Abstract
Background:
Traditionally, the most common diagnostic approach used for diagnosing leptospirosis was the demonstration of immune-seroconversion in acute and convalescent patient serum samples. Recently, a variety of molecular techniques, including conventional and real-time polymerase chain reaction (PCR), have been developed for the specific detection of pathogenic bacteria from the genus Leptospira. PCR is a sensitive, specific, and rapid technique that has been successfully used to detect several microorganisms; including those of clinical significance.
Methods:
In this study, we developed a multiplex PCR (mPCR) assay for detecting Leptospira’s DNA. The mPCR assay detects both the 16S rRNA gene and the major outer membrane lipoprotein gene, which is known as LipL32. Representative serovars were tested from 10 species of Leptospira and 23 other species of bacteria.
Results:
A positive result was obtained from all leptospiral serovars. The amplification sensitivity for the multiplex assay was 21.8 pg and 1 × 103 leptospires/ml. This mPCR assay has the potential to facilitate a rapid and sensitive diagnosis for acute leptospirosis.
Conclusion:
The mPCR assay developed in this study can be used for the early detection of leptospirosis. The LipL32 gene could also serve as another target to aid in the efficient detection of leptospiral infection because using 2 sets of primers in mPCR increases the sensitivity and specificity of the test.
Keywords: 16S rRNA, leptospirosis, LipL32, mPCR, nucleic acid amplification test
Introduction
Leptospirosis is one of the most common zoonoses in the world. Recently, leptospirosis has been recognised as a re-emerging infectious disease among animals and humans (1–3). Although leptospirosis is distributed worldwide (4), it is most common in temperate and tropical climates. However, leptospirosis has the potential to become even more prevalent with the anticipated rise in global temperature (2,5,6). It was reported that the whole region of Southeast Asia is an endemic area for leptospirosis (7), including Malaysia as several outbreaks have been recorded. An outbreak of acute febrile illness was reported among the athletes participating in the Eco-Challenge located in Sabah, and the Segama River was found to be the primary source of infection (8). Furthermore, 46 cases were identified in an outbreak in Kampung, Kebatu, Beaufort, Sabah that was associated with swimming in a creek near an oil palm plantation (9) and a few outbreaks involving individuals (10). Lastly, there was a report of a sudden outbreak of acute febrile illness following the heavy rain and floods in the southern state of Johor (11,12).
Leptospirosis is caused by pathogenic bacteria from the genus Leptospira, which is a spirochete of the family Leptospiraceae and the order Spirochatales. Approximately 10 million people are affected by leptospirosis throughout the year with a 5%–25% fatality rate (5). However, with early detection and treatment, leptospirosis is a treatable disease. Nevertheless, early detection and treatment depends on epidemiological factors, symptoms and laboratory tests. A large array of tests for this disease have been described, including microscopic demonstration, molecular diagnosis, isolation and identification of leptospires, as well as serological diagnosis, such as the microscopic agglutination test (MAT) (3). There are several limitations to MAT, including the false-negative results obtained early in the course of the disease, the cross-reactivity excluding the ability to distinguish between serovars, the highly labour-intensive and time-consuming nature of the technique, and the requirement of a large panel of live leptospires (1). Thus, MAT is a complex test to control, perform, and interpret.
Enzyme-linked immunosorbent assay (ELISA) and micro-capsular agglutination test (MCAT) are serological tests that reduce the false-positives associated with vaccinal responses and provide earlier detection. In Australia, it was found that the IgM ELISA test could exhibit notably high sensitivity (100%) for the detection of acute leptospirosis (13). However, the same commercial assay demonstrated poor diagnostic accuracy (sensitivity of 60.9% and specificity of 65.6%) using admission serum in Laos (14). Bacterial culture, MAT, and ELISA can be applied in well-equipped laboratories by trained staff. However, few diagnostic facilities have the capacity and expertise to perform these tests for leptospirosis.
