Abstract
Background:
This study was performed to compare the oxidative quality of repeatedly heated palm and soybean oils, which were used to fry keropok lekors and potato chips.
Methods:
A kilogramme of keropok lekors or potato chips was fried in 2.5 L of palm or soybean oil at 180 °C for 10 minutes. The frying process was repeated once and four times to obtain twice-heated and five-times-heated oils. The peroxide value and fatty acid composition of the oils were measured.
Results:
Frequent heating significantly increased the peroxide values in both oils, with the five-times-heated oils having the highest peroxide values [five-times-heated palm: 14.26 ± 0.41 and 11.29 ± 0.58 meq/kg vs fresh: 2.13 ± 0.00, F (3,12) = 346.80, P < 0.001; five-times-heated soybean: 16.95 ± 0.39 and 12.90 ± 0.21 meq/kg vs fresh: 2.53 ± 0.00 oils, F (3,12) = 1755, P < 0.001, when used to fry keropok lekors and potato chips, respectively]. Overall, both oils showed significantly higher peroxide values when keropok lekors were fried in them compared with when potato chips were fried. In general, the heated soybean oil had significantly higher peroxide values than the heated palm oil. Fatty acid composition in the oils remained mostly unaltered by the heating frequency.
Conclusion:
keropok lekors, when used as the frying material, increased the peroxide values of the palm and soybean oils. Fatty acid composition was not much affected by the frequency of frying or the fried item used.
Keywords: keropok lekors, peroxide value, palm oil, soybean oil, deep frying
Introduction
Deep frying is one of the oldest practice for food preparation, during which the oil is exposed continuously or repeatedly to high temperature (160–190 °C) in the presence of air and moisture. This leads to a number of chemical reactions in the oil, such as oxidation, hydrolysis and polymerization, which may alter the compositions of the oil leading to production of various types of oxidative products (1). Hydroperoxides and aldehydes are the primary products formed in the initial stages of oxidation and are absorbed into the fried food (2). The extent of oxidation can be measured using the peroxide value of the oil (3–4).
The usage of repeatedly heated cooking oil is common amongst Malaysians who without taking into consideration the effects on health; do this in order to reduce the cost of cooking. Repeated heating can cause changes in the physical appearance of oil, such as increased viscosity, darkening in colour, increased foaming and decrease in the smoke point of the oil (5). Consumption of repeatedly heated oil has been shown to increase the risk of hypertension (6) due to impaired vascular relaxation in rats (7,8).
In many studies involving oil heating, potato chips were the most commonly used food item for deep frying (9–11). The oil quality during deep frying is determined by many factors, such as the type of frying materials and the type of oils used (1,12). In Malaysia, keropok lekors, or traditional fish sticks, are one of the most popular snacks that are commonly deep fried before being served. Different from potatoes, which exclusively contain starch (13), the major compositions of keropok lekors are protein and starch.
Therefore, the objective of this study was to compare the quality of the repeatedly heated palm and soybean oils when using keropok lekors and potato chips as frying items. This study used palm and soybean oil because of their wide usage as cooking oils for deep frying purposes in Malaysia, and the difference in the fatty acid composition (14,15) and antioxidant content (16) of the two oils.
Materials and Methods
Materials
Palm oil (Lam Soon Edible Oil, Malaysia) and soybean oil (Yee Lee Edible Oil, Malaysia) were used in this study. The keropok lekors (Malaysian traditional fish sticks) and potatoes were bought from the same sources at a local market. The size of the food item was standardised for each frying process.
Frying procedure
The potatoes were peeled before being thinly sliced. Two and a half litres of palm oil or soybean oil were used to fry 1 kg of keropok lekors or potato chips in a stainless steel wok for 10 minutes at 180 °C. The frying process was repeated once or four times to obtain twiceheated or five-times-heated oils, respectively, with a cooling interval of 24 hours between repetitions (17). The food quantity was reduced proportionately with the amount of vegetable oil left till the fifth heating. No fresh oil was added between the frying processes. After heating, five samples per group were obtained from different batches for the peroxide value measurement.
