules”.
Nerve-associated intraorbital mass (a)(photo courtesy of Dr. James Garrity), with a plexiform architecture (b). Distinct schwannoma nodules and neurofibroma areas (c,d,e). Neurofilament protein immunostain highlights axons (f), while S100 strongly labels the schwannoma component (g).
A more frequent component of these benign hybrid tumors may be perineurial. In our experience, one manifestation of hybrid nerve sheath tumor is the presence of tumors with the architectural features of soft tissue perineurioma, but with increased S100 expression (Figure 10). Michal and colleagues reported six tumors with hybrid schwannoma and perineurioma components[98]. Hornick and colleagues reported a subsequent series of 42 such cases [63]. Storiform growth and a collagenous stroma were dominant architectural features, typical of perineurioma, but schwannian cytology predominated. Degenerative nuclear atypia was present in a subset of cases. Such tumors have a predilection for superficial (dermal or subcutaneous) locations, usually are unencapsulated and are composed of biphasic, non-overlapping S100 and EMA positive cell components. In addition, the majority of cases expressed GFAP, CD34 and claudin1. In these tumors, the scant neurofilament protein axons present favored a schwannoma over a neurofibroma component. The behavior of such tumors was uniformly benign, with only one recurrence documented. Rare examples with hybrid neurofibroma and perineurioma features have also been reported[69, 124].
Figure 10. Hybrid benign peripheral nerve sheath tumor.
A subset of hybrid benign peripheral nerve sheath tumors present as soft tissue masses with prominent perineurial architecture (a). Areas of increased cellularity may be encountered (b). Strong S100 expression in a large number of cells reflects a Schwannian component (c). The perineurial component expresses EMA (d).
Benign Gastrointestinal Nerve Sheath Tumors
The versatility of nerve sheath tumors has become increasingly evident in recent years, particularly in tumors involving the gastrointestinal tract. The full spectrum of peripheral nerve sheath tumors is represented (Figure 11), including schwannoma, perineurioma[2, 62, 70], granular cell tumor, neurofibroma, as well as hybrid tumors[32] and unusual subtypes such as the recently described microcystic/reticular schwannoma[84]. This has distinctive clinicopathologic features, including a predilection for the intestine (rather than stomach as other GI schwannomas) and presentation in an older age group[3, 84, 132]. In addition, a number of benign polypoid lesions in the GI tract have been proposed as having nerve sheath differentiation, along schwannian or perineurial lines[49, 83].
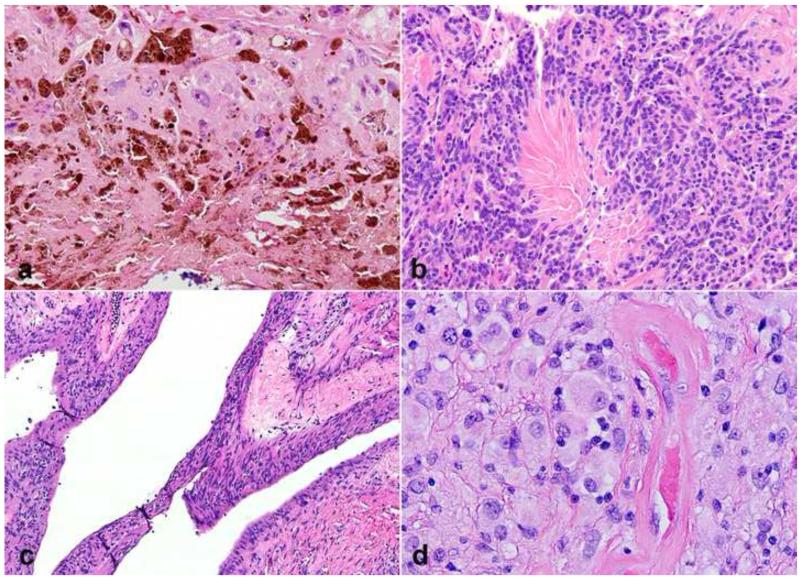
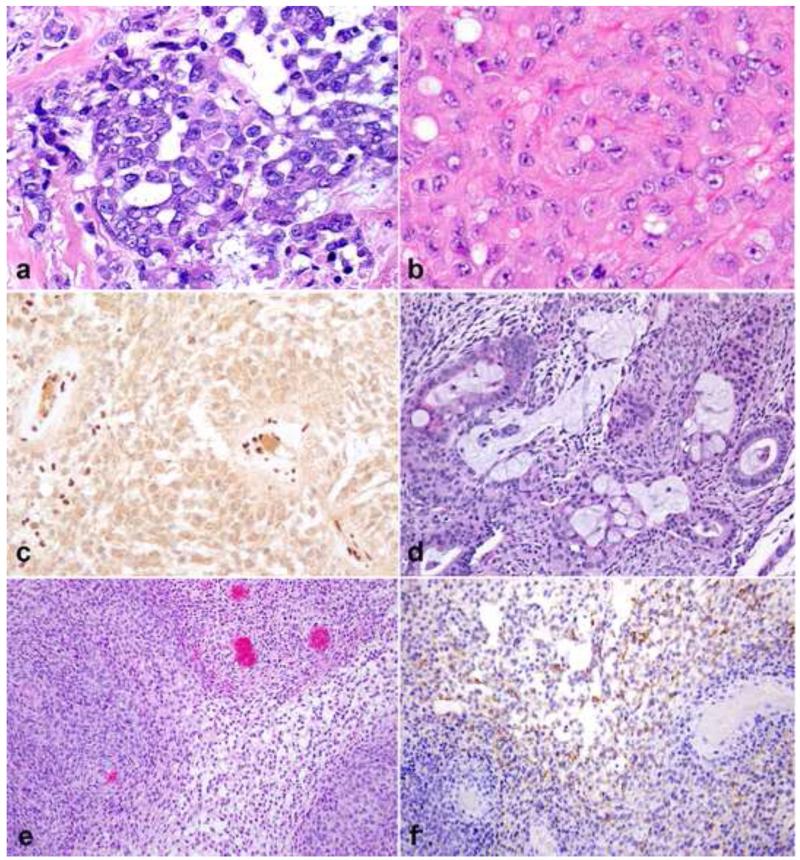
Figure 11. Gastrointestinal (GI) tract-associated benign nerve sheath tumors.
The full range of benign nerve sheath tumors may involve the GI tract. Schwannomas at this site have variable features, which may include marked chronic inflammation (a) and non-specific spindle cell cytology that overlaps with GIST (b). Similarly, perineuriomas may show bland, non-specific spindle cell morphology (c), with specific diagnosis requiring immunohistochemistry for several markers, for example GLUT1 (d). Granular cell tumors represent another subset of neoplasms of presumed nerve sheath origin that favor the GI tract (e). Strong S100 expression is almost universal in these tumors (f).
Granular cell tumors or peripheral nerve sheath tumors with granular cell features represent unique tumors with a GI tract predilection (Figure 11). Rare cases demonstrate an associated lipomatous component in the large bowel, creating a curious “nodule-in-nodule” architectural pattern[103]. It must be noted that some GISTs have been recently recognized to develop granular-epithelioid change[1].
The differential diagnosis of gastrointestinal nerve sheath tumors may be difficult and encompasses GIST and smooth muscle tumors, which are relatively more common[3]. The most frequent locations in a recent study included esophagus and colon[3]. GI nerve sheath tumors may demonstrate variable histology that may differ somewhat from their soft tissue counterparts. For example, in a recent study of gastric schwannomas, classic histologic features of schwannoma in soft tissue and peripheral nerve (i.e. palisades, presence of a capsule, and vascular hyalinization) were infrequent[137]. Of interest, some GI schwannomas may arise in patients with NF1 and even demonstrate loss of heterozygosity of 17q (the NF1 gene locus)[81]. NF2 loss is rarer in GI schwannomas, compared to nerve/soft tissue counterparts [81]. These findings, combined with an increase density of axons and perineurial cells, suggests some biologic overlap with neurofibromas[155].
Rare pseudoneoplastic lesions and mimics of benign nerve sheath tumors
Various rare neoplastic and pseudoneoplastic lesions may involve major peripheral nerves and raise interesting differential diagnoses with peripheral nerve sheath tumors. Neuromuscular choristoma is an exquisitely rare lesion characterized by the presence of mature skeletal muscle fibers within a major peripheral nerve [88] (Figure 12). Although this represents a uniformly benign lesion, recent studies have highlighted a strong association with post-operative aggressive fibromatosis as a sequelae, so the potential for morbidity is high [57].
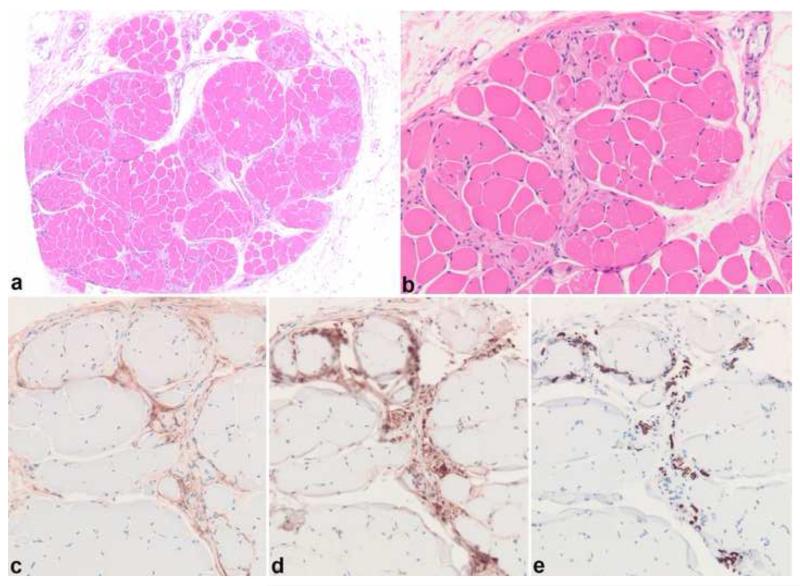
Figure 12. Neuromuscular choristoma.
Neuromuscular choristoma is an exquisitely rare pseudoneoplastic lesion of nerve characterized by intrafascicular replacement of nerve by mature skeletal muscle (a,b). Immunohistochemistry for EMA highlights the outlining perineurium (c), while S100 and neurofilament protein label associated Schwann cells (d) and axons (e).
