Abstract
Context
Autopsy evaluation of the brain of a patient with frontotemporal dementia (FTD) can be daunting to the general pathologist. At some point in their training, most pathologists learn about Pick disease, and can recognize Pick bodies, the morphologic hallmark of Pick disease. Pick disease is a type of frontotemporal lobar degeneration (FTLD), the general category of pathologic process underlying most cases of FTD. The 2 major categories of pathologic FTLD are tauopathies (FTLD-tau) and ubiquitinopathies (FTLD-U). Pick disease is one of the FTLD-tau subtypes and is termed FTLD-tau (PiD).
Objective
To “demystify” FTLDs, and to demonstrate that subtypes of FTLD-tau and FTLD-U can be easily determined by following a logical, stepwise, histochemical, and immunohistochemical investigation of the FTD autopsy brain.
Data Sources
Previously published peer-reviewed articles.
Conclusions
The hope is that this article will be a useful reference for the general pathologist faced with performing a brain autopsy on a decedent with frontotemporal dementia.
The 3 most common pathologic diagnoses in neurodegenerative disorders are Alzheimer disease (AD), dementia with Lewy bodies (DLB or LBD), and frontotemporal lobar degeneration (FTLD). Almost 20 years ago, Mirra et al1 published in the Archives the special article titled “Making the Diagnosis of Alzheimer’s Disease: A Primer for Practicing Pathologists” as a guide for community pathologists in evaluating autopsy brains for the presence of AD. While the criteria for making the pathologic diagnosis of AD have been modified over the years, the basic gross and microscopic workup, outlined in the article listed above remains useful.2–6 Immunostains for abnormal tau and amyloid-β have been added to silver or thioflavin-S stains, for better delineation of the AD pathology.7,8 Likewise, most neuropathologists have replaced ubiquitin immunohistochemistry (IHC) with α-synuclein IHC, for better delineation of the Lewy body pathology, and criteria for the pathologic diagnosis of Lewy body disorders have been more fully developed.9
In the last 10 to 15 years, awareness of the frontotemporal dementia (FTD) has increased. In fact, in demented individuals younger than age 65, FTD and AD have the same prevalence.10 Frontotemporal dementia can be separated into 2 broad categories: behavioral variant FTD and primary progressive aphasia.11,12 Further consideration of the clinical details of FTD is beyond the scope of this article. However, FTD can be inherited in up to 50% of cases, and in order to perform targeted genetic analysis of the patient and potential affected family members, and to investigate biomarkers that will allow antemortem diagnosis upon which to base future targeted therapy, determination of the specific pathologic subtype is important.13 Currently, brain autopsy remains the gold standard for diagnosis of the specific pathologic and molecular FTLD subtype.14,15 (Note: FTD refers to the clinical syndrome and FTLD to the pathologic diagnosis. See the Table for definitions of clinical and pathologic subtypes. Note that this is a fairly comprehensive list of all possible pathologic subtypes and includes many that are uncommon or rare.)
Table.
Definitions of Clinical and Pathologic Subtypes
