Abstract
The WASF3 (WAVE3) gene is an important mediator of cell motility, invasion and metastasis and is expressed at high levels in some advanced stage tumors. In our survey of breast cancer cells we now demonstrate that exposure to hypoxic conditions increases WASF3 expression levels in MDA231, SKBR3 and MCF7 cells. The WASF3 promoter region contains HIF1A response elements (HRE). CHIP assays demonstrate that HIF1A binds to these HRE elements in the promoter region and luciferase reporter assays using the WASF3 gene minimal promoter shows that hypoxia results in its up regulation. Phosphorylation of WASF3 is required for its ability to affect invasion and increased phosphoactivation of WASF3 is also seen in cells challenged with hypoxia. These cells also show increased motility in the scratch wound assay. Cells in which WASF3 has been knocked down show no response to hypoxia as expected, implicating the specificity of the hypoxic response to WASF3. Overall, these experiments demonstrate WASF3 is a HIF1A regulated gene and suggests a mechanism to explain the observation of elevated expression of WASF3 in advanced stage tumors.
Keywords: WASF3, hypoxia, HIF1A, invasion
Introduction
Highly proliferative, solid tumors consistently outgrow their blood supply, which results in transient states of low oxygen tension, or hypoxia, within the tumor [1, 2]. To survive, therefore, they must modify their gene expression profiles to adapt to conditions of low oxygen. Gene expression is typically suppressed in cells under hypoxic conditions in order to conserve cellular energy and only those genes which are critical for survival, are activated [3,4]. We recently demonstrated that the WASF3 gene is up regulated in advanced stage breast cancer [5]. Knockdown of WASF3 in breast and prostate cancer cells results in reduced invasion potential in vitro, and their ability to metastasize in experimental models is almost completely eliminated [5,6]. WASF3 is a member of the WASP/WASF family of proteins, which have been shown to be important in regulating actin cytoskeleton dynamics [7]. In response to extracellular signals, the WASF family of proteins, which contains WASF1, 2, and 3, activate the actin related protein 2/3 (Arp2/3) complex, leading to the induction of actin polymerization and assembly of actin fibers [8,9]. These events play a critical role in cellular processes such as cell motility and invasion. We have reported previously that inactivation of WASF3 results in the down-regulation of matrix metalloproteinases 1 and 9, consistent with the loss of the ability of highly invasive cells to invade a matrigel matrix [6,10]. This loss of WASF3 activity is also associated with loss of phosphorylation of p38 MAP kinase, which is a known intermediate in the pathway that regulates MMP production.
The up regulation of WASF3 in advanced stage tumors [5,11] together with its correlation with metastasis raised the question whether WASF3 expression could be modulated by hypoxia. The major effectors of hypoxia are the HIF1A and EPAS1 (HIF2A) genes [1, 12, 13]. Both genes are induced under conditions of low oxygen and bind consensus sequences in the promoters of their target genes. However, their regulation is not uniform in a given tumor, since both normoxic and hypoxic regions exist. Well-characterized hypoxia response genes include VEGF, which stimulates neovascularization, as well as several cell survival genes such as KDM3A, PGK1, TGFB1, and SLC2A3 (GLUT3), which protect tumor cells against apoptosis [14]. This temporary reversal of the gene expression profile allows the cells to overcome short-term hypoxia and survive. In this report we demonstrate that HIF1A directly up regulates WASF3 expression in breast cancer cells exposed to hypoxia.
Materials and Methods
Cell Culture and Treatments
Cell lines were maintained in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C in 5% CO2. For hypoxia exposure, cells were maintained at a constant gas mixture of 1% oxygen, 94.5% nitrogen, and 5% carbon dioxide in a Hypoxia Workstation (Coy Laboratory Products, Grass Lake, Mich).
For Cycloheximide (20 μg/mL; Sigma) or Actinomycin-D (10 μmol/L) treatments, cells were grown in serum free media for 24 hours and the drugs were added directly to the culture medium following incubation under either normoxic or hypoxic conditions. To determine the effect of blockade of de novo transcription, cells were pretreated with Actinomycin-D (10 μmol/L) or DMSO for 30 min in normoxia before being transferred to the hypoxia chamber.
Immunoprecipitation and Immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously [6], using the following antibodies: WASF3 and HIF1A (both 1:1000, Cell Signaling, Inc.), PY20 (1:1000, Sigma) and β-actin (Sigma). Protein levels were quantified by densitometry using ImageJ Software (NIH).
RNA Extraction and PCR
Total RNA was extracted from breast cancer cell lines using Trizol (Invitrogen, Carlsbad, CA, USA). cDNA was generated using the SuperScript II Reverse Transcriptase kit (Invitrogen). The cDNA generated was used as a template for 30 cycles of PCR amplification for analysis of gene expression using a Thermal Cycler (Bio-Rad). Real-time PCR was carried out with the iCycler iQ System (Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer’s instruction. All amplifications were performed in triplicate and 18SrRNA was used as the internal control.
The primers used were (WASF3) F-TCTCACTACAGGATATCAACATGAAA; R-ATGGTGTCAGGATGTTCAGAG; (HIF1A) F-GCAAGCCCTGAAAGCG; R-GGCTGTCCGACTTTGA; (HIF2A) F-GTCTCTCCACCCCATGTCTC; R-GGTTCTTCATCCGTTTCCAC; (MMP1) F-TTCGGGGAGAAGTGATGTTC; R-AAACTTTGTGGCCAATTCCAG; (MMP3) F-CCTTTTGATGGACCTGGAAA; R-GGAAGAGATGGCCAAAATGA; (MMP9) F-GGCGCTCATGTACCCTATGT; R-TCAAAGACCGAGTCCAGCTT; 18S rRNA:5′-TTGGAGGGCAAGTCTGGTG-3′; 5′-CCGCTCCCAAGATCCAACTA-3. All primers were designed using Primer3 based on the corresponding human mRNA sequences and were obtained from Invitrogen.
