Abstract
Cytochrome P450 2C9 (CYP2C9) c.449G> A (*8) is common in African Americans and is associated with decreased warfarin clearance. We examined the effect of promoter region variants inherited with 449G > A on warfarin clearance, dose requirements, and CYP2C9 expression. In an African American cohort, 449G > A was in linkage disequilibrium with c. – 1766T >C (r2 = 0.89) and c. – 1188T>C (D′ =1). The combination of the – 1766C and 449A alleles with the – 1188CC genotype was associated with lower S-warfarin clearance (0.86±0.22 vs. 1.66±0.75 ml/min/m2; n=48; P <0.01) and dose requirements [33 (25–49) vs. 43 (35–56) mg/week; n= 243; P= 0.03] compared with other genotypes. In liver tissue, alleles with the – 1766C/ – 1188C/449A haplotype showed two-fold decreased mRNA expression compared with reference alleles. In a promoter reporter assay, the – 1766C/ – 1188C haplotype decreased CYP2C9 promoter activity. These data suggest that promoter region polymorphisms inherited with 449G >A decrease CYP2C9 expression and contribute to CYP2C9*8 effects on warfarin clearance and dose requirements.
Keywords: African American, CYP2C9*8, genotype, polymorphism, warfarin
Introduction
Cytochrome P450 (CYP) 2C9 variants leading to reduced S-warfarin clearance increase the risk for supratherapeutic anticoagulation and hemorrhage [1]. The CYP2C9 c.449G>A (rs7900194, R150H) polymorphism occurs in ~12% of African Americans and confers the CYP2C9*8 allele [2]. CYP2C9*8 is associated with reduced S-warfarin clearance and dose requirements, effects partly attributable to decreased warfarin-metabolizing activity with the 449G>A (R150H) variant [3]. The 449G>A allele is reportedly in linkage disequilibrium with c. – 1766T>C (rs9332094) and c. – 1188T>C (rs4918758) in the upstream regulatory region of the gene [4]. We sought to determine the functional consequences of the – 1766T>C and – 1188T>C variants and their contribution to clinical effects attributed to the CYP2C9*8 allele.
Methods
Clinical studies
Genetic samples were collected from 264 African Americans (mean age 56±16 years, 73% women) on a stable warfarin dose. In 48 patients, blood was collected for the determination of warfarin plasma concentrations by a chiral HPLC-based method [5]. The methods for the clinical studies have been detailed elsewhere and were approved by the institutional review board and conducted in accordance with the Declaration of Helsinki [2,3].
Following DNA isolation (Puregene Kit; Qiagen, Valencia, California, USA), samples were genotyped for CYP2C9 coding region variants, as described previously [6]. The – 1766T>C and – 1188T>C variants were determined using PCR and capillary sequencing with primers 5′-ATTGCTTTTCTT-TGCCCTGT-3′ (forward and sequencing) and 5′-CTCCAGACATGGCTGCTT TC-3′ (reverse).
Pharmacokinetic parameters were compared among genotype groups using one-way analysis of variance. Genetic associations with dose requirements were examined using the independent-samples Kruskal–Wallis test.
RNA expression
Liver tissues, snap-frozen immediately after collection, were obtained from Life Technologies (Carlsbad, California, USA) for 34 African Americans (mean age 58±11 years, 65% women), compliant with an institutional review board protocol operating in accordance with the Federal Regulations for the protection of human research participants. Tissues were genotyped for CYP2C9 (as above), and CYP2C9 mRNA levels were determined as described previously [7]. Allele-specific expression of CYP2C9 was also measured according to published methods with modifications, with the 449G>A variant used as the marker [8]. Genomic DNA and RNA were isolated from six samples heterozygous for 449G>A using Qiagen and Trizol (Life Technologies) kits, respectively. A fragment of genomic DNA or RNA (after conversion to cDNA) surrounding the 449G>A polymorphism was PCR amplified (product size 230 bp), followed by a primer extension (SNaPshot; Life Technologies). Then, the primer extension products were analyzed on an ABI 3730 capillary electrophoresis DNA sequencer (Life Technologies). mRNA and genomic DNA expression levels of the 449A allele were measured and normalized by that of the 449G allele.
