Abstract
Telemetric EEG in the rat’s brain has been used for experiments which tests the effects of an antiepileptic compound on it’s antiseizures activity. A simple classification correlating epileptiform discharge and Racine’s behavioral activity is discussed.
Keywords: electroencephalogram, neuroscience, rat, telemetry


Animal models for seizures and epilepsy have played a fundamental role in advancing our understanding of basic mechanisms underlying ictogenesis and epileptogenesis and have been instrumental in the discovery and preclinical development of novel antiepileptic drugs. Despite the successful development of various new antiepileptic drugs in recent decades, the search for new therapies with better efficacy and tolerability remains an important goal (1). The discovery and development of a new antiepileptic drug relies heavily on the preclinical use of animal models to establish efficacy and safety prior to first trials in humans (2).
Racine’s scale is one of the most frequently used tools to determine the intensity of a seizure in rodent models of experiment epilepsy. Racine developed the scale by investigating the relationship between electroencephalogram (EEG)-changes and the development of motor seizures in the amygdala-kindling model, characterized by partial seizures with secondary generalization The motor symptoms include “mouth and facial movements” (intensity stage 1); “head nodding” (stage 2); “forelimb clonus” (stage 3); seizures characterized by rearing, (stage 4) and seizures characterized by rearing and falling (stage 5) (3).
Racine’s scale is frequently applied to many other models for seizures and epilepsy. It is however questionable, whether the use of Racine’s scale for the assessment of seizure intensities in other epilepsy or seizure models is justifiable, given the well known relation between activated brain part and corresponding expressed behavior of Sprague Dawley rats (160–350 g) which are suitable animals to induce status epilepticus models and to record continuous EEG spikes and wave discharges (4–6).
We used a total of 10 male Sprague Dawley and 6 Genetic Absence Epilepsy Rats from Strasbourg (GAERS) rats (7), four to six months of age and weighing 187–325 g. The parent GAERS rats were gifts from the Kyoto University, Japan. The animals were born and raised under environmentally controlled conditions (12 hours light/dark cycles, 20–22 °C in the animal facility house of the Universiti Sains Malaysia Health Campus, with food and water ad libitum. All animals were treated according to the guidelines approved by the Animal Ethics Committee of Universiti Sains Malaysia.
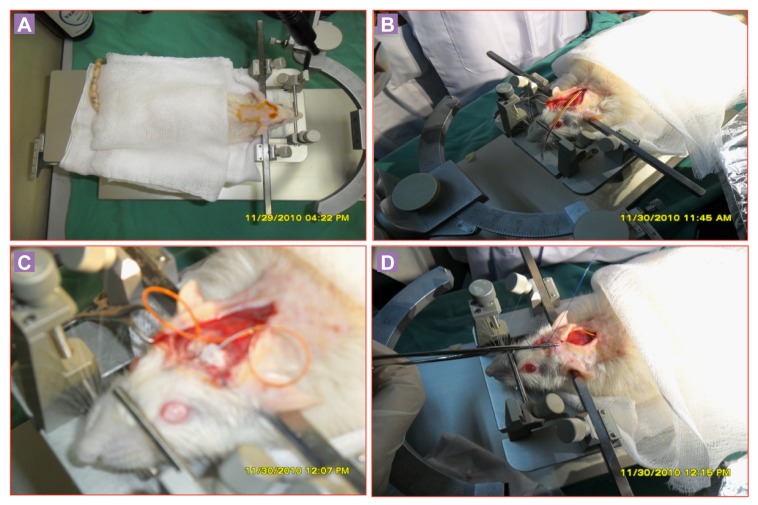
Prior to surgery (5–15 min), the animals were anaesthetized with ketamine and xylazine (80 mg/kg and 7.5 mg/kg, respectively, i.p.) and additional ketamine (5 mg/kg, i.p.) were given during surgery when a sensorial pain stimulus by squeezing the footpad, elicited motor reflexes (8). After proper anaesthesia, the fur on the head and back were clipped rostral to the medial canthus of the eyes to immediately cranial to the last cervical vertebra in a strip approximately 3 cm wide. The animals were placed on a heating pad and secured in a stereotaxic apparatus (Stoelting Model 516 00; Illinois, U.S.A.). The surgical site and surrounding area were swabbed with 70% ethyl alcohol and scrubbed with a 4% chlorohexidine solution. A 3–4 cm mid-sagittal incision was made on the scalp and the skin reflected with haemostats to expose the entire dorsal portion of the skull. The periosteum was removed and haemostasis achieved with sterile cotton-tip applicators. Bregma was marked and two holes bored through the skull with drilling (# 105 drill bit). Stainless steel electrodes (DSI Model F40-EET; St. Paul, MN, U.S.A.) insulated except at the tip were implanted bilaterally into the brain over the parietal cortex. The other two electrodes were placed in the neck muscle of EMG recording to compare with the EEG spikes. The EEG electrodes were fixed to the skull of rat with dental acrylic. The radio telemetry unit was placed subcutaneously into the pocket over and caudal to the scapula. Using blunt-ended scissors, a subcutaneous pocket was made caudally from the incision by pushing aside connective tissue and then skin was sutured (Figure 1A-1D). The method of telemetry implantation was followed from White et al. (2006; 2010). The surgical procedures of our experiment were considered as minimum to mild pain scale according to the pain assessment, and it was managed by local anaesthesia (9).
