Abstract
Stem cells are the seeds of tissue repair and regeneration and a promising source for novel therapies. However, apart from hematopoietic stem cell (HSC) transplantation for hematologic disease, essentially all other stem cell treatments remain experimental. High hopes have inspired numerous clinical trials, but it has been difficult to obtain unequivocal evidence for robust clinical benefit, likely owing to our primitive state of knowledge about therapeutic mechanisms. Outside the standard clinical trial network unproven therapies are being widely practiced in an open market, which threatens the cause of legitimate clinical investigation of the safety and efficacy of stem cell interventions. Here is one practitioner's perspective on the challenges and technical barriers that must be overcome for novel stem cell therapies to achieve meaningful clinical impact.
Cell therapeutics: the current standard of care
In the twentieth century small molecule and protein drugs proved remarkably successful in restoring health and extending lifespan, but in the twenty-first century, our aging population will face an increasing burden of organ failure and neurodegenerative disease. Such conditions are unlikely to be cured by drugs alone, and instead call for restoration of tissue function through novel therapeutic approaches. Transplantation of whole organs—heart, lung, liver, kidney, small bowel, and pancreas—has become routine in modern medicine and has saved countless lives, while grafts of the skin and cornea for burns or ocular injury, and transfusions of red blood cells and platelets for disease-related or chemotherapy-induced cytopenias are likewise widely employed tissue and cell therapies. However, current therapeutic strategies are either limited by donor availability and immunologic barriers, or pertain to only a minor range of conditions. For the many diseases and disorders of aging for which there is no cure, innovative applications of tissue engineering and novel cell therapies derived from pluripotent and tissue-restricted stem cells represent major frontiers for the future.
Hematopoietic stem cells (HSCs), the therapeutic constituents of whole bone marrow and umbilical cord blood, have been the most widely employed stem cell therapy. When successful, HSC transplantation can be curative for scores of genetic blood disorders like thalassemia and immune deficiency and for malignancies like leukemia and lymphoma. HSC transplantation is undoubtedly the most successful application of stem cells in medicine, yet for many conditions success rates remain frustratingly low and morbidity and mortality unacceptably high. The need for precise molecular matching of donor and recipient means that many patients lack a suitable donor, either within their own family or in the public at large, even when databases list many millions of potential unrelated donors. When a match can be found, minor mismatches between donor and recipient frequently incite graft versus host disease (GVHD), an attack of the donor immune effector T cells against host tissues that results in skin rash, mucositis, diarrhea, and liver and lung destruction. GVHD is a major cause of treatment associated morbidity and mortality. Finally, grafts can fail, and disease can relapse. Although it is difficult to give a precise figure for the overall success rate for HSC transplantation, even an optimist would acknowledge that some 50% of patients are left without a cure or with a permanent disability. Thus, even our most successful form of stem cell therapy remains a heroic effort, reserved only for the sickest patients who have no better alternative.
Lessons from the historical development of HSC transplantation
The evolution of HSC transplantation from its experimental origins to its acceptance as a standard of care in medicine is a tale that is both inspiring and cautionary. E. Donnall Thomas and colleagues were the first to perform marrow transplantation for otherwise fatal leukemia in the 1950s (Thomas et al., 1957). The rationale was predicated upon the known capacity for radiation to suppress leukemic hematopoiesis, and studies demonstrating that injections of marrow rescued mice from otherwise lethal radiation exposure (Jacobson et al., 1951; Lorenz et al., 1951). Thomas wrote in a memoir in 2005 “these patients inspired us to speculate that it might be possible to destroy leukemic cells and normal marrow by lethal whole body irradiation, with reconstitution of marrow by marrow transplantation”. Arguably, the first studies in humans were founded upon rather minimal evidence of efficacy in rodent models, and Thomas further noted “we recognized that it would be important to do similar studies in an animal model...[and] decided to move forward with studies of man and dog at the same time” (Thomas, 2005). Indeed, Thomas and colleagues suffered considerable failure in pre-clinical canine models and witnessed the deaths of many scores of patients, which prompted great skepticism about whether the human experiments should continue. Nevertheless, Thomas and his intrepid team of investigators forged ahead. It took almost two decades before advances in research on tissue matching to define compatible donor-recipient pairs, and improved treatment of graft versus host disease and the infectious complications of marrow transplant allowed marrow transplantation to achieve consistent success in the late 1970s.
Some important principles emerge from this lesson in the history of HSC transplantation. First, the risk of the intervention should be commensurate with the severity of the underlying condition to be treated. The aggressively malignant nature of the conditions being treated—fatal leukemia and marrow aplasia—meant that the first practitioners of marrow transplantation were justified and even compelled to attempt heroic and potentially highly toxic interventions for invariably fatal diseases. Second, although human biology is only partially predictable from animal models, pre-clinical animal models remain a key element in the scientific development of novel therapies. At the beginning of human marrow transplantation, it was understood that identical twins accepted skin and solid organ grafts, but only a minority of the time did siblings. Experiments in the murine and canine marrow transplantation models reflected similar transplantation barriers. Notwithstanding these sobering limitations, the early practice of marrow transplant in patients proceeded despite a lack of robust evidence in animal models for graft acceptance between unrelated individuals. Only later were methods for lymphocyte matching developed (the antecedent to HLA typing), which was the key development in advancing the success of marrow transplantation. Finally, important and fundamental insights into therapeutic mechanisms were required before the eventual success of clinical translation of HSC transplantation therapies.
With the benefit of hindsight, one could argue that the earliest human transplants were premature and doomed to fail. One might question whether a therapy as toxic as marrow transplant, with so little evidence for success in animal models prior to testing in humans, could emerge in the current era. Under today's more rigorous regulatory climate, institutional review boards weigh risks and potential benefit on behalf of patients, insist on an impartial process of informed consent to minimize misconceptions about therapeutic potential, and monitor adverse events in the course of clinical trials. Indeed, one might reasonably conclude that today's IRBs might not have approved the early studies of Thomas and colleagues, but if they had, would have interceded to stop the experiments when the high incidence of treatment-related mortality became apparent.