Plasmids and cell transfection
The CYP2C9 expression vector was constructed by subcloning CYP2C9 from BacFast1-CYP2C9 (kindly provided by Dr Allan Rettie, University of Washington) into pcDNA3 (Life Technologies). The 449G>A polymorphism was introduced using a QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, California, USA). Two CYP2C9 expression vectors each carrying cDNA of 449G and 449A were cotransfected into HEK293Tcells in equal amounts. RNAs were isolated from cells using Trizol and treated with DNase I to eliminate plasmid DNA contamination. A portion of untreated total RNA was saved as source of plasmid DNA, serving as internal control for the allele-specific RNA expression experiment. Using the vector-specific primer sets, allelic expression imbalance was examined as described above.
Luciferase reporter assay
The following four luciferase constructs were generated: pCYP2C9 [– 1188T/ – 1766T], pCYP2C9 [– 1188C/ – 1766T], pCYP2C9 [– 1188T/ – 1766C], and pCYP2C9 [– 1188C/ – 1766C]. To generate pCYP2C9, – 1828/+25 of CYP2C9 was PCR amplified using p2C9-5K-Luc plasmid obtained from Dr Dexi Liu (University of Pittsburgh) and cloned into pGL3-basic vector (Promega, Madison, Wisconsin, USA). The – 1766T>C and – 1188T>C polymorphisms were introduced by site-directed mutagenesis, as described above. All insert sequences were confirmed by capillary sequencing. HepG2 cells were transfected with one of the four luciferase vectors and β-galactosidase expression plasmid (for normalization of transfection efficiency) using FuGENE HD transfection reagent (Roche Applied Science, Indianapolis, Indiana, USA). After 48 h, the luciferase assay was performed using kits from Promega.
Results
Clinical studies
The – 1766C, – 1188C, and 449A frequencies were 0.074, 0.37, and 0.074, respectively. The – 1766C variant was in strong linkage disequilibrium with 449A (r2=0.89, D′ =0.95), and – 1188C was always present with – 1766C (r2=0.14, D′ =1) and 449A (r2=0.15, D′ =1), indicating a haplotype containing all three single-nucleotide polymorphisms (SNPs). The following – 1766/ – 1188/449 combinations were identified: TT/TT/GG (designed V0 for no variant, n=116); TT/CT/GG and TT/CC/GG (V1 and V2 for one and two variants, respectively, n=113); CT/CT/AG (V3 for three variants, n=19); CT/CC/AG (V4 for four variants, n=8); and CC/CC/AA (V6 for six variants, n=4). Other combinations, including the V5 combination of five variants (CC/CT/AA) occurred rarely (frequency <0.01). The – 1188C allele also occurred with CYP2C9*2 and *6 (D′ =1); thus, CYP2C9*2 and *6 genotypes (n=21) were excluded from further analyses to eliminate effects associated with their uneven distribution according to – 1188T>C genotype.
Median (interquartile range) weekly warfarin dose requirements were the lowest with V4 or V6 [33 (25–49)mg], intermediate with V3 [38.5 (26–56) mg], and the highest with other genotypes [42.5 (35–56) mg; P=0.036]. Similarly, S-warfarin clearance (ml/min/m2) was lowest with V4 or V6 (0.86±0.22; n=5), intermediate with V3 (1.31±0.32; n=7), and highest with V0 genotype (1.89±0.91; n=9; P=0.025). Clearance with V4 or V6 was also lower than all other genotypes combined (1.66±0.75 ml/min/m2; P<0.01).
CYP2C9 expression
To determine whether CYP2C9 haplotype affects CYP2C9 expression, we examined CYP2C9 mRNA expression in liver tissues. Twenty-eight tissues had V0, five had V3, and one had the rare TT/CT/AG genotype, with 449A occurring without – 1766C. All six samples heterozygous for 449G>A were absent for other CYP2C9 coding region variants. CYP2C9 expression in livers exhibited marked (500-fold) interindividual variation, with no difference in mRNA expression by genotype (data not shown).
