Abstract
Neuroblastoma is the third most common pediatric cancer in the United States and is responsible for 15% of pediatric cancer-related deaths. Despite major advances in multimodal therapy, the clinical outcome for several patients remains poor. Due to the desperate need for innovativation and improved success in the treatment and management of neuroblastoma, research interests in immunotherapy have been on the rise in recent years. Current immunotherapeutic approaches under investigation include antibodies targeting the neuroblastoma antigen GD2, cytokine stimulation of immune cells, use of immunocytokine conjugates, radioimmunotherapy, and tumor-primed dendritic cells. Immunotherapy could serve as a safe alternative or adjunct to current therapeutic protocols and would presumptively have fewer deleterious effects making it more favorable to patients.
Keywords: Neuroblastoma, immunotherapy, cell-mediated therapy, antibody-mediated therapy, cytokine-mediated therapy
INTRODUCTION
Neuroblastoma is the third most common pediatric cancer in the United States as well as the most common extra-cranial solid tumor in children [1]. It is responsible for 15% of childhood cancer-related deaths with over 500 newly diagnosed cases per year [2]. The average age at diagnosis is 17 months and more than 50% of patients have metastatic disease at the time of diagnosis [3]. A unique feature of this disease is that infants less than one year of age may demonstrate spontaneous tumor regression without recurrence and have a favorable prognosis; however, children diagnosed after age 18 months tend to have progressive disease yielding a less favorable prognosis[4].
Neuroblastomas are derived from primitive cells, originating in the neural crest, which migrate during embryogenesis; hence, these tumors are most commonly found in the adrenal medulla, but they may arise at any point along the sympathetic ganglia [5]. Neuroblastomas are heterogeneous tumors, whose pathology and prognosis depend on a variety of factors including age at diagnosis, MYCN gene amplification, vascularization, amount of Schwanian stroma, chromosomal losses/gains, and DNA index abnormalities [5, 6]. Based on these prognostic characteristics, the Children’s Oncology Group (COG) has developed a categorical system which assigns neuroblastoma patients into low, intermediate, and high-risk groups [7, 8].
Currently, the treatment of neuroblastoma is based on COG risk stratification and includes a variable combination of surgery, chemotherapy, and radiation therapy [9]. For instance, patients with low-risk disease are treated with surgery alone, whereas children with intermediate-risk neuroblastoma receive chemotherapy and surgery [9]. In patients with high-risk disease, outcome remains poor with chemotherapy, but is improved for patients treated with intensive induction chemotherapy, surgery, radiation, myeloablative therapy, and 13-cisretinoic acid for minimal residual disease [10]. The mainstay of most neuroblastoma treatment protocols is chemotherapy; however, limitations for pediatric patients, in particular, include appropriate dosage concerns and the toxic side effects [11]. Therefore, despite major advances in multimodality therapy, aggressive neuroblastomas continue to remain refractory to treatment resulting in relapse and poor outcome [12]. As a result, the three year survival rate in patients with advanced disease remains dismal at approximately 30% [10, 13-15].
IMMUNOTHERAPY
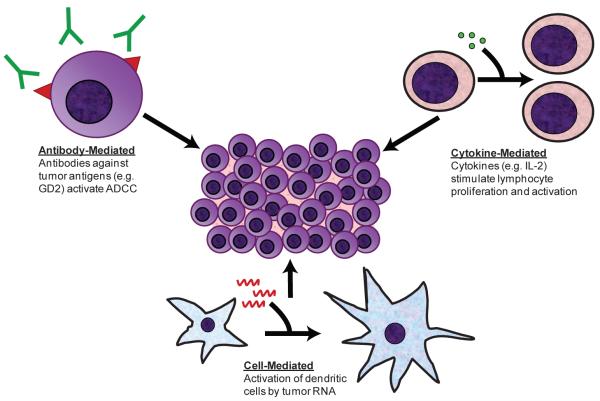
Due to the unsuccessful results of current regimens in treating aggressive disease, there is a tremendous need for innovative treatments involving other therapeutic approaches. Recent studies are focusing their efforts on therapies utilizing biologically active molecules, factors that stimulate differentiation, factors inhibiting angiogenesis, and monoclonal antibodies targeting tumor specific antigens [16]. Earlier investigations have shown that neuroblastoma elicits a strong immune response in vivo [17]. In support of these findings, many infants with neuroblastoma demonstrate spontaneous tumor regression suggesting a role for the immune system in controlling neuroblastoma cell growth [18]. Unlike chemotherapy and radiation, therapies that are toxic to both normal and abnormal tissues, immunotherapy shows promise as tumor-specific therapy while leaving other cells unharmed. Immunotherapy would serve as a safer alternative or adjunct to chemotherapy, surgery and radiation therapy, and would presumptively have fewer deleterious effects thereby making it more favorable and better tolerated by patients. In this monograph, we will highlight a few of the current advances in neuroblastoma immunotherapy (Fig.).