Chromatin Immunoprecipitation (ChIP) Assays
Genomic DNA was cross-linked using 1% formaldehyde at 37 °C for 10 min and then extracted using standard procedures. The DNA was then sheared by sonication on wet ice (Bioruptor, Diagenode Inc, North America) using 5 repeats of a 30 second pulse (set to maximum power) followed by a 60 second pause. This procedure generated fragments of the required length (~200–1000 bp) which were subjected to immunoprecipitation with anti-HIF1A antibodies. The immune complexes were subjected to protein digestion by adding 1.5 μl of proteinase K (20 mg/ml) to each sample followed by overnight incubation at 65°C, and then the immune-precipitated chromatin was purified using Qiaquick PCR purification kits (Qiagen) according to the manufacturer’s instructions. PCR was performed on chromatin using WASF3-specific primers (HRE1) F-ACTCGCCAATCCTGGTGAAG, R-CTGCGCTCTGAGCTCCA-T-3; (HRE4) F-TCTCTTCCTTTCCTTTCCTCCT, (HRE4) R-CCCTGAAACTGCCAGCTAAG.
WASF3 Promoter Activity
MDA-MB-231 cells were plated in triplicate wells of a 24-well plate and cotransfected with 400 ng of the WASF3 promoter plasmids (pGL3-W3-100/+494 and pGL3-W3-747/+494) containing HIF1 binding sites and 8 ng of pRL-TK. After overnight incubation, duplicate plates were incubated either in hypoxia or normoxia for 24 h. Cells were assayed for firefly and renilla luciferase activity using the Dual-Glo Luciferase Assay System (Promega).
siRNA Knockdown
siRNA sequences were designed according to the Stealth RNA system (Invitrogen) for specific silencing of HIF1A and 2A, as previously described [15]. The siRNA target sequences correspond to nucleotides 1302-1322 of the human HIF1A mRNA (5’-TACGTTGTGAGTGGTATTATT-3’) and nucleotides 4740-4760 (5’-CCCGGATAGACTTATT-GCCAA-3’) of HIF2A, respectively. Negative controls were designed with a scrambled sequence which will not lead to the specific degradation of any known cellular mRNA. Cells at 60% confluency were transfected for 48 h with the Stealth RNAs (100 nM) using Lipofectamine (Invitrogen) to determine the efficiency of siRNA knockdown.
VEGF ELISA
VEGF levels were assessed using ELISA (PeproTech, Rocky Hill, NJ). Briefly, the VEGF antibody was bound to the ELISA plate (COSTAR, Corning, NY) overnight at 4°C. The wells were then washed with TBS and cell supernatants (100 μl) were mixed together with a range of concentrations of recombinant human VEGF (0–1,000 pg/ml) for 2 h at room temperature (RT). The wells were washed again and the detection antibody added. After 2 hours at room temperature, the wells were again washed, first with TBS and then with avidin-HRP conjugate followed by an ECL ELISA substrate (Pierce), for 30 min. Finally, chemiluminescence was measured using a plate reader (Bio-Rad).
Cell Proliferation Assay
Cell growth assays were performed using the Promega CellTiter 96R Non-Radioactive Cell Proliferation Assay (MTT) kit (G 4000). Briefly, cells were seeded in 96-well microtiter plates and exposed to 1% O2. At specific time points, the supernatants were aspirated and 3-(4, 5-dimethylthiazol-2-yl) - 2, 5-diphenyltetrazolium bromide (MTT) was added to each well. After incubation at 37°C for 3 hr, the stop solution was added to dissolve the formazan crystals, and absorbance was then measured at 490 nm using a microplate reader (Bio-Rad).
Wound Closure Assay
Cells grown to 80–90% confluence were treated for 48–72 h in a hypoxia chamber. The cell monolayer was then scratched by dragging a sterile pipette tip across the plate to create a cell free area. After marking a specific area of the wound on the underside of the plate it was photographed using an inverted microscope fitted with a CCD camera. Cells were then exposed to either normoxic or hypoxic conditions and after 24h the marked area was re-photographed. The area of the unclosed wound was measured in each case at T=0 and T=24 hours using ImageJ software (NIH). Motility of the cells was defined as the difference in the area of the remaining wound after 24 h as an average derived from five random fields (× 200 magnifications) per well and from triplicate wells. Results were expressed as a percentage of the difference between times 0 h and 24 h..
Matrigel Invasion Assay
Invasive potential was assessed using the Matrigel invasion chambers from BD Biosciences (San Diego, CA). Briefly, cells were cultured in hypoxia for 24 hours in complete media, harvested by trypsinization, and counted. Fifty thousand cells were then placed on the top insert of the chamber in 500 μl of serum-free DMEM media. The well was then filled with 750 μl DMEM, supplemented with 10% fetal bovine serum. After 24 hours incubation in hypoxia at 37°C and 5% CO2, the noninvasive cells present on the upper surface of the filter were removed using a sterile cotton swab. The cells that were able to migrate through the Matrigel onto the lower surface of the filter were fixed and stained with Diff-Quick (American Scientific Products, McGraw Park, IL). The lower surface of the filter was photographed and migrating cells were counted from four fields at 200X magnification.
Results
WASF3 expression is upregulated in response to hypoxia
To investigate whether hypoxia affects expression of the WASF family of proteins, MDA-MB-231 and MCF7 breast carcinoma cell lines were incubated under hypoxic (1.0% oxygen) conditions for different periods of time. Both WASF3 mRNA and protein expression levels increased significantly in response to hypoxia, compared with control cells maintained in normoxia (Figure 1, A, B and C). Using the same approach, no significant change was observed in mRNA expression levels of WASF2, although WASF1 showed a modest increase (Figure1D). Induction of WASF3 expression levels was observed within the first 4 hours of hypoxia, which increased further up to 24 hrs (Figure 1C). Interestingly, the MCF7 cell line, which typically shows low invasion potential, and expresses low endogenous WASF3 levels [16], responded as significantly (p<0.005) to hypoxia as the MDA-MB-231 cells, which show higher endogenous expression of WASF3. To determine whether hypoxic induction of WASF3 expression is a more general phenomenon in breast cancer cells, we also analyzed the MDA 453 and SKBR3 cell lines, where, in both cases, WASF3 protein expression again increased significantly after 24 hours of hypoxia treatment (Figure1E).
Figure 1.
