Abstract
Background
The rise in the number of patients with arthritis coupled with understaffing of medical services has seen the deployment of Clinical Nurse Specialists in running nurse-led clinics alongside the rheumatologist clinics. There are no systematic reviews of nurse-led care effectiveness in rheumatoid arthritis. Few published RCTs exist and they have shown positive results for nurse-led care but they have several limitations and there has been no economic assessment of rheumatology nurse-led care in the UK.
Objective
This paper outlines the study protocol and methodology currently being used to evaluate the outcomes and cost effectiveness for patients attending rheumatology nurse-led clinics.
Design and methods
A multi-centred, pragmatic randomised controlled trial with a non-inferiority design; the null hypothesis being that of ‘inferiority’ of nurse-led clinics compared to physician-led clinics. The primary outcome is rheumatoid arthritis disease activity (measured by DAS28 score) and secondary outcomes are quality of life, self-efficacy, disability, psychological well-being, satisfaction, pain, fatigue and stiffness. Cost effectiveness will be measured using the EQ-5D, DAS28 and cost profile for each centre.
Power calculations
In this trial, a DAS28 change of 0.6 is considered to be the threshold for clinical distinction of ‘inferiority’. A sample size of 180 participants (90 per treatment arm) is needed to reject the null hypothesis of ‘inferiority’, given 90% power. Primary analysis will focus on 2-sided 95% confidence interval evaluation of between-group differences in DAS28 change scores averaged over 4 equidistant follow up time points (13, 26, 39 and 52 weeks). Cost effectiveness will be evaluated assessing the joint parameterisation of costs and effects.
Results
The study started in July 2007 and the results are expected after July 2011.
Trial registration
The International Standard Randomised Controlled Trial Number ISRCTN29803766.
Keywords: Nurse-led care, Clinical nurse specialists, Rheumatoid arthritis, Randomised controlled trial, Economic evaluation, Protocol
What is already known about this topic?
-
•
In the UK, Rheumatology Clinical Nurse Specialists conduct nurse-led clinics which provide follow-up care to patients with rheumatoid arthritis including monitoring, patient education and psychosocial support.
-
•
Research has shown positive outcomes of nurse-led clinics but the UK studies have several limitations and lack generalisability and evidence of cost-effectiveness.
What this paper adds
-
•
This paper outlines a protocol and methodology of a randomised controlled trial being conducted to demonstrate the outcomes and cost-effectiveness of nurse-led care in rheumatoid arthritis.
-
•
This is the first UK multi-centred RCT of effectiveness of nurse-led care in rheumatology.
-
•
This is the first UK study of cost-effectiveness of nurse-led care in rheumatology.
1. Background
During the past 20 years the rise in the number of patients with arthritis, understaffing of medical services and reduced junior hospital doctors’ working hours have prompted the rheumatology community in the United Kingdom (UK) to reassess how patient services are provided. These pressures have meant that whilst essential medical provision remains intact, it is often at the expense of the psychological, social, rehabilitative and educational needs that are so necessary to enhance patient outcomes (Mounce and Ryan, 2001). To counter these problems, rheumatology units increasingly augment the multidisciplinary team with clinical nurse specialists who are senior nurses, specially trained to undertake extended roles. By taking on some of the technical and patient management activities that were previously the sole responsibility of rheumatologists, these nurses allow rheumatologists to concentrate on the more complex tasks such as differential diagnosis for which they are uniquely trained (Bird, 1983).