Figure 1.
Potential Targets for Neuroblastoma Immunotherapy
Antibody-Based Studies
The role of monoclonal antibodies in the search for novel therapies for neuroblastoma has increased significantly in recent years [16]. Monoclonal antibodies exert their effects by attaching to specific tumor surface antigens and subsequently inducing antibody-dependent cell cytotoxicity (ADCC) of the tumor cells. The ADCC response is mediated by neutrophils, natural killer (NK), and lymphokine activated killer cells [19, 20]. Many tumor surface antigens have been used as targets for antibody-mediated therapy including GD2, GD3, GM3, CD56, L1 CAM, GP58 and GP95 [21]. Of these, GD2 has been studied extensively and shows promise as a target for ADCC of neuroblastoma cells.
GD2 is a disialoganglioside that is widely expressed in most tumor cells [22] while its expression in normal tissue is limited to neurons, melanocytes, and peripheral pain fibers [23], making it suitable for targeted anti-tumor therapy. GD2 is thought to be involved in cellular attachment and metastasis; therefore in tumors like neuroblastoma, high levels of GD2 expression has a greater propensity toward metastasis and tumor progression [24]. Several clinical trials have been performed using antibodies either alone or in combination with cytokines targeting the tumor-associated antigen GD2 [13, 25-29]. In early trials, a phase I study using IgG3, a monoclonal antibody specific for GD2, was shown to be active in ADCC, complement activation, and also specifically targeted human neuroblastoma cells in patients with metastatic disease [25]. In some patients complete remission was induced, and the clinical utility for diagnosis and therapy of neuroblastoma was established [25].
Since GD2 is a glycolipid and a T cell-independent antigen it usually evokes a poor immune response in neuroblastoma patients. Riemer et al discovered a way to surpass this problem when they engineered GD2 mimotopes, which are peptide epitopes of the GD2 glycolipid, capable of stimulating the production of IgG, recruiting T-lymphocytes and inducing immune memory [30, 31]. Furthermore, studies have shown that using anti-GD2 antibodies in conjunction with cytokines has been shown to improve the immune response against neuroblastoma tumor cells. Ozkaynak et al demonstrated this after the administration of anti-GD2 antibodies with GM-CSF to children with neuroblastoma. They demonstrated that the combination of GM-CSF and anti-GD2 antibodies mediated ADCC and complement-dependent cytotoxicity [26]. ADCC and complement-dependent cytotoxicity are activated by different leukocyte populations including monocytes and neutrophils. In addition to leukocytes, NK and lymphokine-activated killer cells are also activated which provide a number of mediators to stimulate the innate immune response. Of note, patients only experienced manageable toxicities including neuropathic pain, fever, nausea/vomiting, urticaria, and hypotension. GD2 is highly expressed on nerve tissue; therefore pain after the administration anti-GD2 antibodies is a common problem that is treated with parenteral narcotic analgesics. Furthermore, of the patients that developed hypotension, only a small number required saline or albumin infusion.
Antibody-mediated therapy is promising, but in order to retain function in human models, the formation of human anti-mouse antibodies (HAMA) must be prevented since their production is likely to neutralize the therapeutic effect of anti-GD2 monoclonal antibodies through accelerated clearance and may cause side effects [16]. This was evident when a phase I clinical trial conducted using murine anti-GD2 antibodies resulted in the formation of human anti-mouse antibodies against the anti-GD2 antibodies in all patients studied [32]. Another study evaluating the murine monoclonal antibody 3F8, found that patients treated with 3F8 within 90 days of high-dose cyclophosphamide chemotherapy were less likely to develop HAMA [33]. This has broad implications in regards to the timing of monoclonal antibody administration and the previous notion that chemotherapy would have a detrimental affect on immunity. Since chemotherapy induced granulocytopenia is transient, 3F8 can mediate tumor lysis through granulocytes [34-36]. HAMA production can only be avoided with the use of fully human antibodies [16], for even chimeric (mouse-human) antibodies have been found to induce human anti-chimeric antibodies, although to a lesser degree and without an affect on chimeric antibody levels [37]. Another limitation of antibody-mediated therapy is that patients who have undergone chemotherapy usually have a suppressed immune system and may not be able to mount a sufficient response, and hence may require antibody infusion therapy [31].
