1. STRUCTURE
Besides being a precursor for neurotransmitters, hormones and melanin, tyrosine is a non-essential amino acid that is utilized by the eukaryotic cell to synthesize proteins. Once incorporated into proteins, tyrosine can be post-translationally modified by virtue of its side chain hydroxyl group by nitration, phosphorylation and sulfation. While nitrotyrosine is synthesized through a non-enzymatic reaction, tyrosines are phosphorylated and sulfated through the action of kinases and tyrosylprotein sulfotransferases (TPSTs), respectively. While phosphotyrosine is important in signal transduction, sulfotyrosine is involved in protein-protein interactions (Moore, 2003). Sulfotyrosines are enzymatically generated by two independent TPSTs, TPST-1 and TPST-2 (EC 2.8.2.20), through reactions involving the addition of a sulfate group from 3'-phosphoadenosine 5'-phosphosulfate (PAPS) to protein tyrosyl moieties (Fig. 1A). TPST-1 and TPST-2, whose sequences are 67% identical, are Type II transmembrane proteins that reside in the trans-Golgi compartment. Sulfotyrosines are present in all multicellular eukaryotes, but unicellular eukaryotes such as yeast and prokaryotes are not capable of tyrosine sulfation. While mammals possess two TPSTs, Drosophila and Arabidopsis harbor only one TPST. Unlike tyrosine phosphorylation which is mainly intracellular, sulfotyrosines are observed only on secreted and transmembrane proteins (Moore, 2003). So far, over 62 proteins have been shown to contain sulfotyrosines (Moore, 2003). However, Uniprot predicts that 423 proteins contain sulfotyrosines based on homologies to known sulfotyrosine containing proteins (www.uniprot.org).
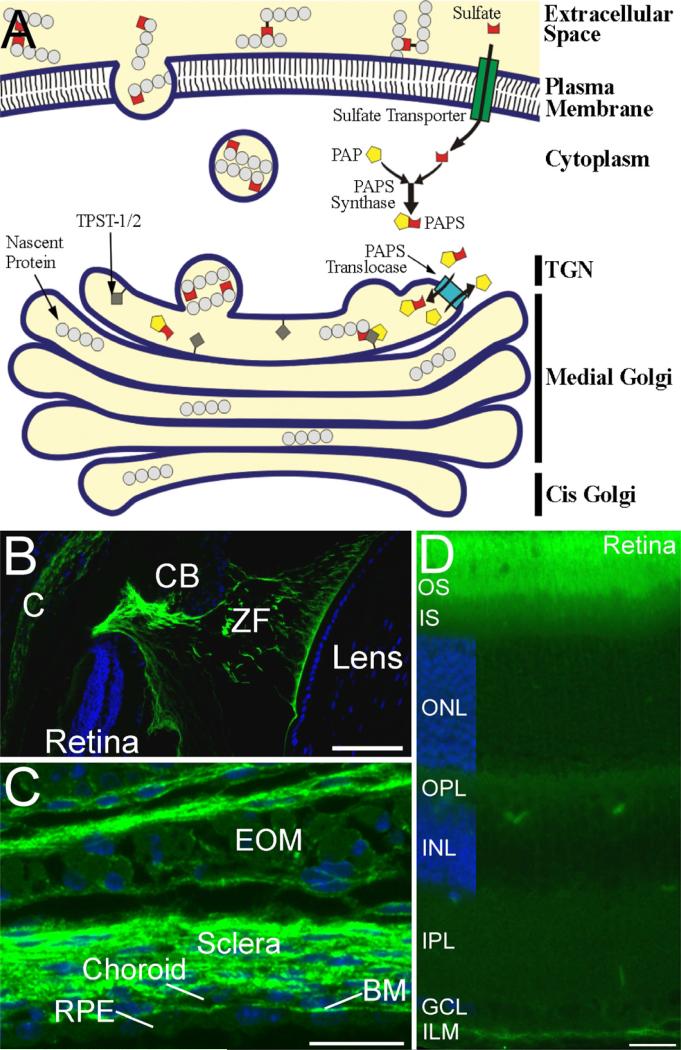
Figure 1.
A. Figure 1. Synthetic pathway for generation of sulfotyrosine and localization of sulfotyrosine proteins in mouse eye. A. The enzymatic generation of sulfotyrosine. Sulfate donor 3'-phosphoadenosine 5'¬phosphosulfate (PAPS) is translocated into the trans-Golgi network (TGN) by the enzyme PAPS translocase from the cytoplasm where it is synthesized. Once inside the TGN, TPST-1/2 located in the TGN membrane incorporates the sulfate residue from PAPS into tyrosine residues of secreted and transmembrane proteins. The sulfotyrosine-containing proteins are then incorporated into vesicles to be transported to their final destination, which may either be the plasma membrane or the extracellular space. B-D. Localization of tyrosine sulfated proteins in ocular tissues. Sections were stained with anti-sulfotyrosine antibody (green) and the cell nuclei were counterstained with DAPI (blue). B. Tyrosine sulfated proteins are present in the cornea (C), and in the zonule fibers (ZF) and their attachments to the ciliary body (CB) and the lens. C. Tyrosine sulfated proteins are abundant in the sclera, choroid, Bruch's membrane (BM), and the connective tissue associated with the extraocular muscles (EOM). The retinal pigmented epithelium (RPE) and skeletal muscle cells of the EOM also show labeling for tyrosine sulfated proteins, but at much lower levels than the surrounding connective tissues. D. Tyrosine sulfated proteins are present in all layers of the retina, but are most abundant in the interphotoreceptor matrix surrounding the photoreceptor outer segments (OS) and at the inner limiting membrane (ILM). IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars = 100 μm for B; 20 μm for C and D.