PCR has been used to detect leptospiral DNA in samples obtained from animals (15,16) and human (17–22). In this study, we have developed an mPCR amplification with 2 sets of primers, which will aid in the specific and sensitive detection of Leptospira species. The mPCR targets the well-known 16s rRNA gene (17), and a newly designed primer that targets the LipL32 gene. The LipL32 gene is a major outer-membrane lipoprotein from the genus Leptospira. Furthermore, the LipL32 gene is an important virulence factor confined to pathogenic strains of all Leptospira species. The mPCR could be used as an alternative to the traditional diagnostic methods, such as leptospiral isolation and the MAT serological assay.
Materials and Methods
Organisms and growth conditions
Leptospiral serovars were obtained from Universiti Putra Malaysia, Selangor. Leptospira were grown in Ellinghausen, McCullough, Johnson, and Harris (EMJH) medium (Becton-Dickinson Biosciences, Detroit, US) at 28 °C and were periodically subcultured in fresh medium. Commonly encountered commensal and pathogenic bacteria cultures from our own stock culture were used as controls.
Genomic DNA isolation and purification
Using an in-house genomic extraction protocol, DNA extraction was carried out on the cell pellets of leptospiral and bacterial cultures. Briefly, 1.5 mL of culture isolate was centrifuged at 13 000 g for 5 minutes to obtain approximately 0.1 μg of pellet. A solution of 200 μL of 20% sucrose, 50 mM Tris-HCl, 1% SDS, 0.2 N NaOH, 25 mM EDTA, and 0.1 M NaCl was added and the mixture was inverted gently. The mixture was subsequently incubated at room temperature for 5 minutes. For protein precipitation, 200 μL of 3 M NaOAc (pH 5.2) was added, and the mixture was gently inverted. The mixture was subsequently spun down at 13 000 g for 5 minutes at 4 °C to separate the supernatant containing the DNA. The clear supernatant was subsequently placed into a fresh tube and 600 μL of ice-cold 95% ethanol was added to precipitate the genomic DNA. The mixture was incubated in liquid nitrogen for 15 minutes and spun down at 13 000 g for 15 minutes at 4 °C to pellet the DNA. Next, the pellet was washed with 1 mL of 80% ethanol, air-dried, and resuspended in 50 μL of sterile distilled water. The concentration of the DNA extract was measured using a spectrophotometer and stored at –20 °C.
Primers design and PCR amplification
Oligonucleotide sequences of Lep F, Lep R, Lau01, and Lau02 used for the mPCR amplification of the Leptospira species are listed in Table 1. Lep F and Lep R primer (17) amplified a 330 bp sequence from the 16S rRNA gene. The Lau01 and Lau02 primer pair was designed by aligning the LipL32 gene (accession number: NC 005823) using the ClustalW programme (EMBL, UK) (23), and primers were selected using the Primer3 programme (Whitehead, US) (24). Lau01 and Lau02 primer pair amplifies a 660 bp sequence of the LipL32 gene. The specificity of the primers was bioinformatically verified using the Primer-Blast programme (NCBI, MD).
Table 1:
Primers for the mPCR amplification of Leptospira species
| Oligonucleotide sequence 5’-3’ | Length (bp) | Amplicon size (bp) | Ref. |
|---|---|---|---|
| Lep F: GGCGGCGCGTCTTAAACATG | 20 | 330 | 17 |
| Lep R: TCCCCCCATTGAGCAAGATT | 20 | 330 | 17 |
| Lau01: ACTCTTTGCAAGCATTACCGC | 21 | 660 | This study |
| Lau02: AGCAGACCAACAGATGCAACG | 21 | 660 | This study |
The final optimised conditions for the mPCR were as follows: PCR was performed with the 1X Colorless GoTaq® Flexi Buffer (Promega, US), 200 μM (each) deoxynucleoside triphosphates, 1.5 mM MgCl2, 1U of GoTaq® Flexi DNA Polymerase (Promega, US), 5 μM of Lau primers, and 10 μM of Lep primers in a total 20 μL reaction volume. Amplification was performed in a thermocycler (Bio-Rad, US) with initial denaturation at 94 °C for 3 minutes followed by 29 cycles of denaturation at 94 °C for 30 seconds, primer annealing at 58 °C for 30 seconds, and DNA extension at 72 °C for 40 seconds before the final extension step at 72 °C for 5 minutes to complete synthesis of all strands.