| FTD 5Clinical frontotemporal dementia syndrome, encompasses: |
| bvFTD: behavioral variant FTD |
| PPA (primary progressive aphasia) and its subtypes: |
| PPA-G: nonfluent/agrammatic PPA |
| PPA-S: semantic dementia (SD), also called semantic PPA |
| PPA-L: logopenic PPA |
| FTLD 5 Pathologic frontotemporal lobar degeneration, encompasses: |
| FTLD-tau (inclusions immunolabeled with antibodies to tau); includes: |
| More common primary diagnoses |
| FTLD-tau (PiD): Pick disease |
| FTLD-tau (CBD): corticobasal degeneration |
| FTLD-tau (PSP): progressive supranuclear palsy |
| Less common primary diagnoses |
| FTLD-tau (AGD): argyrophilic grain disease |
| FTLD-tau (MSTD): multiple-system tauopathy with dementia |
| FTLD-tau (NFT-dementia): tangle predominant senile dementia |
| FTLD-tau (WMT-GGI): white matter tauopathy with globular glial inclusions |
| FTLD-tau (unclassifiable): unclassifiable tauopathies |
| FTLD-U (inclusions labeled by antibodies to ubiquitin and p62); includes: |
| FTLD-TDP |
| Most common FTLD-U |
| Inclusions labeled by antibodies to ubiquitin, p62, and TAR DNA-binding protein of 43 kDa (TDP-43) |
| FTLD-FUS |
| Rare |
| Inclusions immunopositive for fused in sarcoma protein (FUS), ubiquitin, and p62, and negative for TDP-43 |
| Includes: |
| FTLD-FUS (aFTLD-U): atypical FTLD-U |
| FTLD-FUS (NIFID): neuronal intermediate filament inclusion disease |
| FTLD-FUS (BIBD): basophilic inclusion body disease |
| FTLD-UPS |
| Very rare |
| Inclusions labeled by ubiquitin and p62 and negative for tau, TDP-43, and FUS |
| UPS is ubiquitin protease system |
| Includes: |
| FTLD-UPS (FTD-3): familial FTLD associated with CHMP2B mutations |
| FTLD-UPS (sporadic): possibly some sporadic cases of TDP-43 and FUS-negative FTLD-U |
| FTLD-ni |
| Very rare |
| No inclusions seen on immunostains with tau, ubiquitin, p62, TDP-43, or FUS |
EVALUATING THE DEMENTIA BRAIN
Gross Examination
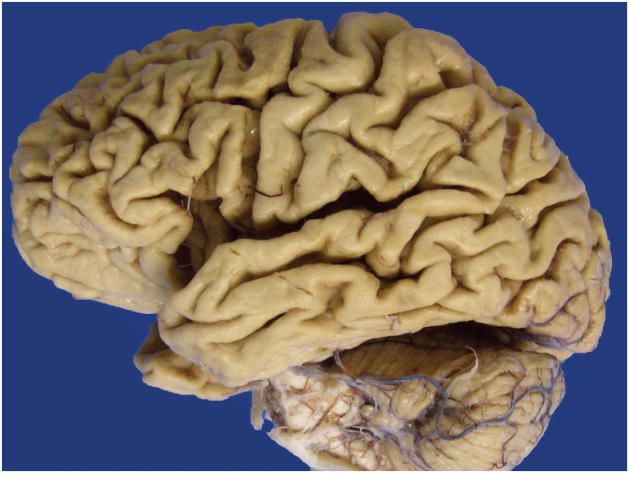
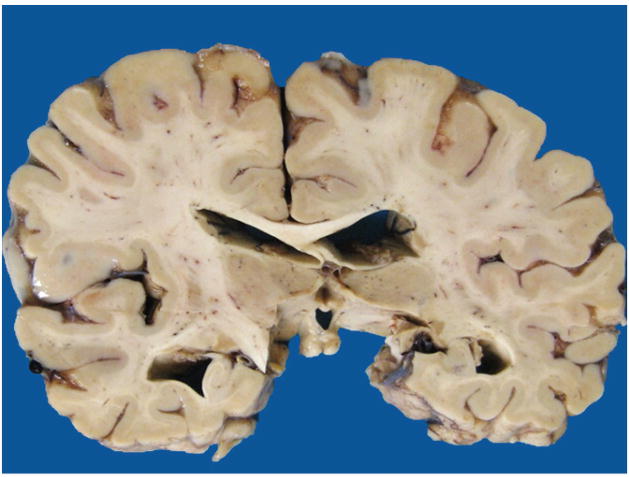
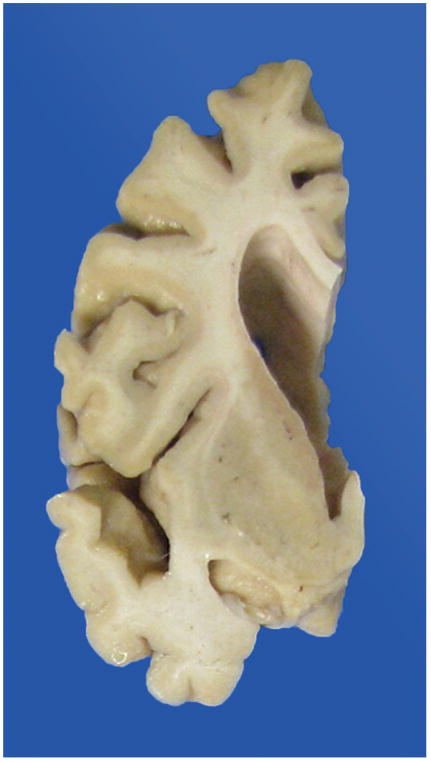
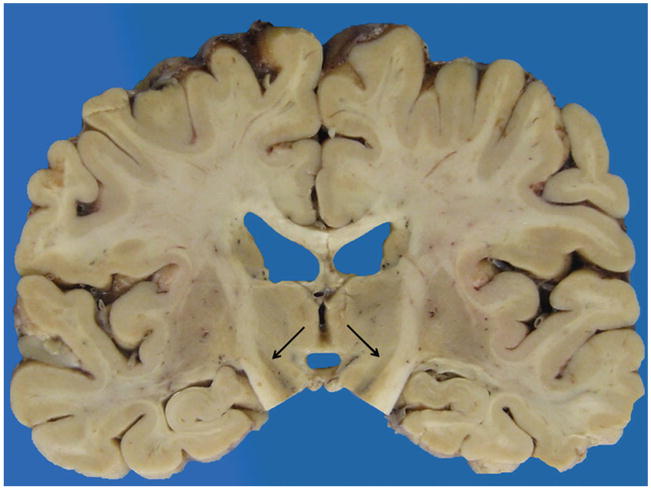
A careful gross examination can reveal a good deal about the likely final neuropathologic diagnosis. Brain weight should of course be recorded, and regional cortical atrophy, as well as atrophy of caudate, hippocampus, brainstem, and cerebellum, should be semiquantitated. The cerebellar dentate nucleus should be examined for atrophy/degeneration, and the pigmented brainstem nuclei, the substantia nigra, and locus coeruleus evaluated for pallor. It should be kept in mind that pathologic AD may be the underlying pathologic process in an FTD brain, so AD must remain on the differential diagnosis.16 In AD, atrophy is usually generalized (Figure 1), involving all cortical regions, most often with prominent atrophy of medial temporal structures including the hippocampus (Figure 2), amygdala, and inferior temporal cortex. In FTLD, cortical atrophy is naturally most often greatest in frontal and temporal lobes (Figure 3) but does not always spare other regions. The FTLD hippocampus is often, but not always, less atrophic than in AD, except in FTLD-tau (PiD), where hippocampal atrophy can be severe.17 Caudate atrophy, often present in FTLD (Figure 4), is uncommon in AD.18 The subthalamic nucleus is quite atrophic virtually only in progressive supranuclear palsy (FTLD-tau [PSP]) (Figure 5), and the cerebellar dentate nucleus is also generally “indistinct” 19 (Figure 6). The substantia nigra and locus coeruleus exhibit pallor in most FTLDs and AD. In AD, however, locus coeruleus pallor is usually greater than substantia nigra pallor, while in FTLD, the reverse is usually seen (personal observation) (Figure 7).