Despite this innovative development, the evidence of effectiveness of nurse-led rheumatology clinics is limited. Our literature search produced no systematic review of nurse-led care effectiveness in rheumatology. Yet there were several systematic reviews of effectiveness in other chronic diseases such as diabetes (Carey and Courtenay, 2007), coronary heart diseases (Page et al., 2005) and chronic obstructive pulmonary disease (Sridhar et al., 2008). Our recent review (Ndosi et al., 2010) revealed seven randomised controlled trials (RCTs) of effectiveness of nurse-led care in rheumatology; four of which were in rheumatoid arthritis (Hill et al., 1994, 2003; Ryan et al., 2006; Tijhuis et al., 2003), two in osteoarthritis (Hill et al., 2009; Victor et al., 2005) and one in fibromyalgia (Kroese et al., 2008). In addition there was an economic evaluation of nurse-led care in rheumatoid arthritis (Van Der Hout et al., 2003). Of the seven RCTs of effectiveness, two were from The Netherlands and five were from the UK. The Dutch team also undertook the economic evaluation.
The majority of the RCTs were in rheumatoid arthritis as this is the disease that the majority of nurses are involved with. The outcomes of patients under nurse-led care were compared to those of the rheumatologist (Hill et al., 1994, 2003), in-patient team and day-patient team care (Tijhuis et al., 2003) and staff nurse working under a rheumatologist (Ryan et al., 2006). The primary outcomes in these studies were disease activity, functional status, health status and coping with rheumatoid arthritis. The results of disease activity showed that the effects of nurse-led care were not significantly different from those of the comparators (Hill et al., 1994, 2003; Tijhuis et al., 2003) and in one study nurse-led care had better effects on disease activity (Ryan et al., 2006). The effects of nurse-led care on functional status were not different from those of in-patient team or day-patient team care (Tijhuis et al., 2003). Nurse-led care demonstrated better effects than standard care on health status and coping with rheumatoid arthritis (Ryan et al., 2006). The economic analysis (Van Der Hout et al., 2003) concluded that nurse-led care provided equivalent quality of life and utility at a lower cost.
The osteoarthritis RCTs demonstrated better nurse-led care effects in pain control (Hill et al., 2009) and no difference in coping with arthritis (Victor et al., 2005). In diagnosing fibromyalgia, nurse-led diagnosis showed excellent agreement with that of the rheumatologist and this agreement was maintained over 24 months (Kroese et al., 2008).
Despite showing positive results, the RCTs of effectiveness of nurse-led care in rheumatoid arthritis have several limitations. The Dutch study (Tijhuis et al., 2003) was the only multicentre study but it did not compare like with like as there was a disparity in favour of day care and in-patient treatments in the number of visits, hours of treatment and intensity of care. Also, the nurse-led care cohort was less impaired and had a better quality of life at the start of the study, making it more difficult to demonstrate a significant difference between the groups on completion. The sequential studies undertaken in Leeds (Hill et al., 1994, 2009, 2003) and Stoke-On-Trent (Ryan et al., 2006) are the only work to date that validates nurse-led rheumatology clinics in the UK. Unfortunately, the Leeds studies also have their limitations, as all were undertaken in one clinic managed by the same rheumatology nurse, one consultant rheumatologist and four junior doctors. In addition, the sample sizes were small and there is no confirmation that these results are reproducible from other nurse-led clinics in the UK. Finally, there has been no economic assessment of nurse-led rheumatology clinics in the UK and so we do not know if they are economically effective.
The aims of this study are to establish whether nurse-led rheumatology clinics are clinically effective and cost effective for patients with rheumatoid arthritis.
