Abstract
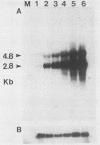
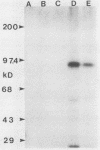
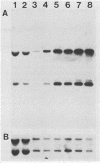
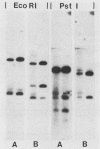
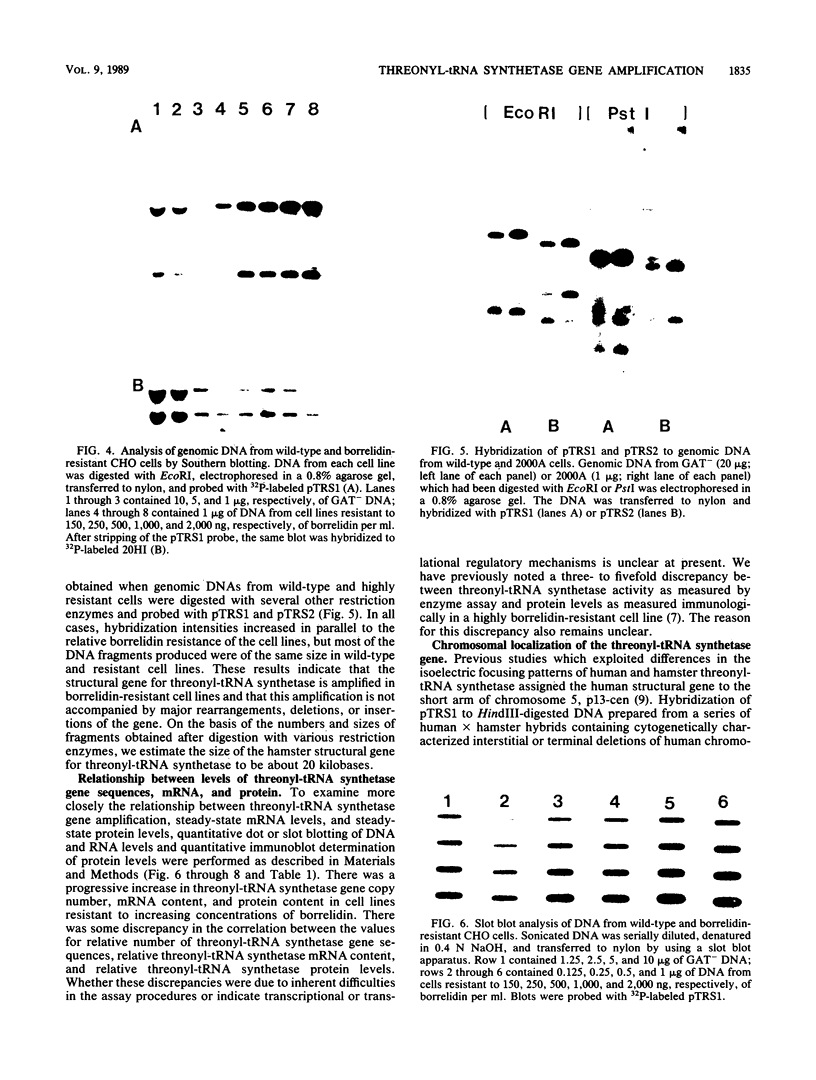
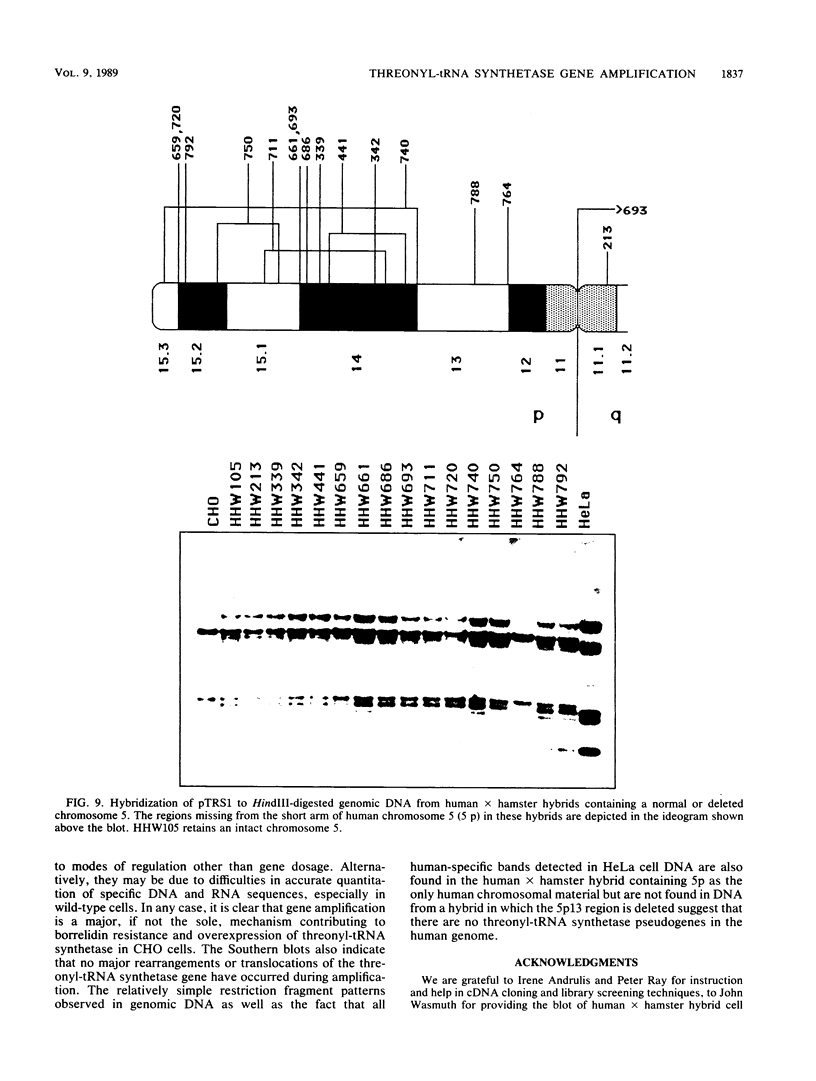
A cDNA for threonyl-tRNA synthetase was isolated from a human placental cDNA lambda gt11 expression library by immunological screening, and its identity was confirmed by hybrid-selected mRNA translation. With this cDNA used as a hybridization probe, borrelidin-resistant Chinese hamster ovary cells that overproduced threonyl-tRNA synthetase were shown to have increased levels of threonyl-tRNA synthetase mRNA and gene sequences. Amplification of the gene did not appear to have been accompanied by any major structural reorganizations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dardel F., Fayat G., Blanquet S. Molecular cloning and primary structure of the Escherichia coli methionyl-tRNA synthetase gene. J Bacteriol. 1984 Dec;160(3):1115–1122. doi: 10.1128/jb.160.3.1115-1122.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gantt J. S., Bennett C. A., Arfin S. M. Increased levels of threonyl-tRNA synthetase in a borrelidin-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5367–5370. doi: 10.1073/pnas.78.9.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken S. C., Arfin S. M. Chinese hamster ovary cells resistant to borrelidin overproduce threonyl-tRNA synthetase. J Biol Chem. 1984 Jul 25;259(14):9202–9206. [PubMed] [Google Scholar]
- Gerken S. C., Arfin S. M. Threonyl-tRNA synthetase from Chinese hamster ovary cells is phosphorylated on serine. J Biol Chem. 1984 Sep 25;259(18):11160–11161. [PubMed] [Google Scholar]
- Gerken S. C., Wasmuth J. J., Arfin S. M. Threonyl-tRNA synthetase gene maps close to leucyl-tRNA synthetase gene on human chromosome 5. Somat Cell Mol Genet. 1986 Sep;12(5):519–522. doi: 10.1007/BF01539923. [DOI] [PubMed] [Google Scholar]
