Abstract
Functional magnetic resonance imaging (fMRI) has become the primary non-invasive method for investigating the human brain function. With an increasing number of ultra-high field MR systems worldwide possibilities of higher spatial and temporal resolution in combination with increased sensitivity and specificity are expected to advance detailed imaging of distinct cortical brain areas and subcortical structures. One target region of particular importance to applications in psychiatry and psychology is the amygdala. However, ultra-high field magnetic resonance imaging of these ventral brain regions is a challenging endeavor that requires particular methodological considerations. Ventral brain areas are particularly prone to signal losses arising from strong magnetic field inhomogeneities along susceptibility borders. In addition, physiological artifacts from respiration and cardiac action cause considerable fluctuations in the MR signal. Here we show that, despite these challenges, fMRI data from the amygdala may be obtained with high temporal and spatial resolution combined with increased signal-to-noise ratio. Maps of neural activation during a facial emotion discrimination paradigm at 7 T are presented and clearly show the gain in percental signal change compared to 3 T results, demonstrating the potential benefits of ultra-high field functional MR imaging also in ventral brain areas.
Keywords: Magnetic resonance imaging (MRI), 7 T, Ventral brain, Amygdala, Emotion discrimination
1. Introduction
1.1. MR imaging at ultra-high fields
Within the last years, ultra-high field MR imaging of human brain structure and function has become increasingly available. The high expectations from the application of scanners with field strengths of 7 T and more [1] have triggered considerable investments by research institutions across the world. Functional MR imaging (fMRI), employing fast imaging sequences, particularly requires increased methodological considerations in hardware design, image acquisition and post-processing to fully benefit from the capacity of the latest advances in scanner engineering.
The most prominent benefit of high magnetic field strengths in MR imaging is the approximately linear enhancement of image signal-to-noise ratio (iSNR) [2]. In addition, BOLD-weighted functional MRI application can also benefit from increased susceptibility contrasts. A significant positive relationship between field strength (comparison of 1.5, 3, and 7 T) and significant voxel counts, t-values, and amplitude of signal change within an a priori defined region of interest has also been reported [3].
The linear increase of iSNR, however, does not directly translate to a linear increase in BOLD sensitivity and temporal SNR (tSNR) at 7 T. While the contributions of thermal noise are reduced, physiological influences (i.e. respiration and cardiac signal) mitigate the measured temporal contrast induced by brain activation [2]. At the same time, higher static magnetic field strengths (B0) lead to an increase in susceptibility-related field inhomogeneities and cause a reduction of relaxation times. The resulting shorter echo times (e.g., cf. typical TE3 T = 42 ms vs. TE7 T = 23 ms) require faster image acquisition techniques, in particular the use of parallel imaging methods (e.g., GRAPPA, SENSE), and optimization of shimming procedures [4].
1.2. Functional MRI of the human amygdala
The ventral brain is particularly affected by both susceptibility-related effects and physiological artifacts. Field inhomogeneities surrounding the orbitofrontal, amygdalar, entorhinal, and inferior temporal regions may cause severe signal dropout via intra-voxel dephasing effects, which make fMRI in these areas a challenging endeavor [5]. These areas are, however, of high relevance in the pathogenesis of a variety of psychiatric diseases including anxiety disorders, major depression, and bipolar disorder. One region of particular interest is the amygdala. This almond-shaped structure within the temporal lobe is considered an important hub in the emotion processing network.
The amygdala consists of several nuclei distinguished by numerous connections to frontal cortical areas, the basal ganglia, the brainstem and olfactory brain regions [6]. Due to its considerable link to other limbic structures such as the hippocampus, the amygdala is mostly described as part of the limbic system. Besides, the amygdala receives various inputs from sensory cortices and mediates a number of autonomous and motor functions via its connections to hypothalamus, thalamus and septal regions [7].
