Abstract
Crystals of the title compound, C14H8Cl6O4S, are twinned by inversion, with unequal components [0.85 (3):0.15 (3)]. The asymmetric unit contains two independent molecules that are related by a pseudo-inversion center. The Car—O [1.393 (9) and 1.397 (9) Å] and ester S—O bond lengths [1.600 (5) and 1.590 (5) Å] of both molecules are comparable to the structurally related 2,3,5,5-trichlorobiphenyl-4-yl 2,2,2-trichloroethyl sulfate. The dihedral angles between the benzene rings in the two molecules are 37.8 (2) and 35.0 (2)°.
Related literature
For related structures of biphenyl-4-yl ester 2,2,2-trichloro-ethyl esters of sulfuric acid, see: Li et al. (2008 ▶, 2010a
▶,b
▶,c
▶). For a review of structures of sulfuric acid aryl mono esters, see: Brandao et al. (2005 ▶); Denehy et al. (2006 ▶). For additional background to sulfate metabolites of polychlorinated biphenyls, see: Liu et al. (2006 ▶, 2009 ▶); Wang et al. (2006 ▶); Dhakal et al. (2012 ▶); Zhai et al. (2013 ▶).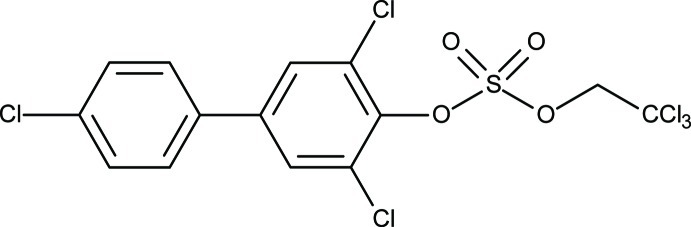
Experimental
Crystal data
C14H8Cl6O4S
M r = 484.96
Orthorhombic,
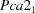
a = 13.993 (3) Å
b = 9.1890 (18) Å
c = 28.778 (6) Å
V = 3700.3 (13) Å3
Z = 8
Cu Kα radiation
μ = 9.71 mm−1
T = 90 K
0.17 × 0.09 × 0.02 mm
Data collection
Bruker X8 Proteum diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2006 ▶) T min = 0.504, T max = 0.830
45894 measured reflections
6651 independent reflections
6238 reflections with I > 2σ(I)
R int = 0.062
Refinement
R[F 2 > 2σ(F 2)] = 0.064
wR(F 2) = 0.161
S = 1.15
6651 reflections
302 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.96 e Å−3
Δρmin = −0.85 e Å−3
Absolute structure: Flack (1983 ▶), 3176 Friedel pairs
Flack parameter: 0.15 (3)
Data collection: APEX2 (Bruker, 2006 ▶); cell refinement: SAINT (Bruker, 2006 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97 and local procedures.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813007976/yk2088sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813007976/yk2088Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813007976/yk2088Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This research was supported by grants ES05605, ES013661 and ES017425 from the National Institute of Environmental Health Sciences, NIH.
supplementary crystallographic information
Comment
Sulfuric acid monoesters of hydroxylated polychlorinated biphenyls (OHPCBs) are emerging as an important class of metabolites of polychlorinated biphenyls (PCBs). Two recent in vivo studies report the formation of PCB sulfates by rats (Dhakal et al., 2012) and poplar plants (Zhai et al., 2013). In vitro studies demonstrate that PCB sulfates are both substrates and inhibitors of mammalian cytosolic sulfotransferases (Liu et al., 2006; Wang et al., 2006; Liu et al., 2009). Only limited structural information about sulfate mono- and diesters of hydroxylated PCBs is available to support structure-activity or structure-property relationship studies. Here we report the structure of the title compound, a biphenyl-4-yl 2,2,2-trichloroethyl sulfate with two chlorine substituents ortho to the sulfate group, to contribute to the number of available crystal structures.
The two independent molecules of the title compound in the asymmetric unit are related by a pseudo-inversion center. The length of the Caromatic—O bonds of the two molecules are 1.393 (9) and 1.397 (9) Å, respectively. These bond lengths are comparable to the Caromatic—O bond length (1.405 Å) reported for the structurally related 2',3,5,5'-trichloro-biphenyl-4-yl 2,2,2-trichloroethyl sulfate (Li et al., 2010b). In contrast, biphenyl-4-yl 2,2,2-trichloroethyl sulfates without electronegative chlorine substituents ortho to the sulfate group have slightly longer Caromatic—O bond length ranging from 1.426 to 1.449 Å (Li et al., 2008; Li et al., 2010b; Li et al., 2010a; Li et al., 2010c).
The lengths of the PCB sulfate ester bond of the title compound (i.e., S1—O1) are 1.600 (5) and 1.590 (5) Å. In contrast, biphenyl-4-yl 2,2,2-trichloroethyl sulfates without chlorine substituents ortho to the sulfate group typically have shorter sulfate ester bond lengths ranging from 1.563 to 1.586 Å (Li et al., 2008; Li et al., 2010b; Li et al., 2010a; Li et al., 2010c). Similar to aromatic sulfate monoesters (Brandao et al., 2005; Denehy et al., 2006), this difference suggests that chlorine substituents ortho to the sulfate group decrease the stability of the S—O ester bond.