Monoclonal antibodies have also been linked to toxins to facilitate selective toxin delivery. Toxin delivery is attractive because it does not require activation of immune effector cells [38]. Several monoclonal antibody-toxin constructs have been applied, but few have been used in vivo. Anti-GD2 antibodies linked to Pseudomonas exotoxin or diphtheria toxin have been found to increase cytotoxicity against neuroblastoma in vitro [39, 40]. Although toxin therapy may be a novel target in the treatment of solid tumors, further in vivo investigation will determine if clinical trials are warranted.
Specific monoclonal antibodies (usually anti-GD2) have also been conjugated with bifunctional chelated α-emitting isotypes, which allows for the formation of new, highly potent and selective α-emitting anticancer drugs [41]. In neuroblastoma, MIBG avidity occurs in 90% of patients which enables the use of radiolabeled MIBG for targeted radiotherapy in these tumors. In patients with advanced neuroblastoma refractory to treatment, 131I-MIBG has proven to be effective. Iodine-131 linked monoclonal antibodies have been compared to 131I-metaiodobenzylguanidine (MIBG) and found that iodine linked antibodies may have clinical utility in detecting metastases which do not accumulate 131I-MIBG, and the antibody may hold potential for radioimmunotherapy [42]. Radiotherapy with 131I-MIBG has been investigated in the treatment of patients with advanced neural crest tumors, including neuroblastoma [43]. In patients with intraperitoneal neuroblastoma, intraperitoneal injection of 131I-MIBG produced considerably higher tumor accumulation in comparison to intravenous injection [44]. Pretreatment with chemotherapy has also been shown to increase neuroblastoma uptake in vitro and in vivo [45, 46]. Radioimmunoconjugate therapy is a promising avenue in the identification of metastasis and treatment of advanced-stage neuroblastoma and is currently under investigation in preclinical trials.
Cytokine-Based Studies
Immunomodulators, also referred to as cytokines, stimulate effector cells. Cytokines stimulate a broad range of immune cells, including T and B lymphocytes, monocytes, macrophages, and NK cells. The tumor-specific delivery of cytokines, achieves high cytokine concentrations within the tumor microenvironment that effectively stimulates cellular immune responses. Immunotherapeutic studies using cytokines, such as interleukin IL-2 and IL-12, to modulate neuroblastoma cell growth have shown success in in vitro models for years [47-53]. IL-2 is a cytokine that stimulates growth, proliferation and activation of the T cell, B cell, and NK cell lineage [47]. IL-12 activates the TH1 inflammatory immune response and is a potent stimulator of NK cells and T-lymphocytes [54]. IL-12 also up-regulates CD25, a transmembrane protein present on the surface of activated lymphocytes which enhances T-cell proliferation in response to IL-2 [50]. It is believed that up-regulation of these cytokines can stimulate a significant anti-tumor response. Lode et al illustrated the effectiveness of IL-12 as an anti-tumor cytokine when murine neuroblastoma cells were transduced with IL-12 [47]. Later, this same group showed that IL-2 could enhance the effects of IL-12 [48]. Subsequent studies elucidated that in NK cells, the anti-tumor activity of IL-12 was actually enhanced by IL-2 via up-regulation of IL-12 receptors [49]. Recently, Neuro-2A murine neuroblastoma cells were induced to express high levels of IL-2 and IL-12, which resulted in the destruction of their tumorigenicty in syngeneic mice [51]. In addition, IL-2 and IL-12 up-regulation stimulated an anti-tumor immune response via CD4 and CD8 positive lymphocytes which lead to complete tumor eradication in a subset of mice [51]. More recently, the same group demonstrated that intratumor injections of syngeneic fibroblasts transfected with IL-2 and IL-12 had significant therapeutic effects, causing reduced growth or complete eradication of tumors in 90% of mice, along with the generation of immunologic memory [52].
Immunocytokine therapy is also under investigation as a way to synergistically increase the immune response with monoclonal antibodies. Immunocytokines are monoclonal antibodies against tumors that are genetically linked with cytokines [55]. Recently, a chimeric human/murine GD2 antibody in combination with IL-2 and GM-CSF was assessed after high-dose chemotherapy and stem-cell rescue [37]. Patients had manageable and reversible toxicities and immune activation was confirmed. Only a small number of patients demonstrated human anti-chimeric antibody production, which did not affect the serum level of antibody. Another phase I clinical trial in pediatric patients with recurrent/refractory neuroblastoma, evaluated a humanized GD2 antibody genetically linked to human IL-2 [56]. A phase II clinical trial is currently underway.