Current standard of knowledge from functional neuroimaging studies outlines that the amygdala is essential to the evaluation and processing of emotional stimuli, notably fear [8]. This theory is supported by the vast amount of fMRI studies using specific paradigms known to actuate amygdala reactivity. The amygdalar BOLD response in healthy subjects has been shown to increase significantly in the presence of a strong trigger, such as fearful faces or negatively affected pictures [8,9]. Moreover, the magnitude of this signal is significantly increased in patients with major depression and anxiety disorders, indicating that the hyperactivation of the amygdala may represent a trait marker of these disease patterns [10]. In addition, these findings are supported by molecular neuroimaging studies determining alterations in the amygdala in patients suffering from major depression and anxiety disorders [11]. Furthermore, neuroscientific data suggest that the function of the amygdala underlies an inhibitory control of frontal brain regions and that disturbances of this functional connectivity may be responsible for the development of several psychiatric disorders, such as posttraumatic stress disorder, social anxiety disorder [12], bipolar disorder and depression. Nonetheless, to date the exact mechanisms underlying the pathogenesis of psychiatric diseases as well as the role of the amygdala in that context remain unclear and further investigations are certainly necessary.
Particularly at very high magnetic field strengths, imaging the ventral brain region entails challenges. The amygdala and other deep-brain structures are located in close vicinity to bone and air-filled cavities (i.e. sinuses). Those areas are known for their vulnerability to susceptibility artifacts caused by local magnetic field inhomogeneities [13] and physiologically induced artifactual signal components [14,15].
On the other hand, the employment of optimized scanning protocols for high spatial resolution can compensate for these challenges. The use of small voxel sizes (such as 1.5 mm × 1.5 mm × 3 mm and smaller) has shown to reliably reduce spin dephasing and signal drop-outs within the amygdala and other parts of the limbic system as well as ventral areas of the orbito-frontal cortex [5]. Furthermore, the combination of high-resolution scanning protocols and high field strength leads to increased relative temporal SNR [2].
In the study presented we demonstrate that applications of ultra-high field fMRI are not limited to functional imaging of primary sensory and motor regions. Sequences and parameters optimized for high-spatial resolution enable reliable signals from ventral brain areas that are highly reproducible at group level.
2. Methods
15 healthy volunteers (6 females, 9 males, mean age ± SD: 29.54 ± 6.65 years) were recruited from the local community via billboard announcements. Inclusion criteria for all subjects were physical and mental health, no history in psychiatric or neurological disorders, no past or current drug abuse, signed written informed consent and age of 18–60 years. Volunteers were instructed to refrain from alcohol on the day of the measurement. All subjects were financially reimbursed for their participation. The study protocol was approved by the ethics committee of the Medical University of Vienna.
2.1. Emotion discrimination and object discrimination task
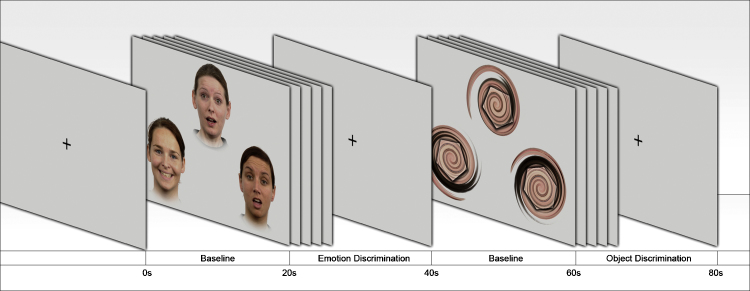
To actuate amygdala reactivity, subjects performed a facial emotion discrimination task (EDT) and an object discrimination task (ODT), adapted from the original paradigm introduced by Hariri et al. [16]. Photos of emotional faces were obtained from the Radboud Faces Database [17]. The contours of regular polygons (3–7 edges) were used for the ODT (Fig. 1).
Fig. 1.
Experimental paradigm. Facial emotion and object discrimination task blocks were alternately presented. Between task conditions, a black fixation cross was presented for 20 s to serve as a baseline condition. Each task block with an individual length of 20 s was repeated four times, yielding a total paradigm length of 340 s.
The two discrimination blocks were alternately presented to the subjects, with four 20 s blocks of each task condition and 20 s fixation cross baseline condition in-between, at the beginning and at the end of the run (total length: 340 s). In the EDT condition, participants were presented with a triplet of faces expressing one of seven emotions (anger, disgust, fear, happiness, sadness, surprise, or calmness). They were instructed to select which of the two emotional faces presented left and right at the bottom of the screen matches the target face at the top, by pressing either the left or the right button of a MR-compatible response pad. In the ODT condition the faces were replaced by contours of geometrical shapes, which were superimposed to a skin colored background. Subjects were told to respond after a waiting period of 2 s to ensure sufficiently long exposure to the stimulus material. The next stimulus was shown, when the subject pressed a button or failed to respond within 7 s.
