Abstract
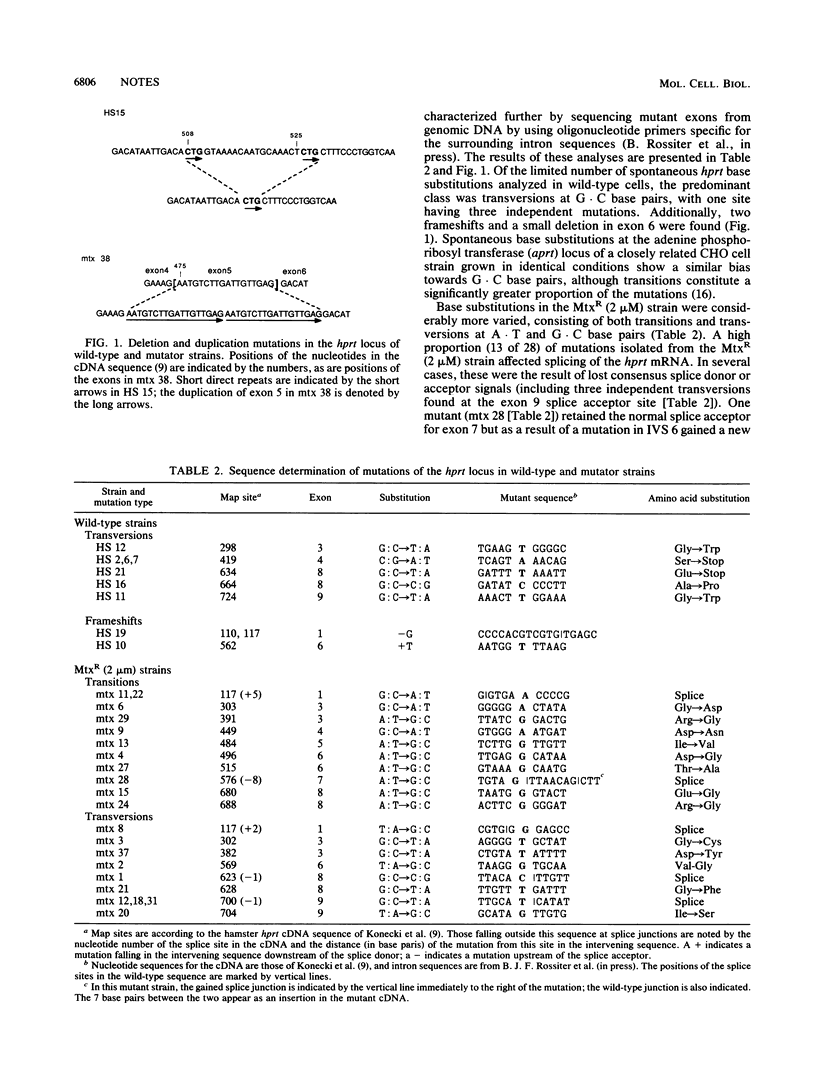
The pattern of mutations produced by a mutator gene (obtained during serial selection for amplification of the dihydrofolate reductase [dhfr] locus) shows a pronounced shift from that found in wild-type cells. The rate of certain types of base substitutions (particularly transitions) is dramatically increased, while gene rearrangements constitute a lower proportion of mutations. These data suggest a lower fidelity of the replication process in the mutator strain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breimer L. H., Nalbantoglu J., Meuth M. Structure and sequence of mutations induced by ionizing radiation at selectable loci in Chinese hamster ovary cells. J Mol Biol. 1986 Dec 5;192(3):669–674. doi: 10.1016/0022-2836(86)90284-6. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Tlsty T. D., Schimke R. T. Enhancement of methotrexate resistance and dihydrofolate reductase gene amplification by treatment of mouse 3T6 cells with hydroxyurea. Mol Cell Biol. 1983 Jun;3(6):1097–1107. doi: 10.1128/mcb.3.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligo M. A., Piras A., Rainaldi G. Time course of sister chromatid exchanges and gene amplification induced by 1-beta-D-arabinofuranosylcytosine in V79-AP4 Chinese hamster cells. Chromosoma. 1988;96(4):306–310. doi: 10.1007/BF00286918. [DOI] [PubMed] [Google Scholar]
- Cox E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- Drobetsky E., Meuth M. Increased mutational rates in Chinese hamster ovary cells serially selected for drug resistance. Mol Cell Biol. 1983 Oct;3(10):1882–1885. doi: 10.1128/mcb.3.10.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser J., Levine A. S., Dixon K. Fidelity of DNA synthesis in a mammalian in vitro replication system. Mol Cell Biol. 1988 Aug;8(8):3267–3271. doi: 10.1128/mcb.8.8.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy C. A., Rice G. C., Kovacs M., Schimke R. T. Over-replication of DNA in S phase Chinese hamster ovary cells after DNA synthesis inhibition. J Biol Chem. 1987 Sep 5;262(25):11927–11934. [PubMed] [Google Scholar]
- Kleinberger T., Etkin S., Lavi S. Carcinogen-mediated methotrexate resistance and dihydrofolate reductase amplification in Chinese hamster cells. Mol Cell Biol. 1986 Jun;6(6):1958–1964. doi: 10.1128/mcb.6.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Exonucleolytic proofreading. Cell. 1988 Jun 17;53(6):837–840. doi: 10.1016/s0092-8674(88)90189-4. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Metherall J. E., Collins F. S., Pan J., Weissman S. M., Forget B. G. Beta zero thalassemia caused by a base substitution that creates an alternative splice acceptor site in an intron. EMBO J. 1986 Oct;5(10):2551–2557. doi: 10.1002/j.1460-2075.1986.tb04534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. DNA mismatch correction. Annu Rev Biochem. 1987;56:435–466. doi: 10.1146/annurev.bi.56.070187.002251. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Meuth M. DNA amplification--deletion in a spontaneous mutation of the hamster aprt locus: structure and sequence of the novel joint. Nucleic Acids Res. 1986 Nov 11;14(21):8361–8371. doi: 10.1093/nar/14.21.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino F. W., Loeb L. A. Differential extension of 3' mispairs is a major contribution to the high fidelity of calf thymus DNA polymerase-alpha. J Biol Chem. 1989 Feb 15;264(5):2898–2905. [PubMed] [Google Scholar]
- Phear G., Armstrong W., Meuth M. Molecular basis of spontaneous mutation at the aprt locus of hamster cells. J Mol Biol. 1989 Oct 20;209(4):577–582. doi: 10.1016/0022-2836(89)90595-0. [DOI] [PubMed] [Google Scholar]
- Phear G., Nalbantoglu J., Meuth M. Next-nucleotide effects in mutations driven by DNA precursor pool imbalances at the aprt locus of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4450–4454. doi: 10.1073/pnas.84.13.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L. Escherichia coli mutT mutator effect during in vitro DNA synthesis. Enhanced A.G replicational errors. J Biol Chem. 1987 Dec 5;262(34):16267–16270. [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Brown P. C., Schimke R. T. UV radiation facilitates methotrexate resistance and amplification of the dihydrofolate reductase gene in cultured 3T6 mouse cells. Mol Cell Biol. 1984 Jun;4(6):1050–1056. doi: 10.1128/mcb.4.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. D., Yu Y. J., Hsie A. W., Caskey C. T., Rossiter B., Gibbs R. A. Deletion screening at the hypoxanthine-guanine phosphoribosyltransferase locus in Chinese hamster cells using the polymerase chain reaction. Teratog Carcinog Mutagen. 1989;9(3):177–187. doi: 10.1002/tcm.1770090306. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Stout J. T., Konecki D. S., Patel P. I., Alford R. L., Caskey C. T. Spontaneous reversion of novel Lesch-Nyhan mutation by HPRT gene rearrangement. Somat Cell Mol Genet. 1988 May;14(3):293–303. doi: 10.1007/BF01534590. [DOI] [PubMed] [Google Scholar]