- Heck J. D., Hatfield G. W. Valyl-tRNA synthetase gene of Escherichia coli K12. Primary structure and homology within a family of aminoacyl-TRNA synthetases. J Biol Chem. 1988 Jan 15;263(2):868–877. [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. A general method for preparing chromatin containing intact DNA. EMBO J. 1985 Apr;4(4):913–918. doi: 10.1002/j.1460-2075.1985.tb03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagus R. Hybrid selection of mRNA and hybrid arrest of translation. Methods Enzymol. 1987;152:567–572. doi: 10.1016/0076-6879(87)52063-8. [DOI] [PubMed] [Google Scholar]
- Jordana X., Chatton B., Paz-Weisshaar M., Buhler J. M., Cramer F., Ebel J. P., Fasiolo F. Structure of the yeast valyl-tRNA synthetase gene (VASI) and the homology of its translated amino acid sequence with Escherichia coli isoleucyl-tRNA synthetase. J Biol Chem. 1987 May 25;262(15):7189–7194. [PubMed] [Google Scholar]
- Lazard M., Mirande M., Waller J. P. Expression of the aminoacyl-tRNA synthetase complex in cultured Chinese hamster ovary cells. Specific depression of the methionyl-tRNA synthetase component upon methionine restriction. J Biol Chem. 1987 Mar 25;262(9):3982–3987. [PubMed] [Google Scholar]
- Lazard M., Mirande M., Waller J. P. Overexpression of mammalian phenylalanyl-tRNA synthetase upon phenylalanine restriction. FEBS Lett. 1987 May 25;216(1):27–30. doi: 10.1016/0014-5793(87)80750-0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Reichlin M., Hughes G. R., Bernstein R. M. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. J Exp Med. 1984 Aug 1;160(2):420–434. doi: 10.1084/jem.160.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overhauser J., Beaudet A. L., Wasmuth J. J. A fine structure physical map of the short arm of chromosome 5. Am J Hum Genet. 1986 Nov;39(5):562–572. [PMC free article] [PubMed] [Google Scholar]