The dihedral angle of the biphenyl moiety of PCB derivatives is a structural parameter associated with the affinity of PCB derivatives for cellular target molecules. The two molecules of the title compound have solid state dihedral angles of 37.8 (2)° and 35.0 (2)°. Similarly, structurally related biphenyl-4-yl 2,2,2-trichloroethyl sulfates have dihedral angles ranging from 4.9° to 41.8° in the solid state (Li et al., 2008; Li et al., 2010a; Li et al., 2010c). The fact that biphenyl-4-yl 2,2,2-trichloroethyl sulfates without ortho chlorine substituents adopt a range of dihedral angles can be explained by crystal packing effects, which force the biphenyl moiety to adopt an energetically less favorable conformation in the solid state.
Experimental
The title compound was synthesized from 3,4',5-trichlorobiphenyl-4-ol and 2,2,2-trichloroethyl sulfonyl chloride using 4-dimethylaminopyridine as catalyst as reported previously (Li et al., 2008). Crystals suitable for X-ray diffraction analysis were obtained by slow evaporation of a methanolic solution.
Refinement
H atoms were found in difference Fourier maps and subsequently placed in idealized positions with constrained distances of 0.99 Å (R2CH2), 0.95 Å (Csp2H), and with Uiso(H) values set to either 1.2Ueq or 1.5Ueq (RCH3, OH) of the attached atom.
The two independent molecules are related by a pseudo-inversion centre, which results in large correlations between the displacement parameters. In order to ensure satisfactory refinement, the displacement parameters of equivalent atoms in each molecule were constrained to be the same using the EADP command of SHELXL97.
Figures
Fig. 1.
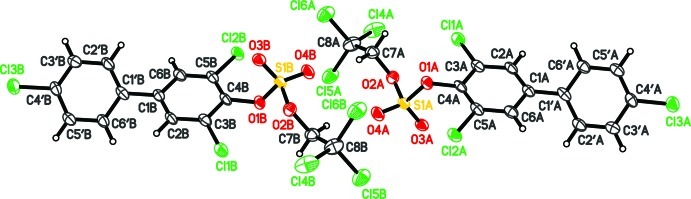
View of the title compound showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 50% probability level.
Crystal data
| C14H8Cl6O4S | F(000) = 1936 |
| Mr = 484.96 | Dx = 1.741 Mg m−3 |
| Orthorhombic, Pca21 | Cu Kα radiation, λ = 1.54178 Å |
| Hall symbol: P 2c -2ac | Cell parameters from 9992 reflections |
| a = 13.993 (3) Å | θ = 3.1–68.3° |
| b = 9.1890 (18) Å | µ = 9.71 mm−1 |
| c = 28.778 (6) Å | T = 90 K |
| V = 3700.3 (13) Å3 | Flake, colourless |
| Z = 8 | 0.17 × 0.09 × 0.02 mm |
Data collection
| Bruker X8 Proteum diffractometer | 6651 independent reflections |
| Radiation source: fine-focus rotating anode | 6238 reflections with I > 2σ(I) |
| Graded multilayer optics monochromator | Rint = 0.062 |
| Detector resolution: 5.6 pixels mm-1 | θmax = 68.4°, θmin = 3.1° |
| φ and ω scans | h = −14→16 |
| Absorption correction: multi-scan (SADABS; Bruker, 2006) | k = −10→11 |
| Tmin = 0.504, Tmax = 0.830 | l = −34→34 |
| 45894 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.064 | H-atom parameters constrained |
| wR(F2) = 0.161 | w = 1/[σ2(Fo2) + (0.0514P)2 + 21.3733P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.15 | (Δ/σ)max < 0.001 |
| 6651 reflections | Δρmax = 0.96 e Å−3 |
| 302 parameters | Δρmin = −0.85 e Å−3 |
| 1 restraint | Absolute structure: Flack (1983), 3176 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.15 (3) |