Determination of peroxide value
Peroxide values of the heated oils were determined according to American Oil Chemists’ Society (AOCS) Official Methods Cd 8-53 (18). Briefly, 5 g of the oil sample was transferred into a 250 mL flask before adding 30 mL of acetic acid-chloroform (3:2). The flask was swirled and then 0.5 mL of saturated potassium iodide was added. Then, the solution was swirled again for 1 minute and 30 mL of distilled water and a few drops of starch solution (10%) were added. The solution was titrated against 0.01 N sodium thiosulphate solution (Na2S2O3), which had been previously standardised using potassium dichromate and potassium iodide, until the blue colour disappeared. The peroxide value was expressed in miliequivalents of peroxide per kg of the sample calculated as:
Peroxide value (meq/kg) = [(Va-Vb) N × 1000]/W
Where;
Va = volume of sodium thiosulphate solution (mL)
Vb = volume of sodium thiosulphate solution (mL) used for the blank
N = normality of sodium thiosulphate
W = weight of the test portion (g)
Fatty acid composition determination
Fatty acid composition of the fresh and heated oils was analysed using gas chromatography (GC-17A, Shimadzu, Japan) with nitrogen at a flow rate of 0.40 mL/min as the carrier gas. The chromatography system consisted of a flame ionisation detector and a BPX 70 capillary column (30 m × 0.25 mm × 0.25 μm), with programmable injector temperature set at 250 °C and detector temperature set at 280 °C. The oil samples (100 μL) were first transesterified to fatty acid methyl ester using 1 mL of 1 M sodium methoxide in 1 mL hexane prior to injection (1 μL) into the gas chromatographic system. Authentic standards were used in the identification of fatty acid methyl ester peaks by comparing their retention times. The readings were obtained based on one sample per group. The fatty acid composition in the oils was expressed as the percentage of the total fatty acids.
Statistical analysis
The data are expressed as mean ± standard error and were analysed using Statistical Package for Social Science version 19. To compare the effect of heating frequency, the data were analysed using a within-subjects analysis of variance for repeated measure. Student’s t test (independent samples) was used for comparison between the type of oil and the food item used for frying at the same heating frequency. Differences were considered significant if P < 0.05.
Results
The results presented in Table 1 show a highly significant increase in peroxide values of palm oil with increasing frequency of heating (once heated: 4.84 ± 0.43 and 3.68 ± 0.26; twice heated: 9.90 ± 0.44 and 4.70 ± 0.16; five times heated: 14.26 ± 0.41 and 11.29 ± 0.58 meq/kg, when used to fry keropok lekors and potato chips, respectively) compared with the fresh oil (2.13 ± 0.00 meq/kg) [F (3,12) = 346.80, P < 0.001]. The same trend was observed in the soybean oil groups [once heated: 6.54 ± 0.19 and 2.74 ± 0.26; twice heated: 7.86 ± 0.07 and 7.12 ± 0.30; five times heated: 16.95 ± 0.39 and 12.90 ± 0.21 meq/kg, when used to fry keropok lekors and potato chips, respectively; F (3,12) = 1755, P < 0.001). Oils heated five times had significantly higher peroxide values than the oils heated twice, which in turn had higher peroxide values than the oils heated once. The peroxide values were significantly higher in heated palm and soybean oils that were used to fry keropok lekors than in the oils used to fry potato chips (P < 0.01). The peroxide values of all soybean oil groups (except oils heated only once to fry potato chips) were significantly higher than those of all palm oil groups regardless of the frying materials used.