One of the main obstacles in neuroblastoma-directed immune therapy has been the inherent lack of tumor immunogenicity [57]. To overcome this obstacle many are investigating the role of cytokines in eliciting immune recognition of neuroblastoma cells. Tumor cells modified to express immunostimulatory molecules can induce specific cytotoxic T-cell responses and tumor rejection in murine models, thus leading the investigation into possible human tumor vaccination [58]. A vaccine targeted specifically against neuroblastoma cells may prove successful in reducing and/or eliminating neuroblastoma cells in vivo. In a study evaluating subcutaneous vaccine injections of lymphotactin and IL-2 secreting allogeneic neuroblastoma cells, there was complete remission in two patients and partial response in one patient [59]. This allogeneic tumor cell vaccine had minimal toxicity and induced an antitumor immune response. Recently, a phase I trial evaluated a vaccine from autologous neuroblastoma cells that were genetically modified to secrete IL-2 and lymphotactin [53]. They found that tumor vaccines containing lymphotactin with IL-2 were safe and did in fact induce an anti-tumor immune response; however, the response failed to overcome active, recurrent neuroblastoma. When given in conjunction with IL-2, Fractalkine, a chemokine known to induce leukocyte adhesion and migration induced an effective anti-tumor response in murine models showing a decrease in primary tumor growth and complete eradication of liver metastases [60]. Autologous neuroblastoma tumor cells have been modified for vaccinations to secrete interleukin IL-2 and found to be safe in patients with advanced neuroblastoma with resultant remission and disease free progression [61].
It has been demonstrated that cytokines are able to stimulate immune cells to mount an anti-tumor response. While cytokines have been shown to be safe for use in human models [53, 62], studies imply that they may not be sufficient to stand alone as a single mode of neuroblastoma therapy. They may, however, serve as an adequate adjunct to other forms of antitumor therapy, as was illustrated in studies using antibodies in conjunction with GM-CSF and anti-GD2 mimotopes with IL-12 and IL-15 [26, 31]. The continued disadvantages that an intact immune system is required [31].
Cell-Mediated Studies
Dendritic cells are antigen-presenting cells which present antigens in MHC class I and II and assist in primary and secondary immune responses. Pulsed dendritic cells have been used in clinical trials with interesting findings. In preclinical trials, Jarnjack-Jancovich et al demonstrated that peripheral blood monocyte-derived dendritic cells pulsed with tumor RNA are able to successfully activate CD4 and CD8 T lymphocytes in vitro [63]. Subsequent clinical trials showed that monocyte-derived dendritic cells pulsed with tumor RNA could induce an anti-tumor immune response and was a safe and feasible therapy in children with high-risk neuroblastoma [64]. In the aforementioned study, some dendritic cells were loaded with apoptotic neuroblastoma cells while others were pulsed with tumor RNA. They found that dendritic cells loaded with tumor RNA expressed more CD25 than dendritic cells loaded with apoptotic tumor cells. This provides evidence that more favorable results would most likely be obtained when dendritic cells are pulsed with tumor RNA rather than loaded with cellular fragments. The most likely reason for this occurrence is that mRNA can mediate specific gene transfer in dendritic cells, whereas apoptotic tumor cells will not generate the amount of antigens needed for effective and sustained immunization. Recently, dendritic cells transfected with tumor RNA were able to recognize neuroblastoma tumor-associated antigens and killed tumor cells. Taken together, these studies show that dendritic cells have promise as potential anti-tumor therapy [3].
There are several benefits of cell-mediated therapies, one of which is that it has a decreased risk of secondary tumor formation, unlike chemotherapy and radiation therapy. This type of therapy might also be successful in cases where conventional methods have failed. Also, a cell-mediated approach is likely to be less toxic and therefore more tolerable to patients than other therapies. One of the major drawbacks, as seen in the other forms of immunotherapy, is that the patient must have an intact, functioning immune system in order to mount a sufficient anti-tumor response. In studies performed by Caruso et al, monocyte-derived dendritic cells pulsed with tumor RNA were able to successfully activate CD4 and CD8 T lymphocytes [64]. Unfortunately, the patients in this study had already undergone chemotherapy and although there was an initial T-cell response to tumor cells, tumor regression was not demonstrated due to insufficient immune function. Another potential problem is that gangliosides are highly expressed in neuroblastoma cells, as previously mentioned, and findings suggest that GM2, specifically, may hinder the development of dendritic cells [65].