Individual stimuli were projected to a semi-transparent screen located at the back end of the scanner bore and presented in true randomized order using Cogent 2000.
2.2. Data acquisition
MRI measurements were performed on an ultra-high field whole-body 7 T MRI scanner (Magnetom 7 T, Siemens Medical, Erlangen, Germany) at the MR Centre of Excellence, Medical University of Vienna, Austria. Subjects were scanned using a 32-channel head array coil (Nova Medical, Wilmington, MA, USA). 245 whole-brain volumes (matrix size: 128 px × 128 px × 32 slices) were obtained at a repetition time of TR = 1.4 s employing a single-shot echo planar imaging (EPI) sequence (TE = 23 ms, FoV = 192 mm × 192 mm, 2 mm slice thickness). In order to reduce MRI signal losses in ventral brain regions caused by intra-voxel dephasing effects due to local magnetic field inhomogeneities, we applied an optimized protocol that provided high in-plane spatial resolution and thin slices with a voxel size of 1.5 mm × 1.5 mm × 2 mm. Parallel imaging (GRAPPA) was used to enable short TE required in ultra-high field fMRI.
2.3. Pre-processing and data analysis
Acquired fMRI data were pre-processed and analyzed in SPM8 (FIL Group, UC London, UK). Preprocessing included correction for slice-timing differences [18], realignment to compensate for bulk head motion, normalization to standard MNI space using an in-house developed EPI template optimized for 7 T EPI imaging. Data were spatially smoothed with an isotropic Gaussian kernel of 8 mm FWHM to reduce the impact of individual anatomical differences in random-effects group analysis and account for the spatial distribution of the BOLD effect. The estimated size of our target regions, particularly the amygdala, was the rationale for the size of the smoothing kernel.
First-level single-subject analysis was conducted using the general linear model (GLM) framework implemented in SPM8. Two boxcar functions of the onsets and durations of the two task conditions were convolved with SPM8's canonical hemodynamic response function. These two separate regressors were designed to model changes in hemodynamic responses during emotional face discrimination and object discrimination. Further, six realignment parameters obtained from SPM8's motion correction algorithm. Time courses from white-matter regions and ventricles were also included in the design matrix to reduce motion and physiological artifacts.
Second-level group analysis was performed by conducting a one-sample t-test on the individuals’ contrasts between emotion and object discrimination (i.e. EDT > ODT). Significance threshold was set to p < 0.05 (FDR corrected for multiple comparisons) and a minimum cluster size of 100 voxels was chosen.
2.4. Comparison of 3 T and 7 T data
For comparison, 15 healthy volunteers (different from the 7 T group; 7 females, 8 females, mean age ± SD: 25.4 ± 3.4 years) participating in an ongoing study on a high field whole body 3 T MRI scanner (Siemens Trio, Siemens Medical, Erlangen, Germany), performed an emotion discrimination task comparable to the design and setup described above (TR = 1.8 s; matrix size: 128 px × 128 px × 20 slices). 3 T data were pre-processed and analyzed using the aforementioned strategy and parameters.
For direct comparison, one subject (female, 25 years) of the 7 T study population underwent 3 T scanning with the identical paradigm used at 7 T (TR = 1.8 s; matrix size: 128 px × 128 px × 23 slices).
3. Results
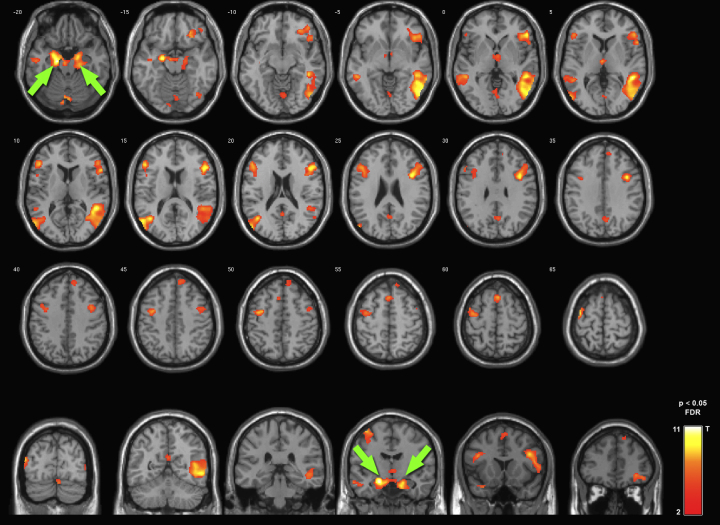
High-quality 7 T BOLD fMRI datasets covering the whole cerebrum including amygdalar and other ventral regions were acquired in all subjects. The result of the second-level group analysis is displayed in Fig. 2 (p < 0.05 FDR corrected, n = 100 voxels minimum cluster size). Global maxima of the task-related contrast (emotion vs. object discrimination) were located in the left amygdala (T = 11.24) and right amygdala (T = 8.66). Other clusters were found within the temporal lobe (fusiform gyrus, middle temporal gyrus), thalamus, insular cortex, posterior cingulate cortex and frontal lobe (bilaterally dorso-lateral prefrontal cortex, medio-frontal cortex).