Table 1:
Peroxide values in (meq/kg) when keropok lekors and potato chips were fried in repeatedly heated palm and soybean oils
| Fresh | Once | Twice | Five times | |
|---|---|---|---|---|
| Palm oil | ||||
| keropok lekors | 2.13 ± 0.00∗ | 4.84 ± 0.43∗ | 9.90 ± 0.44∗∗ | 14.26 ± 0.41∗∗ |
| Potato chips | 3.68 ± 0.26∗# | 4.70 ± 0.16∗∗# | 11.29 ± 0.58∗∗# | |
| Soybean oil | ||||
| keropok lekors | 2.53 ± 0.00∗ | 6.54 ± 0.19∗∗§ | 7.86 ± 0.07∗∗§ | 16.95 ± 0.39∗∗§ |
| Potato chips | 2.74 ± 0.26∗∗#§ | 7.12 ± 0.30∗∗#§ | 12.90 ± 0.21∗∗#§ |
Values represent mean ± sem (n = 5). Significantly different from other groups in the same type of oil and food used with a within-subjects test (∗P = 0.002, ∗∗P = 0.0001), #significantly different from the keropok lekors group fried in the same oil at the same heating frequency (P < 0.05), §significantly different from the palm oil groups at the same heating frequency and same type of food (P < 0.05).
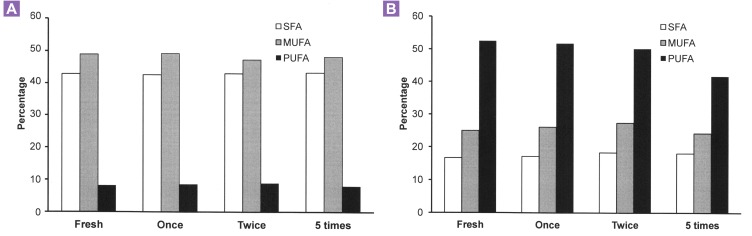
Fatty acid composition of the fresh and heated palm and soybean oils is shown in Figure 1. The percentages of saturated, monounsaturated and polyunsaturated fatty acids in fresh and heated palm oils were similar. Both oils contained about 43% saturated, 49% monounsaturated and 8% polyunsaturated fatty acids. In soybean oil, fresh and heated oils contained similar composition of saturated and monounsaturated fatty acids, which were about 17% and 25%, respectively. It seemed that the polyunsaturated fatty acid content in the five-times-heated soybean oil (42%) was slightly reduced compared to that in other soybean oil groups (50–52%). Generally, palm oil contained higher proportions of saturated and monounsaturated fatty acids but a lower proportion of polyunsaturated fatty acids compared with soybean oil.
Figure 1:
Composition percentage of saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids in fresh, once-, twice-, and five-times-heated palm (panel A) and soybean (panel B) oils. 216 × 227 mm (300 × 300 DPI).
Discussion
The peroxide value is often used as an indicator for the oxidative stability of fats and oils (3,4,19). It was found to be highly correlated with the total concentration of major odorants (including 1-pentanal, pentanoic acid, hexanoic acid and 1-nonanal) and the total concentration of five unsaturated aldehydes (t-2-heptenal, t-2-octenal, t-2-decenal, t-2-undecenal and t,t-2,4-decadienal) in oils. These odorants and aldehydes showed strong cytotoxicity in the heated oils. Moreover, it is relatively easier to measure the peroxide value compared to the direct measurement of odorants in the oils (20). Therefore, the peroxide value is of significance in assessment of quality and stability of foods with high oil content. There are several factors that can affect the quality of oil during the deep frying process, such as the types of food being fried, composition of the oils used, frying temperature, length of frying time, use of a continuous or intermittent frying method and replenishment of fresh oil (1). However, in this study, we investigated only the effect of different types of food items and different types of vegetable oil for the deep frying process on the quality of repeatedly heated oil.