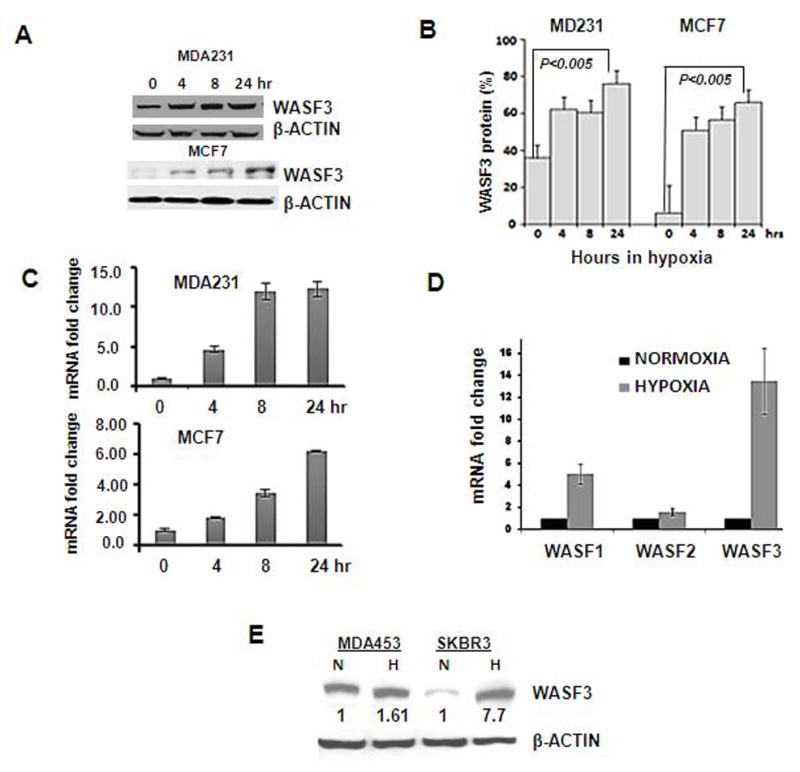
Western blot analysis of breast cancer cell lines MDA 231 and MCF7 (A) demonstrates increased WASF3 levels within 4 hours of hypoxia treatment. These increases are highly significant (P<0.005) after 24 hours when expressed as a percentage of the actin control in each case (B). This increase in WASF3 was also seen at the transcription level as shown by Q-PCR analysis (C). In contrast to the large increase in hypoxia-induced WASF3 expression (D), increased mRNA levels were modest for WASF1 and barely detectable for WASF2. When additional breast cancer cell lines were exposed to hypoxia (E) SKBR3 shows similar induction of WASF3, whereas MDA453 cells showed more modest increases. Relative increases in protein levels following ImageJ analysis are shown numerically below each lane.
Hypoxia regulates WASF3 transcription
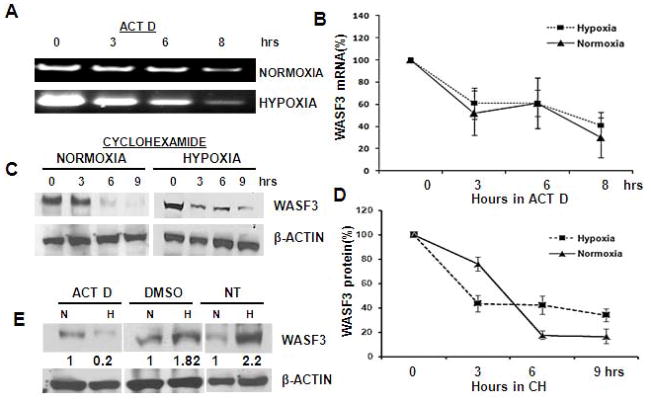
WASF3 mRNA and protein levels are clearly increased by hypoxia. To determine the underlying molecular mechanism behind this phenomenon, we analyzed the stability of both the WASF3 mRNA and protein under normoxic and hypoxic conditions in MDA-MB-231 cells. After 16 hours in hypoxic conditions, the cells were treated with either actinomycin D (ACTD) or cycloheximide (CH) to inhibit transcription or translation, respectively. Following ACTD treatment, no significant change in mRNA stability was observed in response to hypoxia (Figure 2, A and B). Similarly, CH treatment resulted in a decrease in WASF3 protein levels under both normoxic and hypoxic conditions, further suggesting that protein stabilization is unlikely to account for the increase in WASF3 levels (Figure 2C and D). To determine whether blockade of de novo transcription prior to hypoxia exposure affects WASF3 mRNA expression (Figure 2E), we treated MDA-MB-231 cells with ACTD for 30 minutes before subjecting them to hypoxia. ACTD pretreatment reduced hypoxia-induced elevation of WASF3 demonstrating that hypoxia regulates WASF3 expression at the transcription level.
Figure 2.
MDA-MB-231 cells were incubated either in normoxia or hypoxia for 24 hours before actinomycin D (ACTD) was added. Q-PCR analysis (A) demonstrated a proportional decay in mRNA levels over eight hours for both WASF3 and actin (B). When MDA231 cells were similarly grown for 24 hours in hypoxia, and then incubated with cyclohexamide (CH) for nine hours (C), there was also a proportional decline in protein levels for WASF3 compared with actin (D). Pretreatment of MDA231 cells with actinomycin D before hypoxia treatment (E), demonstrates only a limited response of WASF3 induction compared with the DMSO control or untreated cells (NT). Relative increases in protein levels following ImageJ analysis are shown numerically below each lane.
Hypoxia Induction of WASF3 is Dependent on HIF1A
Hypoxia inducible factors (HIF) bind consensus hypoxia response element (HRE) sequences in the promoters of their target genes, which can affect their expression. Since hypoxia leads to up regulation of WASF3, we next determined whether HIF transcription factors were directly involved in hypoxia-induced WASF3 expression. Analysis of the DNA sequence upstream of the WASF3 gene identified several putative HRE that closely resembled the canonical A/G/ CGTG sequence [17]. Of four putative HREs identified in the human WASF3 upstream region (Figure3A), three of them (HRE1, HRE2, HRE3) are located in a cluster (between -27 and -79 bp) close to the first nucleotide of exon1. The other (HRE4) lies more distal (between -721 and -725 bp) in the human WASF3 promoter (Figure3A). In a ChIP assay, we immunoprecipitated chromatin using anti-HIF1A antibodies, to recover DNA binding sequences (Figure 3A). We then used PCR primers which were designed to amplify the regions containing the corresponding WASF3 HREs and demonstrated the presence of the -27/-79 and the -721/-725 regions in the amplified PCR product. These observations demonstrate that HIF1A indeed binds to the HRE in the WASF3 promoter (Figure 3B).
