Summary
A crucial early wound response is the recruitment of inflammatory cells drawn by danger cues released by the damaged tissue. Hydrogen peroxide (H2O2) has recently been identified as the earliest wound attractant in Drosophila embryos and zebrafish larvae [1, 2]. The H2O2 signal is generated by activation of an NADPH oxidase, DUOX, and as a consequence, the first inflammatory cells are recruited to the wound within minutes. To date, nothing is known about how wounding activates DUOX. Here, we show that laser wounding of the Drosophila embryo epidermis triggers an instantaneous calcium flash, which travels as a wave via gap junctions several cell rows back from the wound edge. Blocking this calcium flash inhibits H2O2 release at the wound site and leads to a reduction in the number of immune cells migrating to the wound. We suggest that the wound-induced calcium flash activates DUOX via an EF hand calcium-binding motif and thus triggers the production of the attractant damage cue H2O2. Therefore, calcium represents the earliest signal in the wound inflammatory response.
Highlights
► Wounding the Drosophila embryo epidermis leads to a rapid intracellular Ca2+ wave ► Blocking the Ca2+ response leads to reduced H2O2 and hemocytes at wounds ► DUOX’s EF hand domain is indispensible to interpret wound-induced Ca2+ flashes
Results and Discussion
Wounding the Drosophila Embryo Epidermis Results in an Immediate Calcium Wave
Drosophila embryos are able to heal laser-induced epithelial wounds [3] and in parallel mount a robust inflammatory response with the rapid recruitment of embryonic macrophages, called hemocytes [4, 5]. In Drosophila embryos and zebrafish larvae, hydrogen peroxide (H2O2) synthesized by the NADPH oxidase (NOX) enzyme DUOX appears to be pivotal in the stimulation of this inflammatory response [1, 2], but precisely how DUOX is activated by tissue damage remains unknown. However, DUOX possesses two canonical EF hands on an intracellular loop, which implicates cytosolic calcium as a potential regulator of H2O2 production.
To test for calcium signals in cells at the wound edge—signals similar to those observed in wounded epithelial cells in vitro [6–10], the C. elegans embryo epidermis, [11] or zebrafish larval tissues [12, 13]—we expressed the intracellular calcium reporter GCaMP3 [14] specifically in the epidermis via the GAL4-UAS system [15] by using the e22c-Gal4 driver [16]. We coexpressed mCherry-moesin to visualize cortical actin in epithelial cells.
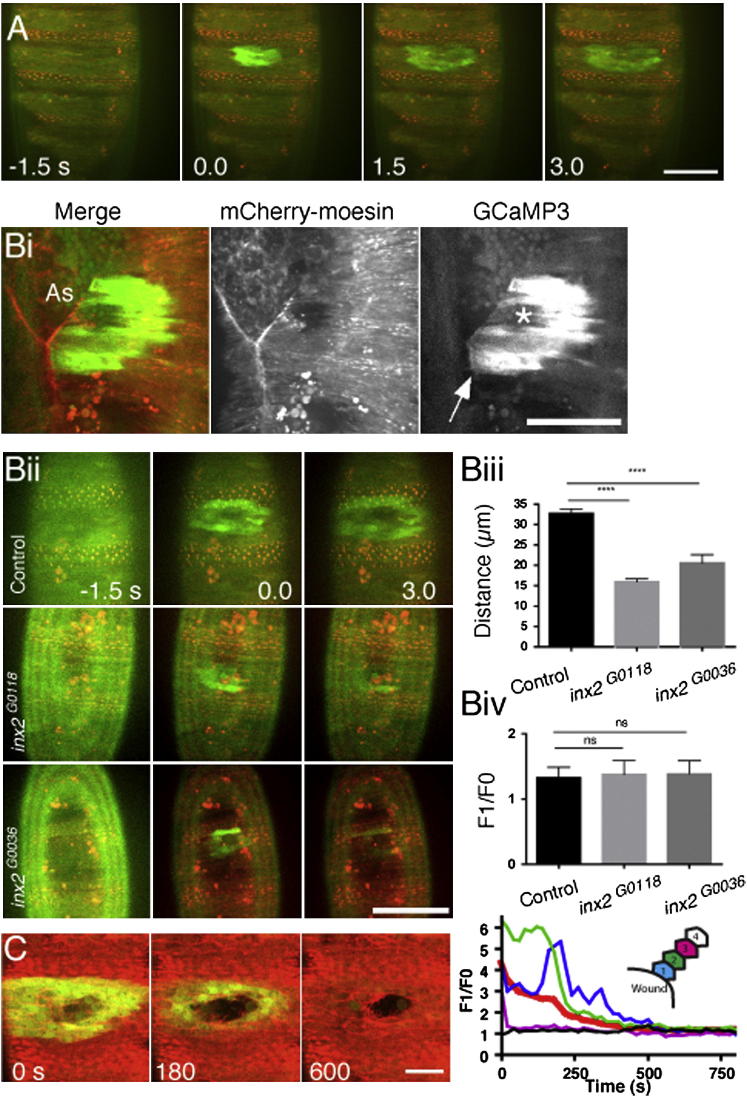
The ventral epithelium of stage 15 embryos was laser wounded and imaged by spinning-disk microscopy. Prior to wounding, GCaMP3 fluorescence, and hence cytosolic calcium levels, did not alter in the epithelial cells; however, laser wounding resulted in a rapid calcium flash extending outward from the point of wounding as a wave to an average maximum distance of 39 ± 4.8 μm from the wound margin (SD, n = 6 movies) at a speed of 6.9 ± 2.5 μm/s (SD, n = 7 movies) across multiple cells (Figure 1A and Movie S1, available online). Ventral epithelial cells are elongated along the dorsoventral axis such that the calcium wave is propagated in a stereotypical ellipsoidal shape. Closer inspection revealed the wave to be traveling through individual cells before activating calcium release in neighboring cells (Figure S1Ai). Plotting the intensity of the GCaMP3 fluorescence in cells at varying distances from the wound edge over time showed that each cell was consecutively activated but that calcium response levels were reduced stepwise in successive rows of cells as cell distance from the wound edge increased (Figure S1Aii) until a threshold such that the wave could not travel further. This threshold was not absolute, given that larger wounds activated coordinately larger calcium waves (Figures S1B–S1D).