CONCLUSIONS
In spite of the major advances in cancer treatment, high-risk neuroblastoma continues to remain a relentless disease claiming many children’s lives. The past two decades of research discoveries have given us many new insights into the pathophysiology, cytogenetics as well as molecular biology of neuroblastoma fostering the quest for more novel ways to treat the disease. The therapies that are currently used to treat neuroblastoma are very general in treatment protocol; hence a major advantage of immunotherapy is that it is tumor-specific. In addition, the effectiveness of immunotherapy in the treatment of cancers has been illustrated in colon cancer [66], lymphoma [67, 68], breast cancer [69, 70], leukemia [71] and pediatric solid tumors including neuroblastoma [72]. Another attribute of immunotherapy is that it can be used as first-line treatment or even second-line therapy in patients who have experienced an unfavorable response to current regimens and tumor recurrence.
Antibody, cytokine, and cell-mediated studies have proven effective in animal models and some human clinical trials [33, 37, 56, 63, 73]. Immunotherapy is based upon the concept of modulating the immune response, therein rests the necessity of a functioning immune system. The main underlying drawback of all modes of immunotherapy is that in order to achieve maximum efficacy, they all require an intact immune system. Monoclonal antibody-toxin delivery and radioimmunotherapy are two therapies that do not rely as much on immune function due to their mechanism of action which focuses on tumor ablation by toxins or radioisotopes. These therapies are currently under investigation, but the majority of immunotherapies require a considerable immune response to be effective. This is especially important for patients who have previously undergone chemotherapy, as would be the case in the majority of patients. While there is still much to be discovered about immunotherapeutic targets for neuroblastoma, as a group they demonstrate great promise as effective treatment for refractory disease and hence, may make a significant impact in patient survival rates.
ACKNOWLEDGEMENTS
The authors thank Karen Martin for manuscript preparation. This work was supported by the grants R01 DK61470 and F31 DK079422 from the National Institutes of Health.
REFERENCES
- [1].Society AC. Cancer Facts & Figures 2008. American Cancer Society. 2008 [Google Scholar]
- [2].Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75:2186–95. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [3].Morandi F, Chiesa S, Bocca P, et al. Tumor mRNA-transfected dendritic cells stimulate the generation of CTL that recognize neuroblastoma-associated antigens and kill tumor cells: immunotherapeutic implications. Neoplasia. 2006;8:833–42. doi: 10.1593/neo.06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brodeur GM. Commentary on Kaneko et al.: Intensified chemotherapy increases the survival rates in patients with stage 4 neuroblastoma with MYCN amplification. J Pediatr Hematol Oncol. 2002;24:608–9. doi: 10.1097/00043426-200211000-00002. [DOI] [PubMed] [Google Scholar]
- [5].Schwab M, Westermann F, Hero B, Berthold F. Neuroblastoma: biology and molecular and chromosomal pathology. Lancet Oncol. 2003;4:472–80. doi: 10.1016/s1470-2045(03)01166-5. [DOI] [PubMed] [Google Scholar]
- [6].Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- [7].Cecchetto G, Mosseri V, De Bernardi B, et al. Surgical risk factors in primary surgery for localized neuroblastoma: the LNESG1 study of the European International Society of Pediatric Oncology Neuroblastoma Group. J Clin Oncol. 2005;23:8483–9. doi: 10.1200/JCO.2005.02.4661. [DOI] [PubMed] [Google Scholar]
- [8].Kushner BH, Cheung NK. Neuroblastoma--from genetic profiles to clinical challenge. N Engl J Med. 2005;353:2215–7. doi: 10.1056/NEJMp058251. [DOI] [PubMed] [Google Scholar]
- [9].London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol. 2005;23:6459–65. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- [10].Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- [11].McCune JS, Salinger DH, Vicini P, et al. Population pharmacokinetics of cyclophosphamide and metabolites in children with neuroblastoma: a report from the Children’s Oncology Group. J Clin Pharmacol. 2009;49:88–102. doi: 10.1177/0091270008325928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Modak S, Cheung NK. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Invest. 2007;25:67–77. doi: 10.1080/07357900601130763. [DOI] [PubMed] [Google Scholar]