2. Methods
2.1. Study design
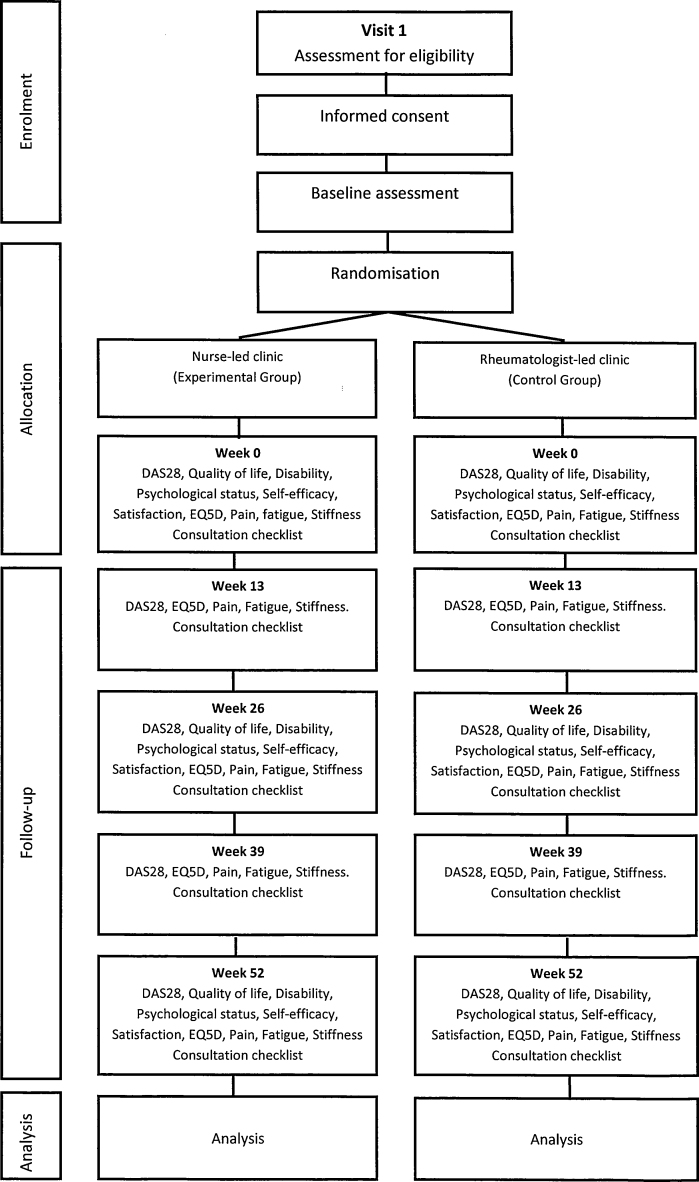
This is a pragmatic trial in a complex clinical environment and is conducted over 4 years as a multi-centred RCT. Patients are randomised using an office hours remote secure telephone randomisation service provided by the Clinical Trials and Research Unit, University of Leeds. Patients who fulfil the eligibility criteria and have provided written informed consent are randomised on a 1:1 basis to either Nurse-led clinic (experimental group) or a rheumatologist-led clinic (control group). Randomisation is by random permuted blocks, using the stratification factors, centre and DAS28 score (low ≤3.2 or moderate to severe >3.2) at baseline. After recruitment, patients have 5 follow-up visits over 12 months. (Fig. 1). The methods are consistent with current guidelines on design, conduct and analysis of pragmatic randomised clinical trials (Moher et al., 2010; Ramsey et al., 2005; Schulz et al., 2010; Zwarenstein et al., 2008), and those explicitly for non-inferiority trials (Bosmans et al., 2008; Piaggio et al., 2006).
Fig. 1.
Study design.
2.2. Study population
The study comprises 180 patients with rheumatoid arthritis recruited from 10 rheumatology centres throughout the UK. Patients with both stable and active disease are included as this reflects the practice of clinical nurse specialists in the UK and Europe and so make the results more meaningful. All patients are seen in one of the 10 participating centres. The centres are distributed throughout the UK and this provides a socio-demographic mix of patients. Inclusion criteria are: a positive diagnosis of rheumatoid arthritis as defined by the American Rheumatism Association (Arnett et al., 1988); aged 18 years or above, and ability to complete questionnaires unaided. Exclusion criteria are: patients unwilling to be randomised to a nurse-led clinic or rheumatologist-led clinic; patients suffering from unstabilised concomitant disease; patients awaiting surgery and patients who have already received care from the practitioners involved in the study.
2.3. Hypothesis
The hypothesis is that the outcomes from nurse-led clinics will not be inferior to those obtained by the rheumatologist-led clinics, but at a lower cost and greater patient satisfaction.