Figure 1.
Wounding of Drosophila Embryos Induces an Immediate Epidermal Calcium Wave
(A) mCherry-moesin (red) and GCaMP3 (green) fluorescence before and after wounding of the Drosophila embryonic epidermis revealed a calcium wave rapidly spreading outward from the wound margin. The scale bar denotes 50 μm, and time is in seconds. See also Movie S1.
(B) In (Bi), a wound made on the dorsal epidermis in an embryo coexpressing GCaMP3 and mCherry-moesin and adjacent to the zipper front of dorsal closure shows no spread of the calcium wave from dorsal epithelium onto amnioserosa (As) or across the seam (the wound position is marked by a white star; the arrow indicates the zippering front where the calcium wave terminates). In (Bii) are stills from movies of wound-induced calcium waves in control and inx2G0118 and inx2G0036 mutant embryos; time is in seconds. Graphs in (Biii) and (Biv) reveal that the distance traveled by calcium waves, but not the initial calcium intensity (F/F0) at the wound edge, was significantly lower in inx2 mutants than in control embryos. (A one-way ANOVA was used with a Bonferroni posttest; n ≥ 18 embryos per genotype). Scale bars in (Bi) and (Bii) denote 50 μm. Error bars in (Biii) and (Biv) represent the SEM. ∗∗∗∗p < 0.0001; ns = nonsignificant.
(C) Resolution of the calcium wave is revealed by GCaMP3-expressing embryos (see also Movie S2). The plot shows fluorescence-intensity change normalized to background fluorescence (F1/F0) for all cells (thick red line) and cells categorized according to their position relative to the wound edge (see schematic inset for the position of cells). The scale bar denotes 20 μm, and time is in seconds.
Previous scratch-wound analyses in cultured cells loaded with calcium reporter dyes showed that similar calcium waves depend upon extracellular diffusible mediators [8, 17]. However, in vivo, the spread of calcium is unlikely to be propagated in this manner, given that wounding the epidermis adjacent to the zippering seam of the dorsal embryonic hole, where cells are yet to be junctionally linked, resulted in immediate termination of the wave (Figure 1Bi), suggesting that cells must be intimately connected for transfer of the calcium wave. Gap junctions allow calcium waves to spread via diffusion of IP3 from cell to cell [18]. Drosophila gap junctions are thought to be composed of innexins, which are analogous to vertebrate connexins; therefore, to determine whether gap junctions are important for the wound-induced calcium wave to spread, we used two innexin 2 (Inx2)-null alleles, inx2G0018 and inx2G0036 [19], because Inx2 is highly expressed in the embryonic epidermis [20]. Compared to the controls, both alleles showed a significantly reduced spread of the calcium wave (Figures 1Bii and 1Biii), and the calcium influx was often restricted to the front row of cells at the wound edge. These alleles, however, did not affect the intensity of the initial calcium flash (Figure 1Biv), suggesting that innexins allow the calcium wave to propagate or maintain the calcium signal as it spreads but are not involved in its initiation.
After the rapid elevation of intracellular calcium, this signal subsequently decayed to background levels of GCaMP3 fluorescence after approximately 15 min (830 ± 360 s, n = 9 wounds; Figure 1C and Movie S2), many minutes prior to the completion of wound closure. Resolution commences in a distal-to-proximal direction, and wound margin cells return to basal levels of calcium last of all (Figure 1C and Movie S2). During the resolution period, around 40% of cells within the calcium flash zone exhibited calcium oscillations (approximately 30% oscillated only once), and no cell underwent greater than three oscillations. Cells rapidly reset their calcium machinery because subsequent wounding (within 20 min of the original wound) again elicited an identical calcium response (Figure S1E).