Figure 1.
Generalized cortical atrophy.
Figure 2.
Hippocampal atrophy, moderate.
Figure 3.
Frontal and superior temporal gyrus atrophy greater than parietal and occipital atrophy.
Figure 4.
Caudate atrophy, severe.
Figure 5.
Subthalamic nucleus atrophy, severe.
Figure 6.
Cerebellar dentate nucleus “indistinct.”
Figure 7.

Nigral greater than locus pallor.
Histologic Workup
The recent National Institute on Aging-Alzheimer’s Association (NIA-AA) revision of criteria for the pathologic diagnosis of AD recommends a minimum of 13 histologic sections to evaluate for Alzheimer disease neuropathologic change (ADNC), Lewy body disease (LBD), vascular brain injury, microvascular lesions, and hippocampal sclerosis.5,6 These same 13 sections may be used for the evaluation of FTLD. The sections include (1) middle frontal gyrus; (2) superior and middle temporal gyri; (3) inferior parietal lobule; (4) occipital cortex; (5) anterior cingulate gyrus; (6) amygdala; (7) hippocampus with dentate gyrus and entorhinal cortex; (8) basal ganglia at the level of the anterior commissure with caudate, putamen, globus pallidus, and the nucleus basalis of Meynert; (9) thalamus with subthalamic nucleus; (10) cerebellar cortex and dentate nucleus; (11) midbrain with substantia nigra; (12) pons with locus coeruleus; (13) and medulla with dorsal motor nucleus of the vagus and hypoglossal nucleus. Figure 8 shows where these sections should be taken. All sections should be stained with routine hematoxylin-eosin (H&E) and evaluated according to NIA-AA revised criteria for regional neuronal loss and gliosis, vascular brain injury, microvascular lesions, and hippocampal sclerosis.5,6 Whereas in AD the most striking neuronal loss and gliosis is in the entorhinal cortex and hippocampus, nucleus basalis, locus coeruleus, and neocortex, neuronal loss and gliosis in FTLD generally parallels the gross atrophy. Neocortical neuronal loss and gliosis is usually most prominent in frontal and temporal regions, but in corticobasal degeneration (FTLD-tau [CBD]), for example, there is often prominent, and sometimes asymmetric, parietal and motor cortex neuronal loss and gliosis as well.20 In most FTLDs, hippocampal neuronal loss and gliosis is less striking than in AD, unless there is also hippocampal sclerosis, which is most often found in frontotemporal lobar degeneration with TAR DNA-binding protein of 43 kDa (TDP-43) proteinopathy (FTLD-TDP).21 The other exception is FTLD-tau (PiD), which may have severe hippocampal neuronal loss and gliosis.17 In FTLD-tau (PSP), there is notable neuronal loss and gliosis in the subthalamic nucleus, lateral thalamic nucleus, globus pallidus, and cerebellar dentate nucleus, where grumose degeneration of presynaptic terminals around dentate neurons is often prominent and can be demonstrated with immunostains for synaptophysin.19
Figure 8.
Illustration showing where to take 13 histologic sections.