Special details
| Experimental. The crystal was twinned by inversion, but with unequal sized pieces of each component. The refined Flack parameter indicates major:minor fractions of 0.85 (3):0.15 (3). |
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against all reflections. The weighted R-value wR and goodness of fit S are based on F2. Conventional R-values R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-values based on F2 are statistically about twice as large as those based on F, and R-values based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1A | 0.77740 (12) | 0.4047 (2) | 0.35290 (7) | 0.0283 (2) | |
| O1A | 0.8439 (4) | 0.2785 (5) | 0.33235 (19) | 0.0273 (7) | |
| O2A | 0.8158 (4) | 0.4167 (6) | 0.40377 (19) | 0.0313 (7) | |
| O3A | 0.7985 (4) | 0.5393 (6) | 0.3314 (2) | 0.0302 (7) | |
| O4A | 0.6838 (4) | 0.3483 (6) | 0.3531 (2) | 0.0349 (7) | |
| Cl1A | 1.03327 (13) | 0.3420 (2) | 0.37524 (7) | 0.0380 (3) | |
| Cl2A | 0.78298 (12) | 0.2737 (2) | 0.23480 (7) | 0.0318 (2) | |
| Cl3A | 1.37897 (13) | 0.5084 (2) | 0.10448 (8) | 0.0378 (3) | |
| Cl4A | 0.8960 (2) | 0.4431 (3) | 0.50012 (9) | 0.0563 (4) | |
| Cl5A | 0.69326 (19) | 0.4810 (2) | 0.48583 (9) | 0.0487 (4) | |
| Cl6A | 0.76595 (19) | 0.2066 (2) | 0.52014 (8) | 0.0489 (4) | |
| C1A | 1.0641 (5) | 0.3630 (7) | 0.2372 (3) | 0.0240 (9) | |
| C2A | 1.0822 (5) | 0.3612 (8) | 0.2847 (3) | 0.0273 (9) | |
| H2A | 1.1456 | 0.3760 | 0.2956 | 0.033* | |
| C3A | 1.0090 (5) | 0.3381 (8) | 0.3166 (3) | 0.0276 (9) | |
| C4A | 0.9162 (5) | 0.3143 (8) | 0.3011 (3) | 0.0252 (9) | |
| C5A | 0.8987 (5) | 0.3119 (7) | 0.2542 (3) | 0.0259 (9) | |
| C6A | 0.9704 (5) | 0.3381 (8) | 0.2223 (3) | 0.0273 (9) | |
| H6A | 0.9560 | 0.3393 | 0.1900 | 0.033* | |
| C7A | 0.7997 (6) | 0.2919 (9) | 0.4344 (3) | 0.0326 (10) | |
| H7A1 | 0.8546 | 0.2241 | 0.4332 | 0.039* | |
| H7A2 | 0.7412 | 0.2386 | 0.4251 | 0.039* | |
| C8A | 0.7887 (7) | 0.3548 (10) | 0.4822 (3) | 0.0431 (12) | |
| C1'A | 1.1400 (5) | 0.3932 (8) | 0.2034 (3) | 0.0284 (9) | |
| C2'A | 1.1233 (5) | 0.4783 (9) | 0.1639 (3) | 0.0314 (11) | |
| H2'A | 1.0607 | 0.5138 | 0.1581 | 0.038* | |
| C3'A | 1.1959 (5) | 0.5118 (9) | 0.1333 (3) | 0.0307 (10) | |
| H3'A | 1.1833 | 0.5691 | 0.1065 | 0.037* | |
| C4'A | 1.2877 (5) | 0.4609 (9) | 0.1420 (3) | 0.0284 (10) | |
| C5'A | 1.3053 (5) | 0.3752 (8) | 0.1800 (3) | 0.0293 (10) | |
| H5'A | 1.3677 | 0.3379 | 0.1850 | 0.035* | |
| C6'A | 1.2329 (5) | 0.3424 (8) | 0.2113 (3) | 0.0285 (9) | |
| H6'A | 1.2464 | 0.2854 | 0.2380 | 0.034* | |
| S1B | 0.48992 (12) | 0.0952 (2) | 0.44025 (7) | 0.0283 (2) | |
| O1B | 0.4243 (3) | 0.2207 (5) | 0.46086 (19) | 0.0273 (7) | |
| O2B | 0.4512 (4) | 0.0835 (6) | 0.38944 (19) | 0.0313 (7) | |
| O3B | 0.4667 (4) | −0.0367 (6) | 0.4615 (2) | 0.0302 (7) | |
| O4B | 0.5834 (4) | 0.1524 (6) | 0.4402 (2) | 0.0349 (7) | |
| Cl1B | 0.23508 (13) | 0.1574 (2) | 0.41731 (7) | 0.0380 (3) | |
| Cl2B | 0.48229 (12) | 0.2267 (2) | 0.55864 (7) | 0.0318 (2) | |
| Cl3B | −0.11866 (13) | 0.0187 (2) | 0.68821 (8) | 0.0378 (3) | |