Reducing the Calcium Flash Impairs the Inflammatory Response of Hemocytes
This calcium flash represents the earliest identified signal following tissue damage and might therefore orchestrate the rapid recruitment of immune cells. To assess the impact of a reduced calcium flash upon the inflammatory response, we monitored the numbers of hemocytes drawn to laser-induced epithelial wounds under a range of pharmacological and genetic treatments.
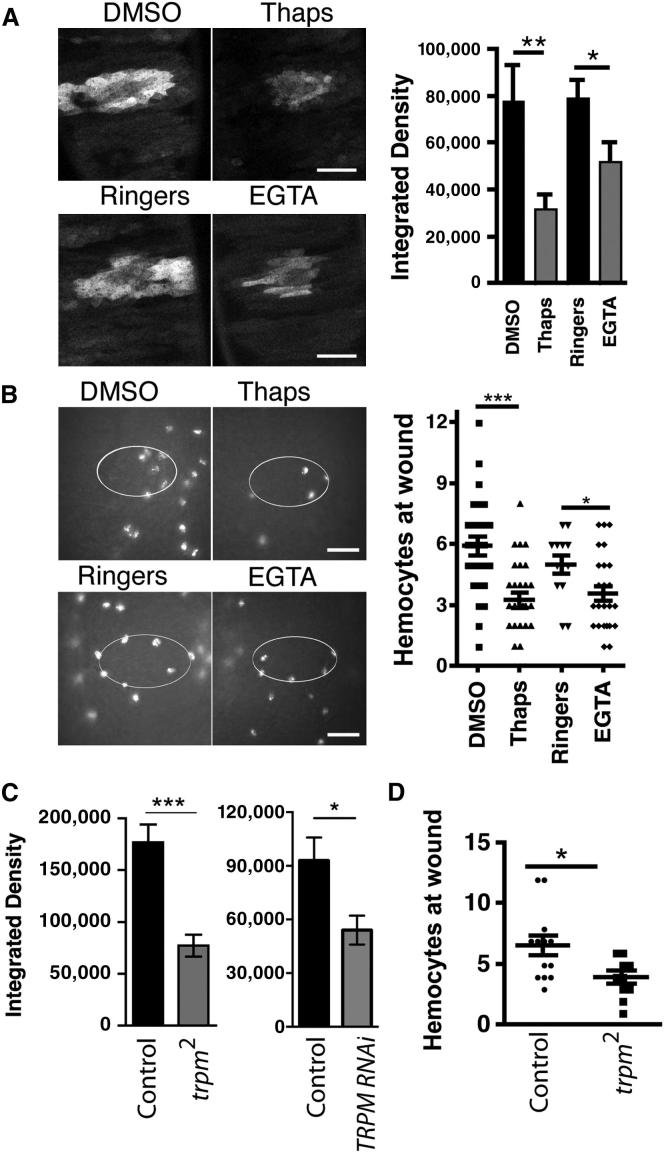
Treatment with 1 μM thapsigargin or 5 mM EGTA to deplete internal stores of calcium or extracellular calcium (present in the vitelline fluid surrounding the embryo at 5 mM [21]), respectively, both significantly decreased the calcium flash (as assessed by integrated density of the calcium flash zone) (Figure 2A and Figure S2A). crq-Gal4,UAS-red stinger transgenic embryos (which allowed us to clearly see the nuclei of hemocytes for accurate counting) were wounded after the same calcium-blocking pharmacological treatments, and the number of hemocytes present at ventral epidermal wounds was assessed after 20 min. Thapsigargin or EGTA treatments reduced the average hemocyte response to 55% or 72%, respectively, of that of negative controls (Figure 2B). Live imaging revealed no difference in the basal developmental migration of hemocytes posttreatment (Figure S2B), suggesting that the reduced recruitment was due to a failure by hemocytes to detect wounds rather than a general migration defect.
Figure 2.
Wound-Induced Calcium Waves Activate the Inflammatory Response
(A) Representative images of epithelial GCaMP3 fluorescence immediately after wounding in embryos treated with 1 μm thapsigargin or 5 mM EGTA reveal that interfering with calcium signaling impaired wound-induced calcium waves. The bar graph shows the mean integrated density of the GCaMP3 signal per embryo (n ≥ 6 embryos per treatment). The scale bars depict 25 μm.
(B) Dampening calcium responses reduced recruitment of red-stinger-labeled hemocytes to wounds (wound edges are indicated by white ellipses). The scatter plot shows quantification of hemocytes per wound (the lines show the mean for ≥13 embryos per treatment). The scale bars depict 20 μm.
(C) The mean integrated density of GCaMP3 fluorescence per embryo immediately after wounding of trpm2 mutants or embryos expressing TRPM RNAi specifically in the epithelium is lower than that for wild-type controls, indicating an epithelial role for TRPM in the generation of a calcium wave (n > 12 embryos per genotype).
(D) Impairment of the calcium wave correlated with a decrease in mean numbers of red-stinger-labeled hemocytes recruited to wounds in trpm2 mutants (n ≥ 10 embryos per genotype).
All error bars represent the SEM, and asterisks denote significance values of p < 0.05 (∗), p < 0.01 (∗∗), and p < 0.001 (∗∗∗) via a Student’s t test.