In addition, a spectrum of benign adipocytic lesions may involve major peripheral nerves, exclusively or in combination with adjacent extraneural tissue. Some authors have recently proposed refinement of classification schemes for these unusual lesions[129]. Although little is known about the true nature of these lesions, they seem to lack the HMGA2 rearrangements characteristic of soft tissue lipoma[116]. A last, apparently reactive lesion of major nerves is inflammatory pseudotumor, which presents as a mononeuropathy and responds favorably to intravenous steroids [91]
Benign non-schwannian neoplasms involving major peripheral nerves are very rare. One unusual mimic is glomus tumor of nerve[121], which usually lacks S100 expression, but may demonstrate pericellular collagen IV reactivity and long spacing collagen on ultrastructural examination, schwannian properties. Curiously, glomus tumors have been recently added to the spectrum of NF1 associated tumors, although they typically do not involve major nerves in this setting[12].
Malignant Peripheral Nerve Sheath Tumors (MPNST)
MPNST are malignant tumors arising from a peripheral nerve or in extraneural soft tissue and showing nerve sheath differentiation[87](Figure 13a,b,c). Arguably, the diagnosis of MPNST has historically suffered from a lack of entirely specific morphological criteria and/or ancillary immunohistochemical or molecular tests. It is thus likely that a variety of non-nerve sheath sarcomas have been placed incorrectly into this category over the years. Most authors would agree that any sarcoma with intrinsic involvement of a major nerve, without a specific diagnosis reflecting an alternative line of differentiation (e.g., intraneural synovial sarcoma, angiosarcoma), or clearly arising from a pre-existing benign nerve sheath tumor should qualify[7, 120, 142]. Similarly, malignant spindled tumors in NF1 patients should be considered MPNST until proven otherwise. More difficult to classify are those tumors that are unrelated to a major nerve, and in these instances a combination of morphologic findings, immunohistochemistry (e.g. partial S100 expression) or ultrastructural features of Schwann cell or perineurial differentiation, must be present[120, 142].
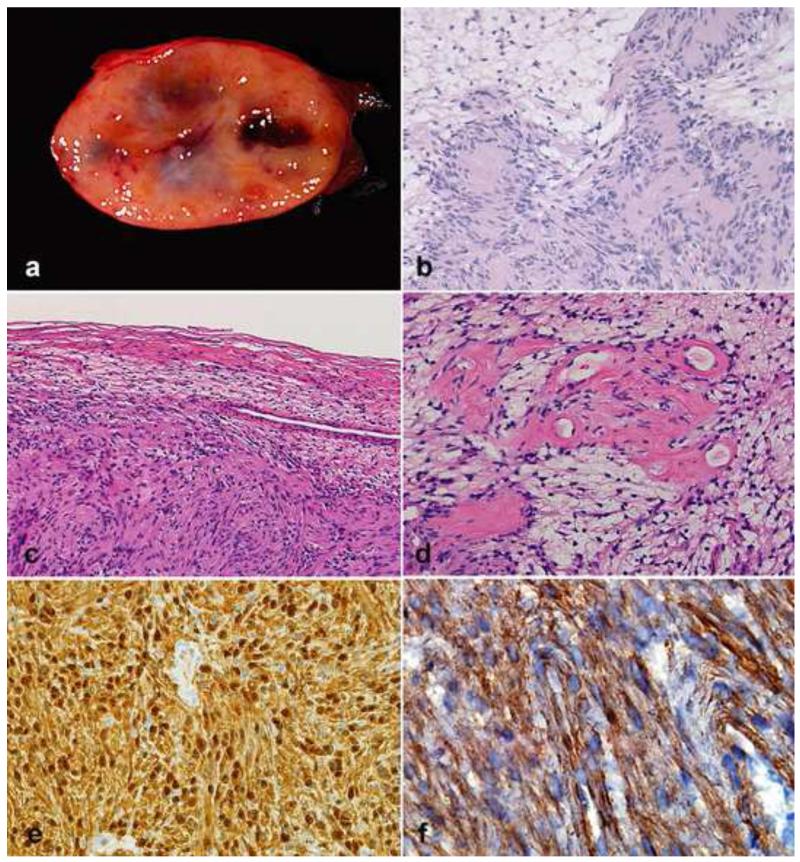
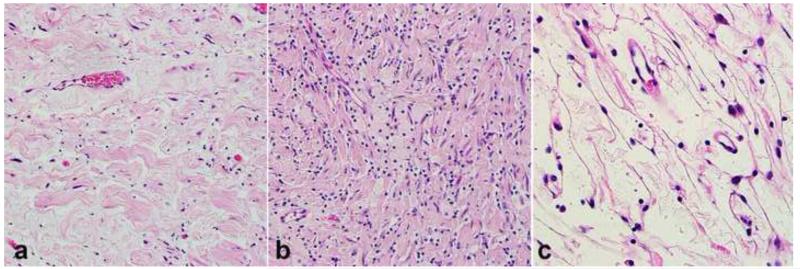
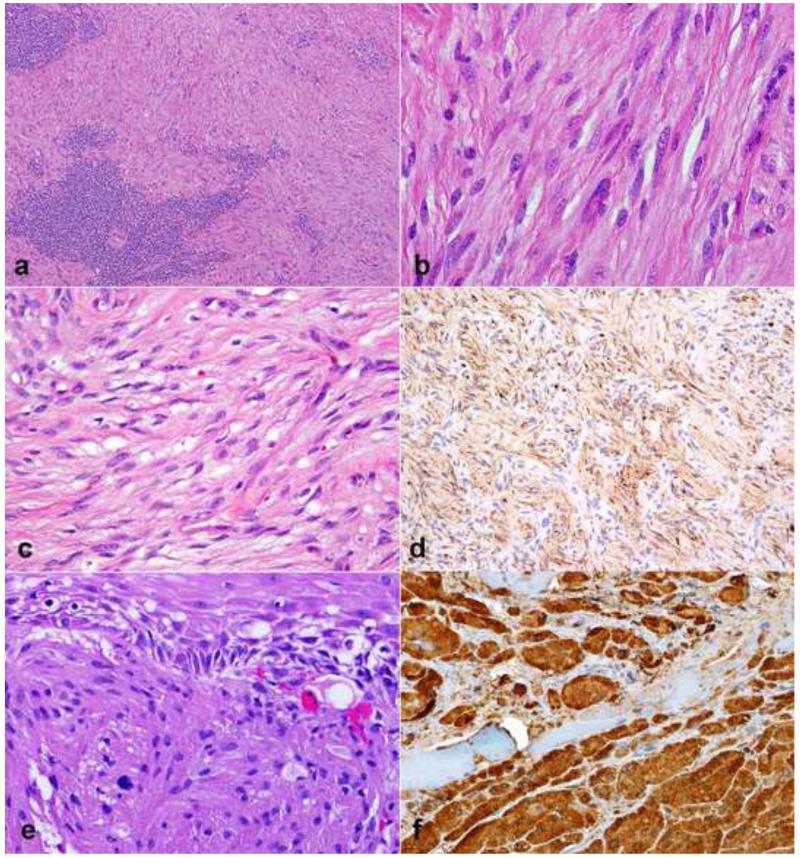
Figure 13. Pathologic features of malignant peripheral nerve sheath tumor (MPNST).
MPNST create fleshy, variegated masses involving large peripheral nerve trunks (a). Multifascicular involvement is usually appreciated on cross sections (b). More often MPNST are characterized by uniform spindle cells with hyperchromatic nuclei arranged in fascicles (c). Heterologous elements, including myogenic differentiation may be present (d) Partial S100 immunoreactivity (e) and desmin expression (f) in a triton tumor.
MPNSTs may show remarkable developmental plasticity, including divergent differentiation. Some arise in the post-irradiation setting [29, 44]. Frequent histologic findings, although not entirely specific, include fascicles of alternating cellularity, whorls, palisades or rosette-like arrangements, perineural/intraneural spread when associated with nerve, subendothelial accentuation of tumor cells, and large areas of geographic-like necrosis[64, 120]. Heterologous differentiation in the form of cartilage and bone, or less commonly skeletal muscle (so called malignant triton tumor)(Figure 13d-e), smooth muscle, angiosarcoma, and even well formed glands occur on occasion (Figure 14), particularly in patients with NF1 [13, 30, 115, 118, 147, 148]. A rare subset of MPNST demonstrates perineurial differentiation, which may be demonstrated by electron microscopy or EMA immunohistochemistry [58, 59](Figure 14).
Figure 14. Rare malignant peripheral nerve sheath tumor (MPNST) variants.
Rare MPNSTs are composed of epithelioid cells that simulate carcinoma or melanoma (a). Rhabdoid cytology (b) and diffuse INI1 protein loss in neoplastic cells with preservation in vessels (c) may be present in rare cases, raising an important differential with epithelioid sarcoma. An even rarer variation in MPNST is overt epithelial differentiation in the form of well formed glands (d). Rare examples of MPNST may lack S100 expression almost entirely, and contain whorls (e) and EMA expression (f) suggestive of perineurial differentiation.
A distinctive, rare subtype of MPNST that raises a completely separate differential diagnosis is characterized by a predominance of large epithelioid cells, i.e. epithelioid MPNST[26, 80, 86](Figure 14a,b). These tumors are more common in superficial sites and in contrast to conventional MPNSTs, express S100 protein strongly and typically, diffusely[80]. For unknown reasons, the great majority of MPNST arising within pre-existing schwannoma (a very rare event) are of epithelioid type. The differential diagnosis of epithelioid MPNST includes melanoma, clear cell sarcoma, epithelioid sarcoma and carcinoma. Lack of expression of melanocytic markers (e.g. MelanA, HMB45, MITF) is very helpful in the distinction of epithelioid MPNST from melanoma and clear cell sarcoma, and absent cytokeratin expression distinguishes them from carcinoma and epithelioid sarcoma. Both epithelioid MPNST and epithelioid sarcoma may show loss of SMARCB1/INI1/BAF47 protein expression[16, 60], a potential diagnostic pitfall in the differential diagnosis with malignant rhabdoid tumor (Figure 14d).
MPNST arising in benign precursors
The main recognizable benign precursor to MPNST is neurofibroma, in particular the plexiform type in the setting of NF1. Malignant transformation into MPNST of schwannoma is a much rarer phenomenon[149], and usually takes the form of epithelioid change, a primitive small cell component or angiosarcoma[93, 133, 149]. One example of rhabdomyoblastic differentiation developing in a schwannoma has also been reported[78]. MPNSTs may also rarely arise in ganglioneuromas/ganglioneuroblastomas[25, 71, 114] and even less commonly, in pheochromocytomas[99, 102]. MPNST arising from benign nerve sheath tumors may be relatively more common in intracranial/cranial nerve examples, with a relatively high association with schwannoma as the precursor, as recently reported[122].