3. Interventions
Following randomisation, patients are given appointments with their respective practitioners for weeks 0, 13, 26, 39 and 52. When patients arrive at the clinic, they are seen by an independent assessor who oversees the completion of pain Visual Analogue Scale (VAS), fatigue VAS, the length of morning stiffness and performs joint counts for Disease Activity Score (DAS28). The independent assessor also gives the patient their blood form, questionnaires in a freepost return envelope and sends them to the waiting area ready to see their allocated practitioner. The joint examination for DAS28 can be prone to inter-observer variation and training or agreement sessions have been shown to minimise this (Grunke et al., 2010; Scott et al., 1996). Therefore a training session was conducted (by MN) with the independent assessors at the study set-up meeting to ensure standardisation. The joint examination technique was based on the European League Against Rheumatism handbook of clinical assessment in rheumatoid arthritis (van Riel and Scott, 2000). Since this is a pragmatic trial, the practitioners in both arms of the trial (Clinical Nurse Specialists and the Rheumatologists) did not receive any more training; they manage their patients according to their normal practice.
During the consultation, the clinical nurse specialists record their interventions in a standard “consultation checklist” especially designed for this study. The nurse-led care interventions may include: pain control, medication and dosage changes, intra-articular or intra-muscular steroid injections, provision of patient education or psychosocial support, prescription of splints, non-protocol blood tests or radiographic examination. Other interventions such as referral to the admission ward, to the rheumatologist, physiotherapists, podiatrist or any other health care professional may be carried out as appropriate. The referrals, conferrals and the length of consultation are also recorded.
Patients randomised to the rheumatologist-led care (control group) also have the same number of study visits and the rheumatologist provides care as per normal practice noting all the interventions and referrals in the consultation checklist.
4. Outcome measures
4.1. Primary and secondary outcomes
The primary outcome measure is the DAS28 (Prevoo et al., 1995), an internationally recognised measurement of disease activity in rheumatoid arthritis. It is a composite measurement comprising objective (number of swollen joints and erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]) and subjective (number of tender joints and patients global assessment) indices. DAS28 score has been shown to be a strong predictor of physical disability and radiological progression and a sensitive discriminator between patients with high and low disease activity (Prevoo et al., 1996; van Gestel et al., 1998). DAS28 score is widely used in making decisions about treatment effectiveness and it forms the basis for the European League against Rheumatism response criteria for rheumatoid arthritis (Van Gestel et al., 1996). Scores can range from 0 to 9.4, assuming that an ESR of 100 is taken as the upper limit. Levels of disease activity are defined as DAS28 ≤3.2 mild; DAS28 >3.2 and ≤5.1 moderate; DAS28 >5.1 severe (van Gestel et al., 1998; Van Gestel et al., 1996). The DAS28 will be measured at baseline and 13, 26, 39 and 52 weeks.
Secondary measures include haematological, clinical and questionnaire data. The haematological and clinical measures comprise: CRP or ESR, pain intensity (using 10 cm VAS), duration of morning stiffness (hours/minutes) and Fatigue (10 cm VAS). The following questionnaires will be administered at baseline, 26 and 52 weeks:
-
•
Health Assessment Questionnaire (Kirwan and Reeback, 1986)
-
•
Hospital Anxiety and Depression Scale (Zigmon and Snaith, 1983)
-
•
Leeds Satisfaction Questionnaire (Hill et al., 1992)
-
•
The Arthritis Self Efficacy Scale (Lorig et al., 1989)
-
•
Rheumatoid Arthritis Quality of Life Questionnaire (De Jong et al., 1997)
An additional questionnaire, the EQ-5D (The Euroqol group, 1990) will be completed at baseline and weeks 13, 26, 39 and 52 to provide health data for the economic analysis
4.2. Power calculations
A change in DAS28 score of 1.2 or more is deemed a clinically significant improvement, and a change of 0.6 or more reflects a moderate improvement (van Riel et al., 1996). A change in DAS28 score of 0.6 is assigned as the ‘inferiority’/’non-inferiority’ margin. Thus:
Null hypothesis (inferiority): mean ΔDAS28RLC − mean ΔDAS28NLC ≥ 0.6.