Similarly, loss-of-function mutant embryos of the TRPM channel (trpm2), whose C. elegans ortholog is important in wound-induced calcium responses [11] and is expressed across epithelial tissues in the embryo [22], displayed both a reduced calcium response after epidermal wounding (Figure 2C and Figure S2C) and reduced hemocyte migration to wounds (Figure 2D). Importantly, loss of TRPM did not perturb basal hemocyte migration or their developmental dispersal (Figure S2D). Significantly, epithelial-specific RNAi-mediated knockdown of TRPM also led to reduction in the calcium wave after wounding (Figure 2C), implicating this channel in the initial influx of calcium into and/or release of calcium from internal stores in epithelial cells.
Calcium Activates DUOX through Its EF Hand at Wound Sites to Direct H2O2 Generation and Attract Hemocytes to Wounds
H2O2 has previously been shown to function as a wound chemoattractant for immune cells in Drosophila and zebrafish [1, 2] and is generated from superoxide by DUOX, a NOX enzyme [23]. More recently, DUOX has been shown to act in concert with calcium and Src family kinases to drive epithelial aspects of the regenerative processes in zebrafish larvae [13].
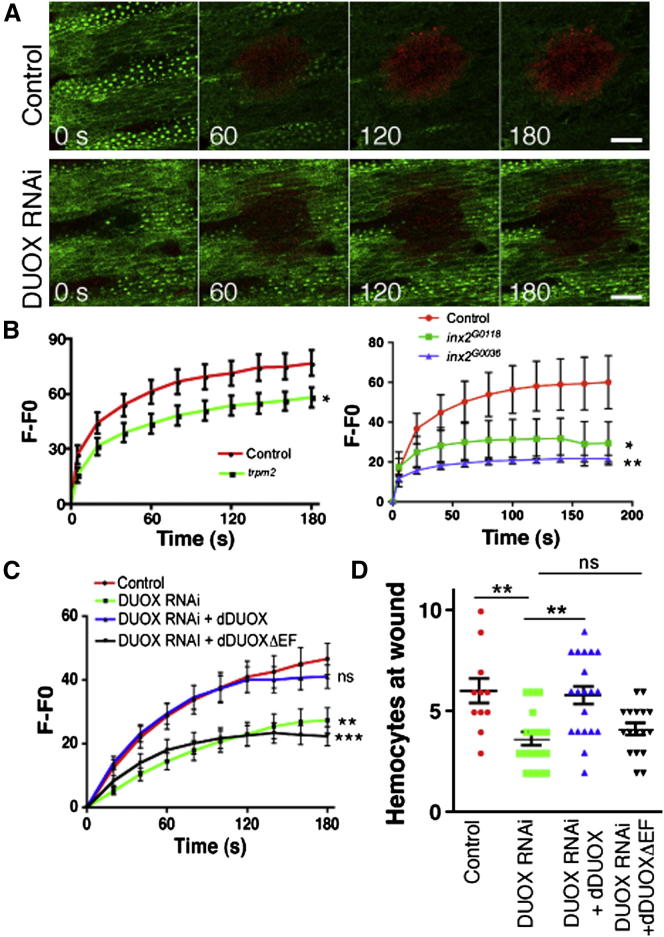
DUOX contains a calcium-binding EF hand domain, which enables calcium to regulate the synthesis of H2O2 [24] and is therefore a good candidate for an effector of the calcium flash. H2O2 production following wounding can be visualized live by the incubation of da-Gal4,UAS-GMA (GFP-tagged actin-binding domain of moesin [25]) embryos in Amplex Ultrared, a fluorigenic reporter that is converted to its fluorescent form specifically by H2O2 in the presence of peroxidase [26] or in fixed tissue by immunospin trapping [27] (Figure S3A). Live imaging of converted Amplex Ultrared revealed a rapid accumulation of H2O2 at the wound site (Figure 3A); this accumulation was similar to that previously seen with other reporters in the Drosophila embryo. As previously described, H2O2 levels appeared to peak at the wound site after about 3 min [1], i.e., shortly after the calcium flash. Coexpressing a previously published DUOX RNAi [28] with da-Gal4,UAS-GMA showed a large reduction in Amplex Ultrared fluorescence at the wound site, confirming DUOX’s role in generating the H2O2 signal (Figure 3A). H2O2 production could not be completely abolished after DUOX RNAi expression. This could be due to either an incomplete knockdown of DUOX or the operation of alternative pathways to synthesize H2O2 (such as via other NOXs or as a consequence of cellular damage itself). As expected, expression of DUOX RNAi did not affect the calcium response (Figure S3B).
Figure 3.
DUOX Interprets the Calcium Wave via Its EF Hand Domain to Drive H2O2 Production and Recruitment of Macrophages
(A) Single confocal slices depicting GMA (actin, green) and Amplex Ultrared (H2O2, red) show that embryos ubiquitously expressing DUOX RNAi and GMA produced less H2O2 at wounds than did wild-type controls, as assayed via Amplex Ultrared fluorescence. The scale bars represent 20 μm.