Figure 3.
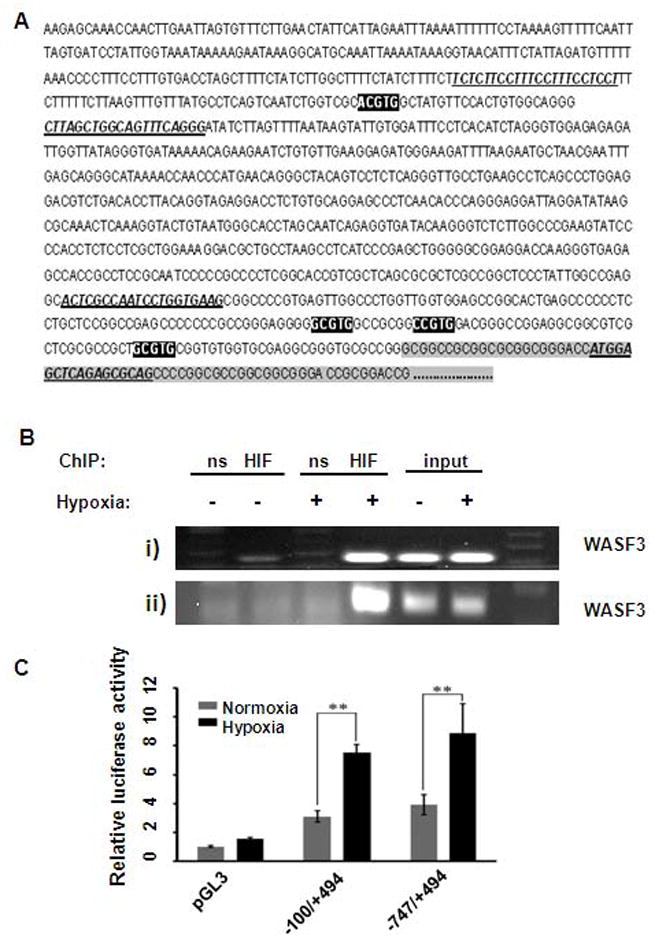
Sequence of the human WASF3 promoter region (A) encompassing ~1000 bp upstream of the start of exon1(grey shaded box). Primer sequences used for the chromatin immunoprecipitation (ChIP) experiments are highlighted and underlined. The putative HRE sequences are shown in black. (B) ChIP was performed using MDA231 cells (i) treated with either normoxia (-) or hypoxia (+). PCR using primers encompassing HRE4 (HRE4WF/HRE4WR) (ii) PCR using primers encompassing three HREs (HRE1WF/HRE1WR). ns: nonspecific control IgG immunoprecipitation. The inputs represent non-immunoprecipitated chromatin. In (C) luciferase activity in MDA-MB-231 cells after transfection with different luciferase reporter constructs was measured after incubation in normoxia or hypoxia for 24 h. The relative luciferase values are shown as the mean of three independent experiments (p <0.001).
As part of our ongoing characterization of the WASF3 promoter (Lesoon, et al, in preparation), two WASF3 deletion constructs (−747/+494 and −100/+494) were generated from within the ~1000 bp upstream of the WASF3 transcription start site. Both constructs drive luciferase expression in cells grown under normoxic conditions. To determine whether the presence of those HREs affects the ability of hypoxia to mediate WASF3 expression, we transiently transfected MDA-MB-231 cells with either of the two luciferase reporter constructs, followed by exposure to hypoxia for 24h. Exposure of MDA-MB-231 cells to hypoxic conditions resulted in a 7 and 9 fold increase in luciferase expression (p = 0.0007) in the presence of the −747/+494 and −100/+494 WASF3 promoter construct respectively, compared to normoxic controls (Figure3C). No activation of the luciferase reporter was observed in response to hypoxia in the presence of the empty vector (Figure 3C). These results support our previous suggestion that HIF1A regulates WASF3 expression under hypoxic conditions by binding directly to HRE sites.
HIF1A is Required for the Regulation of WASF3 Transcription in Response to Hypoxia
The HIF1 and HIF2 transcription factors are considered the most important mediators of physiological changes induced by hypoxic stress. Since accumulation of HIF occurs in response to hypoxia, and we have demonstrated that hypoxia treatment resulted in increased WASF3 activity, we evaluated the role of both HIF1A and HIF2A in this response. Knockdown of HIF1A and HIF2A expression using specific siRNAs against each HIF resulted in a significant down regulation of the respective mRNAs (greater than 80%) in treated MD231 cells, when compared with untreated parental cells (Figure 4A). As expected, siRNA treatment also reduced HIF1A protein levels, as shown by Western blot analysis (Figure 4B). Furthermore, HIF1A knockdown in these cells reduced WASF3 levels in hypoxia treated cells (Figure 4, A and B). Interestingly, no significant changes in mRNA expression of WASF3 were observed when cells were treated with siRNAs targeting HIF2A alone (Figure 4). Since it was clear that knockdown of HIF2A did not affect WASF3 expression appreciably, we did not pursue analysis of HI2A in this system further. Thus, HIF1A appears to be specifically involved in regulating WASF3 expression in MDA-MB-231 breast cancer cells under hypoxic conditions.
Figure 4.