In this study, repeated heating increased the peroxide values of palm and soybean oils. The value increased with the increasing frequency of heating. This indicated that repeated heating of the oils augmented the formation of lipid peroxidation products in the oil, which are potentially harmful to health. Consumption of repeatedly heated vegetable oils has been shown to have detrimental effects on health (7,8,17). A study by Rueda-Claussen et al. (21) demonstrated that repeatedly heated (10 and 20 times) olive, palm and soybean oils had similar acute detrimental effect on the endothelial functions in healthy young subjects. This is contrary to a report by Shuid et al. (22) who found that bone properties of ovariectomized rats fed repeatedly heated palm oil (once and five times) was better than those fed repeatedly heated soybean oil. The discrepancy between these studies could be due to the difference in the heating frequency.
Palm and soybean oils that were used to fry keropok lekors had significantly higher peroxide values compared to the ones that were used to fry potato chips. keropok lekors are primarily made from a combination of pounded fish, mainly mackerel or sardines (about 55–60%) and sago or starch flour (about 40–45%). On the other hand, potatoes only contain about 16% starch, 2% protein and a very small amount of lipids (< 1%) (13). As a marine product, keropok lekors are rich in fish oil. Fish oil is very susceptible to autoxidation because of the high degree of polyunsaturated fatty acids (23). Autoxidation of the fish oil in the keropok lekors during the frying process might have accelerated the oxidative degradation of both vegetable oils and fish oil with the higher production of peroxide content compared to that in the oils used to fry potato chips. This may explain the higher peroxide values of the oils used to fry the keropok lekors. Even in our daily cooking chores, we notice that frying salted fish produces blackish discolouration of the oil.
Previously, no similar study has reported the use of keropok lekors in deep frying. Many studies have used potato chips to suit the Western context to measure the oxidative stability of cooking oils, such as corn and olive oil, either using deep frying or microwave heating (9–11,24). keropok lekors are one of the popular snacks in Malaysia, which are commonly consumed deep-fried and can be easily be found in night markets. Due to the low level of awareness amongst night market food outlet operators regarding the detrimental effects of the use of repeatedly heated cooking oil (25), there is a big possibility that keropok lekors found at the night markets are fried in repeatedly heated cooking oils. This kind of practice could render bad health effects to the public.
Generally, for both types of food (keropok lekors and potato chips), higher peroxide values were found in soybean oil than in palm oil after repeated heating. This suggests that soybean oil undergoes more oxidative modification compared to the palm oil on repeated heating. The unique composition of palm oil allows it to withstand heat better than soybean oil. Palm oil is rich in saturated and monounsaturated fatty acids (MUFA) but has low levels of polyunsaturated fatty acids (PUFA) compared to soybean oil, as shown in the present study. Vegetable oils which are rich in PUFA are more prone and less stable to oxidation compared to those which are rich in MUFA, whereas oils that are rich in MUFA, such as palm oil and olive oil, can better withstand oxidation and form less degradation products on repeated heating (26). In the present study, the percentage of PUFA in five-times-heated soybean oil was 10% less compared to the fresh, once-heated and twice-heated soybean oils. This finding suggests that the unsaturated bonds in PUFA were oxidised due to heating. Previously, the frequency of heating has shown to reduce the unsaturation of fatty acids (27). However, an increase in the saturated fatty acids and MUFA percentage was not obviously seen in the five times-heated soybean oil. The characterisation of individual fatty acids (such as oleic, linoleic and linolenic acids) was not done in this study. Oils that are repeatedly heated at high temperature for a long period will undergo thermal oxidation process with configuration changes in fatty acid from cis to trans isomer (28). Some studies have shown that food containing high trans fatty acid can increase the risk of cardiovascular disease (29) by inducing a proinflammatory response in the endothelial cells (30). The increase in the percentage of saturated fatty acids may be attributed to the cholesterol raising effect of the heated oils.