Special Stains and Immunostains
Alzheimer Disease and LBD Pathology
In addition to evaluating regional neuronal loss and gliosis, microvascular lesions, vascular brain injury, and hippocampal sclerosis, the dementia brain should always be investigated for AD and LBD pathology, regardless of the specific clinical diagnosis. There is often some degree of ADNC in the elderly brain, and combined pathologic processes are not infrequent. Alzheimer disease neuropathologic change can be semiquantitated with Bielschowsky or Gallyas silver or thioflavin-S fluorescent stains of hippocampus and neocortical sections, and the degree of ADNC reported.5,6 Amyloid-β can be demonstrated with an immunostain of hippocampus. If the section includes parahippocampal gyrus, the Thal phase of amyloid deposition can easily be determined, as amyloid plaques appear early in neocortex (phase 1), later in CA1 and the subiculum (phase 2), still later in the fascia dentata (phase 3), then in CA4 (phase 4), and finally in cerebellum and brainstem (phase 5).5,6,22 The LBD pathology can be semiquantitated on H&E sections of brainstem and α-synuclein immunohistochemistry of amygdala. If the amygdala has Lewy bodies, additional α-synuclein immunostaining should be performed on anterior cingulate gyrus, and if Lewy bodies are seen in the cingulate, then additional α-synuclein immunostaining should be performed on frontal, temporal, and parietal neocortex for grading of the Lewy body pathology.5,6
Frontotemporal Lobar Degeneration Pathology
For initial FTLD screening, tau, ubiquitin or p62, and TDP-43 immunostaining should be performed on hippocampus and frontal cortex. Recommended antibodies are the following: for tau, AT8 (Pierce-Endogen, Rockford, Illinois) or PHF-1 (available from Peter Davies, PhD, at Albert Einstein College of Medicine by request); for p62, anti-p62Lck ligand (BD Biosciences, San Jose, California); and for TDP-43, polyclonal antibody to normal TARDBP (Proteintech, Chicago, Illinois) or polyclonal to phosphorylated TARDBP (pS409/410-2) (Cosmo Bio Co, Ltd, Tokyo, Japan). Tau immunostains will highlight the abnormally phosphorylated tau deposits in neurons and glia that are found in FTLD-tau; ubiquitin or p62 immunostains will highlight the abnormal protein deposits in neurons and glia found in FTLD-U; and TDP-43 will demonstrate whether or not the ubiquitin or p62 immunopositive, tau-negative inclusions are those of FTLD-TDP, the most common FTLD-U.14,15
For more specific classification, refer to recent articles describing FTLD subtypes.14,15 Briefly, tau inclusions associated with a specific FTLD-tau, most commonly corticobasal degeneration (FTLD-tau [CBD]), FTLD-tau (PSP), or FTLD-tau (PiD), can be morphologically distinguished. In FTLD-TDP, the antibody to normal TDP-43 will show normal positivity in nuclei of neurons that have no abnormal TDP-43–positive inclusions, and “negative” nuclei in neurons that have inclusions, while the phosphorylated TDP-43 antibody will highlight inclusions only. Should immunostains show ubiquitin- or p62-positive inclusions that are TDP-43 negative, or no tau- or ubiquitin-positive inclusions at all, an additional immunostain can be considered, using an antibody to FUS (also known as FUS/TLS, fused in sarcoma/translocated in liposarcoma protein). If FUS staining is positive, the diagnosis is FTLD-FUS, and the possible, more specific, diagnoses include atypical FTLD-U (FTLD-FUS [aFTLD-U]), neuronal intermediate filament inclusion disease (FTLD-FUS [NIFID]), or basophilic inclusion body disease (FTLD-FUS [BIBD]). If FUS staining is negative, the diagnosis is FTLD involving the ubiquitin proteasome system (FTLD-UPS), and more specifically, it may be associated with mutations in the CHMP2Ba gene (FTD-3) or possibly a FUS-negative sporadic FTLD-UPS. There is also a chance that tau, ubiquitin or p62, and TDP-43 results will all be negative, in which case the possibility that prion disease is responsible for the FTLD should be considered.
The results of the initial screening with these 3 antibodies (tau, ubiquitin or p62, and TDP-43) on 2 sections each, frontal cortex and hippocampus, will in most cases allow the general pathologist to subclassify the FTLD. At this point, screening will likely have indicated the major FTLD disease protein present. In some cases, and certainly for more detailed delineation of the complete pathology present in the case, immunostaining on sections from additional regions may be required. However, the additional sections need only be immunostained with antibodies to the major disease protein. Figure 9 is a flowchart showing the sequence of stains and immunostains that can be followed in working up a case for FTLD.
Figure 9.