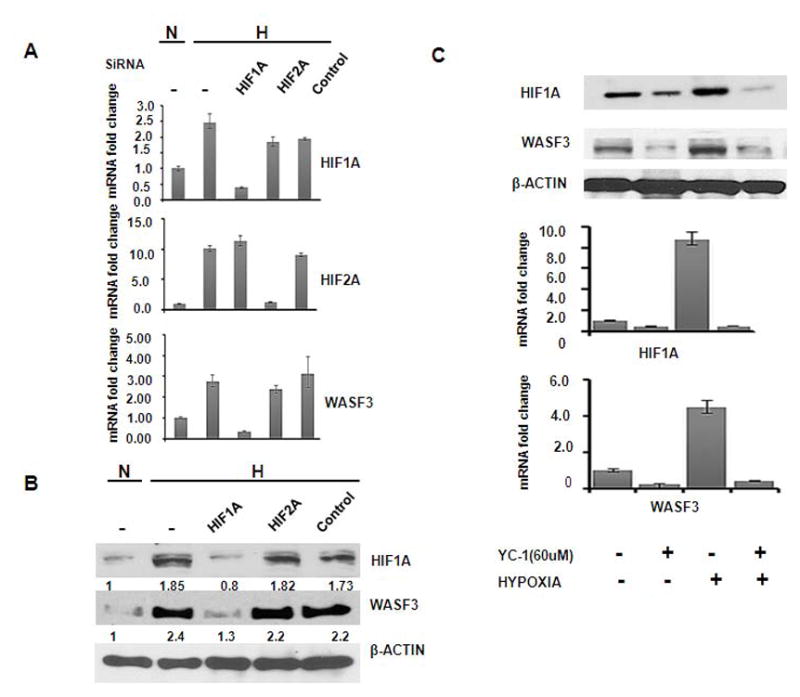
Q-PCR analysis of MDA-MB-231 cells treated with hypoxia (H) following knockdown of HIF1A and HIF2A, using siRNAs (A). Knockdown of HIF1A results in a concomitant reduction in WASF3 levels, although knockdown of HIF2A does not. The resultant effects on protein levels are shown in (B), where the relative changes are shown below the lanes. These observations parallel the mRNA levels. When the YC-1 inhibitor of HIF1A was used (C), in the absence of hypoxia, there was little effect on WASF3 expression. In the presence of hypoxia, however, while WASF3 levels (both mRNA and protein) are normally increased, they are greatly reduced in the presence of the HIF1A inhibitor.
To independently confirm these observations we also used the specific HIF1A inhibitor, YC-1, which blocks endogenous HIF1A expression [18]. When MDA-MB-231 cells were incubated with YC-1 under hypoxic conditions, hypoxia-mediated induction of WASF3 mRNA and protein levels were reduced considerably (Figure 4C), further supporting a direct interaction/relationship of HIF1A with hypoxia-induced WASF3 gene expression.
WASF3 Activation is Increased by Hypoxia
In our previous analysis [5], we demonstrated that WASF3 phosphorylation is required for its interaction with the p85 component of PI3 kinase, as well as WASF3-mediated regulation of lamellipodia formation and cell migration. To investigate whether the enhanced WASF3 activation caused by hypoxia is associated with increased function, we analyzed its phosphorylation status in MDA-MB-231 and MCF7 cells exposed to hypoxia. Immunoprecipitates of WASF3 were subjected to immunoblot analysis with anti-WASF3 and anti-phosphotyrosine (pY) antibodies, which demonstrated increased levels of phosphorylated WASF3 in both cell lines after 24 hours in hypoxia. Quantification of these blots indicates a roughly proportional fold increase of WASF3 protein levels and phospho-WASF3 levels demonstrating that hypoxia enhances WASF3 activation as well.
We have also previously demonstrated that, as a result of WASF3 knockdown, specific matrix metalloproteinases (MMP) were also down regulated, which provided an explanation for the loss of invasion phenotype in these cells (10). Quantitative analysis of MMP expression in MDA-MB-231 cells exposed to hypoxia resulted in a clear increase in the mRNA levels of MMP-1, MMP-3 and MMP-9, compared to cells incubated under normoxic conditions (Figure 5B). In contrast, WASF3-depleted cells did not show any change in MMP expression. These observations also demonstrate that the proportional relationship between WASF3 and MMP expression is maintained under hypoxic conditions. Overall, this series of experiments demonstrates that WASF3 is both functionally active and capable of signaling in hypoxic environments and up regulates downstream targets related to WASF3 activation.
Figure 5.
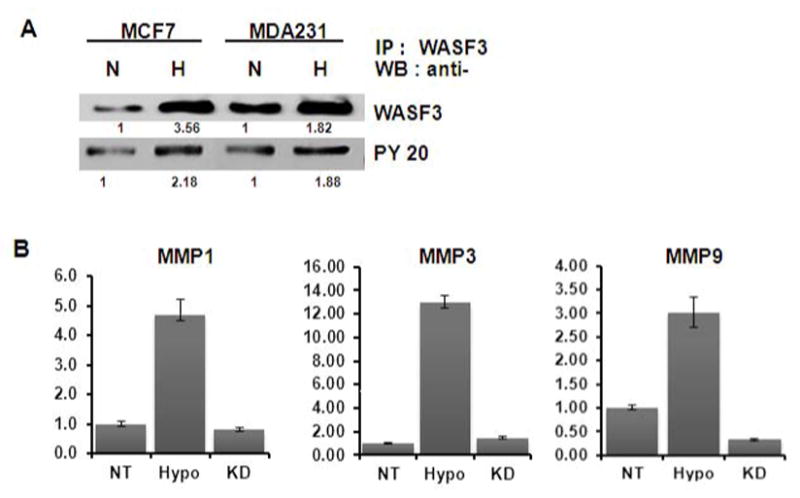
(A) Western blot analysis of WASF3 following immunoprecipitation from MCF7 and MDA-MB-231 lysates, shows increased protein levels following hypoxia (H) treatment compared with cells grown in normoxia (N). Relative intensities are shown below each lane. When the same IPs were assayed for phosphorylated WASF3, using anti-phosphotyrosine antibodies, an increase in activated WASF3 was observed. (B) As a consequence of hypoxia treatment (Hypo), MDA-MB-231 cells show an increase in MMP 1, 3 and 9 mRNA levels compared with cells grown in normoxia (NT) or in WASF3 knockdown cells (KD).