Palm oil also has an abundant content of vitamin E, which may play an important role in its ability to withstand thermal oxidative changes. Inclusion of α-tocopherol to frying oil was found to render PUFA more resistant to oxidation (31). Vitamin E, which effectively protects fatty acids in the oil from oxidation, deteriorates after each frying episode (16). Therefore, repeated heating of frying oils destroys the vitamin E content and exposes the fatty acids to oxidation. The vitamin E content of palm oil mainly consists of tocotrienols, while the vitamin E in soybean oil mainly consists of tocopherols (16). Tocotrienols have better antioxidant capacity than tocopherols (32) and this may have contributed to the better resistance of palm oil to oxidative changes due to repeated heating.
It was also noted that up to two heatings, the peroxide values of the oils were still below the maximum limit of the peroxide value (10 meq/kg), according to the Malaysian Food Act and Regulation 1985 and the AOCS (33). However, if the Food Sanitation Law of Japan guideline (peroxide value ≤ 30 meq/kg oil) is used instead, all fresh and heated oils can be considered safe for human ingestion. In Malaysia, it is very common to use repeatedly heated vegetable oil for frying to cut cost and only be discarded when the colour of the heated oil has darkened. The level of awareness of the general public in this country regarding such usage is influenced by the socioeconomic status, with higher level of awareness in higher income and education level groups (34).
Conclusion
In the present study, it can be concluded that the use of food item with high contents of fish oil, such as keropok lekors, may further increase the peroxide value in thermally oxidised oil, which is influenced by the heating frequency and the type of oil used during the frying process. It is recommended that vegetable oils should not be heated more than two times for safe consumption.
Acknowledgments
The authors wish to thank Puan Azizah Osman and Mr Ng Chun Yi for technical assistance as well as Miss Jurika Sharatun Abdul Wahed for editorial assistance. This work was supported by a grant from Faculty of Medicine, Universiti Kebangsaan Malaysia (FF-292-2010).
Footnotes
Authors’ contributions
Conception and design, final approval of the article: AA, HMSQ,MFNA,KJ
Analysis and interpretation of the data: YK,AA, MFNA,KJ
Conception and design, drafting of the article, critical revision of the article for the important intellectual content: YK
Provision of study materials or patient, Obtaining of funding: KJ
Analysis and interpretation of the data, final approval of the article, drafting of the article, collection and assembly of data: SS, MJN, SYK, NASH
References
- 1.Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007;72(5):77–86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 2.Choe E, Min DB. Chemistry and reactions of reactive oxygen species in foods. J Food Sci. 2005;70(9):142–159. doi: 10.1080/10408390500455474. [DOI] [PubMed] [Google Scholar]
- 3.Palanisamy UD, Sivanathan M, Radhakrishnan AK, Haleagrahara N, Subramaniam T, Chiew GS. An effective ostrich oil bleaching technique using peroxide value as an indicator. Molecules. 2011;16(7):5709–5719. doi: 10.3390/molecules16075709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talpur MY, Sherazi ST, Mahesar SA, Bhutto AA. A simplified UV spectrometric method for determination of peroxide value in thermally oxidized canola oil. Talanta. 2010;80(5):1823–1826. doi: 10.1016/j.talanta.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Rani AKS, Reddy SY, Chetana R. Quality changes in trans and trans free fats/oils and products during frying. Eur Food Res Technol. 2010;230(6):803–811. [Google Scholar]