The conjecture that modern-day IRBs might not approve the early experiments in HSC transplant does not imply that HSC transplant would not emerge under the current regulatory climate. On the contrary, I believe that bone marrow transplant could be developed within today's environment of strict clinical research regulation, although by a more conservative path that would spare considerable patient morbidity and mortality. As we learned from premature attempts at gene therapy in the early 1990s, new therapeutic technologies require considerable understanding of fundamental mechanisms before they can be delivered with confidence. Indeed, roughly 70% of early phase clinical trials of pharmaceuticals fail and over 50% at phase III (Ledford, 2011), and thus it stands to reason that significant resources are squandered because of the imprecision of early stage clinical research. Yet, especially with novel technologies, clinical experimentation proceeds energetically, because hope triumphs over experience. From this author's perspective, a conservative approach to clinical translation of stem cell therapies is warranted at this time, not because stem cell treatments are excessively risky (though some may yet prove to be), but rather because our understanding of the mechanisms by which stem cells might prove useful, and in which diseases, remains grossly inadequate. In a climate where government and philanthropic funds for fundamental research are increasingly scarce, and investment capital from the private sector for biotechnology has dried up, purely empirical attempts at stem cell therapy are difficult to justify, given the high probability of failure. In a 1995 report assessing the investment in gene therapy by the United States National Institutes of Health, a panel chaired by Stuart Orkin and Arno Motulsky recommended “increased emphasis on research dealing with the mechanisms of disease pathogenesis, further development of animal models of disease, enhanced use of preclinical gene therapy approaches in these models, and greater study of stem cell biology in diverse organ systems” (http://oba.od.nih.gov/oba/rac/panelrep.pdf). Similar recommendations regarding the need for proper investments in fundamental aspects of stem cell therapeutics seems warranted and prudent at this time.
Stem cell therapeutics: front-line clinical trials and medical innovations
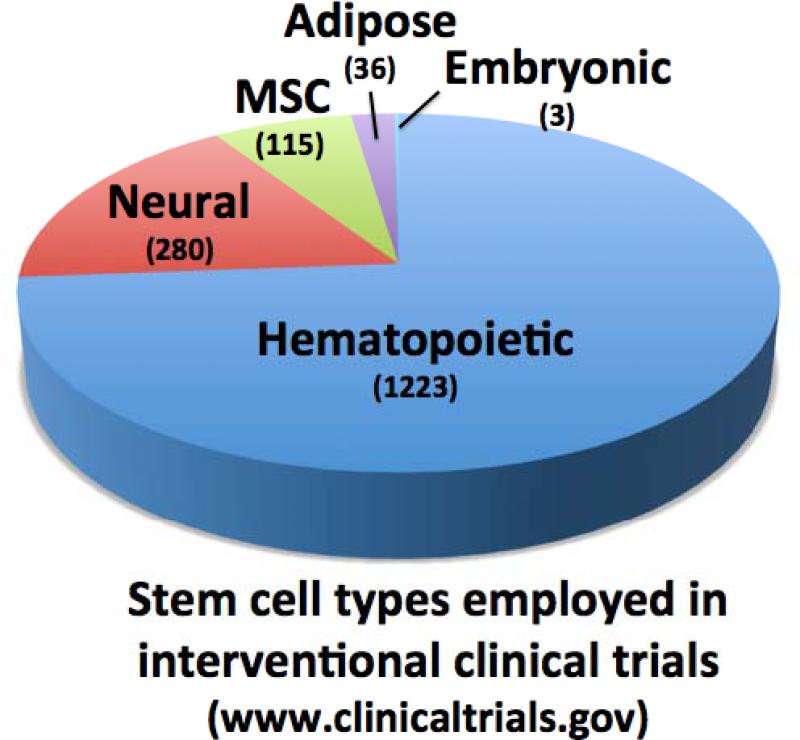
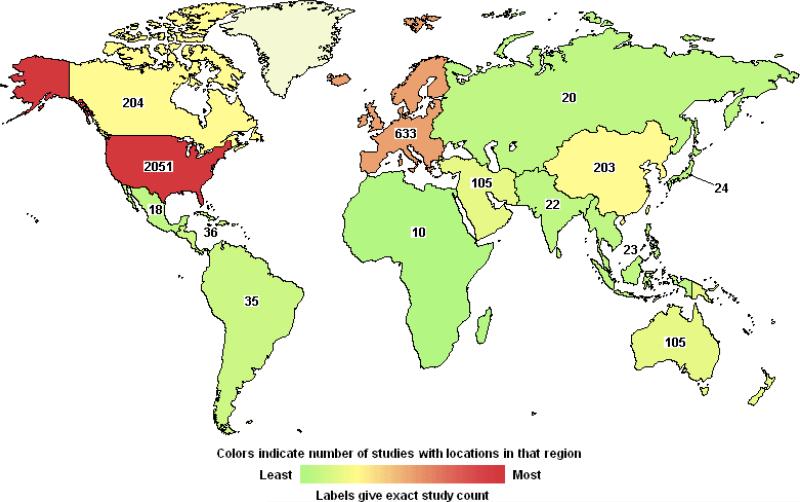
A search of the Unites States government-sponsored website www.clinicaltrials.gov with the term “stem cells” lists over 4000 past, current, and anticipated trials, with over 1750 now open (Figure 1). The vast majority of open trials pertain to variations on HSC transplantation (>1200), including strategies to expand the sub-optimal dose of HSCs within umbilical cord blood, to complement gene defects in HSCs through viral transgene delivery (“gene therapy”), and to engineer T cells to attack malignancy via adoptive immunotherapy. Hundreds more trials are testing mesenchymal (115), adipose-derived stem cells (36), and neural stem cells (280), sometimes in quite bold and unconventional ways that bear little resemblance to the known modes of tissue regeneration or repair associated with these classes of stem cells. As of this writing, three trials pertain to products derived from embryonic stem cells (ESCs). Such studies are being carried out on a global basis on all continents, suggesting widespread clinical interest (Figure 2).
Figure 1. Clinical trials of major stem cell types.
Pie chart indicating the relative numbers of open trials testing clinical interventions for hematopoietic, neural, mesenchymal, adipose, and embryonic stem cells, as listed on the US NIH website clinicaltrials.gov.
Figure 2. World-wide experimental trials of stem cell-based therapies.
World map showing locations of open, closed and pending clinical trials of stem cell-based interventions as listed on US NIH website clinical trials.gov. The relative numbers of trials performed outside of the United States may indeed be markedly understated because of reporting bias at the US government clinical trials website.