High Level WASF3 Expression in Hypoxia is Maintained in the Presence of the VEGF Antagonist SU5416
It has recently been demonstrated that antiangiogenic treatment with VEGF receptor inhibitors, induces a hypoxia response in tumors and liver micrometastases [19]. To investigate whether HIF and VEGF were functionally related to the hypoxia-driven up regulation of WASF3, MDA-MB-231 cells were incubated in hypoxic conditions and challenged with either with the YC-1 (HIF inhibitor) or the SU5416 (inhibitor of VEGF receptor-2 kinase) [20] alone or together (Figure 6A and B). Treatment with 60 μM YC-1 had a profound effect on reducing both WASF3 and HIF1A expression as expected (see above) but cells incubated with 1 μM SU5416 alone maintained continuous high WASF3 expression levels, although secreted VEGF levels were reduced (Figure 6C). Even though HIF1A expression was also significantly reduced after SU5416 treatment, it appears that this was not sufficient to significantly reduce WASF3 expression levels. However, when these cells were treated with both YC1 and SU5416, a significant reduction (p<0.05) of WASF3 expression was seen in a hypoxic environment compared to untreated cells. Since the combined treatment was significantly more effective than YC1 alone in reducing WASF3 levels, it appears that these two agents can act synergistically.
Figure 6.
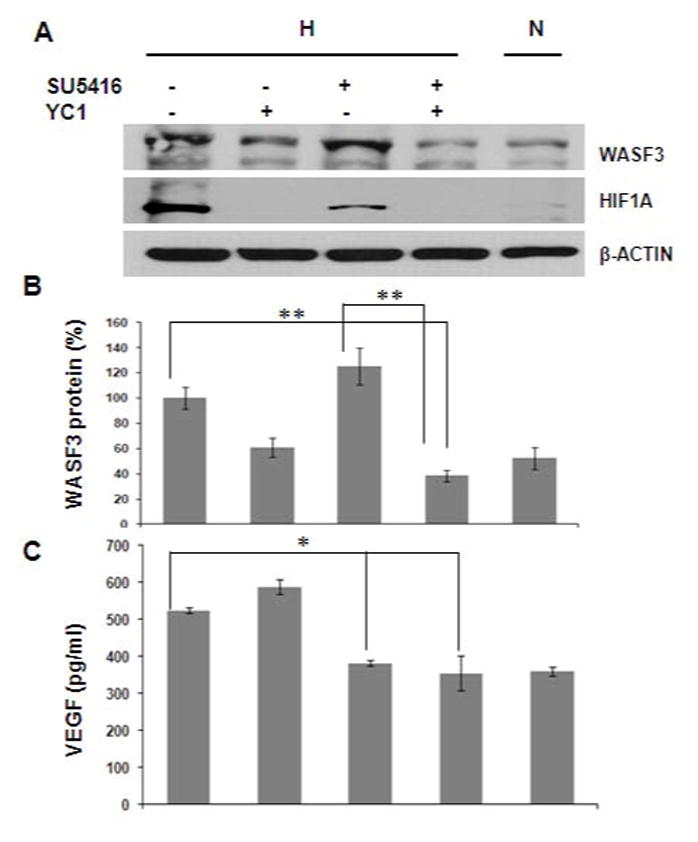
MDA-MB-231 cells were exposed overnight to YC1 (60 μm) or SU5416 (1μm) either alone or together under hypoxic conditions (H). (A) Western blot analysis of WASF3 protein levels is compared with cells under normoxic conditions (N). Actin was used as internal control. In these studies, WASF3 and HIF1 are elevated following hypoxia and treatment with YC1 alone shows loss of HIF1A and reduction in WASF3. In the presence of SU5416 shows induction of HIF1A with a concomitant increase in WASF3 levels. Treatment with both drugs shows essentially the same levels of the two proteins seen in the cells cultured in normoxia. (B) The data were plotted as a percentage of relative protein levels compared with normoxia. (C) VEGF ELISA assays show accumulation of secreted VEGF proteins in serum-free culture media showing significantly reduced VEGF production when treated with SU5416 and YC-1. Data are normalized to the standard curve for purified human VEGF to yield values in pg/ml VEGF. Significant differences are designated by *p < 0.05 or **p < 0.005 as determined by one-way ANOVA.
WASF3 Affects Tumor Cell Behavior in Hypoxia
To determine whether hypoxic induction of WASF3 affects cell phenotype in breast cancer cells, we used MDA-MB-231 cells with stable knockdown of WASF3 [16]. These knockdown cells did not demonstrate an increase in WASF3 expression under hypoxic conditions (Figure 7A). Cells expressing the empty shRNA vector, and knockdown cells re-expressing exogenous WASF3, were then exposed to 1% O2 for 24h and 48 h, and cell viability was analyzed using the MTT assay. Under this hypoxic stress, cell proliferation (Figure 7B) was significantly reduced (p<.005) in knock down cells compared to control cells. To determine whether up regulation of WASF3 expression that is induced under hypoxic condition affects cell motility, we used the wound closure assay that has been widely used as a functional assay of cell motility. When control cells were exposed to hypoxia for 48 hours, the gap was almost closed within 24h, whereas KD cells were less able to close the wound in the same time period (Figure 7C and 7D). We then investigated whether the invasive ability of MD-MBA-231 cells through Matrigel coated membranes was affected by hypoxia. Using transwell invasion assays, overnight incubation in hypoxia significantly increased in the number of cells (p<.05) passing through the filter compared with cells grown in normoxia (Figure 7E and F). Interestingly, in the various experiments described above, the rescued clones showed the same phenotypes as the control cells (W3:EV) demonstrating that the significant effect of hypoxia on cell invasion requires WASF3 expression. These experiments demonstrate that hypoxia is specifically affecting the WASF3 pathway, and any other consequences of hypoxia cannot override the restriction on cell motility and invasion controlled by WASF3.
Figure 7.