Detection of amplified DNAs
PCR products were analysed by gel electrophoresis. Next, 10 μL with 2 μL of gel loading buffer of each sample was loaded onto a 1.2% electrophoresis-grade agarose gel (Bio-Rad, California, US) and run in 1X TAE buffer at 100 V for 45 minutes. Gels were stained with ethidium bromide and visualised using a UV transilluminator (UVP Inc., CA).
Detection limits and specificity of primers
The detection limits and sensitivity of the mPCR was determined using a serial dilution of leptospiral DNA mix and by urinespiking experiments. L. interrogans serovar Icterohaemorrhagiae was used for the spiking of urine sample. Leptospiral cell concentration was measured by spectrophotometry at an optical density (OD) of 0.14 at 420 nm (approximately 5 × 108 cells/ml) (33). Next, 400 μl of the cultured Leptospira in liquid EMJH (number of cells as 2 × 108 cells) was added to 1.6 ml of urine from a healthy human (final concentration of cells is 1 × 108 cells). A 10-fold serial dilution was subsequently prepared by diluting the spiked urine sample with urine of a healthy human. Next, 1 ml of each dilution was used for DNA extraction. Leptospires in each 1 ml dilution were concentrated by centrifugation at 13 000 g for 15 minutes and washed once with 1 ml PBS. Next, 500 μL of chelex slurry (10% 200–400 mesh) (Bio-RAD, US) was added and the solution was vortexed for 15 seconds and quickly spun for 1 minute. The samples were subsequently incubated in a 100 °C water bath for 20 minutes. The samples were vortexed for 15 seconds and spun at 13 000 g for 20 minutes to ensure that all contents were in the bottom of the microcentrifuge tube. Lastly, 5 μL of the supernatant was used for the mPCR.
The specificity of the primers was evaluated with 9 serogroups identified in Malaysia and 23 strains of commensal and pathogenic bacteria (non-Leptospira) commonly encountered in clinical specimens.
Results
Optimisation of mPCR amplification
Because primer-dimers and other nonspecific products may interfere with the specific products of mPCR, the reaction often requires extensive optimisation. Amplification specificity is also influenced by other factors, such as the MgCl2 concentration in the PCR buffers and primer concentration. We split our mPCR optimisation strategy into 3 main steps: annealing temperature, amount of primers used, and concentrations of MgCl2 in the PCR reaction. We began the optimisation strategy by performing the mPCR in standard 20 μL reactions (consisting of 200 mM dNTP, 1.5 mM MgCl2, 10 pmole each of primers, 1X PCR buffer, 1 unit Taq polymerase, and 218 ng of template DNA from a mixture of leptospiral genomic DNA).
Optimisation of annealing temperature
The annealing temperature in a PCR usually depends directly on the length and composition of the primer(s). In our mPCR, we chose to detect 2 targets: 16S rRNA and the leptospiral LipL32 gene. For the 16S rRNA target, we took advantage of previously reported primers (17). Concerning the LipL32 gene, we extracted the sequence from genebank and designed new primers. Recently, the LipL32 gene has been suggested to be a target for nested and real-time PCR (25–27).
We first optimised the multiplex PCR amplification conditions using both the primer sets of Lep-F-Lep-R and Lau01-Lau02 with the genomic DNA mixture from the extracted DNA. A gradient PCR was carried out to determine the best annealing temperature using a BioRAD MyCycler Thermocycler. Figure 1a shows the result of the gradient PCR at an annealing temperature of 50 °C–60 °C. The annealing temperature of 58 °C was chosen as the optimal annealing temperature for the mPCR.