Mesenchymal stem cells (MSCs) are defined by their fibroblast-like morphology, adherence to plastic, expression of a specific set of surface antigens (CD105+, CD90+, CD73+), and capacity for osteogenic, chondrogenic, and adipogenic fates in vitro. MSCs are most often derived from bone marrow but can also be isolated from adipose tissue; adipose-derived stem cells may also consist of pericytes or endothelial progenitors, which may differ somewhat in their properties from MSCs. Easy access to large quantities is an advantage for adipose-derived stem cells, which are being tested for soft-tissue repair and regeneration (Tobita et al., 2011). Both autologous (self) and allogeneic (foreign) MSCs are being tested in vivo to enhance healing that reflects their in vitro potential to form bone or cartilage, as in bone fracture and joint cartilage repair (Griffin et al., 2011). Although such studies are founded on strong pre-clinical evidence and sound scientific and clinical hypotheses, evidence for robust clinical efficacy of MSCs for orthopedic indications has been challenging to confirm, and to date no therapy based on MSCs has yet won approval by the US Food and Drug Administration (FDA). The difficulty in proving the efficacy of regenerative treatments based on the well characterized cellular potentials of MSCs suggests that our understanding of how even familiar stem cells can be exploited therapeutically in vivo remains primitive.
MSCs are being tested in a wide range of clinical indications where the clinical hypotheses are more speculative, the therapeutic mechanisms are incompletely defined, and in some instances the pre-clinical evidence is highly contentious.
For example, from a scientific foundation that can be traced to a highly controversial report that whole bone marrow would regenerate cardiac muscle following transplantation into injured hearts (Orlic et al., 2001), an observation later disproven (Balsam et al., 2004), thousands of patients have been treated in trials worldwide with various cell preparations of bone marrow or MSCs, with the scientific community debating the significance of the results (Choi et al., 2011). Subsequent studies have invoked a variety of contingent mechanisms including salutary paracrine effects on resident cardiomyocytes and putative cardiac stem cells, neoangiogenesis, and biomechanical alterations due to scarring (Gnecchi et al., 2008; Menasche, 2011; Williams et al., 2011). The questions about underlying mechanism notwithstanding, combined meta-analyses of numerous trials has argued for measureable yet quite modest therapeutic effects, which has left practitioners unsure of the significance and robustness of these therapeutic approaches (Tongers et al., 2011).
MSCs have also been widely tested for their capacity to mitigate autoimmunity, following somewhat serendipitous observations that MSCs can interfere with in vitro immunological assays such as mixed lymphocyte reactions and modulate production and function of the major classes of immune cells (Kode et al., 2009; Shi et al., 2011). Although it is unclear whether immune antagonism reflects any native function of MSCs in vivo, ex vivo expanded preparations have been infused in patients in hopes of mitigating transplant-related graft vs host disease and autoimmune conditions like Crohn's disease, multiple sclerosis, and systemic lupus (Kebriaei and Robinson, 2011; Shi et al., 2011). One can find numerous reports of efficacy in the literature, but these are mixed with negative data (Kebriaei and Robinson, 2011). The precise role of MSCs as agents for immune modulation remains to be proven.
When clinical indications stray yet further from the presumptive core functions of MSCs, and therapeutic mechanisms become increasingly speculative, clinical translation is a largely empirical rather than a rational effort. Likewise, while umbilical cord blood (UCB) has emerged as a viable alternative to other sources of HSCs (e.g., mobilized peripheral blood or bone marrow) for the treatment of leukemia and non-malignant hematologic conditions(Rocha et al., 2004) it has also become a common source for experimental interventions in a wide variety of non-hematologic indications as disparate as myocardial infarction, multiple sclerosis, amyotrophic lateral sclerosis, cerebral palsy, traumatic brain injury, stroke, and inherited metabolic disorders (Copeland et al., 2009; Harris, 2009; McKenna and Sheth, 2011; Prasad and Kurtzberg, 2009). Evidence exists that a number of distinct cell types can be cultured from UCB, including multipotential stem cells (Kogler et al., 2004; Pelosi et al., 2012) but it is unclear whether such expandable cell populations exist at appreciable levels in unmanipulated samples. While in theory such cells could mediate therapeutic effects, non-hematologic indications for UCB transplantation have not been widely accepted into standard practice. When clinical investigation proceeds largely empirically, and without a deeper understanding of the basic therapeutic mechanisms, it is difficult to reformulate therapeutic strategies after clinical failures.
Neural stem cells (NSC) can be cultured from fetal and adult brain and demonstrated to differentiate into neurons, oligodendrocytes, and astrocytes in vitro. Given the wide array of neurologic conditions that have devastating clinical consequences, there is considerable interest in the therapeutic potential of neural regeneration therapies. However, neurodegenerative diseases, catastrophic stroke, traumatic brain injury, and spinal paralysis are among the most daunting challenges for regenerative medicine. The development of the brain and peripheral nerves and their interconnectedness with tissues throughout the body requires a remarkably complex choreography during fetal development. The proper milieu for directing the formation of highly specified neuronal sub-types and guiding their projection to and interconnectedness with critical targets is highly unlikely to exist in the adult body. But faced with compelling unmet medical need and desperation on the part of patients, there are hundreds of investigator – initiated clinical trials occurring in academic settings (Figure 1), and several companies have forged efforts to develop novel therapies through involving intracerebral or spinal transplantation of neural stem cells (Trounson et al., 2011). StemCells Inc (California, USA) has tested NSCs in Batten's Disease (neuronal ceroid lipofuscinosis) and was able to document safe delivery but discontinued the trial because of the inability to accrue an adequate number of patients. Their current focus is Pelizaeus-Merzbacher Disease, a myelin disorder, and chronic spinal cord injury. Other companies are testing NSC transplant for stroke (ReNeuron, United Kingdom), Amyotropic Lateral Sclerosis (Neuralstem, Inc, Maryland, USA), and Parkinson's Disease (NeuroGeneration, California, USA). In most of these cases, the clinical hypotheses being tested do not depend upon the generation of neurons de novo, but instead on complementation of enzyme deficiencies, remyelination, or modulation of endogenous repair through neoangiogenesis or neuroprotection.
Although widely publicized, there are comparatively few clinical trials of products derived from human embryonic stem cells (hESC). The first trial conducted in humans delivered oligodendrocyte progenitors for the remyelination of spinal cord axons damaged through crush injury. These studies were based on extensive pre-clinical experience with the derivation and characterization of oligodendrocytes, and their delivery in animal models that showed remyelination and restoration of motor function (Keirstead et al., 2005; Liu et al., 2000; McDonald and Belegu, 2006; McDonald and Howard, 2002; McDonald et al., 1999; Nistor et al., 2005). Moreover, this first trial required a herculean effort to satisfy FDA regulatory oversight, by report entailing the submission of over 20,000 pages of data and documentation. The trial, sponsored by the Geron Corporation (California, USA), enrolled and treated its first four patients before being discontinued due to a decision by company management to focus on alternative corporate priorities (Baker, 2011). No formal results have yet been released regarding the phase 1 clinical trial in this first small cohort of patients, but the primary endpoints were safety of the cells, and at the very least one hopes that some evidence will be gleaned that products of ESCs can be delivered without risk of teratoma, although long-term follow-up of all treated patients will be necessary.