| Cl4B | 0.3715 (2) | 0.0537 (3) | 0.29351 (9) | 0.0563 (4) | |
| Cl5B | 0.50048 (19) | 0.2929 (2) | 0.27257 (8) | 0.0487 (4) | |
| Cl6B | 0.57633 (19) | 0.0222 (3) | 0.30941 (8) | 0.0489 (4) | |
| C1B | 0.2034 (5) | 0.1376 (8) | 0.5547 (3) | 0.0240 (9) | |
| C2B | 0.1855 (5) | 0.1373 (8) | 0.5077 (3) | 0.0273 (9) | |
| H2B | 0.1222 | 0.1221 | 0.4967 | 0.033* | |
| C3B | 0.2590 (5) | 0.1590 (8) | 0.4759 (3) | 0.0276 (9) | |
| C4B | 0.3515 (5) | 0.1866 (8) | 0.4923 (3) | 0.0252 (9) | |
| C5B | 0.3684 (5) | 0.1883 (8) | 0.5392 (3) | 0.0259 (9) | |
| C6B | 0.2963 (5) | 0.1672 (8) | 0.5714 (3) | 0.0273 (9) | |
| H6B | 0.3089 | 0.1725 | 0.6038 | 0.033* | |
| C7B | 0.4686 (6) | 0.2041 (9) | 0.3589 (3) | 0.0326 (10) | |
| H7B1 | 0.5276 | 0.2556 | 0.3684 | 0.039* | |
| H7B2 | 0.4146 | 0.2736 | 0.3604 | 0.039* | |
| C8B | 0.4791 (7) | 0.1461 (10) | 0.3102 (3) | 0.0431 (12) | |
| C1'B | 0.1230 (5) | 0.1095 (8) | 0.5886 (3) | 0.0284 (9) | |
| C2'B | 0.1395 (5) | 0.0364 (9) | 0.6294 (3) | 0.0314 (11) | |
| H2'B | 0.2024 | 0.0042 | 0.6364 | 0.038* | |
| C3'B | 0.0659 (5) | 0.0087 (8) | 0.6607 (3) | 0.0307 (10) | |
| H3'B | 0.0784 | −0.0421 | 0.6888 | 0.037* | |
| C4'B | −0.0243 (5) | 0.0551 (9) | 0.6506 (3) | 0.0284 (10) | |
| C5'B | −0.0442 (5) | 0.1307 (8) | 0.6098 (3) | 0.0293 (10) | |
| H5'B | −0.1072 | 0.1629 | 0.6031 | 0.035* | |
| C6'B | 0.0308 (5) | 0.1580 (8) | 0.5789 (3) | 0.0285 (9) | |
| H6'B | 0.0188 | 0.2102 | 0.5510 | 0.034* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1A | 0.0183 (5) | 0.0276 (5) | 0.0392 (6) | 0.0017 (4) | 0.0002 (4) | 0.0039 (4) |
| O1A | 0.0202 (14) | 0.0205 (15) | 0.0411 (17) | −0.0046 (12) | 0.0037 (13) | 0.0031 (12) |
| O2A | 0.0285 (16) | 0.0267 (16) | 0.0386 (17) | −0.0036 (13) | 0.0023 (13) | 0.0059 (13) |
| O3A | 0.0248 (17) | 0.0217 (16) | 0.0442 (18) | 0.0022 (12) | 0.0006 (13) | 0.0063 (13) |
| O4A | 0.0187 (15) | 0.0370 (18) | 0.0491 (19) | −0.0033 (13) | −0.0001 (14) | 0.0041 (15) |
| Cl1A | 0.0219 (5) | 0.0543 (7) | 0.0379 (6) | −0.0016 (5) | −0.0044 (5) | 0.0037 (5) |
| Cl2A | 0.0167 (5) | 0.0340 (6) | 0.0447 (6) | −0.0060 (4) | −0.0047 (4) | 0.0021 (5) |
| Cl3A | 0.0279 (6) | 0.0455 (7) | 0.0399 (6) | −0.0032 (5) | 0.0037 (5) | 0.0000 (6) |
| Cl4A | 0.0802 (11) | 0.0341 (6) | 0.0545 (8) | −0.0139 (7) | −0.0252 (7) | 0.0035 (5) |
| Cl5A | 0.0709 (11) | 0.0286 (7) | 0.0465 (9) | 0.0093 (7) | 0.0109 (8) | 0.0067 (7) |
| Cl6A | 0.0715 (11) | 0.0299 (8) | 0.0454 (9) | 0.0031 (7) | 0.0054 (8) | 0.0030 (7) |
| C1A | 0.018 (2) | 0.0134 (18) | 0.040 (2) | 0.0007 (16) | 0.0015 (18) | 0.0006 (17) |
| C2A | 0.0137 (19) | 0.023 (2) | 0.045 (3) | −0.0001 (16) | −0.0014 (17) | 0.0013 (19) |
| C3A | 0.019 (2) | 0.020 (2) | 0.044 (2) | 0.0014 (17) | −0.0017 (18) | 0.0002 (18) |
| C4A | 0.0122 (18) | 0.0156 (19) | 0.048 (3) | −0.0009 (15) | −0.0002 (18) | 0.0014 (17) |
| C5A | 0.017 (2) | 0.0120 (18) | 0.048 (3) | 0.0000 (16) | 0.0010 (18) | 0.0004 (17) |
| C6A | 0.020 (2) | 0.022 (2) | 0.040 (2) | −0.0008 (17) | −0.0039 (18) | −0.0016 (18) |