Figure 1a:
The optimisation of the reaction’s annealing temperature. The optimum annealing temperature was 58.0 °C.
Optimisation of primer concentration
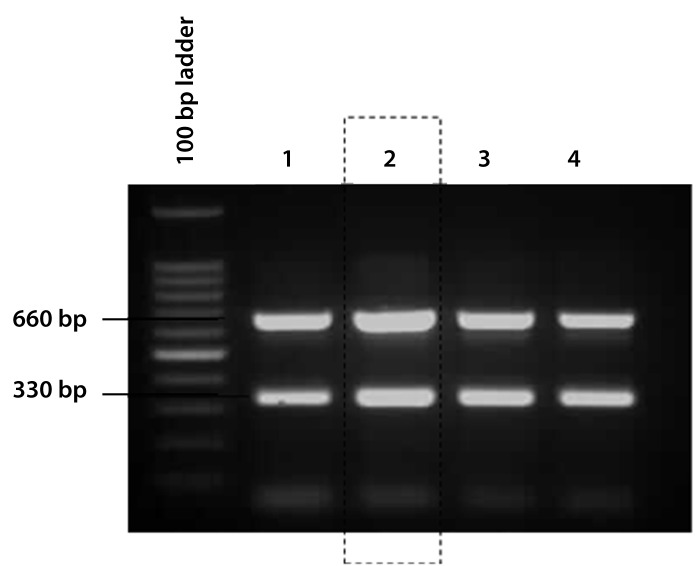
The quantity of DNA primer(s) supplied to the PCR may influence the results of the mPCR. Extremely high or low primer amounts should be avoided because high primer concentrations may inhibit the multiplex reaction, whereas low amounts may not be sufficient. In order to amplify all of the specific PCR products with equal efficiency, the concentrations of the individual primer pairs were optimised. Figure 1c shows that the best combinations of primers were 5 μM and 10 μM for Lau01-Lau02 and Lep-F-Lep-R primers, respectively.
Figure 1c:
The optimisation of the primer concentration. The optimal ratio of primers was 5 μM of Lau primers and 10 μM of Lep primers. Lane 1: Lau 10 μM and Lep 10 μM, Lane 2: Lau 5 μM and Lep 10 μM Lane 3: Lau 5 μM and Lep 15 μM, Lane 4: Lau 5 μM and Lep 20 μM.
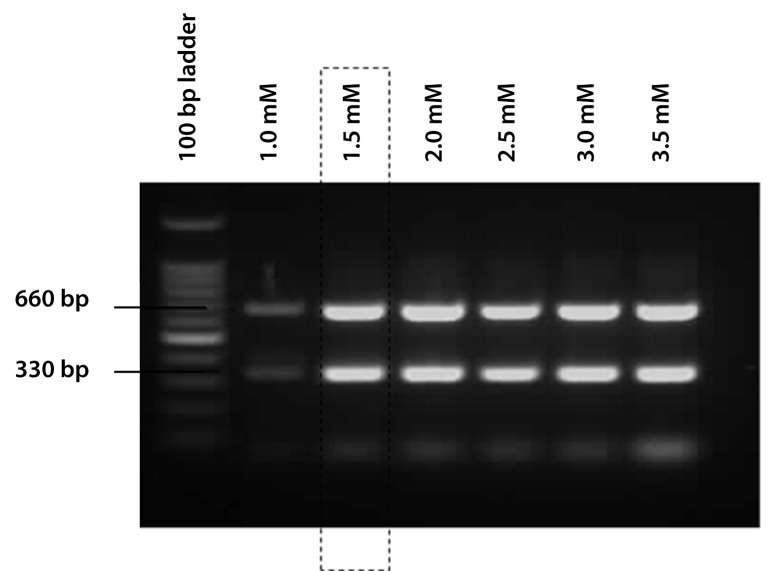
Optimisation of MgCl2 concentration
Finally, optimisation was carried out to determine the best amount of MgCl2 to be added to each reaction. The optimal MgCl2 concentration yields maximal amplification of a specific target with minimal non-specific products and primerdimer formation. In order to find the optimal amount, MgCl2 concentration was varied between 1.5 mM–4.0 mM. As shown in Figure 1b, 1.5 mM of MgCl2 was selected as the optimal concentration.