The only other current clinical trials involve transplantation of hESC-derived cells to treat retinal blindness. This condition takes many forms, both genetic and age-related, and as a group of disorders has many appealing features for stem cell based interventions. The retina is accessible for local delivery of cells, which can then be monitored via direct visualization. The retina may also provide some degree of immune privilege. Very preliminary results of a trial involving the sub-retinal injection of hESC-derived retinal pigment epithelial cells for Stargardt's Macular Degeneration and another for age-related macular degeneration sponsored by the company Advanced Cell Technologies (ACT) were recently reported, despite experience on only one patient in each trail (Schwartz et al., 2012). Only one of the two patients showed evidence of persistent cells but both were reported to show some restoration of visual perception. While it is difficult to draw conclusions from these early trials due to the limited numbers of patients involved and the very brief 4 month period of follow-up, the trials represent milestones in that the investigators succeeded in clearing considerable regulatory hurdles and met very high standards of pre-clinical cell characterization and quality control prior to exposing patients to the risk of ESC-based products. The experience alone, for both investigators and regulators, is an essential albeit small step forward in the long path to establishing ESC-based therapeutics.
While MSCs, NSCs, and products from ESCs are being tested in the context of numerous clinical trials, yet another arm of regenerative medicine—tissue engineering—is co-mingling MSCs or a variety of other cultured cell types with biocompatible materials to solve surgical challenges. Reconstruction of bladders (Aboushwareb and Atala, 2008; Atala, 2011; Tian et al., 2010), tendons (Sun et al., 2011), and complex structures like the trachea (Macchiarini et al., 2008) represent solutions to highly personal needs of specific patients, and are acceptably performed as highly innovative and individualized surgical therapies, part of the long tradition of surgical innovation. The mechanisms for developing such novel interventions and gaining acceptance by the surgical and biomedical communities involve the same core principles required for medical interventions—sound scientific rationale and methods, institutional and practitioner accountability, thorough and rigorous informed consent, patient follow-up, timely reporting of adverse events, peer review of therapeutic claims and publication in the medical literature. The potential for therapeutic innovation at the interface of stem cell biology and tissue engineering is particularly appealing but beyond the scope of this review. I refer the reader instead to excellent recent reviews (Griffin et al., 2011; Peck et al., 2012; Sun et al., 2011).
Anticipated future interventions and opportunities
Among the many disparate conditions, disorders, and diseases for which stem cells have offered promise, a few stand out as particularly compelling. In general, they are conditions where defects are largely cell autonomous, and entail the loss or dysfunction of a single class of cells or a monocellular component of a complex tissue, such that restoration of function through cell replacement would be curative or significantly ameliorate symptoms. Those conditions most amenable to treatment present the least anatomic complexity, and affect tissues that do not typically regenerate spontaneously because they lack endogenous pools of tissue stem cells. We can predict ultimate success with most confidence if some clinical evidence already exists that cell replacement might indeed be therapeutic, for instance through prior assessments of cadaveric or fetal tissue transplantation. For conditions previously treated with cadaveric or fetal material, efficacy may be limited by the inadequate supply or quality of the cells, making pluripotent or reprogrammed cell sources advantageous.
Parkinson's Disease
Although neurologists recognize that Parkinson's disease (PD) has systemic features, the chief deficit remains the loss of a specific sub-type of mid-brain dopaminergic neurons located in a deep brain structure, the substantia nigra, whose many connections to the striatum are responsible for regulating movements, such that PD patients suffer from immobility, rigidity, and tremor. Drug replacement with precursors of dopamine (DA), dopamine agonists, or antagonists of dopamine metabolism serves to ameliorate symptoms but cannot stem the inexorable decline in most patients. Based on decades of experience from several groups with transplantation of fetal tissue sources of DA neurons, deep brain transplantation can indeed restore local DA production and ameliorate symptoms, with some patients showing durable improvement and graft integrity after two decades (Freed et al., 1992; Lindvall et al., 1990; Lindvall et al., 1994; Piccini et al., 1999; Piccini et al., 2005). Functional imaging and post-mortem analysis supports the stable integration and persistence of grafts in some patients, prompting continued enthusiasm for this approach among some practitioners, provided that a suitable source of DA neurons can be defined (Freed et al., 1992; Lindvall et al., 1990; Lindvall et al., 1994; Ma et al., 2010; Nakamura et al., 2001; Piccini et al., 1999; Piccini et al., 2000). Others, however, remain skeptical, in part because a trial of fetal grafts randomized against sham surgery was inconclusive, with some patients sustaining functional decline post-surgery due to dyskinesias as a result of excessive graft function (Freed et al., 2001). Supporters of cell therapy for PD point out that a more reliable, consistent, and defined source of DA neurons would justify further testing of transplantation strategies.
Many groups have differentiated DA neurons from both neural stem cell and pluripotent stem cell sources and proven functional in rodent models (Hargus et al., 2010; Sanchez-Pernaute et al., 2008; Tabar et al., 2008; Wernig et al., 2008). Analysis of this DA neuron production has not always distinguished among the many different classes of neurons that produce DA throughout the neuraxis, but recent advances have made possible the differentiation from pluripotent cell sources of regionally specific mid-brain DA neuronal subtypes whose deficiency is most affected in PD is possible, and such cells have been documented to function in rodent and primate models (Chambers et al., 2009; Fasano et al., 2010; Kriks et al., 2011). Moreover, techniques for producing personalized autologous stem cells via somatic cell reprogramming now exist, and it has been shown that autologous cells function better than cells derived from unrelated donors in rodent models of PD transplant (Tabar et al., 2008). The availability of highly specified, defined, autologous DA neuron preparations creates legitimate opportunities for testing in PD patients, including the testing of specific doses to establish a dose-response curve. Nevertheless, even optimistic accounts identify the significant hurdles that remain (Lindvall and Kokaia, 2010). Notably, any cell therapy must ultimately be superior in safety and efficacy to any drug therapy, and establishing such utility will require large-scale and painstaking prospective trials to be conducted over many years. Thus, despite promise, cell therapy as the standard of care for PD is but a distant horizon.