Alternative hypothesis (non-inferiority): mean ΔDAS28RLC − mean ΔDAS28NLC < 0.6.
Where Δ = change in, RLC = Rheumatologist-led clinic and NLC = Nurse-led clinic.
A total sample size of 180 participants (90 per treatment arm) is needed to reject the null hypothesis of ‘inferiority’, given 90% power and 1-sided statistical testing with 2.5% significance level (and a pre-hypothesized standard deviation in DAS28 change scores of 1.5). This total sample size assumes a 10% drop out/non-response rate. The calculation is based on a repeated-measures (pooled) analysis of between-group differences averaged over 4 equidistant follow up time points (13, 26, 39 and 52 weeks). The sample size calculation assumes that the intra-class correlation coefficient (for the correlation of observations over time within individuals) will be about 0.5.
5. Statistical analysis
5.1. Statistical analysis
Analyses will be carried out using both intention to treat and per protocol methods as advocated in extended CONSORT guidelines (Piaggio et al., 2006). Difference in mean summary scores will be presented with a 95% two-sided confidence interval from which we can draw a conclusion as to whether to accept or reject the null hypothesis of ‘inferiority’ regarding the nurse-led care intervention compared to the rheumatologist-led care. The primary outcome measure (DAS28 change score) and secondary measures will be compared between the two groups using data pooled over time; the evaluation focusing on the comparability of average change in DAS28 over the assessed follow up period. Secondary measurement will focus on individual time points. Analysis will be by hierarchical repeated measures modelling. Analysis will adjust for age, gender, centre, baseline DAS28 (and corresponding baseline values for secondary outcomes). Multiple imputation will be used to address the issue of missing data (Schafer, 1999). Analysis will also be carried out investigating outcome in relation to the interaction of intervention group and specific baseline variables: age, gender and DAS28.
5.2. Economic evaluation
The economic assessment will encompass both a cost utility analysis and a cost effectiveness analysis (Bosmans et al., 2008; Ramsey et al., 2005). A tiered approach to the evaluation will encompass the following economic perspectives: (i) NHS; (ii) healthcare [NHS plus direct patient costs]; (iii) societal [direct (healthcare) plus indirect (productivity) costs].
Healthcare resource use, specifically in relation to rheumatoid arthritis, is derived through clinic audits and follow up patient questionnaires, and embraces health professional consultations (primary and secondary care), hospital admissions (day care, inpatient stays, A&E visits), investigations, and treatments including over-the-counter medications. Costs will be derived from sources of ‘national average’ costs (Curtis, 2009; NHS Executive, 2009), and also by direct elicitation from the self-report questionnaires for private out-of-pocket expenditure on health care service use, travel, medication, aids and special dietary requirements. Data collected from each patient on employment status and job title (classified according to its socioeconomic classification using the Office of National Statistics (ONS) approach (ONS, 2000a,b)) will be used to determine productivity losses using the human capital approach by multiplying a patient's reported number of days off work by the expected average daily wage extracted from National Statistics survey databases (ONS, 2004). Multiple imputation will be used to address missing cost data (Schafer, 1999). Health outcomes will be assessed through QALYs derived from the EQ-5D for the cost utility analysis, and the DAS28 change score for the cost effectiveness analysis (with incremental differences in cost being evaluated in relation to the non-inferiority margin of 0.6 in mean DAS28 change).