- [13].Cheung NK, Kushner BH, Kramer K. Monoclonal antibody-based therapy of neuroblastoma. Hematol Oncol Clin North Am. 2001;15:853–66. doi: 10.1016/s0889-8588(05)70255-0. [DOI] [PubMed] [Google Scholar]
- [14].Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–58. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- [15].Franks LM, Bollen A, Seeger RC, Stram DO, Matthay KK. Neuroblastoma in adults and adolescents: an indolent course with poor survival. Cancer. 1997;79:2028–35. doi: 10.1002/(sici)1097-0142(19970515)79:10<2028::aid-cncr26>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [16].Bestagno M, Occhino M, Corrias MV, Burrone O, Pistoia V. Recombinant antibodies in the immunotherapy of neuroblastoma: perspectives of new developments. Cancer Lett. 2003;197:193–8. doi: 10.1016/s0304-3835(03)00109-5. [DOI] [PubMed] [Google Scholar]
- [17].Hellstrom IE, Hellstrom KE, Pierce GE, Bill AH. Demonstration of cell-bound and humoral immunity against neuroblastoma cells. Proc Natl Acad Sci U S A. 1968;60:1231–8. doi: 10.1073/pnas.60.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hellstrom I, Hellstrom KE, Pierce GE, Yang JP. Cellular and humoral immunity to different types of human neoplasms. Nature. 1968;220:1352–4. doi: 10.1038/2201352a0. [DOI] [PubMed] [Google Scholar]
- [19].Barker E, Mueller BM, Handgretinger R, et al. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51:144–9. [PubMed] [Google Scholar]
- [20].Sabzevari H, Gillies SD, Mueller BM, Pancook JD, Reisfeld RA. A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci U S A. 1994;91:9626–30. doi: 10.1073/pnas.91.20.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheung N-KV, Cohn SL. Neuroblastoma. Springer; Berlin ; New York: 2005. [Google Scholar]
- [22].Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- [23].Svennerholm L, Bostrom K, Fredman P, et al. Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim Biophys Acta. 1994;1214:115–23. doi: 10.1016/0005-2760(94)90034-5. [DOI] [PubMed] [Google Scholar]
- [24].Cheresh DA, Harper JR, Schulz G, Reisfeld RA. Localization of the gangliosides GD2 and GD3 in adhesion plaques and on the surface of human melanoma cells. Proc Natl Acad Sci U S A. 1984;81:5767–71. doi: 10.1073/pnas.81.18.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheung NK, Lazarus H, Miraldi FD, et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–40. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- [26].Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children’s Cancer Group Study. J Clin Oncol. 2000;18:4077–85. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- [27].Cheresh DA, Rosenberg J, Mujoo K, Hirschowitz L, Reisfeld RA. Biosynthesis and expression of the disialoganglioside GD2, a relevant target antigen on small cell lung carcinoma for monoclonal antibody-mediated cytolysis. Cancer Res. 1986;46:5112–8. [PubMed] [Google Scholar]
- [28].Saito M, Yu RK, Cheung NK. Ganglioside GD2 specificity of monoclonal antibodies to human neuroblastoma cell. Biochem Biophys Res Commun. 1985;127:1–7. doi: 10.1016/s0006-291x(85)80117-0. [DOI] [PubMed] [Google Scholar]
- [29].Mueller BM, Romerdahl CA, Gillies SD, Reisfeld RA. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol. 1990;144:1382–6. [PubMed] [Google Scholar]
- [30].Riemer AB, Forster-Waldl E, Bramswig KH, et al. Induction of IgG antibodies against the GD2 carbohydrate tumor antigen by vaccination with peptide mimotopes. Eur J Immunol. 2006;36:1267–74. doi: 10.1002/eji.200535279. [DOI] [PubMed] [Google Scholar]
- [31].Kowalczyk A, Wierzbicki A, Gil M, et al. Induction of protective immune responses against NXS2 neuroblastoma challenge in mice by immunotherapy with GD2 mimotope vaccine and IL-15 and IL-21 gene delivery. Cancer Immunol Immunother. 2007;56:1443–58. doi: 10.1007/s00262-007-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Handgretinger R, Baader P, Dopfer R, et al. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14.G2a. Cancer Immunol Immunother. 1992;35:199–204. doi: 10.1007/BF01756188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kushner BH, Cheung IY, Kramer K, Modak S, Cheung NK. High-dose cyclophosphamide inhibition of humoral immune response to murine monoclonal antibody 3F8 in neuroblastoma patients: broad implications for immunotherapy. Pediatr Blood Cancer. 2007;48:430–4. doi: 10.1002/pbc.20765. [DOI] [PubMed] [Google Scholar]