- 6.Soriguer F, Rojo-Martinez G, Dobarganes MC, García- Almeida JM, Esteva I, Beltrán M, et al. Hypertension is related to the degradation of dietary frying oils. Am J Clin Nutr. 2003;78(6):1092–1097. doi: 10.1093/ajcn/78.6.1092. [DOI] [PubMed] [Google Scholar]
- 7.Leong XF, Mustafa MR, Das S, Jaarin K. Association of elevated blood pressure and impaired vasorelaxation in experimental Sprague-Dawley rats fed with heated vegetable oil. Lipids Health Dis. 2010;9:66. doi: 10.1186/1476-511X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leong XF, Najib MNM, Das S, Mustafa MR, Jaarin K. Intake of repeatedly heated palm oil causes elevation in blood pressure with impaired vasorelaxation in rats. Tohoku J Exp Med. 2009;219(1):71–78. doi: 10.1620/tjem.219.71. [DOI] [PubMed] [Google Scholar]
- 9.Lioumbas JS, Karapantsios TD. Evaporation front compared with crust thickness in potato deep-fat frying. J Food Sci. 2012;77(1):17–25. doi: 10.1111/j.1750-3841.2011.02472.x. [DOI] [PubMed] [Google Scholar]
- 10.Casal S, Malheiro R, Sendas A, Oliveira BP, Pereira JA. Olive oil stability under deep-frying conditions. Food Chem Toxicol. 2010;48(10):2972–2979. doi: 10.1016/j.fct.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 11.Omar MN, Nor-Nazuha MN, Nor-Dalilah MN, Sahri MM. Frying performance of palm-based solid frying shortening. Pak J Biol Sci. 2010;13(6):298–302. doi: 10.3923/pjbs.2010.298.302. [DOI] [PubMed] [Google Scholar]
- 12.Leskova E, Kubikova J, Kovacikova E, Kosicka M, Pobruska J, Holcikova K. Vitamin losses: Retention during heat treatment and continual changes expressed by mathematical models. J Food Comp Anal. 2006;19:252–276. [Google Scholar]
- 13.Burlingame B, Mouillé B, Charrondière R. Nutrients, bioactive non-nutrients and anti-nutrients in potatoes. J Food Compost Anal. 2009;22(6):494–502. [Google Scholar]
- 14.Awney HA. The effects of Bifidobacteria on the lipid profile and oxidative stress biomarkers of male rats fed thermally oxidized soybean oil. Biomarkers. 2011;16(5):445–452. doi: 10.3109/1354750X.2011.590228. [DOI] [PubMed] [Google Scholar]
- 15.Purushothama S, Ramachandran HD, Narasimhamurthy K, Raina PL. Long-term feeding effects of heated and fried oils on hepatic antioxidant enzymes, absorption and excretion of fat in rats. Mol Cell Biochem. 2003;247(1-2):95–99. doi: 10.1023/a:1024194417673. [DOI] [PubMed] [Google Scholar]
- 16.Adam SK, Sulaiman NA, Top AGM, Jaarin K. Heating reduces vitamin E content in palm and soy oils. Malays J Biochem Mol Biol. 2007;15(2):76–79. [Google Scholar]
- 17.Leong XF, Aishah A, Nor Aini U, Das S, Jaarin K. Heated palm oil causes rise in blood pressure and cardiac changes in heart muscle in experimental rats. Arch Med Res. 2008;39(6):567–572. doi: 10.1016/j.arcmed.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 18.4th ed. Champaign: AOCS Press; 2003. Official methods and recommended practices of the American Oil Chemists’ Society. [Google Scholar]
- 19.Karoui IJ, Dhifi W, Jemia MB, Marzouk B. Thermal stability of corn oil flavoured with Thymus capitatus under heating and deep-frying conditions. J Sci Food Agr. 2011;91(5):927–933. doi: 10.1002/jsfa.4267. [DOI] [PubMed] [Google Scholar]
- 20.Shiozawa S, Tanaka M, Ohno K, Nagao Y, Yamada T. Re-evaluation of peroxide value as an indicator of the quality of edible oils. J Food Hyg Soc Jpn (Shokuhin Eiseigaku Zasshi) 2007;48(3):51–57. doi: 10.3358/shokueishi.48.51. [DOI] [PubMed] [Google Scholar]