- Overhauser J., McMahan J., Wasmuth J. J. Identification of 28 DNA fragments that detect RFLPs in 13 distinct physical regions of the short arm of chromosome 5. Nucleic Acids Res. 1987 Jun 11;15(11):4617–4627. doi: 10.1093/nar/15.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L. Regulation of branched-chain amino acid transport in Escherichia coli. J Bacteriol. 1976 Sep;127(3):1225–1238. doi: 10.1128/jb.127.3.1225-1238.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Tsui F. W., Andrulis I. L., Murialdo H., Siminovitch L. Amplification of the gene for histidyl-tRNA synthetase in histidinol-resistant Chinese hamster ovary cells. Mol Cell Biol. 1985 Sep;5(9):2381–2388. doi: 10.1128/mcb.5.9.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui F. W., Siminovitch L. Isolation, structure and expression of mammalian genes for histidyl-tRNA synthetase. Nucleic Acids Res. 1987 Apr 24;15(8):3349–3367. doi: 10.1093/nar/15.8.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui F. W., Siminovitch L. Structural analysis of the 5' region of the chromosomal gene for hamster histidyl-tRNA synthetase. Gene. 1987;61(3):349–361. doi: 10.1016/0378-1119(87)90198-3. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Kurkulos M., Repetto B. Homology of yeast mitochondrial leucyl-tRNA synthetase and isoleucyl- and methionyl-tRNA synthetases of Escherichia coli. J Biol Chem. 1988 Jan 15;263(2):850–856. [PubMed] [Google Scholar]
- Varshavsky A. Diadenosine 5', 5"'-P1, P4-tetraphosphate: a pleiotropically acting alarmone? Cell. 1983 Oct;34(3):711–712. doi: 10.1016/0092-8674(83)90526-3. [DOI] [PubMed] [Google Scholar]
- Webster T., Tsai H., Kula M., Mackie G. A., Schimmel P. Specific sequence homology and three-dimensional structure of an aminoacyl transfer RNA synthetase. Science. 1984 Dec 14;226(4680):1315–1317. doi: 10.1126/science.6390679. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Transcription attenuation. J Biol Chem. 1988 Jan 15;263(2):609–612. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. Diadenosine 5',5"'-P1,P4-tetraphosphate (Ap4A): its role in cellular metabolism. Anal Biochem. 1983 Oct 1;134(1):1–10. doi: 10.1016/0003-2697(83)90255-5. [DOI] [PubMed] [Google Scholar]