Selected Problems in the diagnosis of peripheral nerve sheath tumors
Grading of MPNST
Standardized, reproducible grading systems for MPNST are generally lacking at the present time. A practical approach to MPNST grading is to divide tumors into low grade (roughly 15%) and high grade (roughly 85%). Most MPNST would fall into a high grade category with cytologic atypia, brisk mitotic activity (usually >5 per 10 high power fields in our experience), and hypercellularity with or without necrosis. We usually apply the term low grade MPNST to less patently anaplastic tumors arising in transition from a neurofibroma precursor, which is described further below.
Further splitting of high grade MPNSTs could be accomplished using standard grading schemes. Among the major recognized grading schemes for soft tissue sarcomas, we advocate the French system (Fédération Nationale des Centres de Lutte Contre le Cancer or FNCLCC)[23, 134], given its reproducibility, large numbers of tumors examined and proven value in a variety of soft tissue tumor types. The system is three tiered, and incorporates three histologic parameters (tissue differentiation, mitotic activity and necrosis), to reach a composite score (Table 2). If applied to MPNST a FNCLCC grade 1 (or WHO grade II) may correspond to low grade MPNST, usually arising in transition from neurofibroma in NF1, while highly pleomorphic MPNSTs or those with divergent differentiation, high mitotic rate and necrosis residing at the other end of the spectrum (FNCLCC grade 3 or WHO grade IV).
Table 2.
French system (FNCLCC) for grading soft tissue sarcomas (Modified from Coindre et al. 2006)
| Tumor Differentiation |
| Depends on histologic type/degree of differentiation ranging from well differentiated tumors similar to mature counterparts (Score=1, e.g. well differentiated MPNST arising in transition from neurofibroma); conventional, monomorphous spindle cell MPNST may be assigned a score 2, while highly pleomorphic MPNSTs (often arising in NF1 syndrome), as well as MPNST with divergent differentiation (triton tumor, glandular, osteosarcomatous, chondorsarcomatous, angiosarcomatous) may be assigned a score of 3. |
| Mitotic Count |
| Score 1: Between 0 and 9 mitoses per 10 high power fields (0.1734 mm2) |
| Score 2: Between 10 and 19 mitoses per 10 high power fields |
| Score 3: Greater than 20 mitoses per 10 high power fields |
| Tumor necrosis |
| Score 0: Necrosis absent |
| Score 1: Less than 50% necrosis |
| Score 2: Greater than 50% necrosis |
| GRADE 1 (score 2-3), GRADE 2 (score 4-5), GRADE 3 (score 6-8) |
One caveat is that this system has not been demonstrated to be of prognostic value in MPNST [22], highlighting the need for large multi-institutional studies sufficiently powered to answer the specific question of grading and prognostic relevance, in particular separating a high grade category in two. Perhaps this is not surprising however, since despite its similar spindle cell morphology, MPNST is more accurately classified as a neuroectodermal malignancy rather than a true sarcoma, including considerable genetic and clinicopathological differences from the latter broad category of mesenchymal neoplasms.
MPNST Mimics
The differential diagnosis of MPNST in peripheral nerve and soft tissue is wide and includes a variety of sarcomas, primarily adult-type fibrosarcoma, synovial sarcoma, rhabdomyosarcoma, leiomyosarcoma, dedifferentiated liposarcoma, and clear cell sarcoma. One of the most useful distinctions from benign Schwann cell tumors is the partial or even complete loss of S100 expression in MPNST[24, 140, 145]. Conversely, isolated expression of S100 should not be considered definite evidence of MPNST, since S100 expression may be seen in synovial sarcomas, leiomyosarcomas, and rhabdomyosarcomas, among others [130].
The tumor that perhaps most closely resembles MPNST is synovial sarcoma, in particular its monophasic variant. Synovial sarcomas commonly involve nerves and may even grow in a multinodular or plexiform growth pattern. Very rarely, genetically confirmed, intraneural synovial sarcomas have also been reported, including a recent series of 12 cases[123] (Figure 15). Perhaps the only morphological feature that confidently allows the distinction of MPNST from synovial sarcoma is the presence of pleomorphic cells, essentially never present in synovial sarcoma. Although both synovial sarcoma and MPNST may show glandular differentiation, glandular MPNST tend to show glands resembling enteric epithelium with frequent endocrine differentiation, whereas those of synovial sarcoma are lined by cuboidal cells, often with intraluminal eosinophilic necrotic debris [21, 147]. Obviously, a history of NF1 and/or a co-existing neurofibroma precursor suggest MPNST. By immunohistochemistry, both synovial sarcoma and MPNST may express low molecular weight cytokeratins and EMA, although expression of high molecular weight cytokeratins is seen only in synovial sarcoma. S100 expression may be seen in both tumors, but CD34 expression is not seen in synovial sarcoma[157]. Transducin-like enhancer protein (TLE1) expression is typically diffuse and strong in synovial sarcomas, but may also be present in a somewhat weaker, more variable pattern in some MPNST [47, 74, 131] [75].
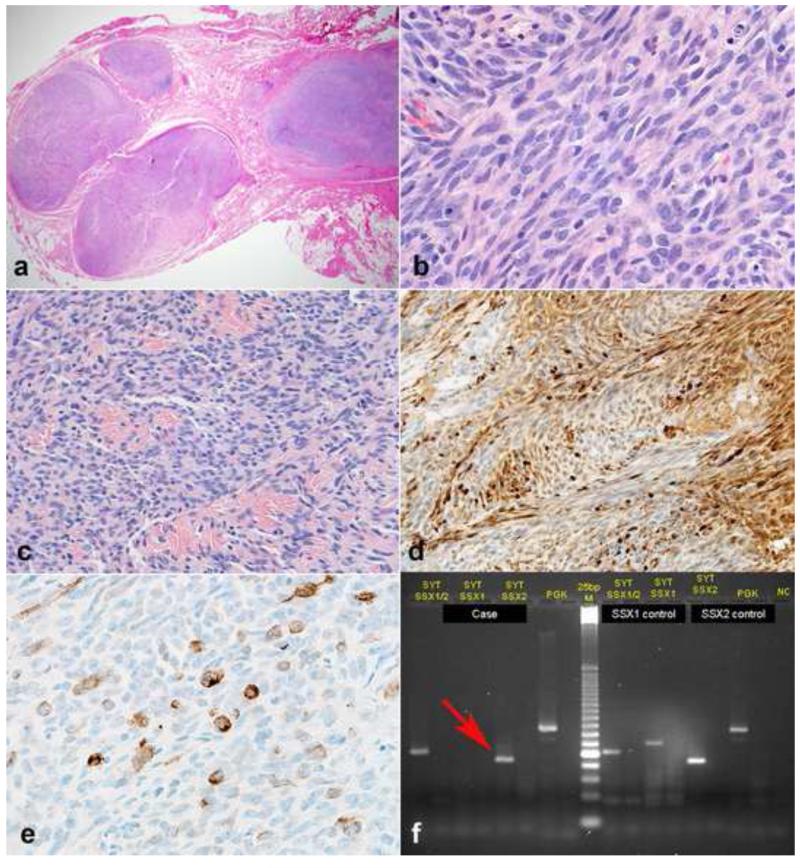
Figure 15. Intraneural synovial sarcoma mimicking malignant peripheral nerve sheath tumor (MPNST).
Synovial sarcoma represents the main entity in the differential diagnosis with MPNST, and similarly may infiltrate numerous nerve fascicles (a). Monotonous spindle cells in fascicles are characteristic (b) as well as variable intratumoral collagen bundles (c). Overt S100 immunoreactivity may be present in some synovial sarcomas (d). Cytokeratin typically labels isolated cells (e). Molecular confirmation may be required in some instances. In this example, RT-PCR reveals a SYT-SSX2 fusion transcript (arrow)(f), which is diagnostic of synovial sarcoma.
Given the limited discriminatory power of histology and immunophenotype in the differential diagnosis of MPNST and synovial sarcoma, demonstration of SS18-SSX1 or SS18-SSX2 gene fusions, usually resulting from a characteristic X;18 translocation[136], may be required for a definitive diagnosis of intraneural synovial sarcoma (Figure 15), as these gene fusions are limited to synovial sarcoma [79]. Conversely, no specific chromosomal rearrangements have been uncovered in MPNST by conventional cytogenetics, but a complex karyotype is usually present [66].
A rare tumor that may occasionally mimic MPNST is the ossifying fibromyxoid tumor of soft parts [46]. This rare neoplasm usually arises in superficial locations, and is characterized by bland cytology, fibromyxoid stroma, peripheral ossification in the form of a shell and frequent S100 expression [34, 100]. Malignant behavior, including the development of metastasis may occur in a small proportion of cases. In a subset of cases a spindle cell component may mimic low grade MPNST[46]. The presence of S100 immunoreactivity further complicates the differential, in particular in the rare examples arising in paraspinal locations[18, 119]. Additional phenotypic features include expression of desmin in a subset of cases, and a patchy (i.e. mosaic) pattern of INI1/BAF47 loss[54].
Although MPNST lack a distinctive cytogenetic signature that allows for a specific diagnosis, molecular alterations including EGFR amplification, and deletions of NF1 or CDKN2A (p16) are supportive in the appropriate setting[77, 97, 106, 112]. The use of high throughput, genomic techniques also has allowed the identification of specific genetic and phenotypic alterations with diagnostic or prognostic relevance that may find increased practical applications in the future[65, 101, 154].
Cellular nerve sheath tumors in childhood
MPNSTs are generally tumors of adulthood and relatively rare in the pediatric population. In 1994 Meis-Kindblom and Enzinger reported a pediatric series of 9 cases of a tumor that they termed “plexiform malignant peripheral nerve sheath tumor of infancy and childhood”[94]. The tumors were unrelated to NF1, except for one case, and were associated with frequent local recurrences.
Subsequently, some authors have interpreted these tumors to represent a subset of plexiform cellular schwannomas, given the consistent absence of aggressive behavior, metastatic disease or deaths after extended clinical follow-up[151]. Histologically the tumors in the pediatric series of Woodruff et al.[151] demonstrated many worrisome features, including hypercellularity, brisk mitotic activity (4-31 per 10 high power fields) and MIB1 labeling indices as high as 30%. However, as it is true of all schwannoma variants, strong uniform S100 immunostaining was demonstrated in all of these tumors.
Although these studies highlight the rarity or even absence of MPNST in the congenital setting, series of MPNST in children exist, with similar malignant behavior as in adults[31, 95].