Figure 1b:
The optimisation of the MgCl2 concentration. The optimal amount of MgCl2 was 1.5 mM.
Detection limits of mPCR
The sensitivity and detection level of the mPCR was determined using a serial dilution of genomic DNA mix from leptospiral strains and by urine-spiking. The PCR was performed using the optimised conditions, as mentioned previously. The lowest limit of detection using genomic DNA of Leptospira in this assay was found to be 21.8 pg, as shown in Figure 2. However, the sensitivity of the test using urine samples spiked with serial dilutions of a pure culture of leptospires was 1 × 103 cells/ml urine (Figure 3).
Figure 2:
The sensitivity of mPCR in terms of the amount of genomic DNA mixture used as template. The agarose gel revealed that the mPCR was able to detect up to 21.8 pg of genomic DNA.
Figure 3:

The sensitivity of the mPCR tested on spiked urine samples. M: PCR marker (NEB), Lane 1: 1 × 108 leptospires/ml, Lane 2: 1 × 107 leptospires/ml, Lane 3: 1 × 106 leptospires/ml, Lane 4: 1 × 105 leptospires/ml,Lane 5: 1 × 104 leptospires/ml, Lane6: 1 × 103 leptospires/ml, Lane 7:1 × 102 leptospires/ml, Lane 8:1 × 10 leptospires/ml, Lane 9:1 leptospires/ml.
Specificity of multiplex PCR
10 leptospiral serovars representative of different serogroups commonly isolated in Malaysia were analysed to determine the specificity of the mPCR. Our results showed that each strain produced a positive signal for both targeted genes (a 330 bp band and a 660 bp band) (Figure 4). The specificity of the oligonucleotide primer sets were also tested against a number of commensal and pathogenic bacteria (Yersinia enterocolitica, Methicillin Resistance Staphylococcus aureus, Psuedomonas aeruginosa, Enterococcus species, Burkholderia pseudomallei, Shigella flexneri, Enteropathogenic Escherichia coli polyval, Shigella boydii, Staphylococcus aureus, Salmonella paratyphi B, Corynebacterium diphtheria, Shigella dysenteriae, Bacillus subtilis, Morganella morganii, Enteropathogenic Escherichia coli, Staphylococcus saprophyticus, Diphteroid, Vibrio cholera, Providencia stuarti, Trepanoma pallidum, Flovobacterium species, Shigella sonnei, and Klebsiella species) commonly encountered in clinical specimens. All of the non-Leptospira bacteria commonly encountered in clinical specimens were negative with both of the primer sets used in the multiplex PCR (results not shown).
Figure 4:
The specificity of the mPCR on Leptospira species. 10 serovars of bacteriologically identified Leptospira were used from Malaysia. Lane M: PCR marker (New England Biolab, US), Lane 1: L. interrogans serovar Canicola, Lane 2: L. interrogans serovar Pomona, Lane 3: L. interrogans serovar Australis, Lane 4: L. interrogans serovar Bataviae, Lane 5: L. interrogans serovar Pyrogenes, Lane 6: L. interrogans serovar Grippotyphosa, Lane 7: L. interrogans serovar Icterohaemorrhagiae, Lane 8: L. interrogans serovar Hebdomadis, Lane 9: L. borgpetersenii serovar Ballum, Lane 10: L. kmetyi serovar Malaysia, Lane 11: Negative control.
Discussion
Detection of the pathogenic Leptospira species is important for the early diagnosis of leptospirosis because it provides unequivocal evidence of active infection. However, the application of this strategy was hampered by the limitation of conventional methods. As a result, clinical diagnosis is often based on serological tests, despite the fact that antibodies begin to appear 8–10 days after the onset of the illness.