| C7A | 0.033 (2) | 0.027 (2) | 0.038 (2) | 0.001 (2) | −0.002 (2) | 0.0039 (19) |
| C8A | 0.060 (3) | 0.028 (2) | 0.041 (3) | −0.002 (2) | −0.007 (3) | 0.000 (2) |
| C1'A | 0.017 (2) | 0.024 (2) | 0.044 (2) | 0.0023 (17) | −0.0018 (18) | −0.0069 (19) |
| C2'A | 0.017 (2) | 0.034 (3) | 0.043 (2) | 0.0000 (19) | −0.0038 (19) | 0.000 (2) |
| C3'A | 0.025 (2) | 0.030 (2) | 0.037 (2) | 0.002 (2) | −0.005 (2) | 0.000 (2) |
| C4'A | 0.019 (2) | 0.026 (2) | 0.040 (2) | −0.0023 (18) | 0.0028 (18) | −0.0037 (19) |
| C5'A | 0.019 (2) | 0.026 (2) | 0.043 (3) | 0.0008 (18) | −0.0034 (18) | −0.003 (2) |
| C6'A | 0.019 (2) | 0.023 (2) | 0.042 (3) | −0.0013 (17) | −0.0001 (19) | 0.001 (2) |
| S1B | 0.0183 (5) | 0.0276 (5) | 0.0392 (6) | 0.0017 (4) | 0.0002 (4) | 0.0039 (4) |
| O1B | 0.0202 (14) | 0.0205 (15) | 0.0411 (17) | −0.0046 (12) | 0.0037 (13) | 0.0031 (12) |
| O2B | 0.0285 (16) | 0.0267 (16) | 0.0386 (17) | −0.0036 (13) | 0.0023 (13) | 0.0059 (13) |
| O3B | 0.0248 (17) | 0.0217 (16) | 0.0442 (18) | 0.0022 (12) | 0.0006 (13) | 0.0063 (13) |
| O4B | 0.0187 (15) | 0.0370 (18) | 0.0491 (19) | −0.0033 (13) | −0.0001 (14) | 0.0041 (15) |
| Cl1B | 0.0219 (5) | 0.0543 (7) | 0.0379 (6) | −0.0016 (5) | −0.0044 (5) | 0.0037 (5) |
| Cl2B | 0.0167 (5) | 0.0340 (6) | 0.0447 (6) | −0.0060 (4) | −0.0047 (4) | 0.0021 (5) |
| Cl3B | 0.0279 (6) | 0.0455 (7) | 0.0399 (6) | −0.0032 (5) | 0.0037 (5) | 0.0000 (6) |
| Cl4B | 0.0802 (11) | 0.0341 (6) | 0.0545 (8) | −0.0139 (7) | −0.0252 (7) | 0.0035 (5) |
| Cl5B | 0.0709 (11) | 0.0286 (7) | 0.0465 (9) | 0.0093 (7) | 0.0109 (8) | 0.0067 (7) |
| Cl6B | 0.0715 (11) | 0.0299 (8) | 0.0454 (9) | 0.0031 (7) | 0.0054 (8) | 0.0030 (7) |
| C1B | 0.018 (2) | 0.0134 (18) | 0.040 (2) | 0.0007 (16) | 0.0015 (18) | 0.0006 (17) |
| C2B | 0.0137 (19) | 0.023 (2) | 0.045 (3) | −0.0001 (16) | −0.0014 (17) | 0.0013 (19) |
| C3B | 0.019 (2) | 0.020 (2) | 0.044 (2) | 0.0014 (17) | −0.0017 (18) | 0.0002 (18) |
| C4B | 0.0122 (18) | 0.0156 (19) | 0.048 (3) | −0.0009 (15) | −0.0002 (18) | 0.0014 (17) |
| C5B | 0.017 (2) | 0.0120 (18) | 0.048 (3) | 0.0000 (16) | 0.0010 (18) | 0.0004 (17) |
| C6B | 0.020 (2) | 0.022 (2) | 0.040 (2) | −0.0008 (17) | −0.0039 (18) | −0.0016 (18) |
| C7B | 0.033 (2) | 0.027 (2) | 0.038 (2) | 0.001 (2) | −0.002 (2) | 0.0039 (19) |
| C8B | 0.060 (3) | 0.028 (2) | 0.041 (3) | −0.002 (2) | −0.007 (3) | 0.000 (2) |
| C1'B | 0.017 (2) | 0.024 (2) | 0.044 (2) | 0.0023 (17) | −0.0018 (18) | −0.0069 (19) |
| C2'B | 0.017 (2) | 0.034 (3) | 0.043 (2) | 0.0000 (19) | −0.0038 (19) | 0.000 (2) |
| C3'B | 0.025 (2) | 0.030 (2) | 0.037 (2) | 0.002 (2) | −0.005 (2) | 0.000 (2) |
| C4'B | 0.019 (2) | 0.026 (2) | 0.040 (2) | −0.0023 (18) | 0.0028 (18) | −0.0037 (19) |
| C5'B | 0.019 (2) | 0.026 (2) | 0.043 (3) | 0.0008 (18) | −0.0034 (18) | −0.003 (2) |
| C6'B | 0.019 (2) | 0.023 (2) | 0.042 (3) | −0.0013 (17) | −0.0001 (19) | 0.001 (2) |
Geometric parameters (Å, º)
| S1A—O4A | 1.408 (5) | S1B—O3B | 1.396 (6) |
| S1A—O3A | 1.414 (6) | S1B—O4B | 1.409 (5) |
| S1A—O2A | 1.563 (6) | S1B—O2B | 1.563 (6) |