Figure 1A-1D:
Implantation of DSI telemetry system into Srague Dawley rat.
Kainic acid was administered intraperitoneally on Sprague Dawley rats (n = 6) to induce an episode of status epilepticus. One to 2 weeks after telemetry implantation, Sprague Dawley rats were injected with kainic acid (5 mg/kg; IP: Sigma, St. Louis, MO) diluted in sterile 0.9% saline at 2.5 mg/ml. Rats were continuously monitored for electroencephalogram and motor seizures. Racine scale was used to characterize motor seizure severity (3). Kainic acid injections were repeated at 1 hours intervals until class III, IV, or V seizures were evoked for at least 3 hrs (i.e., 10 convulsive seizures per hour). If animals were nearing its endpoint, half-doses (2.5 mg/kg) were given to avoid excessive toxicity and mortality. Kainate administration was terminated for animals displaying electrographic seizures with few or no motor seizures after receiving four full doses. Control animals (n = 4) were treated with normal saline with same volume and number of injections. In GAERS rats (n = 6) only DSI telemetry are implanted without injecting kainic acid. All rats were given 5 ml lactated Ringer’s (subcutaneously) and apple slices following treatment.
The EEG activity was acquired by Dataquest DSI telemetry, USA software and analysed offline using Neuro-Score software, DSI, USA, configured for automatic detection and saving of spikes and wave discharges, and seizures. Setting for seizure detection were the following: amplitude threshold, 3; seizures duration, > 2s; detection threshold, 0; minimum frequency, 3 Hz; short burst detection was turned off; length of EEG kept before and after each seizure was 0.5 min. The duration of the acquisition time was 24 hours. Analysis of EEG was performed by a ‘blinded’ unbiased investigator. All seizure EEGs were revised manually. The spike bursts lasting less than 3 s are not counted as seizures.
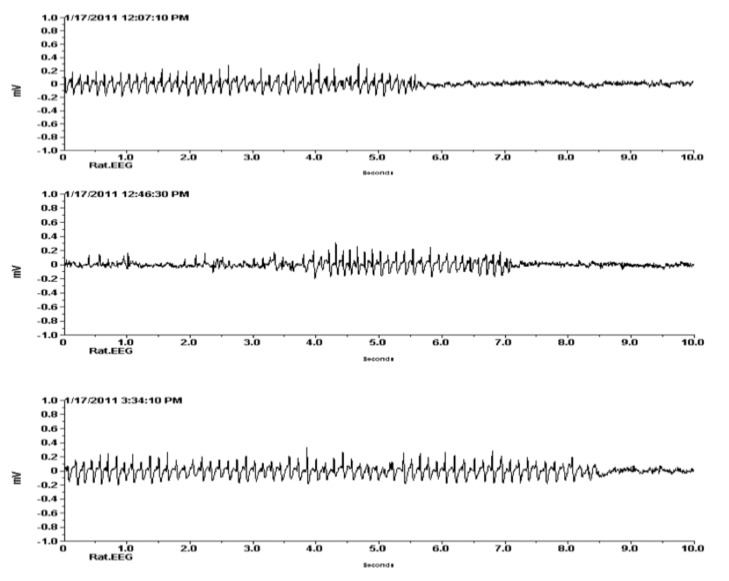
The EEG of GAERS rat was used for positive control in this study. In GAERS rats, the fully developed spike and wave discharges typically have the following features: a fundamental frequency in the range of 7–12 Hz; an amplitude varying from 300 to 1000 µV, and a duration in the range of 1–65 s (Figure 2) that was fully comparable with the previous study (10).