The use of PCR is now gaining popularity for the diagnosis of fastidious organisms, including Leptospira. This method offers several key advantages over the gold standard techniques of culture and MAT, such as a faster turn-around time, less contamination, and elimination of the need for the production of reference hyperimmune antisera to determine the identity of the cultured organism. To date, most of the reported conventional or nested PCR diagnosis are based on the detection of a single target, such as the 16S rRNA gene (17,18,28) or the 23S rDNA (29), or using G1/G2 primer pairs (13,30).
In this report, we described the development of an mPCR for the rapid detection of leptospiral infection based on the amplification of 2 genes: 16S rRNA gene and LipL32 gene. The mPCR used in this study offers further advantage over that of conventional PCR because it uses 2 sets of primers that increase the specific amplification (330 bp and/or 660 bp). Therefore, pathogenic strains can be detected rapidly for diagnostic purposes. It was also shown that the mPCR is a notably sensitive test, detecting up to 21.8 pg of genomic DNA and 1 × 103 leptospires/ml in seeded urine. Although real-time PCR has increasingly gained popularity (due to the elimination of the agarose gel electrophoresis step), the method is still relatively expensive for a small laboratory, especially in the third-world countries where the disease is predominant. Therefore, conventional PCR is still a better choice because this methodology is not technically demanding and uses only commercially available reagents. Therefore, this technique can be applied to diagnostic laboratories to help in identifying pathogenic leptospires, especially in developing countries.
The mPCR uses 2 sets of primers, which are based upon amplification of the Leptospira 16S rRNA gene that is conserved throughout the bacterial kingdom, as well as another set of primers detecting the leptospiral major outer-membrane lipoprotein LipL32 gene. The LipL32 gene is highly specific and is absent from nonpathogenic leptospira or any other commensal pathogenic bacteria. The leptospiral major outer-membrane lipoprotein (LipL32) is expressed during infection by all pathogenic strains and can prove to be an important candidate antigen for the development of a sensitive and specific test for leptospirosis (31,32).
Even though the study started with the detection of Leptospira species using extracted genomic DNA, the results were notably encouraging, and we believe that this method can be used as a valuable tool in the early diagnosis of human leptospirosis. However, clinical evaluation to confirm the potential of the PCR protocol is needed. To monitor the effectiveness of DNA extraction and the presence of any inhibitory factors in the PCR, an internal control that will be co-amplified is to be included in this multiplex PCR. The conversion of the current method into a thermo-stabilised PCR will be undertaken in future work.
Conclusion
In summary, the mPCR was shown to be a promising adjunct to the early diagnosis of leptospirosis. The LipL32 gene can be another target for the efficient detection of leptospiral infection because the 2 sets of primers in an mPCR increases the sensitivity and specificity of the test. On the basis of its sensitivity and specificity, the mPCR method described here should be a useful tool for the early diagnosis of leptospirosis, irrespective of serovar.
Acknowledgments
We gratefully acknowledge and thank Professor Abdul Rani Bahaman, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia for providing leptospiral serovars. This work is dedicated to late Dr. Lau Kwok Leong who initiated the project. The work was supported by Universiti Sains Malaysia short-term grant no. 304/CIPPT/638154.