(B) trpm2 and inx2 mutant embryos with a reduction in their wound-induced calcium responses displayed impaired H2O2 production via Amplex Ultrared (see Experimental Procedures for details of F-F0 quantification). The graphs show mean ± SEM of at least 20 (trpm2) and 7 (Inx2) embryos per genotype.
(C and D) Re-expression of full-length dDUOX, but not a truncated form specifically lacking the EF hand motif (dDUOXΔEF), in embryos with ubiquitous expression of dDUOX RNAi restored wound-induced H2O2 production (C) and hemocyte recruitment (D) to the levels of wild-type controls. The graphs show mean ± SEM of at least 21 (C) and 11 (D) embryos per genotype.
See Movie S3 for a typical example of this data. Hemocyte numbers at wounds were determined from images of hemocytes labeled independently of Gal4 with srp-GMA. The p values were generated with a Student’s t test at the final time point (B [trpm2]), a two-way ANOVA with a Bonferroni posttest (B [inx2] and C), or a one-way ANOVA with a Bonferroni posttest (D). Asterisks denote p < 0.05 (∗), p < 0.01 (∗∗), and p < 0.001 (∗∗∗); ns = not significant.
We then asked whether H2O2 production at wounds could be reduced by blockage of the calcium wave with the use of trpm2, inx2G0118, or inx2G0036 mutant embryos. Compared to controls, all genotypes exhibited a reduction in Amplex Ultrared signals at wound sites (Figure 3B and Movie S3), demonstrating the importance of the calcium flash in generating this potent inflammatory signal.
To test whether H2O2 production via calcium could occur in the absence of wounding, we expressed a temperature-sensitive TRPA channel by using the Gal4/UAS system. This channel opens at 37°C and induces calcium influx into cells [29], which we confirmed by coexpressing TRPA and GCaMP3 in the epidermis (Figure S3C). Treatment of TRPA-expressing embryos for 30 min at 37°C increased the levels of H2O2, as assayed via Amplex Ultrared fluorescence, suggesting that calcium influx is sufficient for the production of H2O2 in the epithelium (Figure S3D). Furthermore, overexpression of TRPA in spiracle bottle cells was sufficient to stimulate hemocyte recruitment to these sites when embryos were shifted to 37°C (Figure S3E).
To directly assess the role of calcium in the activation of DUOX, we knocked down DUOX by using RNAi and then coexpressed either full-length DUOX or a DUOX mutant lacking the calcium-binding EF hands (DUOXΔEF) [28] and monitored H2O2 production via Amplex Ultrared imaging. We were able to rescue H2O2 production in RNAi-treated embryos with full-length DUOX, but not with DUOXΔEF (Figure 3C). Furthermore, there was no difference in the numbers of hemocytes recruited to wounds in DUOX RNAi and DUOXΔEF embryos, whereas full-length DUOX restored the hemocyte response to normal levels (Figure 3D). Immunostaining and western blots using a previously generated DUOX antibody [30] revealed a knockdown of DUOX, whereas neither transgenic form of DUOX was degraded and both displayed wild-type localization within cells (Figure S3F). Taken together, these results suggest that the calcium-binding EF hands of DUOX function as a fundamental link to couple wound-induced calcium signals to the activation of DUOX and to subsequent H2O2-mediated attraction of hemocytes to wounds.
DUOX Activity at the Wound Site Is Dependent on a DUOX Maturation Factor, NIP
In mammalian cells, the maturation factor DUOXA2/NIP is necessary for DUOX2’s folding and translocation to the plasma membrane [31]. The Drosophila ortholog nip/DUOXA/mol is expressed throughout embryogenesis and localizes to the plasma membrane during cellularization [32]. Interestingly, expression of an RNAi targeting NIP transcripts developed fragile wings highly reminiscent of those observed from overexpression of DUOX RNAi in the wing [30].
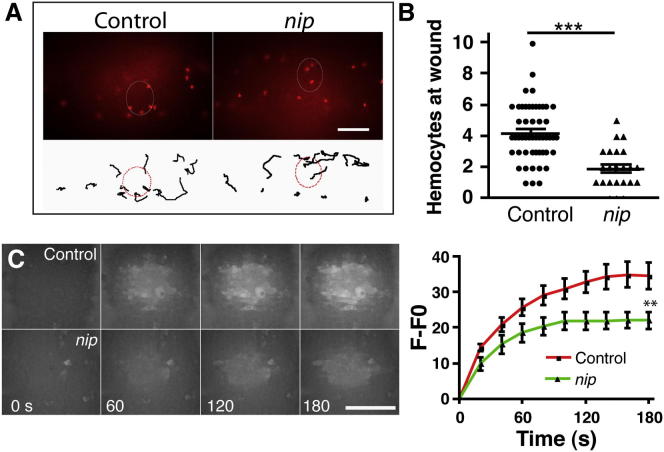
To determine whether production of the wound cue is similarly dependent upon NIP, we analyzed the inflammatory response in a previously characterized nip-null mutant [32]. In nip mutant embryos, hemocytes migrated to their appropriate developmental locations at stage 15 and lay along the midline and lateral lines of the ventral nerve cord (Figure S4A). Furthermore, hemocytes exhibited the same levels of motility as wild-type controls (Figure S4B), suggesting that they were not intrinsically affected by loss of NIP. However, live imaging revealed that hemocytes in nip mutant embryos often ignored laser-induced epithelial wounds (Figures 4A and 4B) such that 20 min after wounding, there were many fewer hemocytes at wounds in nip mutants than in wild-type embryos (Figures 4A and 4B). To test how the loss of nip affects DUOX function, we imaged H2O2 production in nip mutant embryos by using the Amplex Ultrared assay. These mutants showed a reduced H2O2 response (Figure 4C) but a normal calcium flash on wounding (Figure S4C), suggesting that NIP is critical for the wound-associated H2O2 signal. Again, we could not completely block the H2O2 signal, suggesting a contribution from maternal protein or that there are additional alternative pathways at play in the generation of H2O2 at wounds.