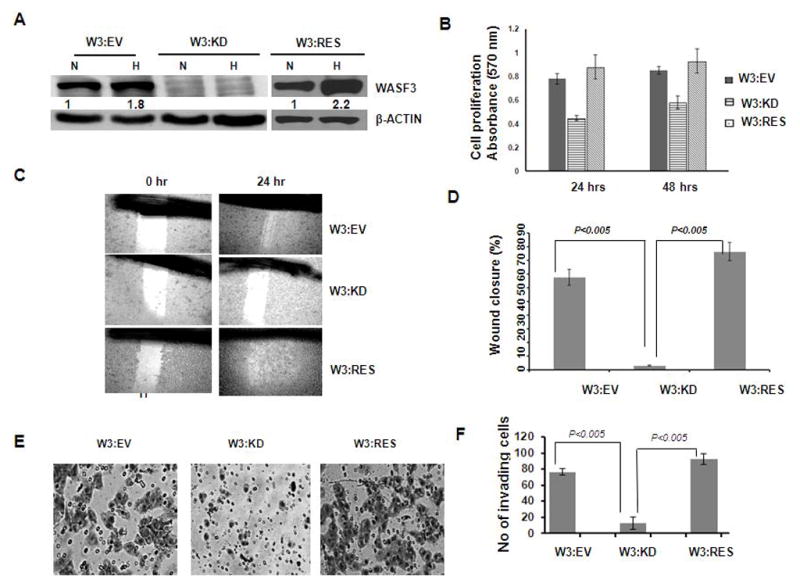
Western blot analysis shows that exposure of MDA-MB-231 cells carrying the empty shRNA vector (EV) to hypoxia (H) leads to increased WASF3 expression (used as control in this specific experiment), which is also seen in cells in which WASF3 has been re-expressed (RES) WASF3 knockdown cells (W3: KD) do not show WASF3 protein under conditions of normoxia (N) and cannot be induced to express WASF3 by hypoxia. In (B) increased cell proliferation was seen in both the control as well as rescued cells treated with hypoxia after both 24 and 48 hours. In (C) the scratch wound assay was used to compare wound closure after 24 hours. Under normoxia (N), MDA 231 cells (EV) close the wound to a large extent. Under hypoxia (24 hr) the wound is closed even more efficiently. In the shRNA knock down clones (W3: KD) the wound closure is inefficient compared with cells transfected with the empty ShRNA vector (W3: EV) although WASF3 rescued cells show a trend similar to the control. These data are graphed in (D). Invasion assays (E, F) demonstrate that cell invasion is significantly reduced as a result of WASF3 knockdown. Knockdown clones rescued with WASF3 recover their invasion phenotype to a level that is not significantly different from the control (EV) cells.
Discussion
It has been well established that tumor cells exposed to hypoxia undergo a variety of biological and molecular responses including the activation of signaling pathways which regulate tumor cell proliferation and migration [21,22]. Many elements of the hypoxia-response pathway [23] are therefore potential candidates for therapeutic targeting, but the mechanism by which hypoxia induces cancer cell migration and invasion is poorly understood. Members of the WASP family of proteins are crucial for actin polymerization dynamics which leads to cell proliferation and migration. It has also been reported that WASF3, a member of WASF family, regulates actin cytoskeleton dynamics, cell motility and invasion involving the p38 MAPK pathway and MMP production [5,24]. In the present study, we have shown that WASF3 is a hypoxia-inducible gene which contributes to breast carcinoma cell survival and invasion in hypoxic environments and we have identified the molecular mechanisms responsible for the up-regulation of WASF3 gene expression through direct binding of HIF1A at HRE sites in the WASF3 promoter. Although it has been suggested that hypoxia can increase mRNA stability of specific genes, such as Sprouty4 [25] and tyrosine hydroxylase [26] by altering their half lives, we were unable to demonstrate any significant difference in WASF3 mRNA stability between normoxia and hypoxia.
The primary requirement of a gene to be hypoxia responsive is that they contain one or more hypoxic responsive elements (HRE), which can bind different transcription factor such HIF1 or HIF2 [27,28]. HIFs bind to the HRE elements of hypoxia-inducible genes, which is essential for transactivating their transcription [29]. Analysis of the human WASF3 promoter region identified multiple sequences that were potential candidates for HIF1 binding. Interestingly analysis of the upstream region of mouse Wasf3 gene (+1 to −1000) also shows multiple putative HRE elements (UCSC Genome Browser, mouse chr5:147,195,561–147,196,561) even though there was not a high homology across this region. This conservation of HRE sequences in the WASF3 promoter adds support to their functional role in the hypoxia induced regulation of WASF3. HIF1A activated both reporter constructs confirming the ability of HIF1A to regulate WASF3 induction. Together these observations demonstrate that the HRE sequences in the WASF3 promoter are responsible for the HIF1A mediated induction of WASF3 expression.
It has been reported previously that ABL kinase-mediated phosphorylation of WASF3 is required for its activation, which promotes cell invasion through regulation of expression of several MMPs [5]. As expected, hypoxia led to an increase in survival of tumor cells and increased invasion which was accompanied in WASF3 phosphorylation and MMP expression, demonstrating increased functional protein as well as increased protein levels. It has previously been demonstrated [10] that the reduction in invasion potential in MDA-MB-231 cells, where WASF3 was knocked down could not be compensated for by the expression of WASF1 and WASF2. The same is true under hypoxic conditions, since suppression of WASF3 significantly impaired the ability of the highly metastatic MDA-MB-231 cell line, which expresses WASF1 and WASF2, to invade and migrate in hypoxic conditions. In addition, when these cells were exposed to hypoxia, although WASF3 expression and protein levels were increased, this was not the case for either WASF1 or WASF2. Sequence analysis of the promoter region of these other two family members demonstrated that WASF2 does not contain any HRE sequences, and only one HRE sequence was found in ~1200 bp upstream of the initiation of transcription site in the WASF1 promoter. Collectively, these observations suggest that the WASF3 family member is likely promoting the invasion phenotype as a result of hypoxia.