- [34].Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73:1936–41. [PubMed] [Google Scholar]
- [35].Munn DH, Cheung NK. Interleukin-2 enhancement of monoclonal antibody-mediated cellular cytotoxicity against human melanoma. Cancer Res. 1987;47:6600–5. [PubMed] [Google Scholar]
- [36].Saarinen UM, Coccia PF, Gerson SL, Pelley R, Cheung NK. Eradication of neuroblastoma cells in vitro by monoclonal antibody and human complement: method for purging autologous bone marrow. Cancer Res. 1985;45:5969–75. [PubMed] [Google Scholar]
- [37].Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children’s Oncology Group. J Clin Oncol. 2009;27:85–91. doi: 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Manzke O, Russello O, Leenen C, et al. Immunotherapeutic strategies in neuroblastoma: antitumoral activity of deglycosylated Ricin A conjugated anti-GD2 antibodies and anti-CD3xanti-GD2 bispecific antibodies. Med Pediatr Oncol. 2001;36:185–9. doi: 10.1002/1096-911X(20010101)36:1<185::AID-MPO1044>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- [39].Tur MK, Sasse S, Stocker M, et al. An anti-GD2 single chain Fv selected by phage display and fused to Pseudomonas exotoxin A develops specific cytotoxic activity against neuroblastoma derived cell lines. Int J Mol Med. 2001;8:579–84. doi: 10.3892/ijmm.8.5.579. [DOI] [PubMed] [Google Scholar]
- [40].Thomas PB, Delatte SJ, Sutphin A, Frankel AE, Tagge EP. Effective targeted cytotoxicity of neuroblastoma cells. J Pediatr Surg. 2002;37:539–44. doi: 10.1053/jpsu.2002.30856. [DOI] [PubMed] [Google Scholar]
- [41].Miederer M, McDevitt MR, Borchardt P, et al. Treatment of neuroblastoma meningeal carcinomatosis with intrathecal application of alpha-emitting atomic nanogenerators targeting disialo-ganglioside GD2. Clin Cancer Res. 2004;10:6985–92. doi: 10.1158/1078-0432.CCR-04-0859. [DOI] [PubMed] [Google Scholar]
- [42].Hoefnagel CA, Rutgers M, Buitenhuis CK, et al. A comparison of targeting of neuroblastoma with mIBG and anti L1-CAM antibody mAb chCE7: therapeutic efficacy in a neuroblastoma xenograft model and imaging of neuroblastoma patients. Eur J Nucl Med. 2001;28:359–68. [PubMed] [Google Scholar]
- [43].Hoefnagel CA, Taal BG, Sivro F, Boot H, Olmos RA Valdes. Enhancement of 131I-MIBG uptake in carcinoid tumours by administration of unlabelled MIBG. Nucl Med Commun. 2000;21:755–61. doi: 10.1097/00006231-200008000-00009. [DOI] [PubMed] [Google Scholar]
- [44].Kinuya S, Li XF, Yokoyama K, et al. Local delivery of (131)I-MIBG to treat peritoneal neuroblastoma. Eur J Nucl Med Mol Imaging. 2003;30:1246–50. doi: 10.1007/s00259-003-1214-1. [DOI] [PubMed] [Google Scholar]
- [45].Armour A, Cunningham SH, Gaze MN, Wheldon TE, Mairs RJ. The effect of cisplatin pretreatment on the accumulation of MIBG by neuroblastoma cells in vitro. Br J Cancer. 1997;75:470–6. doi: 10.1038/bjc.1997.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Meco D, Lasorella A, Riccardi A, et al. Influence of cisplatin and doxorubicin on 125I-meta-iodobenzylguanidine uptake in human neuroblastoma cell lines. Eur J Cancer. 1999;35:1227–34. doi: 10.1016/s0959-8049(99)00078-7. [DOI] [PubMed] [Google Scholar]
- [47].Lode HN, Xiang R, Varki NM, et al. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89:1586–94. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- [48].Lode HN, Xiang R, Dreier T, et al. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–15. [PubMed] [Google Scholar]
- [49].Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000;95:3183–90. [PubMed] [Google Scholar]
- [50].Nguyen T, Wang R, Russell JH. IL-12 enhances IL-2 function by inducing CD25 expression through a p38 mitogen-activated protein kinase pathway. Eur J Immunol. 2000;30:1445–52. doi: 10.1002/(SICI)1521-4141(200005)30:5<1445::AID-IMMU1445>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [51].Siapati KE, Barker S, Kinnon C, et al. Improved antitumour immunity in murine neuroblastoma using a combination of IL-2 and IL-12. Br J Cancer. 2003;88:1641–8. doi: 10.1038/sj.bjc.6600928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Barker SE, Grosse SM, Siapati EK, et al. Immunotherapy for neuroblastoma using syngeneic fibroblasts transfected with IL-2 and IL-12. Br J Cancer. 2007;97:210–7. doi: 10.1038/sj.bjc.6603857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Russell HV, Strother D, Mei Z, et al. Phase I trial of vaccination with autologous neuroblastoma tumor cells genetically modified to secrete IL-2 and lymphotactin. J Immunother. 2007;30:227–33. doi: 10.1097/01.cji.0000211335.14385.57. (1997) [DOI] [PubMed] [Google Scholar]