Figure 2:
Spike and wave discharges (SWDs) of EEG in Genetic Absence Epilepsy Rats from Strasbourg (GAERs) (n = 6). X axis is showing amplitude in mV and Y axis is showing frequency (Hz) in seconds.
We detected a total five different types of electroencephalographic spike and wave epileptic discharges and coupled with the Racine’s behavioural scale (Table 1) throughout the recording periods that were different from the baseline recordings of EEG in Sprague Dawley rats.
Table 1:
Different types of electroencephalographic epileptic discharges in kainic acid-induced Sprague Dawley rats that are coupled with Racine’s scale (n = 6)
| Class | Epileptiform discharge | Racine’s Scale Behavioral Activity |
|---|---|---|
| 1 and 2 | Sharp wave epileptic discharge (sharp/spikes pattern) | Mouth and facial movements (class 1), and Head nodding (wet dog shakes) (class 2) |
| 3 | clonic epileptic discharge (rhythmic pattern/spike and wave) | Forelimb clonus |
| 4 | Tonic epileptic discharge (rapid sharp pattern/poly spikes) | Rearing |
| 5 | Tonic-clonic (irregular pattern/poly spikes and wave) | Rearing and falling |
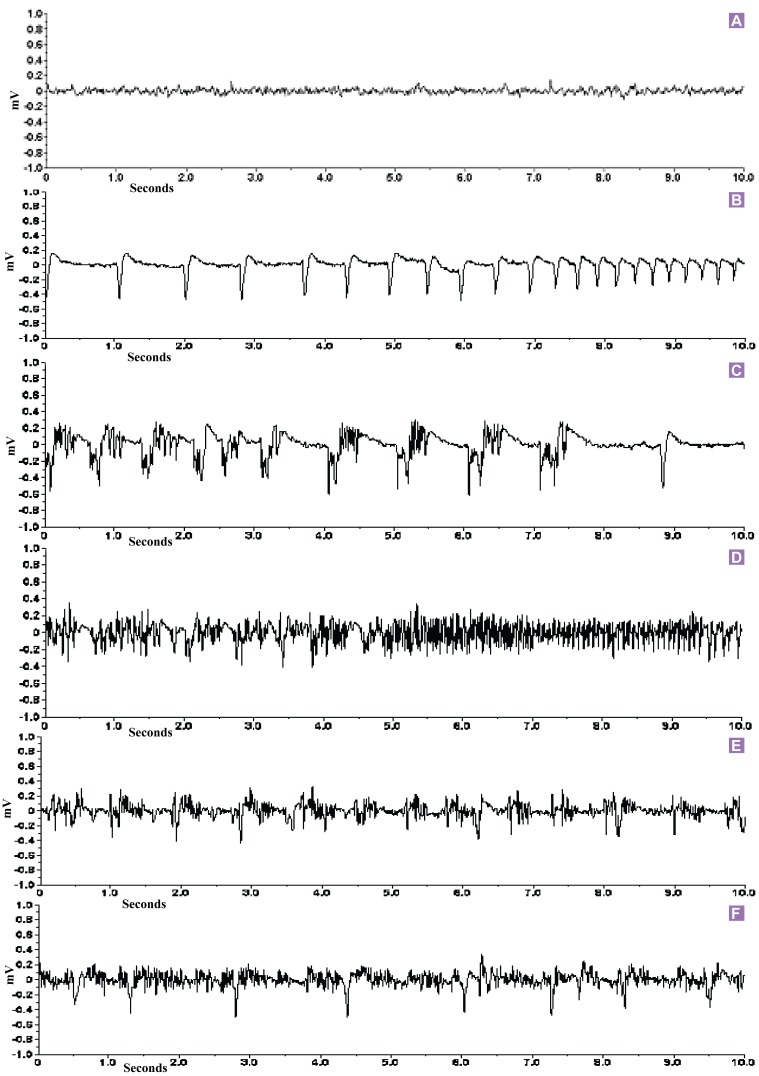
The first epileptic EEG was characterized by sharp-spike discharges with a frequency of 2–4 Hz, a mean duration of 10 s (ranging from 3 s to 1–5 min) (Figure 3A), and a downward spikes amplitude of around 600 µV (baseline up to 200 µV) (Figure 3B shows a close-up view). During this EEG discharges pattern linked to the stage 1 and stage 2 behavioral categories of Racine’s scale. The rats were behaviorally arrested for a while, stiffness of the neck muscles, facial jerking and neck jerks.