Low grade MPNST
The distinction of atypical or cellular neurofibroma from low grade MPNST change is perhaps the most difficult challenge in the pathology of peripheral nerve sheath neoplasms, particularly in the setting of an NF1 patient. Some authors use the term “atypical neurofibroma” to denote neurofibromas with degenerative nuclear changes, analogous to ancient change in schwannoma[120]. These tumors are of little concern. Others, however, have reserved this term for nerve sheath tumors showing worrisome histologic features (e.g., high cellularity, scattered mitotic figures, monotonous cytology or fascicular growth), but not fully meeting criteria for malignancy [120, 142]. Clinically, atypical changes usually develop in large, slowly growing neurofibromas, and pain may be a feature[37]. “Atypical neurofibromas” have generally been regarded as benign[85]. However, a recent study restricted to NF1 patients suggests that atypical neurofibromas, defined as neurofibromas with increased cellularity and nuclear hyperchromasia/enlargement lacking mitotic figures, represent early malignant change in neurofibroma, with CDKN2A/B deletions (seen in MPNST) in the majority (94%) of tested cases[10].
Morphological criteria for the diagnosis of low-grade MPNST arising in neurofibroma, as proposed by Scheithauer and Woodruff, include hypercellularity, nuclear enlargement (~3× the size of a neurofibroma nucleus), and hyperchromasia, independent of the presence of mitotic activity [120](Figure 16). The isolated presence of one of these features is not sufficient for a malignant diagnosis. This is a frequently used scheme and one we routinely follow in our practice. However, some difficulties arise in a case by case basis given the lack of objective criteria for hypercellularity, hyperchromasia, and the extent of changes required before reaching a malignant diagnosis. In our view, one worrisome feature for malignant transformation, given additional abnormal findings, includes the development of a fascicular pattern of growth, usually lacking in conventional neurofibromas. Lack of objective outcome data is another confounding factor in these grading schemes.
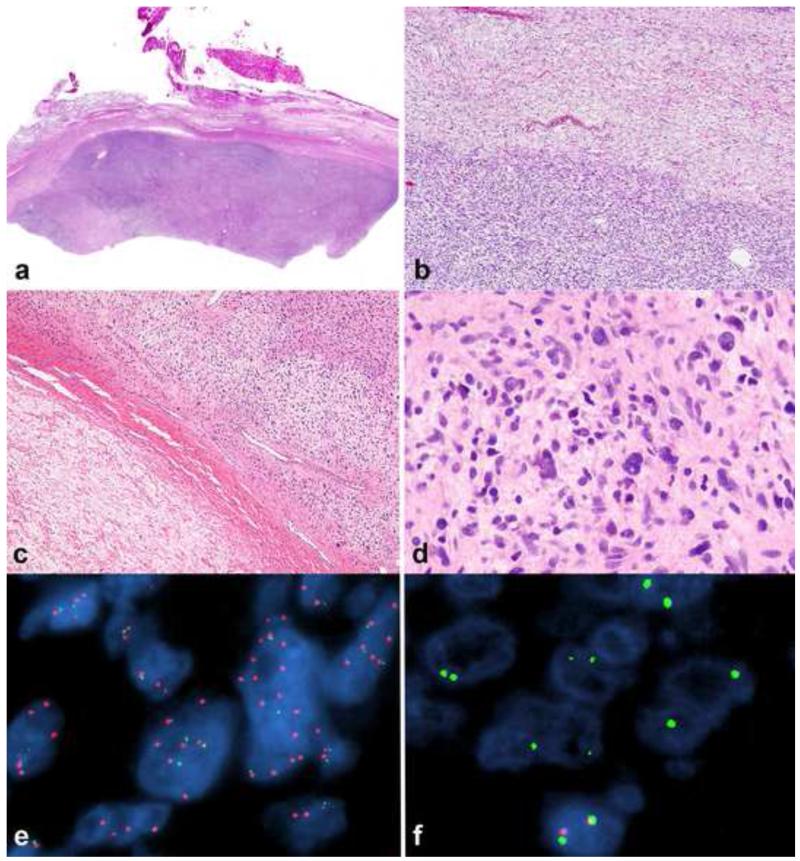
Figure 16. Low grade malignant peripheral nerve sheath tumor (MPNST).
Low grade MPNST represent diagnostically difficult problems but may be suspected by finding areas of hypercellularity within a conventional neurofibroma (a), in particular in the setting of NF1. A sharp interface between the precursor neurofibroma (upper) and low grade MPNST (lower) is usually evident (b). A collagenous interface may be seen in some cases (c). Minimal criteria for low grade MPNST require the presence of nuclear hyperchromasia, enlargement, and crowding (d). Molecular techniques such as fluorescence in situ hybridization (FISH) may be useful in a subset of cases, identifying genetic changes that are frequent in MPNST, including EGFR amplification (e) and homozygous CDKN2A deletions (f; a non-neoplastic cell at bottom serves as a positive internal control).
Ancillary techniques may be of help, in particular increased MIB1 (Ki-67) and p53 nuclear labeling by immunohistochemistry [55, 72]. p16[106] and p27[76] expression is typically present in neurofibromas but absent in MPNSTs, and such areas of loss may highlight foci of malignant change in neurofibromas. Molecular techniques, including fluorescence in situ hybridization[112] and array comparative genomic hybridization[10] may be more objective tools in identifying molecular alterations relatively specific for MPNST, in particular CDKN2A/B deletions as described above (Figure 16).
Recommended updates for the WHO Classification
The spectrum of tumors and pseudoneoplastic lesions that may involve the peripheral nervous system is wide. However, the current WHO Classification of Tumours of the Central Nervous System, Cranial and Paraspinal Nerves is mostly restricted to the larger, traditional categories[87].
Based on our personal experience and review of the spectrum of peripheral nerve sheath neoplasms, categories and entities to incorporate in upcoming classification schemes may include a section clarifying the spectrum of benign hybrid tumors, as discussed above, that do not fit properly in any of the classic tumor types. This may be important, given the strong syndrome association of the traditional benign nerve sheath tumors, specifically those with schwannian features. This issue is more than academic, since it has long term prognostic and even therapeutic implications.
Granular cell tumor is a rare, but major category of tumors with presumed peripheral nerve sheath derivation, based on S100 expression and frequent nerve association including plexiform patterns[6, 139], that may be incorporated in upcoming schemes. Although most behave in a benign fashion, a small subset acts in a bona fide malignant fashion, with scant predictive histologic features. Histologic criteria for malignancy in these tumors, therefore, merits further work.
A number of benign nerve sheath tumors are also not covered in the current WHO classification, including nerve sheath myxoma and palisaded encapsulated neuromas, among others. Despite evidence supporting a nerve sheath origin for most of these lesions, many are not routinely encountered by neuropathologists, but rather are more frequently evaluated in the subspecialty areas of dermatopathology and soft tissue pathology.
Finally, grading of MPNST is a persistent problem that has not been adequately addressed at the present time, which is understandable in part given the absence of concrete outcome data. In our experience, a two tiered scale of “low” and “high” grade represents a practical approach, while further refinement of MPNST grading, in particular to split a high grade category into WHO grades III and IV, may require larger consensus evaluation with critical discussion of standard grading schemes, and/or formal testing in large, multiinstitutional cohorts.
Conclusion
The realm of peripheral nerve sheath tumors is currently a dynamic, evolving area of surgical pathology, with increasing multidisciplinary team collaborations. Morphologic variability of these tumors is wide, and they engender some of the most controversial, difficult differential diagnoses. Some traditional problems continue to plague pathologists, in particular the currently blurred borderzone between benign tumors and low grade MPNST. The recognition of hybrid, non-classic neoplasms defy traditional classification schemes and the spectrum continues to expand. Greater availability of molecular techniques also provides an opportunity to refine morphologic diagnoses and is likely to play an increasing important role in the immediate future.
Table 3.
Possible Updated WHO Classification of Tumours of the Central Nervous System, Tumors of Cranial and Paraspinal Nerves Section
| Schwannoma |
| Conventional schwannoma |
| Cellular schwannoma |
| Plexiform schwannoma |
| Melanotic schwannoma |
| Microcystic/reticular schwannoma |
| Neurofibroma |
| Circumscribed Neurofibroma (dermal/soft tissue/intraneural) |
| Diffuse and Massive Soft Tissue neurofibroma |
| Plexiform neurofibroma |
| Perineurioma |
| Intraneural perineurioma |
| Soft Tissue perineurioma |
| Sclerosing perineurioma |
| Granular cell Tumor |
| Benign granular cell tumor |
| Malignant granular cell tumor |
| Miscellaneous Benign Nerve Sheath Tumors |
| Palisaded encapsulated neuroma |
| Nerve sheath myxoma |
| Benign Hybrid Tumors |
| Hybrid neurofibroma/schwannoma (“neurofibroma with schwannian nodules”) |
| Hybrid schwannoma/perineurioma |
| Hybrid benign nerve sheath tumors NOS |
| Malignant Peripheral Nerve Sheath Tumour (MPNST) |
| Low grade MPNST |
| High grade MPNST |
| Epithelioid MPNST |
| MPNST with divergent differentiation (triton tumor, MPNST with glandular differentiation) |
| MPNST ex Schwannoma |
| Malignant melanotic schwannoma |
| Perineurial MPNST |
| Miscellaneous Malignant Intraneural Neoplasms |
| Synovial sarcoma of nerve |
Acknowledgements
The authors would like to thank Dr. James Woodruff for critically reviewing the manuscript and helpful suggestions. They also thank Norm Baker for assistance with graphics, as well as Drs. Robert Spinner and James Garrity for contributing intraoperative figures.