Flowchart showing sequence of stains and immunostains used to make the diagnosis of a specific frontotemporal lobar degeneration. Abbreviations: Aβ, amyloid-β; AD, Alzheimer disease; aFTLD-U, atypical frontotemporal lobar degeneration; AGD, argyrophilic grain disease; ALS, amyotrophic lateral sclerosis; Amyg, amygdala; α-syn, α-synuclein; BG, basal ganglia; BIBD, basophilic inclusion body disease; Biel, Bielschowsky; CBD, corticobasal degeneration; Cbm, cerebellum; DN, dentate nucleus; FTD-3, frontotemporal dementia associated with chromosome 3; FTLD-FUS, frontotemporal lobar degeneration with fused in sarcoma proteinopathy; FTLD-ni, frontotemporal lobar degeneration with no inclusions; FTLD-tau, frontotemporal lobar degeneration with tauopathy; FTLD-TDP, frontotemporal lobar degeneration with TDP-43 proteinopathy; FTLD-UPS, frontotemporal lobar degeneration with involvement of the ubiquitin proteasome system; FUS, fused in sarcoma protein; Hippo, hippocampus; IHC, immunohistochemistry; IP, inferior parietal region; LBD, Lewy body disease; Med, medulla; MFG, middle frontal gyrus; Mid, midbrain; MSTD, multiple system tauopathy with dementia; NIFID, neuronal intermediate filament inclusion dementia; PiD, Pick disease; PSP, progressive supranuclear palsy; SC, spinal cord; STG, superior temporal gyrus; Str, striatum; TDP-43, transactive response deoxyribose nucleic acid binding protein of 43 kDa; Thal, thalamus; Thio, thioflavin-S; TPSD, tangle predominant senile dementia; Ub, ubiquitin; WMT-GGI, white matter tauopathy with globular glial inclusions.
EXAMPLES
Case 1: FTLD-tau (PiD)
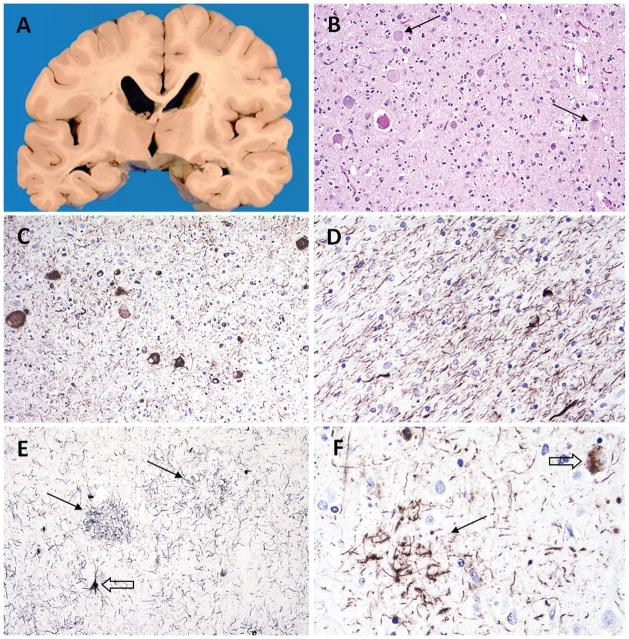
The decedent was a 72-year-old man with an 8-year history of primary progressive aphasia. Brain autopsy revealed asymmetric atrophy of the inferior frontal gyrus with moderate atrophy on the left (Broca region) and mild atrophy on the right. There was also asymmetry of the hippocampal atrophy, which was severe on the left and mild on the right (Figure 10, A). Microscopic examination of H&E sections revealed numerous round, discrete, blue-gray cytoplasmic inclusions in the hippocampal dentate gyrus approximately the size of dentate gyrus neuronal nuclei, compatible with Pick bodies (Figure 10, B). Some of the Pick bodies were labeled with ubiquitin (Figure 10, C). All of them were immunolabeled with antibodies to phosphorylated tau (Figure 10, D). There was severe neuronal loss and gliosis in the left inferior frontal gyrus (Broca region) (Figure 10, E) and ballooned neurons were seen in several cortical and subcortical regions (Figure 10, E, inset). Tau immunostains also labeled Pick bodies and glial inclusions in the frontal cortex (Figure 10, F). Pick bodies were also present in temporal and parietal cortex, caudate and putamen, substantia nigra, locus coeruleus, and pontine nuclei (not shown). They were positive with antibodies to 3R but not 4R tau (not shown).23 TDP-43 immunostaining of frontal cortex and hippocampus was negative.
Figure 10.
Case 1: FTLD-tau (PiD) pathology. A, Coronal section at the level of the posterior hippocampus, showing hippocampal atrophy that is severe on the left and mild on the right. B, Pick bodies in dentate gyrus. C, Some dentate gyrus Pick bodies are immunopositive with ubiquitin. D, All Pick bodies are labeled with tau. E, Severe cortical neuronal loss and gliosis; ballooned neurons (inset). F, Cortical Pick bodies (arrows) and glial inclusions labeled with tau (open arrows) (hematoxylin-eosin, original magnifications ×400 [B], ×50 [E], and ×200 [E, inset]; Dako polyclonal ubiquitin antibody [Carpinteria, California], original magnification ×600 [C]; AT8 antibody [Pierce Endogen, Rockford, Illinois], original magnifications ×600 [D] and ×400 [F]).