Fig. 2.
fMRI group analysis of 7 T dataset. Second level group analysis (t-test for EDT > ODT) revealed significant activations within the left and right amygdalae (p < 0.05 FDR corrected, n = 100 voxels minimum cluster size).
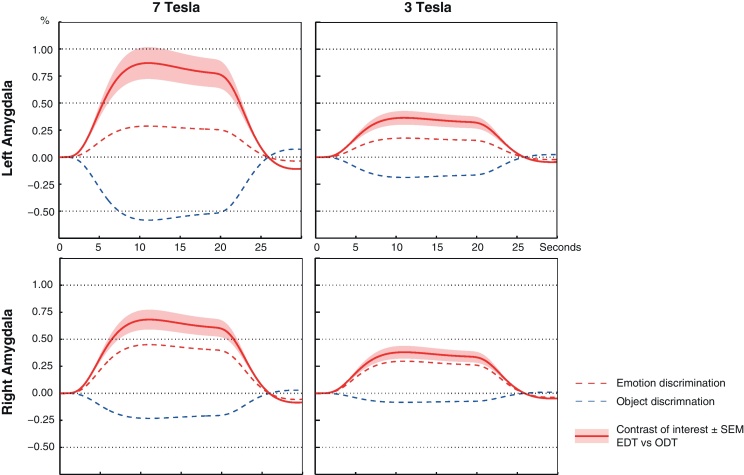
On group level, amplitudes of task-related amygdalar BOLD signal change were compared for 7 T and 3 T data and were significantly higher at 7 T: percent signal change in right amygdala: mean7 T = 0.70%, mean3 T = 0.38%, p = 0.02; percent signal change in left amygdala: mean7 T = 0.88%, mean3 T = 0.38%, p = 0.01 (Fig. 3).
Fig. 3.
Task-related signal change for 15 subjects at 3 and 7 T. Mean and SEM of task-induced signal change in the amygdalae for the contrast of interest (EDT vs. ODT). In comparison with 3 T, parameter estimates of activation change were significantly increased at 7 T.
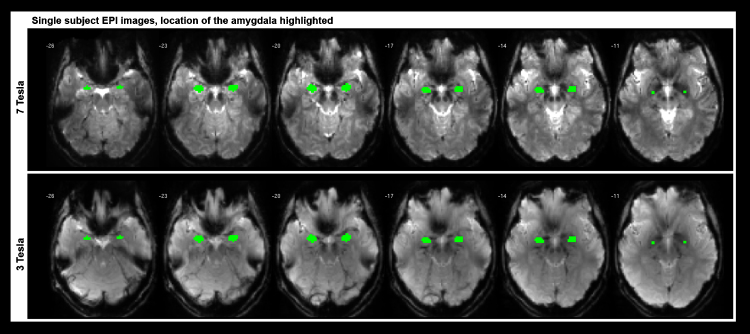
Fig. 4 shows EPI images of the participant measured at 7 T and 3 T.
Fig. 4.
fMRI single subject analysis. These high-resolution EPI images were acquired from a female subject (25 years) at 7 T (upper row) and 3 T (bottom row). Signal dropouts can be seen in close vicinity of the auditory canals and sinuses in both datasets. In addition to the shorter acquisition time (TR = 1.4 s vs. TR = 1.8 s), image contrast was increased in the 7 T images.