References
- 1.Adamiak A, Lee CH, Nielsen TO, Webber D, O’Connell JX. Duodenal epithelioid gastrointestinal stromal tumor with prominent granular cell features. Hum Pathol. 2009;40:599–602. doi: 10.1016/j.humpath.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Agaimy A, Wuensch PH. Perineurioma of the stomach. A rare spindle cell neoplasm that should be distinguished from gastrointestinal stromal tumor. Pathol Res Pract. 2005;201:463–467. doi: 10.1016/j.prp.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Agaimy A, Markl B, Kitz J, Wunsch PH, Arnholdt H, Fuzesi L, Hartmann A, Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411–422. doi: 10.1007/s00428-010-0886-8. [DOI] [PubMed] [Google Scholar]
- 4.Agaram NP, Prakash S, Antonescu CR. Deep-seated plexiform schwannoma: a pathologic study of 16 cases and comparative analysis with the superficial variety. Am J Surg Pathol. 2005;29:1042–1048. [PubMed] [Google Scholar]
- 5.Ahrens WA, Ridenour RV, 3rd, Caron BL, Miller DV, Folpe AL. GLUT-1 expression in mesenchymal tumors: an immunohistochemical study of 247 soft tissue and bone neoplasms. Hum Pathol. 2008;39:1519–1526. doi: 10.1016/j.humpath.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Aldabagh B, Azmi F, Vadmal M, Neider S, Usmani AS. Plexiform pattern in cutaneous granular cell tumors. J Cutan Pathol. 2009;36:1174–1176. doi: 10.1111/j.1600-0560.2009.01253.x. [DOI] [PubMed] [Google Scholar]
- 7.Allison KH, Patel RM, Goldblum JR, Rubin BP. Superficial malignant peripheral nerve sheath tumor: a rare and challenging diagnosis. Am J Clin Pathol. 2005;124:685–692. doi: 10.1309/V8XM-K5R7-8Q96-V090. [DOI] [PubMed] [Google Scholar]
- 8.Antonescu CR, Woodruff JM, Scheithauer BW. Tumors of the Peripheral Nervous System. 4th edition edn. American Registry of Pathology; Washington DC: 2012. [Google Scholar]
- 9.Ariza A, Bilbao JM, Rosai J. Immunohistochemical detection of epithelial membrane antigen in normal perineurial cells and perineurioma. Am J Surg Pathol. 1988;12:678–683. doi: 10.1097/00000478-198809000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Beert E, Brems H, Daniels B, De Wever I, Van Calenbergh F, Schoenaers J, Debiec-Rychter M, Gevaert O, De Raedt T, Van Den Bruel A, de Ravel T, Cichowski K, Kluwe L, Mautner V, Sciot R, Legius E. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer. 2011;50:1021–1032. doi: 10.1002/gcc.20921. [DOI] [PubMed] [Google Scholar]
- 11.Berg JC, Scheithauer BW, Spinner RJ, Allen CM, Koutlas IG. Plexiform schwannoma: a clinicopathologic overview with emphasis on the head and neck region. Hum Pathol. 2008;39:633–640. doi: 10.1016/j.humpath.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Brems H, Park C, Maertens O, Pemov A, Messiaen L, Upadhyaya M, Claes K, Beert E, Peeters K, Mautner V, Sloan JL, Yao L, Lee CC, Sciot R, De Smet L, Legius E, Stewart DR. Glomus tumors in neurofibromatosis type 1: genetic, functional, and clinical evidence of a novel association. Cancer Res. 2009;69:7393–7401. doi: 10.1158/0008-5472.CAN-09-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RW, Tornos C, Evans HL. Angiosarcoma arising from malignant schwannoma in a patient with neurofibromatosis. Cancer. 1992;70:1141–1144. doi: 10.1002/1097-0142(19920901)70:5<1141::aid-cncr2820700519>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol. 1990;14:206–222. [PubMed] [Google Scholar]
- 15.Carroll S. Molecular mechanisms promoting the pathogenesis of Schwann cell neoplasms. Acta Neuropathol. 2012 doi: 10.1007/s00401-011-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter JM, O’Hara C, Dundas G, Gilchrist D, Collins MS, Eaton K, Judkins AR, Biegel JA, Folpe AL. Epithelioid Malignant Peripheral Nerve Sheath Tumor Arising in a Schwannoma, in a Patient With “Neuroblastoma-like“ Schwannomatosis and a Novel Germline SMARCB1 Mutation. Am J Surg Pathol. 2011 doi: 10.1097/PAS.0b013e3182380802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casadei GP, Scheithauer BW, Hirose T, Manfrini M, Van Houton C, Wood MB. Cellular schwannoma. A clinicopathologic, DNA flow cytometric, and proliferation marker study of 70 patients. Cancer. 1995;75:1109–1119. doi: 10.1002/1097-0142(19950301)75:5<1109::aid-cncr2820750510>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Cha JH, Kwon JW, Cho EY, Lee CS, Yoon YC, Choi SH. Ossifying fibromyxoid tumor invading the spine: a case report and review of the literature. Skeletal Radiol. 2008;37:1137–1140. doi: 10.1007/s00256-008-0562-0. [DOI] [PubMed] [Google Scholar]
- 19.Chaubal A, Paetau A, Zoltick P, Miettinen M. CD34 immunoreactivity in nervous system tumors. Acta Neuropathol. 1994;88:454–458. doi: 10.1007/BF00389498. [DOI] [PubMed] [Google Scholar]
- 20.Chen JY, Hruby G, Scolyer RA, Murali R, Hong A, Fitzgerald P, Pham TT, Quinn MJ, Thompson JF. Desmoplastic neurotropic melanoma: a clinicopathologic analysis of 128 cases. Cancer. 2008;113:2770–2778. doi: 10.1002/cncr.23895. [DOI] [PubMed] [Google Scholar]
- 21.Christensen WN, Strong EW, Bains MS, Woodruff JM. Neuroendocrine differentiation in the glandular peripheral nerve sheath tumor. Pathologic distinction from the biphasic synovial sarcoma with glands. Am J Surg Pathol. 1988;12:417–426. doi: 10.1097/00000478-198806000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchere D, Sastre X, Vilain MO, Bonichon F, N’Guyen Bui B. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91:1914–1926. doi: 10.1002/1097-0142(20010515)91:10<1914::aid-cncr1214>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130:1448–1453. doi: 10.5858/2006-130-1448-GOSTSR. [DOI] [PubMed] [Google Scholar]
- 24.Daimaru Y, Hashimoto H, Enjoji M. Malignant peripheral nerve-sheath tumors (malignant schwannomas). An immunohistochemical study of 29 cases. Am J Surg Pathol. 1985;9:434–444. doi: 10.1097/00000478-198506000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Damiani S, Manetto V, Carrillo G, Di Blasi A, Nappi O, Eusebi V. Malignant peripheral nerve sheath tumor arising in a “de novo” ganglioneuroma. A case report. Tumori. 1991;77:90–93. doi: 10.1177/030089169107700121. [DOI] [PubMed] [Google Scholar]
- 26.DiCarlo EF, Woodruff JM, Bansal M, Erlandson RA. The purely epithelioid malignant peripheral nerve sheath tumor. Am J Surg Pathol. 1986;10:478–490. doi: 10.1097/00000478-198607000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Downs-Kelly E, Goldblum JR, Patel RM, Weiss SW, Folpe AL, Mertens F, Hartke M, Tubbs RR, Skacel M. The utility of fluorescence in situ hybridization (FISH) in the diagnosis of myxoid soft tissue neoplasms. Am J Surg Pathol. 2008;32:8–13. doi: 10.1097/PAS.0b013e3181578d5a. [DOI] [PubMed] [Google Scholar]
- 28.Doyle LA, Moller E, Dal Cin P, Fletcher CD, Mertens F, Hornick JL. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am J Surg Pathol. 2011;35:733–741. doi: 10.1097/PAS.0b013e318210c268. [DOI] [PubMed] [Google Scholar]
- 29.Ducatman BS, Scheithauer BW. Postirradiation neurofibrosarcoma. Cancer. 1983;51:1028–1033. doi: 10.1002/1097-0142(19830315)51:6<1028::aid-cncr2820510610>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Ducatman BS, Scheithauer BW. Malignant peripheral nerve sheath tumors with divergent differentiation. Cancer. 1984;54:1049–1057. doi: 10.1002/1097-0142(19840915)54:6<1049::aid-cncr2820540620>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM. Malignant peripheral nerve sheath tumors in childhood. J Neurooncol. 1984;2:241–248. doi: 10.1007/BF00253276. [DOI] [PubMed] [Google Scholar]
- 32.Emanuel P, Pertsemlidis DS, Gordon R, Xu R. Benign hybrid perineurioma-schwannoma in the colon. A case report. Ann Diagn Pathol. 2006;10:367–370. doi: 10.1016/j.anndiagpath.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Emory TS, Scheithauer BW, Hirose T, Wood M, Onofrio BM, Jenkins RB. Intraneural perineurioma. A clonal neoplasm associated with abnormalities of chromosome 22. Am J Clin Pathol. 1995;103:696–704. doi: 10.1093/ajcp/103.6.696. [DOI] [PubMed] [Google Scholar]
- 34.Enzinger FM, Weiss SW, Liang CY. Ossifying fibromyxoid tumor of soft parts. A clinicopathological analysis of 59 cases. Am J Surg Pathol. 1989;13:817–827. doi: 10.1097/00000478-198910000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Fanburg-Smith JC, Majidi M, Miettinen M. Keratin expression in schwannoma; a study of 115 retroperitoneal and 22 peripheral schwannomas. Mod Pathol. 2006;19:115–121. doi: 10.1038/modpathol.3800489. [DOI] [PubMed] [Google Scholar]
- 36.Feany MB, Anthony DC, Fletcher CD. Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: a conceptual challenge. Histopathology. 1998;32:405–410. doi: 10.1046/j.1365-2559.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferner RE, Golding JF, Smith M, Calonje E, Jan W, Sanjayanathan V, O’Doherty M. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol. 2008;19:390–394. doi: 10.1093/annonc/mdm450. [DOI] [PubMed] [Google Scholar]
- 38.Ferry JA, Dickersin GR. Pseudoglandular schwannoma. Am J Clin Pathol. 1988;89:546–552. doi: 10.1093/ajcp/89.4.546. [DOI] [PubMed] [Google Scholar]
- 39.Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433–1442. doi: 10.1097/00000478-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331–343. doi: 10.1097/00000478-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Fine SW, McClain SA, Li M. Immunohistochemical staining for calretinin is useful for differentiating schwannomas from neurofibromas. Am J Clin Pathol. 2004;122:552–559. doi: 10.1309/AGBG-TBRJ-4W0B-C7LN. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher CD, Davies SE. Benign plexiform (multinodular) schwannoma: a rare tumour unassociated with neurofibromatosis. Histopathology. 1986;10:971–980. doi: 10.1111/j.1365-2559.1986.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher CD, Davies SE, McKee PH. Cellular schwannoma: a distinct pseudosarcomatous entity. Histopathology. 1987;11:21–35. doi: 10.1111/j.1365-2559.1987.tb02606.x. [DOI] [PubMed] [Google Scholar]