Case 2: FTLD-tau (CBD)
A brain autopsy was performed on a 67-year-old woman who died after an 8-year history of primary progressive aphasia. Gross examination revealed absent to mild symmetric cortical and hippocampal atrophy and moderate dilatation of the lateral ventricles (Figure 11, A). Hematoxylin-eosin sections revealed neuronal loss and gliosis that was greatest in language regions, and numerous ballooned neurons in cortical and subcortical regions and brainstem nuclei (Figure 11, B) that were labeled with antibodies to phosphorylated tau (Figure 11, C). There were numerous tau-positive threads in cortex (Figure 11, C) and white matter (Figure 11, D). Astrocytic plaques and threads in cortex were also highlighted by Gallyas stains (Figure 11, E). A higher-power image of a tau-labeled astrocytic plaque and neuronal inclusion is seen in Figure 11, F. TDP-43 immunostaining of frontal cortex and hippocampus was negative.
Figure 11.
Case 2: FTLD-tau (CBD) pathology. A, Coronal section at the level of the posterior hippocampus, showing no cortical asymmetry and moderate ventricular dilatation. B, Ballooned neurons in motor cortex; arrows point to two. C, Ballooned neurons and cortical threads labeled with tau. D, White matter threads and glial inclusions labeled with tau. E, Astrocytic plaques (arrows), neuronal inclusions (open arrow), and cortical threads stain positively with silver stain. F, Astrocytic plaque (arrow) and neuronal inclusion (open arrow) labeled with tau (hematoxylin-eosin, original magnification ×200 [B]; AT8 [Pierce Endogen, Rockford, Illinois], original magnifications ×200 [C] and ×400 [D and F]; Gallyas stain, original magnification ×200 [E]).
Case 3: FTLD-tau (PSP)
A 74-year-old man with a 9-year history of FTD died, and brain autopsy revealed moderate frontal atrophy, severe ventricular dilatation, severe atrophy of the head of the caudate, and mild atrophy and yellowish discoloration of the globus pallidus (Figure 12, A). Sections of globus pallidus immunostained with tau showed neuronal inclusions and tufted astrocytes (Figure 12, B). A H&E section of cerebellar dentate nucleus showed moderate neuronal loss and gliosis (Figure 12, C) and a synaptophysin immunostain of this section highlighted “grumose” degeneration (Figure 12, C, inset).24 Only rare dentate nucleus neurons were tau positive (Figure 12, D). There were tau-positive neurons and tufted astrocytes in frontal, temporal, parietal, and motor cortex (Figure 12, E; positive with Gallyas stain in Figure 12, E, inset), nucleus basalis, caudate, putamen (Figure 12, F), globus pallidus, thalamus, subthalamic nucleus (Figure 12, G), and many brainstem nuclei. In some regions, such as the putamen, tufted astrocytes predominated (Figure 12, F), while in others, such as the subthalamic nucleus, tau-positive neurons predominated (Figure 12, G). Tau inclusions were labeled with antibodies to 4R but not 3R tau (not shown).23 TDP-43 immunostaining of frontal cortex and hippocampus was negative.
Figure 12.
Case 3: FTLD-tau (PSP) pathology. A, Coronal section at the level of the anterior hippocampus, showing mild to moderate cortical atrophy, severe ventricular dilatation, severe atrophy of the head of the caudate (white arrows), and mild atrophy and yellowish discoloration of the globus pallidus (black arrows). B, Globus pallidus with tau-positive neuronal inclusion (arrow) and several tau-positive tufted astrocytes (open arrow). C, Cerebellar dentate nucleus showing moderate neuronal loss and gliosis; synaptophysin highlights grumose degeneration (arrows) in dentate nucleus (inset). D, Rare tau-positive neurons in dentate nucleus. E, Tau-positive neurons and tufted astrocytes in cortex; silver-positive tufted astrocyte (inset). F, Tau-positive tufted astrocytes in putamen. G, Tau-positive neurons in subthalamic nucleus (AT8 [Pierce Endogen, Rockford, Illinois], original magnifications ×200 [B and G], ×100 [D and F], and ×50 [E]; hematoxylin-eosin, original magnification ×200 [C]; Dako [Carpinteria, California] monoclonal synaptophysin antibody SY38, original magnification ×200 [C, inset]; Gallyas stain, original magnification ×200 [E, inset]).
Case 4: FTLD-TDP
An autopsy of the brain of a 63-year-old man who had a 6-year history of FTD with corticobasal syndrome revealed marked asymmetry of frontal, temporal, and parietal atrophy—severe on the right and mild to moderate on the left, with relative sparing of motor and sensory gyri (Figure 13, A and B). Coronal sections at the level of the anterior hippocampus also showed severe right and moderate left ventricular dilatation (Figure 13, C). Tau immunostaining of frontal cortex and hippocampus was negative. A TDP-43 immunostain of frontal cortex showed cytoplasmic and intranuclear inclusions and short dystrophic neurites predominantly in upper layers of cortex (Figure 13, D). There were sparse cytoplasmic inclusions in hippocampal dentate gyrus (not shown). This is an example of FTLD-TDP type A, as described in the recent article on the “harmonized” classification scheme for FTLD-TDP.25 For comparison, Figure 13, E and F, shows FTLD-TDP type B; and Figure 13, G, shows FTLD-TDP type C.