| S1A—O1A | 1.600 (5) | S1B—O1B | 1.590 (5) |
| O1A—C4A | 1.393 (9) | O1B—C4B | 1.397 (9) |
| O2A—C7A | 1.464 (9) | O2B—C7B | 1.434 (9) |
| Cl1A—C3A | 1.722 (9) | Cl1B—C3B | 1.720 (8) |
| Cl2A—C5A | 1.749 (7) | Cl2B—C5B | 1.726 (7) |
| Cl3A—C4'A | 1.730 (8) | Cl3B—C4'B | 1.739 (8) |
| Cl4A—C8A | 1.783 (10) | Cl4B—C8B | 1.793 (10) |
| Cl5A—C8A | 1.772 (10) | Cl5B—C8B | 1.755 (9) |
| Cl6A—C8A | 1.774 (9) | Cl6B—C8B | 1.774 (10) |
| C1A—C2A | 1.391 (11) | C1B—C2B | 1.378 (11) |
| C1A—C6A | 1.400 (10) | C1B—C6B | 1.412 (10) |
| C1A—C1'A | 1.467 (10) | C1B—C1'B | 1.510 (10) |
| C2A—C3A | 1.390 (11) | C2B—C3B | 1.389 (11) |
| C2A—H2A | 0.9500 | C2B—H2B | 0.9500 |
| C3A—C4A | 1.390 (10) | C3B—C4B | 1.401 (10) |
| C4A—C5A | 1.373 (11) | C4B—C5B | 1.370 (11) |
| C5A—C6A | 1.381 (11) | C5B—C6B | 1.384 (11) |
| C6A—H6A | 0.9500 | C6B—H6B | 0.9500 |
| C7A—C8A | 1.500 (12) | C7B—C8B | 1.509 (12) |
| C7A—H7A1 | 0.9900 | C7B—H7B1 | 0.9900 |
| C7A—H7A2 | 0.9900 | C7B—H7B2 | 0.9900 |
| C1'A—C6'A | 1.399 (10) | C1'B—C2'B | 1.373 (12) |
| C1'A—C2'A | 1.400 (12) | C1'B—C6'B | 1.393 (10) |
| C2'A—C3'A | 1.380 (12) | C2'B—C3'B | 1.391 (12) |
| C2'A—H2'A | 0.9500 | C2'B—H2'B | 0.9500 |
| C3'A—C4'A | 1.389 (11) | C3'B—C4'B | 1.364 (10) |
| C3'A—H3'A | 0.9500 | C3'B—H3'B | 0.9500 |
| C4'A—C5'A | 1.369 (12) | C4'B—C5'B | 1.392 (12) |
| C5'A—C6'A | 1.388 (11) | C5'B—C6'B | 1.399 (11) |
| C5'A—H5'A | 0.9500 | C5'B—H5'B | 0.9500 |
| C6'A—H6'A | 0.9500 | C6'B—H6'B | 0.9500 |
| O4A—S1A—O3A | 121.2 (3) | O3B—S1B—O4B | 122.7 (3) |
| O4A—S1A—O2A | 109.9 (3) | O3B—S1B—O2B | 105.6 (3) |
| O3A—S1A—O2A | 106.0 (3) | O4B—S1B—O2B | 110.3 (3) |
| O4A—S1A—O1A | 106.0 (3) | O3B—S1B—O1B | 109.4 (3) |
| O3A—S1A—O1A | 110.5 (3) | O4B—S1B—O1B | 105.4 (3) |
| O2A—S1A—O1A | 101.4 (3) | O2B—S1B—O1B | 101.4 (3) |
| C4A—O1A—S1A | 119.3 (4) | C4B—O1B—S1B | 120.0 (4) |
| C7A—O2A—S1A | 117.1 (5) | C7B—O2B—S1B | 117.5 (5) |
| C2A—C1A—C6A | 118.1 (7) | C2B—C1B—C6B | 120.1 (7) |
| C2A—C1A—C1'A | 121.5 (7) | C2B—C1B—C1'B | 119.9 (6) |
| C6A—C1A—C1'A | 120.3 (7) | C6B—C1B—C1'B | 120.0 (7) |
| C3A—C2A—C1A | 121.1 (7) | C1B—C2B—C3B | 120.8 (7) |
| C3A—C2A—H2A | 119.5 | C1B—C2B—H2B | 119.6 |
| C1A—C2A—H2A | 119.5 | C3B—C2B—H2B | 119.6 |
| C4A—C3A—C2A | 120.0 (8) | C2B—C3B—C4B | 119.3 (8) |
| C4A—C3A—Cl1A | 120.1 (6) | C2B—C3B—Cl1B | 119.9 (6) |
| C2A—C3A—Cl1A | 119.8 (6) | C4B—C3B—Cl1B | 120.7 (6) |
| C5A—C4A—C3A | 119.0 (7) | C5B—C4B—O1B | 120.6 (6) |
| C5A—C4A—O1A | 120.1 (6) | C5B—C4B—C3B | 119.5 (7) |
| C3A—C4A—O1A | 120.5 (7) | O1B—C4B—C3B | 119.8 (7) |
| C4A—C5A—C6A | 121.5 (7) | C4B—C5B—C6B | 122.2 (7) |
| C4A—C5A—Cl2A | 118.8 (6) | C4B—C5B—Cl2B | 118.7 (6) |
| C6A—C5A—Cl2A | 119.7 (6) | C6B—C5B—Cl2B | 119.0 (6) |
| C5A—C6A—C1A | 120.3 (8) | C5B—C6B—C1B | 118.1 (7) |
| C5A—C6A—H6A | 119.9 | C5B—C6B—H6B | 121.0 |
| C1A—C6A—H6A | 119.9 | C1B—C6B—H6B | 121.0 |
| O2A—C7A—C8A | 105.4 (6) | O2B—C7B—C8B | 108.2 (7) |
| O2A—C7A—H7A1 | 110.7 | O2B—C7B—H7B1 | 110.0 |
| C8A—C7A—H7A1 | 110.7 | C8B—C7B—H7B1 | 110.0 |
| O2A—C7A—H7A2 | 110.7 | O2B—C7B—H7B2 | 110.0 |