Footnotes
Authors’ contributions
Conception and design: MS, THT, KLL
Obtaining of funding, analysis and interpretation of the data: THT
Provision of study materials or patients, collection and assembly of the data: MR, SAA
Drafting of the article: DAS, SAA
Critical revision of the article: SAA
Final approval of the article: DAS, THT
Administrative, technical or logistic support: MS, CHH
References
- 1.Plank R, Dean D. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 2000;2(10):1265–1276. doi: 10.1016/s1286-4579(00)01280-6. [DOI] [PubMed] [Google Scholar]
- 2.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levett PN. Leptospirosis: A forgotten zoonosis? Clin App Immunol Rev. 2004;4(6):435–448. [Google Scholar]
- 4.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 5.Ko AI, Reis MG, Dourado CMR, Johnson Jr WD, Riley LW, the Salvador Leptospirosis Study Group Urban epidemic of severe leptospirosis in Brazil. Lancet Infect Dis. 1999;354(9181):820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 6.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: Worldwide incidence trends. Int J Infect Dis. 2008;12(4):351–357. doi: 10.1016/j.ijid.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Laras K, Van CB, Bounlu K, Tien NTK, Olson JG, Thongchanh S, et al. The importance of leptospirosis in Souteast Asia. Am J Trop Med Hyg. 2002;67(3):278–286. doi: 10.4269/ajtmh.2002.67.278. [DOI] [PubMed] [Google Scholar]
- 8.Sejvar J, Bancroft E, Winthrop K, Bettinger J, Bajani M, Bragg S, et al. Leptospirosis in “Eco-Challenge” athletes, Malaysian Borneo, 2000. Emerg Infect Dis. 2003;9(6):702–707. doi: 10.3201/eid0906.020751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koay TK, Nirmal S, Noitie L, Tan E. An epidemiological investigation of an outbreak of leptospirosis associated with swimming, Beaufort, Sabah. Med J Malaysia. 2004;59(4):5. [PubMed] [Google Scholar]
- 10.Thiruventhiran T, Tan SY. The patient who had a picnic at a waterfall and presented with haemoptysis and renal failure. Nephrol Dial Transpl. 2000;15(5):727–728. doi: 10.1093/ndt/15.5.727. [DOI] [PubMed] [Google Scholar]
- 11.Al-Jazeera Asia-Pacific News. Fatal floods strike south Malaysia. Doha (QA): Al-Jazeera English; 2007. [cited 2007 Jan 15]. [Internet] Available from: http://english.aljazeera.net/news/asia-pacific/2007/01/2008525131241424879.html . [Google Scholar]
- 12.Agence France-Presse (AFP). Malaysia battles outbreaks of disease in flood zone. Reliefweb: 2007. [cited 2007 17 Jan]. [Internet] Available from: http://www.reliefweb.int/rw/rwb.nsf/db900SID/LSGZ-6XJDMG?OpenDocument&RSS20=03 . [Google Scholar]
- 13.Winslow WE, Merry DJ, Pirc ML, Devine PL. Evaluation of a commercial enzyme-llinked immunosorbent assay for detection of immunoglobulin M antibody in diagnosis of human leptospiral infection. J Clin Microb. 1997;35(8):1938–1942. doi: 10.1128/jcm.35.8.1938-1942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blacksell SD, Smythe L, Phetsouvanh R, Dohnt M, Hartskeerl R, Symonds M, et al. Limited diagnosic capacities of two commercial assays for the detection of Leptospira immunoglobulin M antibodies in Laos. Clin Vaccine Immunol. 2006;13(10):1166–1169. doi: 10.1128/CVI.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bomfim MRQ, Barbosa-Stancioli EF, Koury MC. Detection of pathogenic leptospires in urine from naturally infected cattle by nested PCR. Vet J. 2008;178(2):251–256. doi: 10.1016/j.tvjl.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Lilenbaum W, Varges R, Brandao FZ, Cortez A, de Souza SO, Brandao PE, et al. Detection of Leptospira spp. in semen and vaginal fluids of goats and sheep by polymerase chain reaction. Theriogenology. 2008;69(7):837–842. doi: 10.1016/j.theriogenology.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Merien F, Amouriaux P, Perolat P, Baranton G, Girons IS. Polymerase chain reaction for detection of Leptospira spp. in clinical samples. J Clin Microbiol. 