- [54].Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–96. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- [55].Lode HN, Xiang R, Becker JC, Gillies SD, Reisfeld RA. Immunocytokines: a promising approach to cancer immunotherapy. Pharmacol Ther. 1998;80:277–92. doi: 10.1016/s0163-7258(98)00033-3. [DOI] [PubMed] [Google Scholar]
- [56].Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res. 2006;12:1750–9. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Prigione I, Corrias MV, Airoldi I, et al. Immunogenicity of human neuroblastoma. Ann N Y Acad Sci. 2004;1028:69–80. doi: 10.1196/annals.1322.008. [DOI] [PubMed] [Google Scholar]
- [58].Jaffee EM, Lazenby A, Meurer J, et al. Use of murine models of cytokine-secreting tumor vaccines to study feasibility and toxicity issues critical to designing clinical trials. J Immunother Emphasis Tumor Immunol. 1995;18:1–9. doi: 10.1097/00002371-199507000-00001. [DOI] [PubMed] [Google Scholar]
- [59].Rousseau RF, Haight AE, Hirschmann-Jax C, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101:1718–26. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- [60].Zeng Y, Huebener N, Fest S, et al. Fractalkine (CX3CL1)- and interleukin-2-enriched neuroblastoma microenvironment induces eradication of metastases mediated by T cells and natural killer cells. Cancer Res. 2007;67:2331–8. doi: 10.1158/0008-5472.CAN-06-3041. [DOI] [PubMed] [Google Scholar]
- [61].Russell HV, Strother D, Mei Z, et al. A phase 1/2 study of autologous neuroblastoma tumor cells genetically modified to secrete IL-2 in patients with high-risk neuroblastoma. J Immunother. 2008;31:812–9. doi: 10.1097/CJI.0b013e3181869893. [DOI] [PubMed] [Google Scholar]
- [62].Atzpodien J, Reitz M. GM-CSF plus antigenic peptide vaccination in locally advanced melanoma patients. Cancer Biother Radiopharm. 2007;22:551–5. doi: 10.1089/cbr.2007.376. [DOI] [PubMed] [Google Scholar]
- [63].Jarnjak-Jankovic S, Pettersen RD, Saeboe-Larssen S, et al. Preclinical evaluation of autologous dendritic cells transfected with mRNA or loaded with apoptotic cells for immunotherapy of high-risk neuroblastoma. Cancer Gene Ther. 2005;12:699–707. doi: 10.1038/sj.cgt.7700820. [DOI] [PubMed] [Google Scholar]
- [64].Caruso DA, Orme LM, Amor GM, et al. Results of a Phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with Stage 4 neuroblastoma. Cancer. 2005;103:1280–91. doi: 10.1002/cncr.20911. [DOI] [PubMed] [Google Scholar]
- [65].Wolfl M, Batten WY, Posovszky C, Bernhard H, Berthold F. Gangliosides inhibit the development from monocytes to dendritic cells. Clin Exp Immunol. 2002;130:441–8. doi: 10.1046/j.1365-2249.2002.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Riethmuller G, Holz E, Schlimok G, et al. Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol. 1998;16:1788–94. doi: 10.1200/JCO.1998.16.5.1788. [DOI] [PubMed] [Google Scholar]
- [67].Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15:3266–74. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- [68].McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- [69].Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- [70].Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- [71].Jurcic JG, DeBlasio T, Dumont L, Yao TJ, Scheinberg DA. Molecular remission induction with retinoic acid and anti-CD33 monoclonal antibody HuM195 in acute promyelocytic leukemia. Clin Cancer Res. 2000;6:372–80. [PubMed] [Google Scholar]
- [72].Geiger JD, Hutchinson RJ, Hohenkirk LF, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–9. [PubMed] [Google Scholar]
- [73].Wierzbicki A, Gil M, Ciesielski M, et al. Immunization with a mimotope of GD2 ganglioside induces CD8+ T cells that recognize cell adhesion molecules on tumor cells. J Immunol. 2008;181:6644–53. doi: 10.4049/jimmunol.181.9.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]