4. Discussion and outlook
In principle, functional imaging at 7 T allows for faster image acquisition, higher spatial resolution and stronger BOLD-weighting. This increased contrast-to-noise ratio has been repeatedly demonstrated in fMRI studies at 7 T (cf. [3]). These studies, however, were limited to non-ventral brain areas. Here we have shown that ultra-high field MR can be successfully applied to obtain functional MR images with high temporal and spatial resolution not only in primary sensory and motor regions, but also in ventral brain areas including the amygdala.
The methods necessary for high-quality 7 T fMRI in ventral brain regions, in particular the amygdala, need to target the two most challenging features: (1) signal loss from susceptibility-related field inhomogeneities and (2) strong temporal signal fluctuations due to motion and physiological artifacts. We have used an approach based on high spatial resolution to reduce signal losses due to intra-voxel spin dephasing. Smaller voxel reduce intra-voxel field inhomogeneities and thus lead to increased MR signals. At 3 T, higher spatial resolution implies prolonged image acquisition periods and thus reduced temporal resolution. At 7 T, however, we can benefit from the increased MR signal strength and use parallel imaging with high GRAPPA factors to speed up acquisition.
The second challenge for 7 T amygdala fMRI lies in the strong signal fluctuations found in ventral brain regions. These fluctuations arise from head motion and physiological artifacts.
The use of both high spatial resolution and nuisance regressors has enabled us to increase fMRI sensitivity to a level where amygdalar activation can be easily observed. In fact, activation maxima in our target contrast (emotion vs. object discrimination task) were indeed located within the amygdala and other limbic regions. A group comparison of percental signal changes in the amygdala at 3 T and 7 T has shown an increase of up to 100% for the 7 T fMRI study.
In the individual subject scanned on both systems the 7 T MR images provide excellent contrast and, therefore, enabled proper visual discrimination of structural details. The 3 T images were free of geometric distortions. However, in comparison, the image contrast was only sufficient to identify gross structures and did not allow for exact anatomical localization of the amygdala.
The amygdala has been classified as an important hub in the emotion processing network and plays an important role in the pathogenesis of psychiatric disorders. With a stimulus design that triggers amygdalar activation and sequence parameters optimized for ventral brain regions, high spatial accuracy of single-subject functional brain maps can be established, which contribute to a robust and reliable group-level analysis.
In addition to their important contribution to fundamental research on brain function, current and future studies target the neuronal correlates of treatment response in various disorders. Assuming amygdala dysfunction represents a decisive promoting factor for the development of psychiatric phenotypes, a treatment response may be accompanied by a resolution of this disturbance. Depressive patients show a greater amygdalar BOLD response in the presence of sad faces compared to healthy subjects, whereas the latter seem to display an increased signal in view of happy faces. After treatment with selective serotonin reuptake inhibitors, the first-line treatment in major depression, the negative bias in affected subjects seems to reverse and adjust to the amygdala activity in healthy controls [19]. Several fMRI studies corroborate this suggestion, demonstrating normalized activation in several brain regions in major depression after antidepressant treatment, in particular a decrease of amygdala activation and an increase of activation in frontal areas [19,20]. These significant findings represent a major landmark in the understanding of various disease patterns as well as the effects of commonly used medication.
It should be noted that these insights only represent a first part on the way of conceiving the human brain's complexity; research methods using the advantage of high spatial and temporal resolution provided by ultra-high field MRI seem necessary to gain more precise data, in particular in view of small brain regions such as the amygdala with its differentiation in several nuclear structures. Recent developments in structural imaging techniques suggest improved tissue contrast, ideal for segmentation. However, its applicability to small structures in the ventral brain strongly depends on the increased signal strength achieved with ultra-high field MR imaging. In standard high-field MR scanning on 1.5–3 T systems, on the contrary, the amygdala is imaged as a single, relatively homogenous portion of grey matter, therefore not allowing for a distinction between individual clusters.