1992;30(9):2219–2224. doi: 10.1128/jcm.30.9.2219-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bal AE, Gravekamp C, Hartskeerl RA, Meza-Brewster JD, Korver H, Terpstra WJ. Detection of leptospires in urine by PCR for early diagnosis of leptospirosis. J Clin Microbiol. 1994;32(8):1894–1898. doi: 10.1128/jcm.32.8.1894-1898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero EC, Billerbeck AEC, Lando VS, Camargo ED, Souza CC, Yasuda PH. Detection of Leptospira DNA in patients with aseptic meningitis by PCR. J Clin Microbiol. 1998;36(5):1453–1455. doi: 10.1128/jcm.36.5.1453-1455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugathan S, Varghese TP. Multiplex PCR on leptospiral isolates from Kolenchery, Kerala, India. Indian J Med Microbiol. 2005;23(2):114–116. doi: 10.4103/0255-0857.16051. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca C de A, Teixeira MMG, Romero EC, Tengan FM, da Silva MV, Shikanai-Yasuda MA. Leptospira DNA detection for the diagnosis of human leptospirosis. J Infect. 2006;52(1):15–22. doi: 10.1016/j.jinf.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Ooteman MC, Vago AR, Koury MC. Evaluation of MAT, IgM ELISA and PCR methods for the diagnosis of human leptospirosis. J Microbiol Methods. 2006;65(2):247–257. doi: 10.1016/j.mimet.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 24.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols: Methods in molecular biology. New Jersey: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 25.Levett PN, Morey RE, Galloway RL, Turner DE, Steigerwalt AG, Mayer LW. Detection of pathogenic leptospires by real-time quantitative PCR. J Med Microbiol. 2005;54(1):45–49. doi: 10.1099/jmm.0.45860-0. [DOI] [PubMed] [Google Scholar]
- 26.Tansuphasiri U, Chanthadee R, Phulsuksombati D, Sangjun N. Development of a duplex-polymerase chain reaction for rapid detection of pathogenic Leptospira. Southeast Asian J Trop Med Public Health. 2006;37(2):297–308. [PubMed] [Google Scholar]
- 27.Cheema PS, Srivastava SK, Amutha R, Singh S, Singh H, Sandey M. Detection of pathogenic leptospires in animals by PCR based on lipL21 and lipL32 genes. Indian J Exp Biol. 2007;45(6):568–573. [PubMed] [Google Scholar]
- 28.Smythe LD, Smith IL, Smith GA, Dohnt MF, Symonds ML, Barnett LJ, et al. A quatitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infect Dis. 2002;2(13):1–7. doi: 10.1186/1471-2334-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kositanont U, Rugsasuk S, Leelaporn A, Phulsuksombati D, Tantitanawat S, Naigowit P. Detection and differentiation between pathogenic and saprophytic Leptospira spp. by multiplex polymerase chain reaction. Diagn Microbiol Infect Dis. 2007;57(2):117–122. doi: 10.1016/j.diagmicrobio.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira MAA, Caballero OL, Vago AR, Harskeerl RA, Romanha AJ, Pena DJ, et al. Low-stringency single specific primer PCR for identification of Leptospira. J Med Microbiol. 2003;52(2):127–135. doi: 10.1099/jmm.0.04923-0. [DOI] [PubMed] [Google Scholar]
- 31.Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M, et al. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun. 2000;68(4):2276–2285. doi: 10.1128/iai.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CW, Wu MS, Pan MJ, Hsoeh WJ, Vandewalle A, Huang CC. The Leptospira outer membrane protein LipL32 induces tubulointerstitial nephritis-mediated gene expression in mouse proximal tubule cells. J Am Soc Nephrol. 2002;13(8):2037–2045. doi: 10.1097/01.asn.0000022007.91733.62. [DOI] [PubMed] [Google Scholar]
- 33.Schreier S, Triampo W, Doungchawee G, Triampo D, Chadsuthi S. Leptospirosis research:Fast, easy and reliable enumeration of mobile leptospires. Biol Res. 2009;42(1):5–12. [PubMed] [Google Scholar]