Figure 4.
NIP Is Required for DUOX Maturation and Activation after Wounding
(A) Tracking red-stinger-labeled hemocytes for 20 min after wounding revealed that fewer hemocytes migrated to wounds (indicated by white ellipses; scale bar shows 40 μm) in nip mutant embryos than in controls. The upper panel shows the final frame of a hemocyte movie at 20 min; the lower panel displays hemocyte tracks from the movies above and indicates that hemocytes tend to ignore wounds in nip mutant embryos and instead remain in their developmental positions. The wound is highlighted by the red dashed line (representative of at least three movies per genotype).
(B) Quantification of mean hemocyte numbers at wounds after 20 min in wild-type versus nip mutant embryos; n ≥ 24 embryos per genotype. Error bars show the SEM; asterisks denote p < 0.001 (∗∗∗) and p < 0.01 (∗∗), generated via a Student’s t test.
(C) Single confocal slice images of Amplex Ultrared at wounds and quantification of mean levels of fluorescence demonstrate compromised H2O2 production in nip mutant embryos compared to wild-type controls (see Experimental Procedures for details of quantification; n ≥ 11 embryos per genotype; scale bar shows 50 μm; time is in seconds). Error bars show the SEM; asterisks denote p < 0.001 (∗∗∗) and p < 0.01 (∗∗), generated via a two-way ANOVA with a Bonferroni posttest.
The importance of DUOX has been previously implicated in the synthesis of the H2O2 signal that draws immune cells to wounds [1, 2]. However, how DUOX is activated upon wounding was previously unknown. We have shown that a calcium wave, induced upon wounding the Drosophila embryo epidermis, leads to activation of DUOX via its canonical EF hands and the subsequent production of H2O2. Blocking wound-induced calcium flashes causes hemocytes to fail to detect wounds. We therefore believe that calcium represents the earliest key event in activation of the inflammatory response.
Experimental Procedures
Fly Lines and Genetics
Flies were raised and embryos were imaged at room temperature. Embryos for experiments involving RNA interference were raised at 29°C. Genotypes used are outlined in the Supplemental Experimental Procedures and Table S1.
Imaging and Wounding
Embryos collected from overnight apple juice agar plates were dechorionated in bleach and mounted ventral side up (they were mounted dorsal side up when they were wounded near the dorsal hole) on Greiner Lumox gas-permeable culture dishes (Sigma) in halocarbon oil 700 (Sigma) or glass slides with double-sided sticky tape with voltalef oil (VWR International); see Evans et al. [33] for a video protocol. Calcium and Amplex Ultrared (Invitrogen) imaging was performed on a Zeiss LSM510 confocal microscope or a Leica DMI6000B Ultraview Vox spinning-disk system (Perkin Elmer) fitted with Micropoint nitrogen ablation lasers (Andor) for wounding; red-stinger-labeled hemocytes were imaged on a Zeiss Axioplan 2 widefield imaging system and were again wounded with a nitrogen ablation laser (Spectra-Physics). Cell tracking (manual tracking and chemotaxis plugins) and image quantification were performed with ImageJ. Prism for Mac (Graph Pad) was used for statistical analysis. For detailed description of image analyses, see the Supplemental Experimental Procedures.
Drug and Amplex Ultrared Treatments
Dechorionated stage 15 embryos were incubated in a mixture of 1:1 heptane:drug (or Amplex Ultrared) solution in a glass vial for 30 min on a shaker. For TRPA overexpression experiments, embryos were incubated at 37°C in water prior to further treatments. Drug or Amplex Ultrared solutions consisted of Drosophila Ringers solution, composed of 128 mM NaCl (Fisher Scientific), 2 mM KCl (Sigma), 35.5 mM sucrose (Fisher Scientific), 5 mM HEPES (Sigma), and 4 mM MgCl2 (Fisher Scientific), as well as 5 mM EGTA (Sigma), 1 μM thapsigargin (1 mM stock dissolved in DMSO; Sigma), or 50 μM Amplex Ultrared. DMSO (Sigma) was added to Ringers at a 1:1,000 dilution as a negative control for thapsigargin treatments. After incubation, embryos were transferred from the heptane-aqueous interface to halocarbon oil 700 and were then mounted and imaged as above.