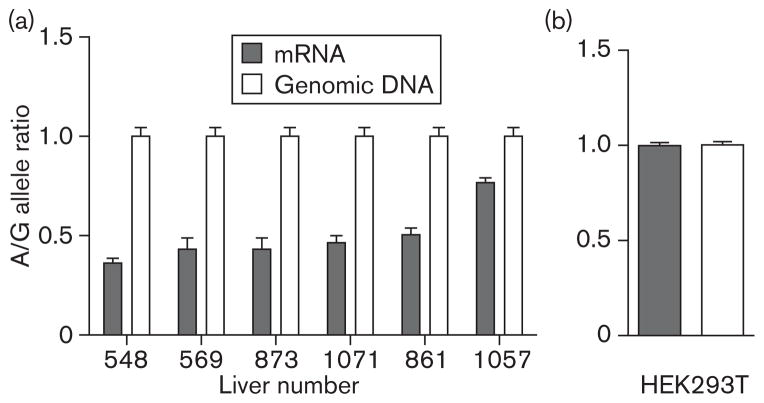
To eliminate the effects of unknown clinical factors (e.g. liver function) on CYP2C9 expression, allele-specific expression was determined for the six heterozygous livers [8]. As allele-specific RNA expression measures the relative amount of RNA derived from each of the two alleles in the same cells from the same individual, the influence from trans-acting factors (e.g. environmental and clinical factors) can be eliminated. Therefore, allele-specific RNA expression is a more accurate and sensitive measure than total RNA expression for detecting the effects of cis-acting regulatory polymorphisms on RNA expression. As expected from A/G heterozygosity, the 449A/ G allele ratio for genomic DNA was close to 1 (Fig. 1a). However, mRNA of 449G and 449A revealed allele-specific expression imbalance; there was significantly lower expression with 449A compared with 449G; the A/G allele ratios ranged from 0.36 to 0.77 (Fig. 1a). The sample with the – 1766 T/T rather than T/C genotype exhibited the least expression imbalance (A/G ratio 0.77).
Fig. 1.
(a) Allele-specific expression of cytochrome P450 2C9 (CYP2C9) in human livers. Allele-specific mRNA expression of CYP2C9 was obtained for each allele using 449A>G as a marker in six heterozygous livers. Results represent the ratio between 449A and 449G for mRNA and genomic DNA (mean±SD from triplicate or quadruplicate measurement in each liver sample). Samples were also heterozygous for – 1766T>C and – 1188T >C, except for liver 1057, which was homozygous for – 1766TT. (b) Allele-specific mRNA expression of CYP2C9 was obtained in HEK293T cells cotransfected with CYP2C9 449G and 449A cDNA.
To rule out potential contribution of the coding SNP in the – 1766C/ – 1188C/449A haplotype to allele-specific expression imbalance in A/G livers, expression of 449G and 449A was examined after transfection of expression vectors in human cells. The results showed that allele-specific expression levels of 449G and 449A were similar (Fig. 1b), suggesting that the associated promoter region SNPs are responsible for decreased allele-specific expression of – 1766C/ – 1188C/449A haplotype.
Promoter activity
To determine whether – 1766C and – 1188C decrease CYP2C9 expression through effects on promoter activity, luciferase assays were performed. The T>C polymorphism at – 1766 or – 1188 led to enhanced luciferase activity (2.6-fold and 1.7-fold induction, respectively), whereas polymorphisms at both – 1766 and – 1188 (i.e., the variants associated with CYP2C9*8) led to five-fold reduction in activity (Fig. 2). These results indicate that the – 1766C and – 1188C alleles that occur commonly in 449A (150H) carriers lead to decreased CYP2C9 promoter activity.
Fig. 2.
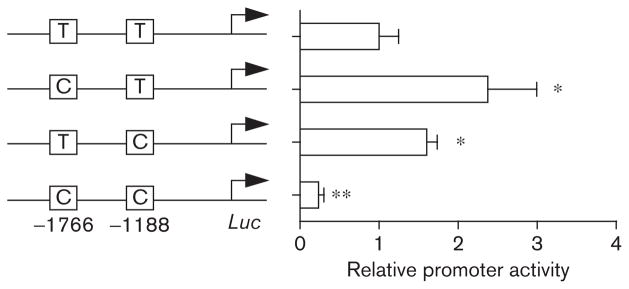
Effects of – 1766T >C and – 1188T >C promoter region single-nucleotide polymorphisms on promoter activity of cytochrome P450 2C9 (CYP2C9). HepG2 cells were transfected with a luciferase (Luc) construct harboring the promoter SNPs of CYP2C9, and β-galactosidase expression plasmid. After 48 h post-transfection, luciferase assay was performed. Results represent the relative luciferase activity in comparison with the wild-type sequence of – 1766T/ – 1188T (mean±SD; n=3). *P<0.05; **P<0.01 compared with wild type.
Discussion
The combination of the – 1766C and 449A variants with the – 1188CC genotype conferred reduced warfarin clearance and dose requirements. In line with these data, the – 1766C/ – 1188C/449A haplotype was associated with reduced mRNA expression. Finally, promoter region activity was reduced with the – 1766C/ – 1188C haplotype.