Cell therapy for PD will need to be efficacious and safe to compete with the highly effective drug treatments that currently exist (Hjelmgren et al., 2006). In contrast, a condition like Huntington's Disease, which has no viable drug therapy and is invariably fatal, is an appealing alternative therapeutic target for cell transplantation therapies derived from NSCs and ESCs. Intrastriatal transplantation of homotypic fetal tissues has shown graft durability and reports of amelioration of symptoms in HD patients(Gallina et al., 2010; Nicoleau et al., 2011). As for PD, an improved cell source would facilitate the necessary studies to optimize the dose and target region for cell transplantation. Techniques for directed differentiation of ESCs into relevant medium spiny neurons and amelioration of rodent models of HD have been reported and bode well for future translational clinical studies (Benraiss and Goldman, 2011).
Autoimmune Diabetes Mellitus
Type 1 diabetes (T1D; insulin-dependent, juvenile onset) is an autoimmune condition that involves active immune destruction of the beta cells of the islets of Langerhans of the pancreas, leaving the patient with inadequate supplies of insulin and susceptibility to hyperglycemic crises characterized by life-threatening ketoacidosis. At diagnosis, patients harbor depleted pools of beta cells and are unable to mount a regenerative response to restore beta cell mass, even if their autoimmune response can be controlled. Whether beta cells regenerate after injury in the adult pancreas has been vigorously debated (Bonner-Weir and Weir, 2005; Dor et al., 2004; Dor and Melton, 2008), but endogenous regeneration under pathologic conditions is not robust, and alternative sources of beta cells would therefore be required. Deriving fully functional beta cells in vitro from pluripotent stem cells has proved challenging, but a group from the biotechnology company Novocell did report successful derivation of precursors in vitro that appear to fully differentiate and mature after transplantation in vivo (D'Amour et al., 2006; Kroon et al., 2008). In a more recent advance, Gadue and colleagues have derived a stably expandable endodermal progenitor that is more efficient at producing beta cells than if one proceeds directly from ESC (Cheng et al., 2012). If a reliable source of beta cells can be produced in vitro, a credible path towards clinical development could be envisioned. We know that transplantation of whole pancreas, or infusion of islet preparations from cadaveric sources in the context of a corticosteroid-sparing regimen of immune suppression (the “Edmondton Protocol”) can restore glycemic control for extended time periods (Shapiro et al., 2000; Shapiro et al., 2006). Although patients later relapse, the potential for repeated cell infusions would be greatly facilitated by a more abundant source of beta cells, and deriving purified beta cells from pluripotent stem cell sources thus remains a much sought after goal in stem cell biology. As T1D is an autoimmune disorder, it seems unlikely that autologous cells would be a preferable source of material to allogeneic cells, as immune suppression to protect the beta cells would still be required in either scenario. Attempts to convert exocrine pancreatic tissue into beta-like endocrine cells through ectopic expression of transcription factors, a type of direct reprogramming of cell fates in situ, is a new therapeutic concept with provocative appeal(Zhou et al., 2008).
Other treatable conditions on the horizon
Corneal injury that leads to scarring and blindness has prompted efforts to culture and expand limbal stem cells into corneal patches in vitro, followed by corneal grafting. Recent reports confirm several independent studies that corneal grafting using alternative sources of epithelial cells can restore vision, and appears to be a promising novel stem –cell based treatment for a grave but rare human condition (Nishida et al., 2004; Rama et al., 2010; Tsai et al., 2000; Tsubota et al., 1999). Liver transplantation cannot meet the demands of patients suffering from liver failure around the globe, and production of hepatocyte-like cells from pluripotent stem cells sources has been reported by several groups. Despite considerable similarity to native hepatocytes, the in vitro derived cells have not yet been reported to be fully functional in animal models, and considerable challenges remain for achieving functional integration of in vitro derived hepatocytes, especially for conditions like cirrhosis that already entail markedly altered liver anatomy and compromised circulation. Similarly, production of cardiomyocytes appears to be robust in the petri dish, but achieving engraftment in the damaged heart of a clinically meaningful dose of cells, together with integration in a manner that restores pump function, remains a major challenge. In this case, clever engineering of biomaterials might enable the creation of contractile cardiac patches that could be sewn onto the heart. Finally, producing HSCs from personalized pluripotent stem cells, coupled to gene repair, is an appealing strategy for dozens of genetic disorders of the bone marrow including immune deficiency, hemoglobinopathy, and genetic marrow failure syndromes. Still other potential indications for tissue replacement therapies involve in vitro production of endothelial cells and potentially even human gametes, but none appear to have imminent clinical application. All cell replacement therapies face similar challenges of graft integration into the host environment, which entails trafficking, homing, and integration into native niches or microenvironments, connection to a host blood supply, immune compatibility, and graft durability. Solving such challenges will engage the research community for decades to come.
Who will translate stem cell science into regenerative medicine?
Scientific advances in stem cell biology are being driven by the current intellectual ferment and excitement of the field, but when and how these advances will be translated into successful treatments remain fertile questions for debate. Will cell therapies remain a highly patient-focused endeavor performed solely in academic medical centers, akin to bone marrow or solid organ transplantation? Or will stem cells ever become commercial, pharmaceutical grade “off-the-shelf” products?
One might imagine a future in which medical centers offer highly customized, patient-focused approaches to stem cell treatments, perhaps utilizing the products of personalized induced pluripotent stem cells (see Yamanaka, this volume, 2012). IPS cells have enormous theoretical appeal as vehicles for combined gene repair and cell replacement therapy for genetic disease (Daley and Scadden, 2008). Newer forms of stem cell transplant could replicate the current status of bone marrow transplantation, which has developed into a remarkably complex infrastructure for capturing cellular and molecular information in international registries for literally millions of potential donors, and entails lengthy, costly, and risky interventions in intensive clinical care settings. Given the imperative of treating patients in need, stem cell transplants for genetic and acquired diseases will emerge from academic centers because clinician investigators will develop them and patients will demand them. Like gene repair (“gene therapy”), cell replacement therapies will likely serve rare conditions first, and pertain to small numbers of patients receiving highly individualized treatments, perhaps coupling gene repair with autologous cell replacement approaches, for example for blood diseases. Such small-scale applications will dominate until and unless generic interventions and off-the-shelf approaches prove feasible.