- 21.Rueda-Clausen CF, Silva FA, Lindarte MA, Villa-Roel C, Gomez E, Gutierrez R, et al. Olive, soybean and palm oils intake have a similar acute detrimental effect over the endothelial function in healthy young subjects. Nutr Metab Cardiovasc Dis. 2007;17:50–57. doi: 10.1016/j.numecd.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Shuid AN, Chuan H, Mohamed N, Jaarin K, Fong YS, Soelaiman IN. Recycled palm oil is better than soy oil in maintaining bone properties in a menopausal syndrome model of ovariectomized rat. Asia Pac J Clin Nutr. 2007;16(3):393–402. [PubMed] [Google Scholar]
- 23.Medina I, Lois S, Alcántara D, Lucas R, Morales JC. Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-inwater emulsions. J Agr Food Chem. 2009;57(20):9773–9779. doi: 10.1021/jf9023867. [DOI] [PubMed] [Google Scholar]
- 24.Malheiro R, Oliveira I, Vilas-Boas M, Falcão S, Bento A, Pereira JA. Effect of microwave heating with different exposure times on physical and chemical parameters of olive oil. Food Chem Toxicol. 2009;47(1):92–97. doi: 10.1016/j.fct.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Azman, SM Shahrul, XS Chan, AP Noorhazliza, M Khairunnisak, MF Nur Azlina, HMS Qodriyah, et al. Level of knowledge, attitude and practice of night market food outlet operators in Kuala Lumpur regarding the usage of repeatedly heated cooking oil. Med J Malays. 2012;67(1):97–107. [PubMed] [Google Scholar]
- 26.Kochhar SP, Henry CJ. Oxidative stability and shelflife evaluation of selected culinary oils. Inter J Food Sci Nutr. 2009;60(7 Suppl):289–296. doi: 10.1080/09637480903103774. [DOI] [PubMed] [Google Scholar]
- 27.Moya Moreno MC, Mendoza Olivares D, Amézquita López FJ, Gimeno Adelantado JV, Bosch Reig F. Analytical evaluation of polyunsaturated fatty acids degradation during thermal oxidation of edible oils by Fourier transform infrared spectroscopy. Talanta. 1999;50(2):269–275. doi: 10.1016/s0039-9140(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 28.Velasco J, Marmesat S, Bordeaux O, Márquez-Ruiz G, Dobarganes C. Formation and evolution of monoepoxy fatty acids in thermoxidized olive and sunflower oils and quantitation in used frying oils from restaurants and fried-food outlets. J Agr Food Chem. 2004;52(14):4438–4443. doi: 10.1021/jf030753f. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willet WC. Trans fatty acids and cardiovascular disease. New Engl J Med. 2006;354(15):1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 30.Bryk D, Zapolska-Downar D, Malecki M, Hajdukiewicz K, Sitkiewicz D. Trans fatty acids induce a proinflammatory response in endothelial cells through ROS-dependent nuclear factor-κB activation. J Physiol Pharmacol. 2011;62(2):229–238. [PubMed] [Google Scholar]
- 31.Quiles JL, Ramírez-Tortosa MC, Ibáñez S, Alfonso González J, Duthie GG, Huertas JR, et al. Vitamin E supplementation increases the stability and the in vivo antioxidant capacity of refined olive oil. Free Radic Res. 1999;31:S129–S135. doi: 10.1080/10715769900301421. [DOI] [PubMed] [Google Scholar]
- 32.Bardhan J, Chakraborty R, Raychaudhuri U. The 21st century form of vitamin E - Tocotrienol. Curr Pharm Des. 2011;17(21):2196–2205. doi: 10.2174/138161211796957472. [DOI] [PubMed] [Google Scholar]
- 33.Matthaus B. Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. Eur J Lipid Sci Tech. 2006;108(3):200–211. [Google Scholar]
- 34.Abdullah A, Suondoh MS, Xuan CS, Patah NA, Mokhtar K, Fahami NAM, et al. Level of awareness amongst the general public regarding usage of repeatedly heated cooking oil in Kuala Lumpur, Malaysia. Inter Med J. 2010;17:310–311. [Google Scholar]