Figure 13.
Case 4: FTLD-TDP pathology. Marked asymmetry of cortical atrophy, with severe right (A) and mild to moderate left (B) frontal, temporal, and parietal atrophy, demonstrated in coronal section at the level of the anterior hippocampus (C), which also shows severe right and moderate left ventricular dilatation. Note relative sparing of motor and sensory gyri. TDP-43–positive cytoplasmic and intranuclear inclusions and short dystrophic neurites consistent with harmonized FTLD-TDP type A (D). A different case, showing FTLD-TDP type B pathology in cortex, with predominantly granular cytoplasmic inclusions (E). FTLD-TDP type B pathology in dentate gyrus. Note that antibody to normal TDP-43 highlights normal nuclear TDP-43 in neurons that do not have inclusions (F). Last, harmonized FTLD-TDP type C pathology (G) (phosphorylated TDP-43 antibody S409/410-2 [Cosmo Bio, Carlsbad, California], original magnifications ×600 [D] and ×200 [G]; antibody to normal TDP-43 [ProteinTech, Chicago Illinois], original magnifications ×400 [E and F]).
Case 5: FTLD-FUS (BIBD)
The decedent was a 55-year-old man with a 7-year history of FTD. Brain autopsy revealed moderate to severe right frontal (Figure 14, A) and mild left frontal atrophy (Figure 14, B). Distinct basophilic inclusions were seen in frontal, motor, and parietal cortex, red nucleus, hypoglossal nucleus, pontine nuclei, and superior colliculus. One such inclusion in motor cortex is shown in Figure 14, C. Basophilic inclusions stained negatively with ubiquitin immunostain (Figure 14, D) and antibodies to tau and TDP-43 (not shown). FUS immunostains strongly labeled basophilic inclusions (Figure 14, E) and also demonstrated numerous cytoplasmic inclusions in superficial cortical layers that were not seen on H & E stains (Figure 14, F), as has been described in basophilic inclusion body disease.26
Figure 14.
Case 5: FTLD-FUS (BIBD) pathology. Asymmetry of frontal cortex atrophy with moderate to severe right (A) and mild left (B) frontal atrophy. Basophilic inclusion in motor cortex neuron (C). Basophilic inclusions are unstained by ubiquitin (D) but are strongly labeled with FUS antibody (E). The FUS antibody also highlights numerous cytoplasmic inclusions in superficial frontal and motor cortex that are not apparent on hematoxylin-eosin stains (F) (hematoxylin-eosin, original magnification ×400 [C]; Dako ubiquitin antibody [Dako, Carpinteria California], original magnification ×600 [D]; FUS antibody [ProteinTech, Chicago Illinois original magnifications ×600 [E] and ×400 [F]).
SUMMARY OF THE PATHOLOGIC APPROACH
Pathologic classification of the FTLD brain can be approached in a logical, stepwise manner by using immunohistochemistry and the morphologic characteristics of the inclusions and their distribution. Using tau, ubiquitin or p62, and TDP-43 immunostains of frontal cortex and hippocampus, the pathologist will in most cases be able to classify the FTLD into either a tauopathy or a non-tau FTLD. Approximately one-half of FTLDs are tauopathies and the other half, TDP-43 proteinopathies.
In the case of a tauopathy, the presence of round, tau-positive Pick bodies characterizes the case as FTLD-tau (PiD). If tau-positive inclusions, but not Pick bodies, are present, the most common alternative tauopathies are FTLD-tau (CBD) and FTLD-tau (PSP). Both have tau-positive neuronal and astrocytic inclusions. The distinguishing astrocytic inclusions are the astrocytic plaques of FTLD-tau (CBD) and tufted astrocytes of FTLD-tau (PSP). Astrocytic plaques are characterized by small, homogeneously sized tau-positive inclusions in peripheral astrocytic processes, and the central astrocytic nucleus may or may not be seen in the plane of the section. The parts of the astrocytic processes close to the nucleus are not tau positive. In contrast, tau positivity in tufted astrocyte processes is strongest closest to the astrocytic nucleus and becomes weaker in the distal processes before it disappears, and the nucleus is generally visible. In FTLD-TDP, tau staining is negative, and TDP-43 immunostains label neuronal cytoplasmic inclusions, intranuclear inclusions, and short dystrophic neurites in type A; predominantly cytoplasmic inclusions in type B; and predominantly long dystrophic neurites in type C. In FTLD-FUS, tau and TDP-43 staining is negative. Ubiquitin staining is usually positive in FTLD-FUS (aFTLD-U), is positive in some of the inclusions found in FTLD-FUS (NIFID), and is usually negative in FTLD-FUS (BIBD). FUS inclusions are generally strongly positive in all these cases. However, all types of FTLD-FUS are rare.