- 44.Foley KM, Woodruff JM, Ellis FT, Posner JB. Radiation-induced malignant and atypical peripheral nerve sheath tumors. Ann Neurol. 1980;7:311–318. doi: 10.1002/ana.410070405. [DOI] [PubMed] [Google Scholar]
- 45.Folpe AL, Billings SD, McKenney JK, Walsh SV, Nusrat A, Weiss SW. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620–1626. doi: 10.1097/00000478-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Folpe AL, Weiss SW. Ossifying fibromyxoid tumor of soft parts: a clinicopathologic study of 70 cases with emphasis on atypical and malignant variants. Am J Surg Pathol. 2003;27:421–431. doi: 10.1097/00000478-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Foo WC, Cruise MW, Wick MR, Hornick JL. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol. 2011;135:839–844. doi: 10.1309/AJCP45SSNAOPXYXU. [DOI] [PubMed] [Google Scholar]
- 48.Giannini C, Scheithauer BW, Jenkins RB, Erlandson RA, Perry A, Borell TJ, Hoda RS, Woodruff JM. Soft-tissue perineurioma. Evidence for an abnormality of chromosome 22, criteria for diagnosis, and review of the literature. Am J Surg Pathol. 1997;21:164–173. doi: 10.1097/00000478-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Gibson JA, Hornick JL. Mucosal Schwann cell “hamartoma“: clinicopathologic study of 26 neural colorectal polyps distinct from neurofibromas and mucosal neuromas. Am J Surg Pathol. 2009;33:781–787. doi: 10.1097/PAS.0b013e31818dd6ca. [DOI] [PubMed] [Google Scholar]
- 50.Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–1630. [PMC free article] [PubMed] [Google Scholar]
- 51.Goldblum JR, Beals TF, Weiss SW. Neuroblastoma-like neurilemoma. Am J Surg Pathol. 1994;18:266–273. doi: 10.1097/00000478-199403000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Campora R, Delgado MD, Amate AH, Gallardo SP, Leon MS, Beltran AL. Old and new immunohistochemical markers for the diagnosis of gastrointestinal stromal tumors. Anal Quant Cytol Histol. 2011;33:1–11. [PubMed] [Google Scholar]
- 53.Graadt van Roggen JF, McMenamin ME, Belchis DA, Nielsen GP, Rosenberg AE, Fletcher CD. Reticular perineurioma: a distinctive variant of soft tissue perineurioma. Am J Surg Pathol. 2001;25:485–493. doi: 10.1097/00000478-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Graham RP, Dry S, Li X, Binder S, Bahrami A, Raimondi SC, Dogan A, Chakraborty S, Souchek JJ, Folpe AL. Ossifying fibromyxoid tumor of soft parts: a clinicopathologic, proteomic, and genomic study. Am J Surg Pathol. 2011;35:1615–1625. doi: 10.1097/PAS.0b013e3182284a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halling KC, Scheithauer BW, Halling AC, Nascimento AG, Ziesmer SC, Roche PC, Wollan PC. p53 expression in neurofibroma and malignant peripheral nerve sheath tumor. An immunohistochemical study of sporadic and NF1-associated tumors. Am J Clin Pathol. 1996;106:282–288. doi: 10.1093/ajcp/106.3.282. [DOI] [PubMed] [Google Scholar]
- 56.Hebert-Blouin MN, Amrami KK, Scheithauer BW, Spinner RJ. Multinodular/plexiform (multifascicular) schwannomas of major peripheral nerves: an underrecognized part of the spectrum of schwannomas. J Neurosurg. 2010;112:372–382. doi: 10.3171/2009.5.JNS09244. [DOI] [PubMed] [Google Scholar]
- 57.Hebert-Blouin MN, Scheithauer BW, Amrami KK, Durham SR, Spinner RJ. Fibromatosis: a potential sequela of neuromuscular choristoma. J Neurosurg. 2011 doi: 10.3171/2011.6.JNS102171. [DOI] [PubMed] [Google Scholar]
- 58.Hirose T, Sumitomo M, Kudo E, Hasegawa T, Teramae T, Murase M, Higasa Y, Ikata T, Hizawa K. Malignant peripheral nerve sheath tumor (MPNST) showing perineurial cell differentiation. Am J Surg Pathol. 1989;13:613–620. doi: 10.1097/00000478-198907000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Hirose T, Scheithauer BW, Sano T. Perineurial malignant peripheral nerve sheath tumor (MPNST): a clinicopathologic, immunohistochemical, and ultrastructural study of seven cases. Am J Surg Pathol. 1998;22:1368–1378. doi: 10.1097/00000478-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol. 2011;35:e47–63. doi: 10.1097/PAS.0b013e31822b325b. [DOI] [PubMed] [Google Scholar]
- 61.Hornick JL, Fletcher CD. Soft tissue perineurioma: clinicopathologic analysis of 81 cases including those with atypical histologic features. Am J Surg Pathol. 2005;29:845–858. doi: 10.1097/01.pas.0000155166.86409.d2. [DOI] [PubMed] [Google Scholar]
- 62.Hornick JL, Fletcher CD. Intestinal perineuriomas: clinicopathologic definition of a new anatomic subset in a series of 10 cases. Am J Surg Pathol. 2005;29:859–865. doi: 10.1097/01.pas.0000154130.87219.2c. [DOI] [PubMed] [Google Scholar]
- 63.Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma/perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554–1561. doi: 10.1097/PAS.0b013e3181accc6c. [DOI] [PubMed] [Google Scholar]
- 64.Hruban RH, Shiu MH, Senie RT, Woodruff JM. Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer. 1990;66:1253–1265. doi: 10.1002/1097-0142(19900915)66:6<1253::aid-cncr2820660627>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 65.Hummel TR, Jessen WJ, Miller SJ, Kluwe L, Mautner VF, Wallace MR, Lazaro C, Page GP, Worley PF, Aronow BJ, Schorry EK, Ratner N. Gene expression analysis identifies potential biomarkers of neurofibromatosis type 1 including adrenomedullin. Clin Cancer Res. 2010;16:5048–5057. doi: 10.1158/1078-0432.CCR-10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jhanwar SC, Chen Q, Li FP, Brennan MF, Woodruff JM. Cytogenetic analysis of soft tissue sarcomas. Recurrent chromosome abnormalities in malignant peripheral nerve sheath tumors (MPNST) Cancer Genet Cytogenet. 1994;78:138–144. doi: 10.1016/0165-4608(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 67.Jokinen CH, Dadras SS, Goldblum JR, van de Rijn M, West RB, Rubin BP. Diagnostic implications of podoplanin expression in peripheral nerve sheath neoplasms. Am J Clin Pathol. 2008;129:886–893. doi: 10.1309/M7D5KTVYYE51XYQA. [DOI] [PubMed] [Google Scholar]
- 68.Kawahara E, Oda Y, Ooi A, Katsuda S, Nakanishi I, Umeda S. Expression of glial fibrillary acidic protein (GFAP) in peripheral nerve sheath tumors. A comparative study of immunoreactivity of GFAP, vimentin, S-100 protein, and neurofilament in 38 schwannomas and 18 neurofibromas. Am J Surg Pathol. 1988;12:115–120. doi: 10.1097/00000478-198802000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Kazakov DV, Pitha J, Sima R, Vanecek T, Shelekhova K, Mukensnabl P, Michal M. Hybrid peripheral nerve sheath tumors: Schwannoma-perineurioma and neurofibroma-perineurioma. A report of three cases in extradigital locations. Ann Diagn Pathol. 2005;9:16–23. doi: 10.1016/j.anndiagpath.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Kelesidis T, Tarbox A, Lopez M, Aish L. Perineurioma of esophagus: a first case report. Am J Med Sci. 2009;338:230–232. doi: 10.1097/MAJ.0b013e3181a59053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keller SM, Papazoglou S, McKeever P, Baker A, Roth JA. Late occurrence of malignancy in a ganglioneuroma 19 years following radiation therapy to a neuroblastoma. J Surg Oncol. 1984;25:227–231. doi: 10.1002/jso.2930250402. [DOI] [PubMed] [Google Scholar]
- 72.Kindblom LG, Ahlden M, Meis-Kindblom JM, Stenman G. Immunohistochemical and molecular analysis of p53, MDM2, proliferating cell nuclear antigen and Ki67 in benign and malignant peripheral nerve sheath tumours. Virchows Arch. 1995;427:19–26. doi: 10.1007/BF00203733. [DOI] [PubMed] [Google Scholar]
- 73.Kindblom LG, Meis-Kindblom JM, Havel G, Busch C. Benign epithelioid schwannoma. Am J Surg Pathol. 1998;22:762–770. doi: 10.1097/00000478-199806000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Knosel T, Heretsch S, Altendorf-Hofmann A, Richter P, Katenkamp K, Katenkamp D, Berndt A, Petersen I. TLE1 is a robust diagnostic biomarker for synovial sarcomas and correlates with t(X;18): analysis of 319 cases. Eur J Cancer. 2010;46:1170–1176. doi: 10.1016/j.ejca.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 75.Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol. 2009;22:872–878. doi: 10.1038/modpathol.2009.47. [DOI] [PubMed] [Google Scholar]
- 76.Kourea HP, Cordon-Cardo C, Dudas M, Leung D, Woodruff JM. Expression of p27(kip) and other cell cycle regulators in malignant peripheral nerve sheath tumors and neurofibromas: the emerging role of p27(kip) in malignant transformation of neurofibromas. Am J Pathol. 1999;155:1885–1891. doi: 10.1016/S0002-9440(10)65508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kourea HP, Orlow I, Scheithauer BW, Cordon-Cardo C, Woodruff JM. Deletions of the INK4A gene occur in malignant peripheral nerve sheath tumors but not in neurofibromas. Am J Pathol. 1999;155:1855–1860. doi: 10.1016/S0002-9440(10)65504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurtkaya-Yapicier O, Scheithauer BW, Woodruff JM, Wenger DD, Cooley AM, Dominique D. Schwannoma with rhabdomyoblastic differentiation: a unique variant of malignant triton tumor. Am J Surg Pathol. 2003;27:848–853. doi: 10.1097/00000478-200306000-00020. [DOI] [PubMed] [Google Scholar]
- 79.Ladanyi M, Woodruff JM, Scheithauer BW, Bridge JA, Barr FG, Goldblum JR, Fisher C, Perez-Atayde A, Dal Cin P, Fletcher CD, Fletcher JA, Re: O’Sullivan MJ, Kyriakos M, Zhu X, Wick MR, Swanson PE, Dehner LP, Humphrey PA, Pfeifer JD. malignant peripheral nerve sheath tumors with t(X;18). A pathologic and molecular genetic study. Mod pathol. 2001;13:1336–46. doi: 10.1038/modpathol.3880381. 2000. Mod Pathol 14:733-737. [DOI] [PubMed] [Google Scholar]