Acknowledgments
We would like to thank John Gillespie and James Hodge (University of Bristol, UK), Won-Jae Lee (Seoul National University, South Korea) and Pauline Phelan (University of Kent, UK) for fly lines, Poonam Ghai (University of Bath, UK) for technical assistance, and Yi Feng (University of Bristol, UK) and Karl Swann (University of Cardiff, UK) for helpful discussion. We would also like to thank Anna Huttenlocher (University of Wisconsin-Madison, USA) for sharing data ahead of publication and Kaeko Kamei (Department of Applied Biology, University of Kyoto, Japan) for giving us the dDUOX antisera. This work would not have been possible without the use of the Wolfson Bioimaging Facility (University of Bristol, UK) and the Bioimaging facility at the University of Bath (CEOS, University of Bath, UK), the Bloomington Stock Centre (University of Indiana, USA), and Flybase. W.R., W.W., and P.M. are funded by the Wellcome Trust.
Published: February 7, 2013
Footnotes
Supplemental Information includes four figures, one table, Supplemental Experimental Procedures, and three movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.01.058.
Contributor Information
Paul Martin, Email: paul.martin@bristol.ac.uk.
Will Wood, Email: w.wood@bath.ac.uk.
Supplemental Information
Movie showing immediate induction of a calcium wave after wounding of an embryo expressing epithelial mCherry-moesin (red) and GCaMP3 (green). An intracellular calcium wave is transmitted several cells back from the wound margin. Wounding occurs at 0 min and is marked by the white asterisk. Images taken from this movie correspond to those shown in Figure 1A. The genotype of the embryo is w;e22c-Gal4,UAS-GCaMP3,UAS-mCherry-moesin. The scale bar represents 50 μm.
Movie showing resolution of the calcium flash in an w;e22c-Gal4,UAS-GCaMP3,UAS-mCherry-moesin embryo after wounding (red is mCherry-moesin, green is GCaMP3). The calcium wave is switched off in a distal-proximal direction such that the wound-margin cells extinguish their calcium response last. The scale bar represents 20 μm; time is in seconds.
Amplex Ultrared live imaging over time in wounded control versus trpm2 mutant embryos shows a reduced signal when the calcium wave is perturbed after loss of TRPM. The scale bar represents 25 μm; time is in seconds.
References
- 1.Moreira S., Stramer B., Evans I., Wood W., Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 2010;20:464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Niethammer P., Grabher C., Look A.T., Mitchison T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood W., Jacinto A., Grose R., Woolner S., Gale J., Wilson C., Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat. Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 4.Wood W., Faria C., Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stramer B., Wood W., Galko M.J., Redd M.J., Jacinto A., Parkhurst S.M., Martin P. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinman L.E., Beilman G.J., Groehler K.E., Sammak P.J. Wound-induced calcium waves in alveolar type II cells. Am. J. Physiol. 1997;273:L1242–L1248. doi: 10.1152/ajplung.1997.273.6.L1242. [DOI] [PubMed] [Google Scholar]
- 7.Leiper L.J., Walczysko P., Kucerova R., Ou J.X., Shanley L.J., Lawson D., Forrester J.V., McCaig C.D., Zhao M., Collinson J.M. The roles of calcium signaling and ERK1/2 phosphorylation in a Pax6+/- mouse model of epithelial wound-healing delay. BMC Biol. 2006;4:27. doi: 10.1186/1741-7007-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shabir S., Southgate J. Calcium signalling in wound-responsive normal human urothelial cell monolayers. Cell Calcium. 2008;44:453–464. doi: 10.1016/j.ceca.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Sung Y.J., Sung Z.L., Ho C.L., Lin M.T., Wang J.S., Yang S.C., Chen Y.J., Lin C.H. Intercellular calcium waves mediate preferential cell growth toward the wound edge in polarized hepatic cells. Exp. Cell Res. 2003;287:209–218. doi: 10.1016/s0014-4827(03)00160-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z.Q., Walczysko P., Zhao M. Intracellular Ca2+ stores are essential for injury induced Ca2+ signaling and re-endothelialization. J. Cell. Physiol. 2008;214:595–603. doi: 10.1002/jcp.21248. [DOI] [PubMed] [Google Scholar]
- 11.Xu S., Chisholm A.D. A Gαq-Ca2+ signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr. Biol. 2011;21:1960–1967. doi: 10.1016/j.cub.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieger D., Moritz C., Ziegenhals T., Prykhozhij S., Peri F. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev. Cell. 2012;22:1138–1148. doi: 10.1016/j.devcel.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Yoo S.K., Freisinger C.M., LeBert D.C., Huttenlocher A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J. Cell Biol. 2012;199:225–234. doi: 10.1083/jcb.201203154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian L., Hires S.A., Mao T., Huber D., Chiappe M.E., Chalasani S.H., Petreanu L., Akerboom J., McKinney S.A., Schreiter E.R. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 16.Dierick H.A., Bejsovec A. Functional analysis of Wingless reveals a link between intercellular ligand transport and dorsal-cell-specific signaling. Development. 1998;125:4729–4738. doi: 10.1242/dev.125.23.4729. [DOI] [PubMed] [Google Scholar]