CONCLUSION
Pathology of the FTLD brain is not easily subclassified in every case, but in most cases the approach is straightforward and logical. In most cases, the results of the workup will allow the general pathologist to synthesize the pathologic diagnosis of a specific FTLD subtype (Figure 15). Hopefully, this article will be a useful reference when faced with the autopsy of a decedent with frontotemporal dementia.
Figure 15.
Graphic display of frontotemporal lobar degeneration (FTLD) pathologic subtypes, with currently known genetic associations. Abbreviations: aFTLD-U, atypical frontotemporal lobar degeneration; AGD, argyrophilic grain disease; ALS, amyotrophic lateral sclerosis; BIBD, basophilic inclusion body disease; CBD, corticobasal degeneration; CTE, chronic traumatic encephalopathy; DLDH, dementia lacking distinctive histology; FTD-3, frontotemporal dementia associated with chromosome 3; FTLD-FUS, frontotemporal lobar degeneration with fused in sarcoma proteinopathy; FTLD-ni, frontotemporal lobar degeneration with no inclusions; FTLD-tau, frontotemporal lobar degeneration with tauopathy; FTLD-TDP, frontotemporal lobar degeneration with TDP-43 proteinopathy; FTLD-UPS, frontotemporal lobar degeneration with involvement of the ubiquitin proteasome system; FUS, fused in sarcoma protein; MSTD, multiple system tauopathy with dementia; NIFID, neuronal intermediate filament inclusion dementia; PiD, Pick disease; PSP, progressive supranuclear palsy; TDP-43, transactive response deoxyribose nucleic acid binding protein of 43 kDa; TPSD, tangle predominant senile dementia; Ub, ubiquitin; WMT-GGI, white matter tauopathy with globular glial inclusions; 3R, 3 microtubule-binding repeats, 4R, 4 microtubule-binding repeats.
Acknowledgments
This work was supported in part by NIA grant AG 13854. We would like to thank the patients and their families who, through their generous participation, make studies like this possible.
Footnotes
The author has no relevant financial interest in the products or companies described in this article.
References
- 1.Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer’s disease: a primer for practicing pathologists. Arch Pathol Lab Med. 1993;117(2):132–144. [PubMed] [Google Scholar]
- 2.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), II: standardization of neuropathological assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 4.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alafuzoff I, Arzberger T, Al-Sarraj S, et al. Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol. 2008;18(4):484–496. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alafuzoff I, Thal DR, Arzberger T, et al. Assessment of beta-amyloid deposits in human brain: a study of the BrainNet Europe Consortium. Acta Neuropathol. 2009;117(3):309–320. doi: 10.1007/s00401-009-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies; third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 10.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 11.Piquet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10(2):162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- 12.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafson L. Frontal lobe degeneration of non-Alzheimer type. II: clinical picture and differential diagnosis. Arch Gerontol Geriatr. 1987;6(3):209–223. doi: 10.1016/0167-4943(87)90022-7. [DOI] [PubMed] [Google Scholar]
- 14.Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigio EH, Mishra M, Hatanpaa KJ, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol. 2010;120(1):43–54. doi: 10.1007/s00401-010-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson DW. Neuropathology of Pick’s disease. Neurology. 2001;56(suppl 4):S16–S20. doi: 10.1212/wnl.56.suppl_4.s16. [DOI] [PubMed] [Google Scholar]
- 18.Garibotto V, Borroni B, Agosti C, et al. Subcortical and deep cortical atrophy in frontotemporal lobar degeneration. Neurobiol Aging. 2011;32(5):875–884. doi: 10.1016/j.neurobiolaging.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17(1):74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitani K, Uchihara T, Tamaru F, Endo K, Tsukagoshi H. Corticobasal degeneration: clinico-pathological studies on two cases [in Japanese] Rinsho Shinkeigaku. 1993;33(2):155–161. [PubMed] [Google Scholar]
- 21.Josephs KA, Dickson DW. Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging. 2007;28(11):1718–1722. doi: 10.1016/j.neurobiolaging.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Thal DR, Rub U, Orantes M, Braak H. Phases of Abeta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 23.de Silva R, Lashley T, Gibb G, et al. Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol Appl Neurobiol. 2003;29(3):288–302. doi: 10.1046/j.1365-2990.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- 24.Arai N. “Grumose degeneration” of the dentate nucleus: a light an electron microscopic study in progressive supranuclear palsy and dentatoru-bropallidoluysial atrophy. J Neurol Sci. 1989;90(2):131–145. doi: 10.1016/0022-510x(89)90096-8. [DOI] [PubMed] [Google Scholar]
- 25.Mackenzie IRA, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122(1):111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz DG, Neumann M, Kusaka H, et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 2009;118(5):617–627. doi: 10.1007/s00401-009-0598-9. [DOI] [PubMed] [Google Scholar]