| C8A—C7A—H7A2 | 110.7 | C8B—C7B—H7B2 | 110.0 |
| H7A1—C7A—H7A2 | 108.8 | H7B1—C7B—H7B2 | 108.4 |
| C7A—C8A—Cl5A | 112.5 (6) | C7B—C8B—Cl5B | 108.6 (6) |
| C7A—C8A—Cl6A | 106.7 (6) | C7B—C8B—Cl6B | 108.2 (6) |
| Cl5A—C8A—Cl6A | 109.3 (5) | Cl5B—C8B—Cl6B | 110.8 (6) |
| C7A—C8A—Cl4A | 110.8 (7) | C7B—C8B—Cl4B | 109.5 (7) |
| Cl5A—C8A—Cl4A | 108.6 (5) | Cl5B—C8B—Cl4B | 110.0 (5) |
| Cl6A—C8A—Cl4A | 108.8 (5) | Cl6B—C8B—Cl4B | 109.7 (5) |
| C6'A—C1'A—C2'A | 118.2 (7) | C2'B—C1'B—C6'B | 118.9 (7) |
| C6'A—C1'A—C1A | 120.1 (7) | C2'B—C1'B—C1B | 120.7 (6) |
| C2'A—C1'A—C1A | 121.6 (6) | C6'B—C1'B—C1B | 120.4 (7) |
| C3'A—C2'A—C1'A | 121.3 (7) | C1'B—C2'B—C3'B | 121.2 (7) |
| C3'A—C2'A—H2'A | 119.3 | C1'B—C2'B—H2'B | 119.4 |
| C1'A—C2'A—H2'A | 119.3 | C3'B—C2'B—H2'B | 119.4 |
| C2'A—C3'A—C4'A | 119.3 (8) | C4'B—C3'B—C2'B | 119.4 (8) |
| C2'A—C3'A—H3'A | 120.3 | C4'B—C3'B—H3'B | 120.3 |
| C4'A—C3'A—H3'A | 120.3 | C2'B—C3'B—H3'B | 120.3 |
| C5'A—C4'A—C3'A | 120.3 (7) | C3'B—C4'B—C5'B | 121.3 (8) |
| C5'A—C4'A—Cl3A | 120.7 (6) | C3'B—C4'B—Cl3B | 120.7 (7) |
| C3'A—C4'A—Cl3A | 118.9 (7) | C5'B—C4'B—Cl3B | 118.0 (6) |
| C4'A—C5'A—C6'A | 120.7 (7) | C4'B—C5'B—C6'B | 118.5 (7) |
| C4'A—C5'A—H5'A | 119.6 | C4'B—C5'B—H5'B | 120.8 |
| C6'A—C5'A—H5'A | 119.6 | C6'B—C5'B—H5'B | 120.8 |
| C5'A—C6'A—C1'A | 120.0 (8) | C1'B—C6'B—C5'B | 120.6 (8) |
| C5'A—C6'A—H6'A | 120.0 | C1'B—C6'B—H6'B | 119.7 |
| C1'A—C6'A—H6'A | 120.0 | C5'B—C6'B—H6'B | 119.7 |
| O4A—S1A—O1A—C4A | 138.7 (5) | O3B—S1B—O1B—C4B | −4.9 (6) |
| O3A—S1A—O1A—C4A | 5.6 (6) | O4B—S1B—O1B—C4B | −138.6 (6) |
| O2A—S1A—O1A—C4A | −106.4 (5) | O2B—S1B—O1B—C4B | 106.3 (6) |
| O4A—S1A—O2A—C7A | 45.2 (6) | O3B—S1B—O2B—C7B | −177.9 (5) |
| O3A—S1A—O2A—C7A | 177.8 (5) | O4B—S1B—O2B—C7B | −43.3 (6) |
| O1A—S1A—O2A—C7A | −66.7 (5) | O1B—S1B—O2B—C7B | 68.0 (6) |
| C6A—C1A—C2A—C3A | −1.2 (11) | C6B—C1B—C2B—C3B | 3.1 (11) |
| C1'A—C1A—C2A—C3A | 177.5 (7) | C1'B—C1B—C2B—C3B | −178.2 (7) |
| C1A—C2A—C3A—C4A | 0.9 (11) | C1B—C2B—C3B—C4B | −2.3 (11) |
| C1A—C2A—C3A—Cl1A | −178.0 (6) | C1B—C2B—C3B—Cl1B | 179.7 (6) |
| C2A—C3A—C4A—C5A | 1.0 (11) | S1B—O1B—C4B—C5B | 91.6 (8) |
| Cl1A—C3A—C4A—C5A | 179.9 (5) | S1B—O1B—C4B—C3B | −92.2 (7) |
| C2A—C3A—C4A—O1A | 174.4 (6) | C2B—C3B—C4B—C5B | 1.6 (11) |
| Cl1A—C3A—C4A—O1A | −6.7 (10) | Cl1B—C3B—C4B—C5B | 179.5 (6) |
| S1A—O1A—C4A—C5A | −92.0 (7) | C2B—C3B—C4B—O1B | −174.6 (6) |
| S1A—O1A—C4A—C3A | 94.7 (7) | Cl1B—C3B—C4B—O1B | 3.3 (10) |
| C3A—C4A—C5A—C6A | −2.6 (11) | O1B—C4B—C5B—C6B | 174.4 (6) |
| O1A—C4A—C5A—C6A | −176.0 (6) | C3B—C4B—C5B—C6B | −1.8 (11) |
| C3A—C4A—C5A—Cl2A | 177.1 (5) | O1B—C4B—C5B—Cl2B | −2.3 (10) |
| O1A—C4A—C5A—Cl2A | 3.7 (9) | C3B—C4B—C5B—Cl2B | −178.5 (5) |
| C4A—C5A—C6A—C1A | 2.3 (11) | C4B—C5B—C6B—C1B | 2.5 (11) |
| Cl2A—C5A—C6A—C1A | −177.4 (5) | Cl2B—C5B—C6B—C1B | 179.2 (5) |
| C2A—C1A—C6A—C5A | −0.4 (11) | C2B—C1B—C6B—C5B | −3.2 (11) |
| C1'A—C1A—C6A—C5A | −179.1 (6) | C1'B—C1B—C6B—C5B | 178.2 (6) |
| S1A—O2A—C7A—C8A | −148.3 (6) | S1B—O2B—C7B—C8B | 148.2 (6) |