- 17.Klepeis V.E., Cornell-Bell A., Trinkaus-Randall V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J. Cell Sci. 2001;114:4185–4195. doi: 10.1242/jcs.114.23.4185. [DOI] [PubMed] [Google Scholar]
- 18.Paemeleire K., Martin P.E.M., Coleman S.L., Fogarty K.E., Carrington W.A., Leybaert L., Tuft R.A., Evans W.H., Sanderson M.J. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Mol. Biol. Cell. 2000;11:1815–1827. doi: 10.1091/mbc.11.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer R., Lehmann C., Fuss B., Eckardt F., Hoch M. The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J. Cell Sci. 2002;115:1859–1867. doi: 10.1242/jcs.115.9.1859. [DOI] [PubMed] [Google Scholar]
- 20.Bauer R., Lehmann C., Martini J., Eckardt F., Hoch M. Gap junction channel protein innexin 2 is essential for epithelial morphogenesis in the Drosophila embryo. Mol. Biol. Cell. 2004;15:2992–3004. doi: 10.1091/mbc.E04-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meer J.M., Jaffe L.F. Elemental composition of the perivitelline fluid in early Drosophila embryos. Dev. Biol. 1983;95:249–252. doi: 10.1016/0012-1606(83)90025-8. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann T., Chubanov V., Chen X., Dietz A.S., Gudermann T., Montell C. Drosophila TRPM channel is essential for the control of extracellular magnesium levels. PLoS ONE. 2010;5:e10519. doi: 10.1371/journal.pone.0010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedard K., Krause K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 24.Rigutto S., Hoste C., Grasberger H., Milenkovic M., Communi D., Dumont J.E., Corvilain B., Miot F., De Deken X. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J. Biol. Chem. 2009;284:6725–6734. doi: 10.1074/jbc.M806893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutta D., Bloor J.W., Ruiz-Gomez M., VijayRaghavan K., Kiehart D.P. Real-time imaging of morphogenetic movements in Drosophila using Gal4-UAS-driven expression of GFP fused to the actin-binding domain of moesin. Genesis. 2002;34:146–151. doi: 10.1002/gene.10113. [DOI] [PubMed] [Google Scholar]
- 26.Zhou M.J., Diwu Z.J., Panchuk-Voloshina N., Haugland R.P. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Mejiba S.E., Zhai Z., Akram H., Deterding L.J., Hensley K., Smith N., Towner R.A., Tomer K.B., Mason R.P., Ramirez D.C. Immuno-spin trapping of protein and DNA radicals: “tagging” free radicals to locate and understand the redox process. Free Radic. Biol. Med. 2009;46:853–865. doi: 10.1016/j.freeradbiomed.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha E.-M., Lee K.-A., Park S.H., Kim S.-H., Nam H.-J., Lee H.-Y., Kang D., Lee W.-J. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev. Cell. 2009;16:386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Kang K., Panzano V.C., Chang E.C., Ni L., Dainis A.M., Jenkins A.M., Regna K., Muskavitch M.A.T., Garrity P.A. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anh N.T.T., Nishitani M., Harada S., Yamaguchi M., Kamei K. Essential role of Duox in stabilization of Drosophila wing. J. Biol. Chem. 2011;286:33244–33251. doi: 10.1074/jbc.M111.263178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasberger H., Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J. Biol. Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 32.Xie X., Hu J., Liu X., Qin H., Percival-Smith A., Rao Y., Li S.S.C. NIP/DuoxA is essential for Drosophila embryonic development and regulates oxidative stress response. Int. J. Biol. Sci. 2010;6:252–267. doi: 10.7150/ijbs.6.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans I.R., Zanet J., Wood W., Stramer B.M. Live imaging of Drosophila melanogaster embryonic hemocyte migrations. J. Vis. Exp. 2010 doi: 10.3791/1696. Published online February 12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie showing immediate induction of a calcium wave after wounding of an embryo expressing epithelial mCherry-moesin (red) and GCaMP3 (green). An intracellular calcium wave is transmitted several cells back from the wound margin. Wounding occurs at 0 min and is marked by the white asterisk. Images taken from this movie correspond to those shown in Figure 1A. The genotype of the embryo is w;e22c-Gal4,UAS-GCaMP3,UAS-mCherry-moesin. The scale bar represents 50 μm.
Movie showing resolution of the calcium flash in an w;e22c-Gal4,UAS-GCaMP3,UAS-mCherry-moesin embryo after wounding (red is mCherry-moesin, green is GCaMP3). The calcium wave is switched off in a distal-proximal direction such that the wound-margin cells extinguish their calcium response last. The scale bar represents 20 μm; time is in seconds.
Amplex Ultrared live imaging over time in wounded control versus trpm2 mutant embryos shows a reduced signal when the calcium wave is perturbed after loss of TRPM. The scale bar represents 25 μm; time is in seconds.