- 80.Laskin WB, Weiss SW, Bratthauer GL. Epithelioid variant of malignant peripheral nerve sheath tumor (malignant epithelioid schwannoma) Am J Surg Pathol. 1991;15:1136–1145. doi: 10.1097/00000478-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Lasota J, Wasag B, Dansonka-Mieszkowska A, Karcz D, Millward CL, Rys J, Stachura J, Sobin LH, Miettinen M. Evaluation of NF2 and NF1 tumor suppressor genes in distinctive gastrointestinal nerve sheath tumors traditionally diagnosed as benign schwannomas: s study of 20 cases. Lab Invest. 2003;83:1361–1371. doi: 10.1097/01.lab.0000087591.29639.e3. [DOI] [PubMed] [Google Scholar]
- 82.Le LQ, Shipman T, Burns DK, Parada LF. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewin MR, Dilworth HP, Abu Alfa AK, Epstein JI, Montgomery E. Mucosal benign epithelioid nerve sheath tumors. Am J Surg Pathol. 2005;29:1310–1315. doi: 10.1097/01.pas.0000162762.03068.7a. [DOI] [PubMed] [Google Scholar]
- 84.Liegl B, Bennett MW, Fletcher CD. Microcystic/reticular schwannoma: a distinct variant with predilection for visceral locations. Am J Surg Pathol. 2008;32:1080–1087. doi: 10.1097/PAS.0b013e318160cfda. [DOI] [PubMed] [Google Scholar]
- 85.Lin BT, Weiss LM, Medeiros LJ. Neurofibroma and cellular neurofibroma with atypia: a report of 14 tumors. Am J Surg Pathol. 1997;21:1443–1449. doi: 10.1097/00000478-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Lodding P, Kindblom LG, Angervall L. Epithelioid malignant schwannoma. A study of 14 cases. Virchows Arch A Pathol Anat Histopathol. 1986;409:433–451. doi: 10.1007/BF00705415. [DOI] [PubMed] [Google Scholar]
- 87.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer; Lyon: 2007. [Google Scholar]
- 88.Maher CO, Spinner RJ, Giannini C, Scheithauer BW, Crum BA. Neuromuscular choristoma of the sciatic nerve. Case report. J Neurosurg. 2002;96:1123–1126. doi: 10.3171/jns.2002.96.6.1123. [DOI] [PubMed] [Google Scholar]
- 89.Matsuyama A, Hisaoka M, Shimajiri S, Hayashi T, Imamura T, Ishida T, Fukunaga M, Fukuhara T, Minato H, Nakajima T, Yonezawa S, Kuroda M, Yamasaki F, Toyoshima S, Hashimoto H. Molecular detection of FUS-CREB3L2 fusion transcripts in low-grade fibromyxoid sarcoma using formalin-fixed, paraffin-embedded tissue specimens. Am J Surg Pathol. 2006;30:1077–1084. doi: 10.1097/01.pas.0000209830.24230.1f. [DOI] [PubMed] [Google Scholar]
- 90.Mauermann ML, Amrami KK, Kuntz NL, Spinner RJ, Dyck PJ, Bosch EP, Engelstad J, Felmlee JP. Longitudinal study of intraneural perineurioma--a benign, focal hypertrophic neuropathy of youth. Brain. 2009;132:2265–2276. doi: 10.1093/brain/awp169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mauermann ML, Scheithauer BW, Spinner RJ, Amrami KK, Nance CS, Kline DG, O’Connor MI, Dyck PJ, Engelstad J. Inflammatory pseudotumor of nerve: clinicopathological characteristics and a potential therapy. J Peripher Nerv Syst. 2010;15:216–226. doi: 10.1111/j.1529-8027.2010.00273.x. [DOI] [PubMed] [Google Scholar]
- 92.McCarron KF, Goldblum JR. Plexiform neurofibroma with and without associated malignant peripheral nerve sheath tumor: a clinicopathologic and immunohistochemical analysis of 54 cases. Mod Pathol. 1998;11:612–617. [PubMed] [Google Scholar]
- 93.McMenamin ME, Fletcher CD. Expanding the spectrum of malignant change in schwannomas: epithelioid malignant change, epithelioid malignant peripheral nerve sheath tumor, and epithelioid angiosarcoma: a study of 17 cases. Am J Surg Pathol. 2001;25:13–25. doi: 10.1097/00000478-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 94.Meis-Kindblom JM, Enzinger FM. Plexiform malignant peripheral nerve sheath tumor of infancy and childhood. Am J Surg Pathol. 1994;18:479–485. doi: 10.1097/00000478-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Meis JM, Enzinger FM, Martz KL, Neal JA. Malignant peripheral nerve sheath tumors (malignant schwannomas) in children. Am J Surg Pathol. 1992;16:694–707. doi: 10.1097/00000478-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Mennemeyer RP, Hallman KO, Hammar SP, Raisis JE, Tytus JS, Bockus D. Melanotic schwannoma. Clinical and ultrastructural studies of three cases with evidence of intracellular melanin synthesis. Am J Surg Pathol. 1979;3:3–10. doi: 10.1097/00000478-197902000-00001. [DOI] [PubMed] [Google Scholar]
- 97.Menon AG, Anderson KM, Riccardi VM, Chung RY, Whaley JM, Yandell DW, Farmer GE, Freiman RN, Lee JK, Li FP, et al. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990;87:5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michal M, Kazakov DV, Belousova I, Bisceglia M, Zamecnik M, Mukensnabl P. A benign neoplasm with histopathological features of both schwannoma and retiform perineurioma (benign schwannoma-perineurioma): a report of six cases of a distinctive soft tissue tumor with a predilection for the fingers. Virchows Arch. 2004;445:347–353. doi: 10.1007/s00428-004-1102-5. [DOI] [PubMed] [Google Scholar]
- 99.Miettinen M, Saari A. Pheochromocytoma combined with malignant schwannoma: unusual neoplasm of the adrenal medulla. Ultrastruct Pathol. 1988;12:513–527. doi: 10.3109/01913128809032236. [DOI] [PubMed] [Google Scholar]
- 100.Miettinen M, Finnell V, Fetsch JF. Ossifying fibromyxoid tumor of soft parts--a clinicopathologic and immunohistochemical study of 104 cases with long-term follow-up and a critical review of the literature. Am J Surg Pathol. 2008;32:996–1005. doi: 10.1097/PAS.0b013e318160736a. [DOI] [PubMed] [Google Scholar]
- 101.Miller SJ, Jessen WJ, Mehta T, Hardiman A, Sites E, Kaiser S, Jegga AG, Li H, Upadhyaya M, Giovannini M, Muir D, Wallace MR, Lopez E, Serra E, Nielsen GP, Lazaro C, Stemmer-Rachamimov A, Page G, Aronow BJ, Ratner N. Integrative genomic analyses of neurofibromatosis tumours identify SOX9 as a biomarker and survival gene. EMBO Mol Med. 2009;1:236–248. doi: 10.1002/emmm.200900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Min KW, Clemens A, Bell J, Dick H. Malignant peripheral nerve sheath tumor and pheochromocytoma. A composite tumor of the adrenal. Arch Pathol Lab Med. 1988;112:266–270. [PubMed] [Google Scholar]
- 103.Mori T, Orikasa H, Shigematsu T, Yamazaki K. An ultrastructural and immunohistochemical study of a combined submucosal granular cell tumor and lipoma of the colon showing a unique nodule-in-nodule structure: putative implication of CD34 or prominin-2-positive stromal cells in its histopathogenesis. Virchows Arch. 2006;449:137–139. doi: 10.1007/s00428-006-0210-9. [DOI] [PubMed] [Google Scholar]
- 104.Naber U, Friedrich RE, Glatzel M, Mautner VF, Hagel C. Podoplanin and CD34 in peripheral nerve sheath tumours: focus on neurofibromatosis 1-associated atypical neurofibroma. J Neurooncol. 2011;103:239–245. doi: 10.1007/s11060-010-0385-4. [DOI] [PubMed] [Google Scholar]
- 105.Nascimento AF, Fletcher CD. The controversial nosology of benign nerve sheath tumors: neurofilament protein staining demonstrates intratumoral axons in many sporadic schwannomas. Am J Surg Pathol. 2007;31:1363–1370. doi: 10.1097/PAS.0b013e318031bc0c. [DOI] [PubMed] [Google Scholar]
- 106.Nielsen GP, Stemmer-Rachamimov AO, Ino Y, Moller MB, Rosenberg AE, Louis DN. Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am J Pathol. 1999;155:1879–1884. doi: 10.1016/S0002-9440(10)65507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 108.Ogawa K, Oguchi M, Yamabe H, Nakashima Y, Hamashima Y. Distribution of collagen type IV in soft tissue tumors. An immunohistochemical study. Cancer. 1986;58:269–277. doi: 10.1002/1097-0142(19860715)58:2<269::aid-cncr2820580212>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 109.Panagopoulos I, Storlazzi CT, Fletcher CD, Fletcher JA, Nascimento A, Domanski HA, Wejde J, Brosjo O, Rydholm A, Isaksson M, Mandahl N, Mertens F. The chimeric FUS/CREB3l2 gene is specific for low-grade fibromyxoid sarcoma. Genes Chromosomes Cancer. 2004;40:218–228. doi: 10.1002/gcc.20037. [DOI] [PubMed] [Google Scholar]
- 110.Perentes E, Nakagawa Y, Ross GW, Stanton C, Rubinstein LJ. Expression of epithelial membrane antigen in perineurial cells and their derivatives. An immunohistochemical study with multiple markers. Acta Neuropathol. 1987;75:160–165. doi: 10.1007/BF00687077. [DOI] [PubMed] [Google Scholar]
- 111.Perry A, Roth KA, Banerjee R, Fuller CE, Gutmann DH. NF1 deletions in S-100 protein-positive and negative cells of sporadic and neurofibromatosis 1 (NF1)-associated plexiform neurofibromas and malignant peripheral nerve sheath tumors. Am J Pathol. 2001;159:57–61. doi: 10.1016/S0002-9440(10)61673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perry A, Kunz SN, Fuller CE, Banerjee R, Marley EF, Liapis H, Watson MA, Gutmann DH. Differential NF1, p16, and EGFR patterns by interphase cytogenetics (FISH) in malignant peripheral nerve sheath tumor (MPNST) and morphologically similar spindle cell neoplasms. J Neuropathol Exp Neurol. 2002;61:702–709. doi: 10.1093/jnen/61.8.702. [DOI] [PubMed] [Google Scholar]
- 113.Plaza JA, Wakely PE, Jr., Suster S. Lipoblastic nerve sheath tumors: report of a distinctive variant of neural soft tissue neoplasm with adipocytic differentiation. Am J Surg Pathol. 2006;30:337–344. doi: 10.1097/01.pas.0000194297.81721.1c. [DOI] [PubMed] [Google Scholar]