Between-group economic comparisons will focus on the joint estimation of incremental costs and effects. The precision of the estimates will be ascertained by calculating confidence intervals around effect and cost differences, derived through adjusted linear regression modelling. Data for costs are usually right-skewed, and will therefore be analysed using the preferred bootstrap technique (Heyse et al., 2001; Lambert et al., 1998; Mullner, 2003). Uncertainty around the cost-effect estimates will be shown graphically using cost effectiveness/utility planes (Briggs and Fenn, 1998). Cost-acceptability curves will be used to help make informative decisions regarding the cost-effectiveness of the nurse clinics at variable ceiling willingness-to-pay cost thresholds (Stinnett and Mullahy, 1998) Sensitivity analyses will establish the robustness of findings to various assumptions (e.g. imputed versus complete-case data).
Funding: This research is carried out by funding from the Arthritis Research UK grant. It is hosted by the University of Leeds and it has been adopted by the National Institute for Health Research Clinical Research Network.
Conflict of interest: None declared.
Study registration and ethical approval: The study is registered as a clinical trial at the International Standard Randomised Controlled Trial Number Register (ISRCTN29803766). Multicentre ethical approval was obtained from the Leeds West Research Ethics Committee and site specific approvals were obtained from Local Research Ethics Committees of the 10 participating centres. The study is being conducted in accordance with good clinical practice in research to the Research Governance Framework for Health and Social Care (Department of Health, 2005).
References
- Arnett F., Edworthy S., Bloch D. The American Rheumatism Association – 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bird H.A. Divided rheumatological care: the advent of the nurse practitioner? Annals of Rheumatic Diseases. 1983;42(3):354–355. doi: 10.1136/ard.42.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmans J.E., De Bruijne M.C., Van Hout H.P.J., Hermens M.L.M., Adèr H.J., Van Tulder M.W. Practical guidelines for economic evaluations alongside equivalence trials. Value in Health. 2008;11(2):251–258. doi: 10.1111/j.1524-4733.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- Briggs A., Fenn P. Confidence intervals or surfaces? Uncertainty on the cost-effectiveness plane. Health Economics. 1998;7(8):723–740. doi: 10.1002/(sici)1099-1050(199812)7:8<723::aid-hec392>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Carey N., Courtenay M. A review of the activity and effects of nurse-led care in diabetes. Journal of Clinical Nursing. 2007;16(11c):296–304. doi: 10.1111/j.1365-2702.2007.01969.x. [DOI] [PubMed] [Google Scholar]
- Curtis L. Personal Social Services Research Unit, University of Kent; Canterbury: 2009. Unit Costs of Health and Social Care. [Google Scholar]
- De Jong Z., Van Der Heijde D., McKenna S. The reliability and construct validity of the RAQoL: a rheumatoid arthritis-specific quality of life instrument. British Journal of Rheumatology. 1997;36:878–883. doi: 10.1093/rheumatology/36.8.878. [DOI] [PubMed] [Google Scholar]
- Department of Health . Crown Copyright; London: 2005. Research Governance Framework for Health and Social Care. [Google Scholar]
- Grunke M., Antoni C., Kavanaugh A., Hildebrand V., Dechant C., Schett G., Manger B., Ronneberger M. Standardization of joint examination technique leads to a significant decrease in variability among different examiners. The Journal of Rheumatology. 2010;37(4):860. doi: 10.3899/jrheum.090195. [DOI] [PubMed] [Google Scholar]
- Heyse J., Cook J., Carides G. Statistical considerations in analysing healthcare resource utilization and cost data. In: Drummond M., McGuire A., editors. Economic Evaluation in Healthcare Merging Theory with Practice. Oxford University Press; Oxford: 2001. [Google Scholar]