Figure 3:
EGG records in Sprague Dawley rats (n = 6). (A) Baseline EEG recording in Sprague Dawley rats before kainic acid injection. (B) Downward sharp epileptic EEG discharges in kainiteinduced rats. (C) Clonic epileptic EEG discharges. (D) Tonic epileptic EEG discharges. (E) Tonic-clonic epileptic EEG discharges. (F) Irregular epileptic EEG recording of kainiteinduced Sprague Dawley rats. X axis is showing amplitude in mV and Y axis is showing frequency (Hz) in seconds.
The second epileptic EEG discharge (Figure 3C shows a close-up view) consisted of clonic epileptic discharges with a frequency of 2 to 3 Hz and poly spikes and waves amplitude was around 600 µV (baseline up to 200 µV). These discharges had irregular spikes, sometimes consisted of poly spikes, and the spikes were less sharp than those of the first type of discharge pattern. Forelimb clonus symptoms were observed in this stage.
The third epileptiform EEG discharge pattern was long lasting characterized by a frequency of 6 to 10 Hz sharp irregular spikes, sometimes with poly-spikes, and a spike amplitude of around 800 µV. A close-up look of this EEG deviation is found in Figure 3D. The tonic behavioral symptoms, rearing were observed in kainic acid induced epileptic rats.
Another distinct tonic-clonic epileptic EEG abnormality consisted of very characteristic high amplitude poly spikes (up to 900 µV), which were sometimes embedded in some irregular spikes of smaller amplitude (Figure 3E).
A last irregular pattern of epileptic EEG discharge found consisted mostly of long lasting (1 to 5 min), high amplitude single spiking with short amplitude poly-spikes, and sometimes it followed by high frequency oscillations. These high frequency oscillations ranged from 10 to 20 Hz. A close-up look at this type of EEG deviation can be found in Figure 3F.
This classification will be able to guide us when one observes the EEG changes when the effects of the proposed antiseizure compound is being monitored.
Acknowledgments
We acknowledge the distributor of above GAERS rats used in this experiments from the Institute of Laboratory Animal, Graduate School of Medicine, Kyoto University, Yoshidakonoecho, Sakyo-ku, Japan.
References
- 1.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 2.White A.M., Williams P.A., Ferraro D.J., Clark S, Kadam S.D., Dudek F.E., et al. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. Neurosci Methods. 2006;152:255–266. doi: 10.1016/j.jneumeth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Racine R.J. Modification of seizure activity by electrical stimulation. I. After-discharge threshold II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:269–294. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y., Pang J.R., Zheng J.O., Deng X.Q., Liang X.L., Li J.Q. Effects of topiramate on hippocampal neuronal apoptosis in rats after kainic acid-evoked seizures. Neural Regen Res. 2008;3:212–215. [Google Scholar]
- 5.White H.S., Perucca E., Privitera M.D. Investigational Drugs: Brivaracetam, Carisbamate, Eslicarbazepine Fluorofelbamate, Gamaxolone, Isovaleramide, Lacosamide (Harkoseride; SPM 927), Losigamine, Retigabine, Safinamide, Seletracetam, Stiripentol, Talampanel, and Valrocemide. In: Engel J Jr, Pedley T.A., editors. Epilepsy: A Comprehensive Textbook. 2nd ed. United States (US): Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 6.White A., Williams P. A., Hellier J. L., Clark S., Dudek F. E., Staley K. J. EEG spike activity precedes epilepsy after kainite-induced status epilepticus. Epilepsia. 2010;51:371–383. doi: 10.1111/j.1528-1167.2009.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danober L., Deransart C., Depaulis A., Vergnes M., Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol. 1998;55(1):27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- 8.Dedeurwaerdere S., Boon P., De Smedt T., Claeys P., Raedt R., Bosman T., et al. Chronic levetiracetam treatment early in the life decreases epileptiform events in young GAERS, but does not prevent the expression of spike and wave discharges during adulthood. Seizure. 2005;14(6):403–411. doi: 10.1016/j.seizure.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Kohn D.F., Martin T.E., Foley P.L., Morris T.H., Swindle M.M., Vogler G.A., et al. Public statement: guidelines for the assessment and management of pain in rodents and rabbits. J Am Assoc Lab Anim Sci. 2007;46:97–108. [PubMed] [Google Scholar]
- 10.Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg-A review. J Neural Transmiss. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]