The mechanism underlying potential synergistic effects with – 1766C and – 1188C is unknown, but possibly related to recruitment of different homeodomain transcription factors and coregulators. In-silico analysis of CYP2C9 upstream regulatory region using MatInspector (Genomatix Software GmbH, Munich, Germany) predicted that transcription factors in homeodomain protein family bind to – 1766 and – 1188, regardless of the presence of the variant alleles (data not shown). Homeodomain transcription factors behave as activators or repressors of target gene expression and are involved in regulation of developmental processes in eukaryotes [9,10]. Different members of homeodomain transcription factors are known to show similar DNA-binding specificity, and yet regulate a distinct set of target genes by selective interaction with coregulators [9,10]. It appears possible that subtle changes in recruitment of homeodomain transcription factors and coregulators are responsible for the decreased CYP2C9 promoter activity from – 1766C and – 1188C variants. Similar subtle changes in actions of homeodomain transcription factors may be responsible for the increased CYP2C9 promoter activity upon polymorphism at – 1766 or – 1188 alone.
Conclusion
Our data suggest that the combination of – 1188C and – 1766C decreases CYP2C9 mRNA expression, likely due to reduced CYP2C9 promoter activity. Decreased expression from promoter region SNPs, in addition to the decreased warfarin-metabolizing activity with 449G>A in the gene coding region [3], provides a mechanistic explanation for the association between CYP2C9*8 and decreased warfarin dose requirements. In other words, our data suggest that the effect of CYP2C9*8 is not solely conferred by the R150H amino acid change, but further enhanced by SNPs located in the gene promoter.
Acknowledgments
This work was supported by grants from the American Heart Association Midwest Affiliate (10GRNT3750024) (L.H.C.), NIH U01 (GM092655) (D.W.), NIH R21 (HL106097) (M.A.P.), and NIH K23 (HL089808–01A2) (M.A.P.).
Footnotes
Conflicts of interest
L.H.C. is coinventor for US Utility Patent Application No. 12/572908, titled ‘CYP2C9*8 alleles correlate with decreased warfarin metabolism and increased warfarin sensitivity’. Published: 27 May 2010; Pub. No. US 2010/0130599. For the remaining authors there are no conflicts of interest.
References
- 1.Limdi NA, McGwin G, Goldstein JA, Beasley TM, Arnett DK, Adler BK, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther. 2008;83:312–321. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavallari LH, Langaee TY, Momary KM, Shapiro NL, Nutescu EA, Coty WA, et al. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin Pharmacol Ther. 2010;87:459–464. doi: 10.1038/clpt.2009.223. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Jeong H, Takahashi H, Drozda K, Patel SR, Shapiro NL, et al. Decreased warfarin clearance associated with the CYP2C9 R150H (*8) polymorphism. Clin Pharmacol Ther. 2012;91:660–665. doi: 10.1038/clpt.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaisdell J, Jorge-Nebert LF, Coulter S, Ferguson SS, Lee SJ, Chanas B, et al. Discovery of new potentially defective alleles of human CYP2C9. Pharmacogenetics. 2004;14:527–537. doi: 10.1097/01.fpc.0000114759.08559.51. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi H, Kashima T, Kimura S, Muramoto N, Nakahata H, Kubo S, et al. Determination of unbound warfarin enantiomers in human plasma and 7-hydroxywarfarin in human urine by chiral stationary-phase liquid chromatography with ultraviolet or fluorescence and on-line circular dichroism detection. J Chromatogr B Biomed Sci Appl. 1997;701:71–80. doi: 10.1016/s0378-4347(97)00346-0. [DOI] [PubMed] [Google Scholar]
- 6.Cavallari LH, Butler C, Langaee TY, Wardak N, Patel SR, Viana MAG, et al. Association of apolipoprotein E genotype with duration of time to achieve a stable warfarin dose in African-American patients. Pharmacotherapy. 2011;31:785–792. doi: 10.1592/phco.31.8.785. [DOI] [PubMed] [Google Scholar]
- 7.Choi SY, Koh KH, Jeong H. Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos. 2012 doi: 10.1124/dmd.112.046276. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J. 2011;11:274–286. doi: 10.1038/tpj.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee-Basu S, Baxevanis AD. Molecular evolution of the homeodomain family of transcription factors. Nucleic Acids Res. 2001;29:3258–3269. doi: 10.1093/nar/29.15.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CC, Herr W. Differential control of transcription by homologous homeodomain coregulators. Mol Cell Biol. 1996;16:2967–2976. doi: 10.1128/mcb.16.6.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]