Furthermore, additional evaluation of ultra-high field MR data quality and comparison with 3 T datasets are indicated and currently in preparation. Based on these assessments, new quality estimations can be given to drive MR sequence development, improve coil designs, and optimize post-processing strategies. Given the increased contrasts at 7 T, novel insights into the temporal and spatial nature of the BOLD effect in the human brain can be expected. Combining the recent methodological and hardware improvements would enable us to further investigate the central role of delicate structures, such as the amygdala, and their associated networks in healthy brain function, as well as the pathogenesis and treatment of neuropsychiatric disorders.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
This research was funded by the Austrian Science Fund (FWF P23021) to Rupert Lanzenberger, the Austrian National Bank (ONB P12982) to Christian Windischberger, and via an unlimited research grant by Siemens Medical Solutions to Ewald Moser. This experiment was realised using Cogent 2000 developed by the Cogent 2000 team at the FIL and the ICN and Cogent Graphics developed by John Romaya at the LON at the Wellcome Department of Imaging Neuroscience. We thank Andreas Hahn and Jan Losak for the implementation and testing of the paradigm in Cogent and the clinical staff of the Department of Psychiatry and Psychotherapy.
Contributor Information
Ronald Sladky, Email: ronald.sladky@meduniwien.ac.at.
Christian Windischberger, Email: christian.windischberger@meduniwien.ac.at.
References
- 1.Moser E., Stahlberg F., Ladd M., Trattnig S. 7 Tesla MR from research to clinical applications? NMR in Biomedicine. 2011;24 doi: 10.1002/nbm.1794. [DOI] [PubMed] [Google Scholar]
- 2.Triantafyllou C., Hoge R.D., Krueger G. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. NeuroImage. 2005;26:243–250. doi: 10.1016/j.neuroimage.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 3.van der Zwaag W., Francis S., Head K. fMRI at 1.5, 3 and 7 T: characterising BOLD signal changes. NeuroImage. 2009;47:1425–1434. doi: 10.1016/j.neuroimage.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Balteau E., Hutton C., Weiskopf N. Improved shimming for fMRI specifically optimizing the local BOLD sensitivity. NeuroImage. 2010;49:327–336. doi: 10.1016/j.neuroimage.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson S., Windischberger C., Rauscher A., Moser E. Optimized 3 T EPI of the amygdalae. NeuroImage. 2004;22:203–210. doi: 10.1016/j.neuroimage.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 6.Amunts K., Kedo O., Kindler M. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson K.A.E., Windischberger C., Gerstl F., Mayr W., Siegel J.M., Moser E. Modulation of hypothalamus and amygdalar activation levels with stimulus valence. NeuroImage. 2010;51:324–328. doi: 10.1016/j.neuroimage.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breiter H.C., Etcoff N.L., Whalen P.J. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 9.Derntl B., Windischberger C., Robinson S. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009;34:687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Phan K.L., Fitzgerald D.A., Nathan P.J., Tancer M.E. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Lanzenberger R., Mitterhauser M., Kranz G.S. Progesterone level predicts serotonin-1A receptor binding in the male human brain. Neuroendocrinology. 2011;94:84–88. doi: 10.1159/000328432. [DOI] [PubMed] [Google Scholar]
- 12.Hahn A., Stein P., Windischberger C. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 13.Merboldt K.D., Fransson P., Bruhn H., Frahm J. Functional MRI of the human amygdala? NeuroImage. 2001;14:253–257. doi: 10.1006/nimg.2001.0802. [DOI] [PubMed] [Google Scholar]
- 14.Biswal B., Deyoe E., Hyde J. Reduction of physiological fluctuations in fMRI using digital filters. Magnetic Resonance Imaging. 1996;35:107–113. doi: 10.1002/mrm.1910350114. [DOI] [PubMed] [Google Scholar]
- 15.Windischberger C., Langenberger H., Sycha T. On the origin of respiratory artifacts in BOLD-EPI of the human brain. Magnetic Resonance Imaging. 2002;20:575–582. doi: 10.1016/s0730-725x(02)00563-5. [DOI] [PubMed] [Google Scholar]
- 16.Hariri A., Mattay V., Tessitore A. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 17.Langner O., Dotsch R., Bijlstra G., Wigboldus D., Hawk S., Van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition & Emotion. 2010;00:1–12. [Google Scholar]
- 18.Sladky R., Friston K.J., Tröstl J., Cunnington R., Moser E., Windischberger C. Slice-timing effects and their correction in functional MRI. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Windischberger C., Lanzenberger R., Holik A. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. NeuroImage. 2010;49:1161–1170. doi: 10.1016/j.neuroimage.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Delaveau P., Jabourian M., Lemogne C., Guionnet S., Bergouignan L., Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. Journal of Affective Disorders. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]