- 114.Ricci A, Jr., Parham DM, Woodruff JM, Callihan T, Green A, Erlandson RA. Malignant peripheral nerve sheath tumors arising from ganglioneuromas. Am J Surg Pathol. 1984;8:19–29. doi: 10.1097/00000478-198401000-00002. [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez FJ, Scheithauer BW, Abell-Aleff PC, Elamin E, Erlandson RA. Low grade malignant peripheral nerve sheath tumor with smooth muscle differentiation. Acta Neuropathol. 2007;113:705–709. doi: 10.1007/s00401-006-0171-8. [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez FJ, Erickson-Johnson MR, Scheithauer BW, Spinner RJ, Oliveira AM. HMGA2 rearrangements are rare in benign lipomatous lesions of the nervous system. Acta Neuropathol. 2008;116:337–338. doi: 10.1007/s00401-008-0412-0. [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez FJ, Stratakis CA, Evans DG. Genetic predisposition to peripheral nerve neoplasia: Diagnostic criteria and pathogenesis of neurofibromatoses, Carney complex, and related syndromes. Acta Neuropathol. 2012 doi: 10.1007/s00401-011-0935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rose DS, Wilkins MJ, Birch R, Evans DJ. Malignant peripheral nerve sheath tumour with rhabdomyoblastic and glandular differentiation: immunohistochemical features. Histopathology. 1992;21:287–290. doi: 10.1111/j.1365-2559.1992.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 119.Sangala JR, Park P, Blaivas M, Lamarca F. Paraspinal malignant ossifying fibromyxoid tumor with spinal involvement. J Clin Neurosci. 2010;17:1592–1594. doi: 10.1016/j.jocn.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 120.Scheithauer BW, Woodruff JM, Erlandson RA. Tumors of the Peripheral Nervous System. Armed Forces Institute of Pathology; Washington D.C.: 1997. [Google Scholar]
- 121.Scheithauer BW, Rodriguez FJ, Spinner RJ, Dyck PJ, Salem A, Edelman FL, Amrami KK, Fu YS. Glomus tumor and glomangioma of the nerve. Report of two cases. J Neurosurg. 2008;108:348–356. doi: 10.3171/JNS/2008/108/2/0348. [DOI] [PubMed] [Google Scholar]
- 122.Scheithauer BW, Erdogan S, Rodriguez FJ, Burger PC, Woodruff JM, Kros JM, Gokden M, Spinner RJ. Malignant peripheral nerve sheath tumors of cranial nerves and intracranial contents: a clinicopathologic study of 17 cases. Am J Surg Pathol. 2009;33:325–338. doi: 10.1097/PAS.0b013e31818d6470. [DOI] [PubMed] [Google Scholar]
- 123.Scheithauer BW, Amrami KK, Folpe AL, Silva AI, Edgar MA, Woodruff JM, Levi AD, Spinner RJ. Synovial sarcoma of nerve. Hum Pathol. 2011;42:568–577. doi: 10.1016/j.humpath.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 124.Shelekhova KV, Danilova AB, Michal M, Kazakov DV. Hybrid neurofibroma-perineurioma: an additional example of an extradigital tumor. Ann Diagn Pathol. 2008;12:233–234. doi: 10.1016/j.anndiagpath.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 125.Shiurba RA, Eng LF, Urich H. The structure of pseudomeissnerian corpuscles. An immunohistochemical study. Acta Neuropathol. 1984;63:174–176. doi: 10.1007/BF00697200. [DOI] [PubMed] [Google Scholar]
- 126.Slomiany MG, Dai L, Bomar PA, Knackstedt TJ, Kranc DA, Tolliver L, Maria BL, Toole BP. Abrogating drug resistance in malignant peripheral nerve sheath tumors by disrupting hyaluronan-CD44 interactions with small hyaluronan oligosaccharides. Cancer Res. 2009;69:4992–4998. doi: 10.1158/0008-5472.CAN-09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith TW, Bhawan J. Tactile-like structures in neurofibromas. An ultrastructural study. Acta Neuropathol. 1980;50:233–236. doi: 10.1007/BF00688760. [DOI] [PubMed] [Google Scholar]
- 128.Spinner RJ, Scheithauer BW, Perry A, Amrami KK, Emnett R, Gutmann DH. Colocalized cellular schwannoma and plexiform neurofibroma in the absence of neurofibromatosis. Case report. J Neurosurg. 2007;107:435–439. doi: 10.3171/JNS-07/08/0435. [DOI] [PubMed] [Google Scholar]
- 129.Spinner RJ, Scheithauer BW, Amrami KK, Wenger DE, Hebert-Blouin MN. Adipose lesions of nerve: the need for a modified classification. J Neurosurg. 2011 doi: 10.3171/2011.8.JNS101292. [DOI] [PubMed] [Google Scholar]
- 130.Swanson PE, Stanley MW, Scheithauer BW, Wick MR. Primary cutaneous leiomyosarcoma. A histological and immunohistochemical study of 9 cases, with ultrastructural correlation. J Cutan Pathol. 1988;15:129–141. doi: 10.1111/j.1600-0560.1988.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 131.Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, Downs-Kelly E, Corless CL, Rubin BP, van de Rijn M, Ladanyi M, Nielsen TO. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007;31:240–246. doi: 10.1097/01.pas.0000213330.71745.39. [DOI] [PubMed] [Google Scholar]
- 132.Tozbikian G, Shen R, Suster S. Signet ring cell gastric schwannoma: report of a new distinctive morphological variant. Ann Diagn Pathol. 2008;12:146–152. doi: 10.1016/j.anndiagpath.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 133.Trassard M, Le Doussal V, Bui BN, Coindre JM. Angiosarcoma arising in a solitary schwannoma (neurilemoma) of the sciatic nerve. Am J Surg Pathol. 1996;20:1412–1417. doi: 10.1097/00000478-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 134.Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 135.Tsang WY, Chan JK, Chow LT, Tse CC. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756–763. doi: 10.1097/00000478-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 136.Turc-Carel C, Dal Cin P, Limon J, Rao U, Li FP, Corson JM, Zimmerman R, Parry DM, Cowan JM, Sandberg AA. Involvement of chromosome X in primary cytogenetic change in human neoplasia: nonrandom translocation in synovial sarcoma. Proc Natl Acad Sci U S A. 1987;84:1981–1985. doi: 10.1073/pnas.84.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2011 doi: 10.1016/j.humpath.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Watabe K, Kumanishi T, Ikuta F, Oyake Y. Tactile-like corpuscles in neurofibromas: immunohistochemical demonstration of S-100 protein. Acta Neuropathol. 1983;61:173–177. doi: 10.1007/BF00691982. [DOI] [PubMed] [Google Scholar]
- 139.Weinreb I, Bray P, Ghazarian D. Plexiform intraneural granular cell tumour of a digital cutaneous sensory nerve. J Clin Pathol. 2007;60:725–726. doi: 10.1136/jcp.2005.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weiss SW, Langloss JM, Enzinger FM. Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest. 1983;49:299–308. [PubMed] [Google Scholar]
- 141.Weiss SW, Nickoloff BJ. CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am J Surg Pathol. 1993;17:1039–1045. doi: 10.1097/00000478-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 142.Weiss SW, Goldblum JR, editors. Enzinger & Weiss’s Soft Tissue Tumors. 5th edn. Mosby Elsevier; 2007. [Google Scholar]
- 143.West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R, Goldblum JR, Brown PO, Heinrich MC, van de Rijn M. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.White W, Shiu MH, Rosenblum MK, Erlandson RA, Woodruff JM. Cellular schwannoma. A clinicopathologic study of 57 patients and 58 tumors. Cancer. 1990;66:1266–1275. doi: 10.1002/1097-0142(19900915)66:6<1266::aid-cncr2820660628>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 145.Wick MR, Swanson PE, Scheithauer BW, Manivel JC. Malignant peripheral nerve sheath tumor. An immunohistochemical study of 62 cases. Am J Clin Pathol. 1987;87:425–433. doi: 10.1093/ajcp/87.4.425. [DOI] [PubMed] [Google Scholar]
- 146.Woodruff JM, Marshall ML, Godwin TA, Funkhouser JW, Thompson NJ, Erlandson RA. Plexiform (multinodular) schwannoma. A tumor simulating the plexiform neurofibroma. Am J Surg Pathol. 1983;7:691–697. doi: 10.1097/00000478-198310000-00009. [DOI] [PubMed] [Google Scholar]
- 147.Woodruff JM, Christensen WN. Glandular peripheral nerve sheath tumors. Cancer. 1993;72:3618–3628. doi: 10.1002/1097-0142(19931215)72:12<3618::aid-cncr2820721212>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 148.Woodruff JM, Perino G. Non-germ-cell or teratomatous malignant tumors showing additional rhabdomyoblastic differentiation, with emphasis on the malignant Triton tumor. Semin Diagn Pathol. 1994;11:69–81. [PubMed] [Google Scholar]
- 149.Woodruff JM, Selig AM, Crowley K, Allen PW. Schwannoma (neurilemoma) with malignant transformation. A rare, distinctive peripheral nerve tumor. Am J Surg Pathol. 1994;18:882–895. doi: 10.1097/00000478-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 150.Woodruff JM. Pathology of tumors of the peripheral nerve sheath in type 1 neurofibromatosis. Am J Med Genet. 1999;89:23–30. doi: 10.1002/(sici)1096-8628(19990326)89:1<23::aid-ajmg6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 151.Woodruff JM, Scheithauer BW, Kurtkaya-Yapicier O, Raffel C, Amr SS, LaQuaglia MP, Antonescu CR. Congenital and childhood plexiform (multinodular) cellular schwannoma: a troublesome mimic of malignant peripheral nerve sheath tumor. Am J Surg Pathol. 2003;27:1321–1329. doi: 10.1097/00000478-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 152.Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D, Stemmer-Rachamimov AO, Cancelas JA, Ratner N. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625–630. doi: 10.1111/j.1600-0560.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- 154.Yu J, Deshmukh H, Payton JE, Dunham C, Scheithauer BW, Tihan T, Prayson RA, Guha A, Bridge JA, Ferner RE, Lindberg GM, Gutmann RJ, Emnett RJ, Salavaggione L, Gutmann DH, Nagarajan R, Watson MA, Perry A. Array-based comparative genomic hybridization identifies CDK4 and FOXM1 alterations as independent predictors of survival in malignant peripheral nerve sheath tumor. Clin Cancer Res. 2011;17:1924–1934. doi: 10.1158/1078-0432.CCR-10-1551. [DOI] [PubMed] [Google Scholar]
- 155.Zamecnik M, Mukensnabl P, Sokol L, Michal M. Perineurial cells and nerve axons in gastrointestinal schwannomas: a similarity with neurofibromas. An immunohistochemical study of eight cases. Cesk Patol. 2004;40:150–153. [PubMed] [Google Scholar]
- 156.Zheng H, Chang L, Patel N, Yang J, Lowe L, Burns DK, Zhu Y. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 157.Zhou H, Coffin CM, Perkins SL, Tripp SR, Liew M, Viskochil DH. Malignant peripheral nerve sheath tumor: a comparison of grade, immunophenotype, and cell cycle/growth activation marker expression in sporadic and neurofibromatosis 1-related lesions. Am J Surg Pathol. 2003;27:1337–1345. doi: 10.1097/00000478-200310000-00006. [DOI] [PubMed] [Google Scholar]