- Hill J., Bird H., Harmer R. An evaluation of the effectiveness, safety and acceptability of a nurse practitioner in a rheumatology outpatient clinic. British Journal of Rheumatology. 1994;33:283–288. doi: 10.1093/rheumatology/33.3.283. [DOI] [PubMed] [Google Scholar]
- Hill J., Bird H., Hopkins R. Audit of satisfaction with care in a rheumatology outpatient clinic. Annals of Rheumatic Diseases. 1992;51:195–197. doi: 10.1136/ard.51.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Lewis M., Bird H. Do OA patients gain additional benefit from care from a clinical nurse specialist?—a randomized clinical trial. Rheumatology. 2009 doi: 10.1093/rheumatology/kep049. [DOI] [PubMed] [Google Scholar]
- Hill J., Thorpe R., Bird H. Outcomes for patients with RA: a rheumatology nurse practitioner clinic compared to standard outpatient care. Musculoskeletal Care. 2003;1:5–20. doi: 10.1002/msc.35. [DOI] [PubMed] [Google Scholar]
- Kirwan J.R., Reeback J.S. Stanford health assessment questionnaire modified to assess disability in british patients with rheumatoid arthritis. Rheumatology. 1986;25(2):206–209. doi: 10.1093/rheumatology/25.2.206. [DOI] [PubMed] [Google Scholar]
- Kroese M., Schulpen G.J.C., Bessems M.C.M., Severens J.L., Nijhuis F.J., Geusens P.P., Landewé R.B. Substitution of specialized rheumatology nurses for rheumatologists in the diagnostic process of fibromyalgia: a randomized controlled trial. Arthritis Care and Research. 2008;59(9):1299–1305. doi: 10.1002/art.24018. [DOI] [PubMed] [Google Scholar]
- Lambert C.M, Hurst N.P., Forbes J.F., Lochhead A., Macleod M., Nuki G. Is day care equivalent to inpatient care for active rheumatoid arthritis? Randomised controlled clinical and economic evaluation. British Medical Journal. 1998;316(7136):965. doi: 10.1136/bmj.316.7136.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig K., Chastain R., Ung E. Development and evaluation of a scale to measure perceived self efficacy in people with arthritis. Arthritis and Rheumatism. 1989;32:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P.J., Elbourne D., Egger M., Altman D.G. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010:340. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounce K., Ryan S. Defining the Extended Clinical Role for Allied Health Professionals in Rheumatology. Arthritis Research Campaign; Chesterfield: 2001. The Historical Development of Extended Clinical Roles in Rheumatology. pp. 9–10. [Google Scholar]
- Mullner M. Commentary: bootstrapping simplifies appreciation of statistical inferences. British Medical Journal. 2003;326(7395):914. doi: 10.1136/bmj.326.7395.900/b. [DOI] [PubMed] [Google Scholar]
- Ndosi M., Vinall K., Hale C., Bird H., Hill J. Is nurse-led care effective in rheumatology? A systematic review. Rheumatology. 2010;49(Suppl. 1):i14. [Google Scholar]
- NHS Executive . Crown; London: 2009. National Schedule of Reference Costs 2008. [Google Scholar]
- ONS . Office of National Statistics; London: 2004. Annual Survey of Hours and Earnings (ASHE) [Google Scholar]
- ONS . The Stationery Office; London: 2000. Standard Occupational Classification 200: Volume 1. Structure and Descriptions of Unit Groups. [Google Scholar]
- ONS . The Stationery Office; London: 2000. Standard Occupational Classification 200: Volume 2. The Coding Index. [Google Scholar]
- Page T., Lockwood C., Conroy-Hiller T. Effectiveness of nurse-led cardiac clinics in adult patients with a diagnosis of coronary heart disease. International Journal of Evidence-Based Healthcare. 2005;3:2–26. doi: 10.1111/j.1479-6988.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- Piaggio G., Elbourne D.R., Altman D.G., Pocock S.J., Evans S.J.W., for the C.G. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295(10):1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- Prevoo M., Van Gestel A., Van’t Hof M., Van Rijswijk M., Van de Putte L., Van Riel P. Remission in a prospective study of patients with rheumatoid arthritis. British Journal of Rheumatology. 1996;35:1101–1105. doi: 10.1093/rheumatology/35.11.1101. [DOI] [PubMed] [Google Scholar]