| O2A—C7A—C8A—Cl5A | 58.7 (8) | O2B—C7B—C8B—Cl5B | −180.0 (5) |
| O2A—C7A—C8A—Cl6A | 178.5 (5) | O2B—C7B—C8B—Cl6B | −59.6 (8) |
| O2A—C7A—C8A—Cl4A | −63.2 (7) | O2B—C7B—C8B—Cl4B | 59.9 (8) |
| C2A—C1A—C1'A—C6'A | 36.2 (11) | C2B—C1B—C1'B—C2'B | 145.8 (8) |
| C6A—C1A—C1'A—C6'A | −145.1 (7) | C6B—C1B—C1'B—C2'B | −35.5 (11) |
| C2A—C1A—C1'A—C2'A | −140.7 (8) | C2B—C1B—C1'B—C6'B | −34.4 (11) |
| C6A—C1A—C1'A—C2'A | 38.0 (11) | C6B—C1B—C1'B—C6'B | 144.2 (7) |
| C6'A—C1'A—C2'A—C3'A | 0.1 (12) | C6'B—C1'B—C2'B—C3'B | 0.8 (12) |
| C1A—C1'A—C2'A—C3'A | 177.0 (7) | C1B—C1'B—C2'B—C3'B | −179.4 (7) |
| C1'A—C2'A—C3'A—C4'A | −0.5 (12) | C1'B—C2'B—C3'B—C4'B | 0.0 (13) |
| C2'A—C3'A—C4'A—C5'A | 1.7 (12) | C2'B—C3'B—C4'B—C5'B | −0.5 (12) |
| C2'A—C3'A—C4'A—Cl3A | −177.9 (6) | C2'B—C3'B—C4'B—Cl3B | 178.5 (6) |
| C3'A—C4'A—C5'A—C6'A | −2.4 (12) | C3'B—C4'B—C5'B—C6'B | 0.3 (12) |
| Cl3A—C4'A—C5'A—C6'A | 177.1 (6) | Cl3B—C4'B—C5'B—C6'B | −178.8 (6) |
| C4'A—C5'A—C6'A—C1'A | 2.0 (12) | C2'B—C1'B—C6'B—C5'B | −1.1 (12) |
| C2'A—C1'A—C6'A—C5'A | −0.8 (11) | C1B—C1'B—C6'B—C5'B | 179.2 (7) |
| C1A—C1'A—C6'A—C5'A | −177.8 (7) | C4'B—C5'B—C6'B—C1'B | 0.5 (11) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: YK2088).
References
- Brandao, T. A. S., Priebe, J. P., Damasceno, A. S., Bortoluzzi, A. J., Kirby, A. J. & Nome, F. (2005). J. Mol. Struct. 734, 205–209.
- Bruker (2006). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Denehy, E., White, J. M. & Williams, S. J. (2006). Chem. Commun. pp. 314–316. [DOI] [PubMed]
- Dhakal, K., He, X., Lehmler, H. J., Teesch, L. M., Duffel, M. W. & Robertson, L. W. (2012). Chem. Res. Toxicol. 25, 2796–2804. [DOI] [PMC free article] [PubMed]
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Li, X., Parkin, S., Duffel, M. W., Robertson, L. W. & Lehmler, H.-J. (2010a). Acta Cryst. E66, o1073. [DOI] [PMC free article] [PubMed]
- Li, X., Parkin, S., Duffel, M. W., Robertson, L. W. & Lehmler, H.-J. (2010b). Environ. Int. 36, 843–848. [DOI] [PMC free article] [PubMed]
- Li, X., Parkin, S., Duffel, M. W., Robertson, L. W. & Lehmler, H.-J. (2010c). Acta Cryst. E66, o1615–o1616. [DOI] [PMC free article] [PubMed]
- Li, X., Parkin, S., Robertson, L. W. & Lehmler, H.-J. (2008). Acta Cryst. E64, o2464. [DOI] [PMC free article] [PubMed]
- Liu, Y., Apak, T. I., Lehmler, H.-J., Robertson, L. W. & Duffel, M. W. (2006). Chem. Res. Toxicol. 19, 1420–1425. [DOI] [PubMed]
- Liu, Y., Smart, J. T., Song, Y., Lehmler, H.-J., Robertson, L. W. & Duffel, M. W. (2009). Drug Metab. Dispos. 37, 1065–1072. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wang, L.-Q., Lehmler, H.-J., Robertson, L. W. & James, M. O. (2006). Chem. Biol. Interact. 159, 235–246. [DOI] [PubMed]
- Zhai, G., Lehmler, H. J. & Schnoor, J. L. (2013). Environ. Sci. Technol. 47, 557–562. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813007976/yk2088sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813007976/yk2088Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813007976/yk2088Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report