The prospects for more widespread stem cell-based treatments depends on either solving the immune rejection barrier, through advances in promoting immune tolerance to allogeneic tissues, or accepting the use of immune suppression—even lifelong—to facilitate allogeneic cell therapies. Immune suppression is already standard for organ transplantation, so we know that its use to facilitate life-sustaining cell therapy is feasible. Because cell manufacture is likely to be the most costly and time-consuming aspect limiting cell therapies, the prospects for realizing economies of scale would seem to call for the establishment of master cell banks that could be the source of cells “off-the-shelf”. The polymorphism of histocompatibility genes and the resulting variety of tissue types is far too great in human populations to expect banks to be able to supply perfect tissue matches for all potential patients. Instead, one might envision banks of cells derived from donors with highly common genotypes of the histocompatibility genes. This type of approach would be greatly facilitated by cell strains with homozygosity of histocompatibility loci. Past approximations of the number of cell lines that would be needed in such a repository or master cell bank, based on modeling data from pools of kidney transplant patients and recipients in the United Kingdom and Japan, have suggested that a bank comprised on the order of 10-50 cell lines might effectively provide a single HLA antigen match (deemed a minimal requirement for acceptable solid organ transplantation) for approximately 80% of the local population (Gourraud et al., 2012; Nakajima et al., 2007; Taylor et al., 2011; Taylor et al., 2005). While encouraging, these numbers suggest that some kind of dual system might well be needed in which the vast majority of individuals can benefit from off-the-shelf therapies, but personalized autologous cells derived via reprogramming would be needed for those with difficult-to-match tissue types.
Alternatives to cell therapy
Because of the significant hurdles that remain in terms of cell manufacture, delivery, anatomical integration, and immune suppression for all but highly personalized therapies, it is entirely possible that more traditional modes of treatment will evolve from stem cell research and ultimately prove the most feasible. Indeed, the generation of patient-derived stem cells holds the most immediate promise for advancing traditional drug discovery paradigms (for a recent review, please see (Grskovic et al., 2011). Capturing diseases in a dish promises to enable cell-based phenotypic assays that could yield new drugs that repair cell and tissue defects, or perhaps act on endogenous pools of stem cells, stimulating repair and regeneration. For tissues that do not readily regenerate from endogenous pools of stem cells, such as the majority of the brain, the heart, and the kidney, another provocative possibility is the direct conversion of one cell or tissue identity to another that has been depleted by disease or injury. A host of such conversions have been realized in vitro, converting fibroblasts into cells that resemble and exhibit some functions like neurons, cardiomyocytes, and hepatocytes (Vierbuchen and Wernig, 2011). Cell conversion has considerable theoretical advantages, but whether this new cellular alchemy can be harnessed for therapeutic end remains almost science fiction at present, although it is clearly worthy of deeper exploration.
Threats to clinical translation and to the integrity of regenerative medicine
Translating the basic discoveries of stem cell biology into robust, effective, and safe new modalities of care will mean solving new challenges; before success, regenerative medicine will suffer many setbacks. While translating too timidly might deprive needy patients of precious time and life quality, testing cells in patients before a deeper understanding of how stem cells work is risky too. We need to be confident that we understand the full spectrum of safety concerns and can therefore avoid placing patients at undue risk. We also need to design rigorous controlled trials where evidence for clinical efficacy can be defined precisely, rather than depend upon anecdote and clinical observation alone. Given that patients and practitioners may carry unrealistic expectations of clinical efficacy, there is a high likelihood for a robust placebo effect as well as interpretive bias in reporting of clinical results. We also need to be conscious of not exhausting resources that would be better spent on more practical health care needs. Premature application runs the risk of high-profile failure that would sully the credibility of this still-developing field.
With the goal of advancing clinical investigation while preserving rigor, promoting medical innovation while protecting patients, and ensuring integrity in regenerative medicine while respecting autonomy of individual practitioners and patients, the International Society for Stem Cell Research (ISSCR) assembled an international group of scientists, surgeons, gene therapists, bioethicists, patient advocates, and attorneys and composed “The ISSCR Guidelines for the Clinical Translation of Stem Cells” (Hyun et al., 2008). These “Guidelines” articulated principles and standards as a roadmap for practitioners and regulatory bodies when considering if, when, and how to allow tests of experimental stem cell therapies in actual patients. The Guidelines call for independent and rigorous analysis of the decision to test novel treatments in patients, by reviewers with relevant area-specific expertise, who are free of conflicts of interest that might lead to positive or negative bias. Expert judgment about the reliability and rigor of the pre-clinical evidence for efficacy and safety of cellular products is essential for weighing the potential risks against the potential benefits before launching a clinical trial.
Because no pre-clinical animal or cellular model is entirely predictive of outcomes in patients, a credible and rigorous process of informed consent is essential to protecting the autonomy of patients and their thoughtful engagement in the research process, where they consent to participate without heightened expectations or therapeutic misconception; such wishful thinking renders patients vulnerable to exploitation and contaminates interpretations of therapeutic efficacy. Given the enormous attention given to stem cells in the media over the last decade, stem cell-based interventions are certain to elicit a strong placebo effect, which makes it that much more critical to supplant anecdotal evidence for clinical efficacy with rigorous, blinded, and randomized data on patient outcomes.
Medical innovations outside of clinical trials
Many in the medical field recognize the value of innovation outside the context of a clinical trial. However, especially if incorporating the use of highly manipulated cell preparations, such innovative attempts at therapy in the United States would fall under the jurisdiction of the Food and Drug Administration. To comply with accepted professional standards governing the practice of medicine, highly novel uses of any cellular product should not be performed on more than a small number of patients before such use is subject to independent review of the scientific rationale, informed consent, close patient follow-up, and reporting of adverse events. Any attempt to extend the innovative therapy to a larger group of patients should be preceded by a more standard clinical trial. Although some may contend that requiring approval for the practice of novel clinical treatments from an independent body undermines the autonomy of practitioners to provide care to their patients, independent peer review ensures that the rationale for treatment sound, and represents a defensible community standard of medical practice.
Premature clinical translation
The traditional strategy for proving that a medical intervention works and is safe requires rigorous clinical trial design, and thus can be frustratingly slow, costly, and is generally best suited to highly organized medical settings. However, the history of even legitimate medical practice is rife with examples of instances whereby trust in medical intuition alone, or reliance on uncontrolled retrospective or purely observational studies, has led to mistaken assumptions about medical efficacy, only to be corrected when rigorous blinded, randomized trials proved our assumptions to be false (for example, high dose chemotherapy and autologous marrow rescue for metastatic breast cancer, post-menopausal hormone replacement therapy and cardiovascular risk, to name just two).