The microenvironment surrounding a tumor is characterized by regions of hypoxic and nutrient depletion [30].Although most translation processes are expected to be down-regulated in this type of microenvironment, genes crucial for adapting to hypoxic conditions that ensure survival of the tumor cell undergo distinct metabolic changes that secure continued translation of genes such as those encoding HIF1 and VEGF [31], and now WASF3. Interestingly, there are reports which show that VEGF may also influence cell invasion through actin reorganization by forming a complex with N-WASP [32], a member of the WASF3 family. It is possible, therefore, that VEGF and WASF3 may act in a coordinated manner in response to hypoxia to promote tumor cell invasion. VEGF also promotes angiogenesis [33]. Conventional anti-angiogenic therapy may promote an unwanted induction of hypoxia due to the disruption of blood flow, oxygen depletion [19,34] and the resultant hypoxic environment that is created, which in turn may inadvertently facilitate up regulation of WASF3 followed by metastasis. The synergistic effect of HIF and VEGF inhibitors to suppress WASF3 expression to normal levels after hypoxia, offers a possible way to combat angiogenic effects caused by hypoxia. Understanding how WASF3 regulates invasion and metastasis under hypoxic conditions may provide alternative ways of treating tumors beyond conventional anti-angiogenic therapy.
In summary, our findings demonstrate that hypoxia stimulates WASF3 expression in breast cancer cells, and up regulation of WASF3 involves activation of its promoter by HIF1A. Further, the present work provides new insight into the mechanism behind the invasion phenotype modulated by induced WASF3 in hypoxia and may form the basis for new inhibitory therapies.
Novelty
Increased expression of the WASF3 gene leads to increased invasion and metastasis potential. We have now shown that WASF3 is inducible by HIF1A following exposure to hypoxia, which enhances invasion of breast cancer cells.
Impact
Enhancement of a metastasis promoting gene by hypoxia provides a mechanism to account for the progression and increased invasion of solid tumors which often experience a hypoxic environment.
Acknowledgments
We are grateful to Drs. In-Kiu Kwon and Darren Browning for assistance with the hypoxia experiments.
Grant support: This work was supported in part by a grant CA120510 from the National Institutes of Health. Dr Cowell is supported by the Georgia Cancer Coalition as a Distinguished Cancer Scholar.
Footnotes
Conflict of interests
The authors declare no conflicts of interest associated with this manuscript.
References
- 1.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 2.Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18:2183–94. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29 :297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 4.Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland A, Børresen-Dale AL, Giaccia A, Longaker MT, Hastie T, Yang GP, van de Vijver MJ, Brown PO. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sossey-Alaoui K, Safina A, Li X, Vaughan MM, Hicks DG, Bakin AV, Cowell JK. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am J Pathol. 2007;170:2112–21. doi: 10.2353/ajpath.2007.060975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng Y, Ren MQ, Cheney R, Sharma S, Cowell JK. Inactivation of the WASF3 gene in prostate cancer cells leads to suppression of tumorigenicity and metastases. Br J Cancer. 2010;103:1066–75. doi: 10.1038/sj.bjc.6605850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suetsugu S, Takenawa T. Regulation of cortical actin networks in cell migration. Int Rev Cytol. 2003;229:245–86. doi: 10.1016/s0074-7696(03)29006-9. [DOI] [PubMed] [Google Scholar]
- 8.Pollard TD, Borisy GG. Cellular motility driven by assembly anddisassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 9.Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci. 2001;114:1801–09. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- 10.Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK. WAVE3promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res. 2005;308:135–45. doi: 10.1016/j.yexcr.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Fernando HS, Sanders AJ, Kynaston HG, Jiang WG. WAVE3 is associated with invasiveness in prostate cancer cells. Urol Oncol. 2010;28:320–27. doi: 10.1016/j.urolonc.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Carroll VA, Ashcroft M. Targeting the molecular basis for tumour hypoxia. Expert Rev Mol Med. 2005;7:1–16. doi: 10.1017/S1462399405009117. [DOI] [PubMed] [Google Scholar]
- 13.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 14.Bando H, Toi M, Kitada K, Koike M. Genes commonly upregulated by hypoxia in human breast cancer cells MCF-7 and MDA-MB-231. Biomedicine & Pharmacotherapy. 2003;57:333–40. doi: 10.1016/s0753-3322(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 15.Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1A versus HIF-2A in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I,or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–70. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- 16.Teng Y, Liu M, Cowell JK. Functional interrelationship between the WASF3 and KISS1 metastasis-associated genes in breast cancer cells. Int J Cancer. 2001;129:2825–35. doi: 10.1002/ijc.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun HL, Liu YN, Huang YT, Pan SL, Huang DY, Guh J-H, Lee F-Y, Kuo S-C, Teng C-M. YC-1 inhibits HIF-1 expression in prostate cancer cells: contribution of Akt/NF-kappaB signaling to HIF-1alpha accumulation during hypoxia. Oncogene. 2007;26:3941–51. doi: 10.1038/sj.onc.1210169. [DOI] [PubMed] [Google Scholar]
- 19.Páez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 21.Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wouters BG, Koritzinsky M. Hypoxia signaling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–64. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 24.Sossey-Alaoui K, Su G, Malaj E, Roe B, Cowell JK. WAVE3, anactin-polymerization gene, is truncated and inactivated as a result of a constitutional t(1;13)(q21;q12) chromosome translocation in a patient with ganglioneuroblastoma. Oncogene. 2002;21 :5967–74. doi: 10.1038/sj.onc.1205734. [DOI] [PubMed] [Google Scholar]
- 25.Haigl B, Mayer CE, Siegwart G, Sutterlüty H. Sprouty4 levels are increased under hypoxic conditions by enhanced mRNA stability and transcription. Biol Chem. 2010;391:813–21. doi: 10.1515/BC.2010.082. [DOI] [PubMed] [Google Scholar]
- 26.Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells. J Biol Chem. 1994;269:760–64. [PubMed] [Google Scholar]
- 27.Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–16. [PubMed] [Google Scholar]
- 28.Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1a, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–20. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 29.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia inducible factor-1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–14. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leo C, Giaccia AJ, Denko NC. The hypoxic tumor microenvironment and gene expression. Semin Radiat Oncol. 2004;14:207–14. doi: 10.1016/j.semradonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Vasseura S, Afzalb S, Tardivel-Lacombea J, Park D, Iovannaa J, Makb T. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci. 2009;106:1111–16. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong CV, Stoletov KV, Terman BI. VEGF treatment induces signaling pathways that regulate both actin polymerization and depolymerization. Angiogenesis. 2004;7:313–21. doi: 10.1007/s10456-004-7960-2. [DOI] [PubMed] [Google Scholar]
- 33.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis aftershort-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–39. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]