- Prevoo M.L., van’t Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., van Riel P.L. Modified disease activity scores that include twenty-eight-joint counts Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis and Rheumatism. 1995;38(1):44. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- Ramsey S., Willke R., Briggs A., Brown R., Buxton M., Chawla A., Cook J., Glick H., Liljas B., Petitti D. Good research practices for cost effectiveness analysis alongside clinical trials: the ISPOR RCT CEA task force report. Value in Health. 2005;8(5):521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- Ryan S., Hassell A.B., Lewis M., Farrell A. Impact of a rheumatology expert nurse on the wellbeing of patients attending a drug monitoring clinic. Journal of Advanced Nursing. 2006;53(3):277–286. doi: 10.1111/j.1365-2648.2006.03725.x. [DOI] [PubMed] [Google Scholar]
- Schafer J.L. Multiple imputation: a primer. Statistical Methods in Medical Research. 1999;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- Schulz K., Altman D., Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine. 2010;8(1):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D., Choy E., Greeves A., Isenberg D., Kassinor D., Rankin E., Smith E. Standardising joint assessment in rheumatoid arthritis. Clinical Rheumatology. 1996;15(6):579–582. doi: 10.1007/BF02238547. [DOI] [PubMed] [Google Scholar]
- Sridhar M., Taylor R., Dawson S., Roberts N.J., Partridge M.R. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2008;63(3):194. doi: 10.1136/thx.2007.077578. [DOI] [PubMed] [Google Scholar]
- Stinnett A.A., Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Medical Decision Making. 1998;18(2):S68. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- The Euroqol group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Tijhuis G.J., Zwinderman A.H., Hazes J.M.W., Breedveld F.C., Vlieland P.M.T.V. Two-year follow-up of a randomized controlled trial of a clinical nurse specialist intervention, inpatient, and day patient team care in rheumatoid arthritis. Journal of Advanced Nursing. 2003;41(1):34–43. doi: 10.1046/j.1365-2648.2003.02503.x. [DOI] [PubMed] [Google Scholar]
- Van Der Hout W, Tijhuis G., Hazes J. Cost effectiveness and cost utility analysis of multidisciplinary team care in patients with rheumatoid arthritis: a randomised comparison of clinical nurse specialist care, inpatient team care, and day patient team care. Annals of Rheumatic Diseases. 2003;62:308–315. doi: 10.1136/ard.62.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gestel A., Haagsma C., van Riel P. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis and Rheumatism. 1998;41(10):1845–1850. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Van Gestel A., Prevoo M., van′t Hof M., van Rijswijk M., van de Putte L., van Riel P. Development and validation of the European League against rheumatism response criteria for rheumatoid arthritis. Arthritis and Rheumatism. 1996;39:34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- van Riel P., Scott D. Van Zuiden Communications; Alphen Aan Den Rijn, The Netherlands: 2000. EULAR Handbook of Clinical Assessment in Rheumatoid Arthritis. [Google Scholar]
- van Riel P.L.C.M., van Gestel A.M., van de Putte L.B.A. Development and validation of response criteria in rheumatoid arthritis: steps towards an international consensus on prognostic markers. Rheumatology. 1996;35(Suppl. 2):4–7. doi: 10.1093/rheumatology/35.suppl_2.4. [DOI] [PubMed] [Google Scholar]
- Victor C.R., Triggs E., Ross F., Lord J., Axford J.S. Lack of benefit of a primary care-based nurse-led education programme for people with osteoarthritis of the knee. Clinical Rheumatology. 2005;24(4):358–364. doi: 10.1007/s10067-004-1001-9. [DOI] [PubMed] [Google Scholar]
- Zigmon A., Snaith R. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zwarenstein M., Treweek S., Gagnier J.J., Altman D.G., Tunis S., Haynes B., Oxman A.D., Moher D. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. British Medical Journal. 2008;337(November):a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]