The fledgling field of stem cells is already suffering from the taint of illegitimate clinical translation. A quick Google search for “stem cell treatments” returns a plethora of sponsored websites peddling cures for ailments as diverse as Alzheimer's disease and autism. As documented by Caulfield and colleagues, such websites systematically overpromise the potential efficacy of stem cells and trivialize the potential risks (Lau et al., 2008). Sadly, even sophisticated patients or their families can be misled by the veneer of scientific credibility on such websites.
As stated previously, apart from treatments using HSCs for blood diseases, and various dermal and corneal indications, essentially all other treatments based on stem cells must be considered experimental medical research, and should be administered exclusively in organized clinical trials. Subjects in medical research are generally not required to pay for unproven interventions.
Administering interventions outside of controlled clinical trials threatens patients and jeopardizes the integrity of and public trust in medical research, compromising legitimate efforts to advance knowledge. Because of the particular vulnerabilities of patients, many governments have enacted laws to protect patients from exploitation and risk, but some practitioners see such regulation as burdensome and unwarranted restraints on their trade. The threat of litigation for medical malpractice serves as an additional constraint on unwarranted medical practice. Recently, the German government shut down the Xcell Clinic in the wake of a child's death after receiving intracranial injections of cord blood in an unproven intervention. A recent report documented the development of glioneural masses in the brain and spinal cord of a child who was treated with intrathecal infusions of what were reportedly neural stem cells for Ataxia Telangectasia, a genetic movement disorder (Amariglio et al., 2009). While one hopes that most stem cell interventions are benign, the safety data are still rudimentary. The history of “gene therapy” was shaped in a deleterious way by the untimely death of a young man, Jesse Gelsinger, in an FDA-approved clinical study. James Wilson, the physician responsible for the gene therapy clinical trial in question, has written a compelling admonition to practitioners of stem cell therapies, warning that much of the history that prompted premature clinical translation of gene therapy is being repeated by the practitioners of stem cell therapy (Wilson, 2009). He sees the same assumptions of a “simplistic, theoretical model indicating that the approach “ought to work”, “a large population of patients with disabling or lethal diseases ... harboring fervent hopes”; and “unbridled enthusiasm of some scientists in the field, fueled by uncritical media coverage”. He ends with “I am concerned that expectations for the timeline and scope of clinical utility of hESCs have outpaced the field's actual state of development and threaten to undermine its success”. The warning is just as appropriate for all kinds of stem cells—umbilical cord blood, neural stem cell, mesenchymal stem cells.
Conclusions
The maturation of new therapeutics takes decades. If one examines the history of any of the recent new thrusts in biomedicine—recombinant DNA, monoclonal antibodies, gene therapy, or RNAi—the vanguard treatments were introduced within a decade but twenty years passed before the full impact of the new form of medicine was felt widely in clinical medicine; for RNAi, we are still waiting for clinical success. 50 years after the first attempts at HSC transplantation, and even with all the improved understanding we now have of both HSCs and immunological mismatch, our success rates are still woefully inadequate. Although the development of novel stem cell based therapies will benefit greatly from the collective failures and acquired experience of marrow transplantation, our ignorance of the challenges of applying stem cells in distinct tissues with far greater anatomic complexity than the blood should give us pause as practitioners, and inspire humility. Realistically, we should anticipate that new therapies based on stem cells for other tissues will likewise take decades to mature. In the short term, there will likely be more failures than successes, and one can only hope that the new field of regenerative medicine can learn the lessons of the past and proceed with prudence and caution.
Acknowledgements
GQD is supported by grants from the NIH (R24DK092760, UO1-HL100001, RC4-DK090913, P50HG005550, and special funds from the ARRA stimulus package-RC2-HL102815), the Roche Foundation for Anemia Research, Alex's Lemonade Stand, Ellison Medical Foundation, Doris Duke Medical Foundation, and the Harvard Stem Cell Institute. GQD is an affiliate member of the Broad Institute and an investigator of the Howard Hughes Medical Institute and the Manton Center for Orphan Disease Research.
Footnotes
Disclosure:
GQD is a member of the scientific advisory board and receives consulting fees and holds equity in the following companies that work with stem cells: Johnson & Johnson, Verastem, iPierian, and MPM Capital.
References
- Aboushwareb T, Atala A. Stem cells in urology. Nat Clin Pract Urol. 2008;5:621–631. doi: 10.1038/ncpuro1228. [DOI] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atala A. Tissue engineering of human bladder. Br Med Bull. 2011;97:81–104. doi: 10.1093/bmb/ldr003. [DOI] [PubMed] [Google Scholar]
- Baker M. Stem-cell pioneer bows out. Nature. 2011;479:459. doi: 10.1038/479459a. [DOI] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Goldman SA. Cellular therapy and induced neuronal replacement for Huntington's disease. Neurotherapeutics. 2011;8:577–590. doi: 10.1007/s13311-011-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Ying L, Lu L, Galvao AM, Mills JA, Lin HC, Kotton DN, Shen SS, Nostro MC, Choi JK, et al. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell. 2012;10:371–384. doi: 10.1016/j.stem.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Kurtz A, Stamm C. Mesenchymal stem cells for cardiac cell therapy. Hum Gene Ther. 2011;22:3–17. doi: 10.1089/hum.2010.211. [DOI] [PubMed] [Google Scholar]
- Copeland N, Harris D, Gaballa MA. Human umbilical cord blood stem cells, myocardial infarction and stroke. Clin Med. 2009;9:342–345. doi: 10.7861/clinmedicine.9-4-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Dor Y, Melton DA. Facultative endocrine progenitor cells in the adult pancreas. Cell. 2008;132:183–184. doi: 10.1016/j.cell.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, Lone T, Zhang YB, Snyder JA, Wells TH, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. N Engl J Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Gallina P, Paganini M, Lombardini L, Mascalchi M, Porfirio B, Gadda D, Marini M, Pinzani P, Salvianti F, Crescioli C, et al. Human striatal neuroblasts develop and build a striatal-like structure into the brain of Huntington's disease patients after transplantation. Exp Neurol. 2010;222:30–41. doi: 10.1016/j.expneurol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourraud PA, Gilson L, Girard M, Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells. 2012;30:180–186. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- Griffin M, Iqbal SA, Bayat A. Exploring the application of mesenchymal stem cells in bone repair and regeneration. J Bone Joint Surg Br. 2011;93:427–434. doi: 10.1302/0301-620X.93B4.25249. [DOI] [PubMed] [Google Scholar]
- Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells--opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011;10:915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DT. Non-haematological uses of cord blood stem cells. Br J Haematol. 2009;147:177–184. doi: 10.1111/j.1365-2141.2009.07767.x. [DOI] [PubMed] [Google Scholar]
- Hjelmgren J, Ghatnekar O, Reimer J, Grabowski M, Lindvall O, Persson U, Hagell P. Estimating the value of novel interventions for Parkinson's disease: an early decision-making model with application to dopamine cell replacement. Parkinsonism Relat Disord. 2006;12:443–452. doi: 10.1016/j.parkreldis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Hyun I, Lindvall O, Ahrlund-Richter L, Cattaneo E, Cavazzana-Calvo M, Cossu G, De Luca M, Fox IJ, Gerstle C, Goldstein RA, et al. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008;3:607–609. doi: 10.1016/j.stem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Jacobson LO, Simmons EL, Marks EK, Eldredge JH. Recovery from radiation injury. Science. 1951;113:510–511. doi: 10.1126/science.113.2940.510. [DOI] [PubMed] [Google Scholar]
- Kebriaei P, Robinson S. Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy. 2011;13:262–268. doi: 10.3109/14653249.2010.549688. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Lau D, Ogbogu U, Taylor B, Stafinski T, Menon D, Caulfield T. Stem cell clinics online: the direct-to-consumer portrayal of stem cell medicine. Cell Stem Cell. 2008;3:591–594. doi: 10.1016/j.stem.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Ledford H. Translational research: 4 ways to fix the clinical trial. Nature. 2011;477:526–528. doi: 10.1038/477526a. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Sawle G, Widner H, Rothwell JC, Bjorklund A, Brooks D, Brundin P, Frackowiak R, Marsden CD, Odin P, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson's disease. Ann Neurol. 1994;35:172–180. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;97:6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E, Uphoff D, Reid TR, Shelton E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J Natl Cancer Inst. 1951;12:197–201. [PubMed] [Google Scholar]
- Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, Freed C, Dhawan V, Eidelberg D. Dopamine cell implantation in Parkinson's disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med. 2010;51:7–15. doi: 10.2967/jnumed.109.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23:345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Howard MJ. Repairing the damaged spinal cord: a summary of our early success with embryonic stem cell transplantation and remyelination. Prog Brain Res. 2002;137:299–309. doi: 10.1016/s0079-6123(02)37023-7. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- McKenna D, Sheth J. Umbilical cord blood: current status & promise for the future. Indian J Med Res. 2011;134:261–269. [PMC free article] [PubMed] [Google Scholar]
- Menasche P. Cardiac cell therapy: lessons from clinical trials. J Mol Cell Cardiol. 2011;50:258–265. doi: 10.1016/j.yjmcc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Nakajima F, Tokunaga K, Nakatsuji N. Human leukocyte antigen matching estimations in a hypothetical bank of human embryonic stem cell lines in the Japanese population for use in cell transplantation therapy. Stem Cells. 2007;25:983–985. doi: 10.1634/stemcells.2006-0566. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Dhawan V, Chaly T, Fukuda M, Ma Y, Breeze R, Greene P, Fahn S, Freed C, Eidelberg D. Blinded positron emission tomography study of dopamine cell implantation for Parkinson's disease. Ann Neurol. 2001;50:181–187. doi: 10.1002/ana.1075. [DOI] [PubMed] [Google Scholar]
- Nicoleau C, Viegas P, Peschanski M, Perrier AL. Human pluripotent stem cell therapy for Huntington's disease: technical, immunological, and safety challenges human pluripotent stem cell therapy for Huntington's disease: technical, immunological, and safety challenges. Neurotherapeutics. 2011;8:562–576. doi: 10.1007/s13311-011-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Peck M, Gebhart D, Dusserre N, McAllister TN, L'Heureux N. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs. 2012;195:144–158. doi: 10.1159/000331406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E, Castelli G, Testa U. Human umbilical cord is a unique and safe source of various types of stem cells suitable for treatment of hematological diseases and for regenerative medicine. Blood Cells Mol Dis. 2012 doi: 10.1016/j.bcmd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- Piccini P, Lindvall O, Bjorklund A, Brundin P, Hagell P, Ceravolo R, Oertel W, Quinn N, Samuel M, Rehncrona S, et al. Delayed recovery of movement-related cortical function in Parkinson's disease after striatal dopaminergic grafts. Ann Neurol. 2000;48:689–695. [PubMed] [Google Scholar]
- Piccini P, Pavese N, Hagell P, Reimer J, Bjorklund A, Oertel WH, Quinn NP, Brooks DJ, Lindvall O. Factors affecting the clinical outcome after neural transplantation in Parkinson's disease. Brain. 2005;128:2977–2986. doi: 10.1093/brain/awh649. [DOI] [PubMed] [Google Scholar]
- Prasad VK, Kurtzberg J. Umbilical cord blood transplantation for non-malignant diseases. Bone Marrow Transplant. 2009;44:643–651. doi: 10.1038/bmt.2009.290. [DOI] [PubMed] [Google Scholar]
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, Jacobsen N, Ruutu T, de Lima M, Finke J, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Lee H, Patterson M, Reske-Nielsen C, Yoshizaki T, Sonntag KC, Studer L, Isacson O. Parthenogenetic dopamine neurons from primate embryonic stem cells restore function in experimental Parkinson's disease. Brain. 2008;131:2127–2139. doi: 10.1093/brain/awn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Liu W, Zhou G, Zhang W, Cui L, Cao Y. Tissue engineering of cartilage, tendon and bone. Front Med. 2011;5:61–69. doi: 10.1007/s11684-011-0122-1. [DOI] [PubMed] [Google Scholar]
- Tabar V, Tomishima M, Panagiotakos G, Wakayama S, Menon J, Chan B, Mizutani E, Al-Shamy G, Ohta H, Wakayama T, et al. Therapeutic cloning in individual parkinsonian mice. Nat Med. 2008;14:379–381. doi: 10.1038/nm1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CJ, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011;366:2312–2322. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- Thomas ED. Bone marrow transplantation from the personal viewpoint. Int J Hematol. 2005;81:89–93. doi: 10.1532/ijh97.04197. [DOI] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL, Jr., Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- Tian H, Bharadwaj S, Liu Y, Ma PX, Atala A, Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. 2010;16:1769–1779. doi: 10.1089/ten.tea.2009.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11:160–170. [PubMed] [Google Scholar]
- Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, Shimazaki J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–1703. doi: 10.1056/NEJM199906033402201. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol. 2011;29:892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM. Medicine. A history lesson for stem cells. Science. 2009;324:727–728. doi: 10.1126/science.1174935. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]