Sulfation of tyrosine is irreversible. No enzyme has been identified that removes sulfate from tyrosine residues. The sulfate group on a sulfotyrosine is very stable at neutral and basic pH, but labile at acidic pH.
However, sulfotyrosines are labile to electrospray and matrix-associated laser desorption/ionization processes in positive ion MS/MS, making it difficult to detect sulfotyrosines using this method. To overcome this obstacle, a method has been developed in which unsulfated tyrosines are blocked by acetylation using sulfosuccinimidyl acetate. Sulfotyrosines can then be detected by MS/MS in positive ion mode as unmodified tyrosines.
2. FUNCTION IN OCULAR TISSUES
Immunoblot analyses of bovine ocular tissues using the anti-sulfotyrosine antibody, PSG2, have revealed that proteins containing sulfotyrosine residues are present in the lens, cornea, iris, sclera, RPE, retina, vitreous, and aqueous humor with highest levels in the vitreous humor and lowest levels in the lens (Kanan et al; 2012). Immunohistochemical analyses of the mouse eye show that sulfotyrosine proteins are expressed in all tissues of the eye including cornea, sclera, choroid, Bruch's membrane, RPE, interphotoreceptor matrix surrounding the photoreceptor and inner limiting membrane of the retina (Fig. 1B-D).
Clues to the function of sulfotyrosine-containing proteins in the eye have emerged from studies of Tpst1&2 double knockout mice (DKO). Most (~94%) of newborn DKO mice die by postnatal day 5 and of those that survive, none have lived beyond postnatal day 31. Histological and MRI studies of newborn DKO pups delivered by C-section show that these mice exhibit hypothyroidism and often die as a result of cardiopulmonary insufficiency. At postnatal day 21, Tpst1&2 DKO mice exhibit visual defects characterized by reduced scotopic ERG responses that are ~25% of normal and severely reduced photopic b-waves that are approximately 15% of normal. In addition to functional defects, the rod photoreceptors in the retinas of these animals exhibit structural defects. The ultrastructure of the outer segments of the rod cells is abnormal with large inter-and intradiscal spaces and evaginations of the rod discs into the surrounding extracellular space. In contrast, the ultrastructure of the cone outer segments appears normal suggesting that the reduced photopic responses recorded from these animals may result from abnormal synaptic transmission. This possibility is supported by the observations that cone, as well as rod, photoreceptor terminal ultrastructure is abnormal including malformed synaptic ribbons and free-floating synaptic ribbons, and diminished neuronal plexes in the synaptic layers (Sherry et al; 2010). The retinal phenotypes exhibited by Tpst1&2 DKO mice indicate that sulfotyrosine-containing proteins play a significant role in retinal homeostasis and processing, although specific functions for these proteins remain to be defined.
3.DISEASE INVOLVEMENT
Sulfotyrosines have been identified in proteins that have been implicated in human diseases including age-related macular degeneration (AMD) and diabetes. In AMD, excessive activation of complement proteins is accompanied by the appearance of extracellular deposits called drusen that contain an abundance of the sulfotyrosine-containing protein vitronectin. One of the many functions of vitronectin is to modulate the activation of the complement system by associating with the complement complex C5b-7, rendering it unable to insert into membranes and cause cellular damage. Therefore, elevated vitronectin levels associated with AMD may result from an attempt to protect the retina from damage caused by excessive complement activation.
In diabetic retinopathy, the earliest hallmarks of the disease are thickening of the basement membrane (BM) and vascular leakage. It is well known that hyperglycemic conditions up-regulate expression of the sulfotyrosine-containing basement membrane protein fibronectin that contributes to BM thickening. It is not yet clear how thickening of the BM relates to vascular leakage, however, down-regulation of fibronectin expression has been shown to inhibit BM thickening in retinal capillaries and reduce vascular leakage.
At present, the only reported disease-causing sulfotyrosine mutation is a naturally occurring mutation in factor VIII. This mutation changes the sulfotyrosine at residue 1680 to phenylalanine and causes inefficient binding of factor VIII to von Willebrand factor that induces mild to moderate hemophilia (Higuch et al; 1990).
4.FUTURE STUDIES
Sulfotyrosines mediate interactions between proteins and other molecules and are therefore positioned to play significant roles in ocular biology. Current studies are aimed at identifying sulfotyrosine-containing proteins and their binding partners in the eye. Once identified, it will be possible to carry out functional studies of these protein complexes using mutation, knock-in, and knock-out techniques the results of which will help improve our understanding of the roles that these proteins play in ocular function.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Higuchi M, Wong C, Kochhan L, Olek K, Aronis S, Kasper CK, Kazazian HH, Jr., Antonarakis SE. Characterization of mutations in the factor VIII gene by direct sequencing of amplified genomic DNA. Genomics. 1990;6:65–71. doi: 10.1016/0888-7543(90)90448-4. [DOI] [PubMed] [Google Scholar]
- Kanan Y, Hamilton RA, Moore KL, Al-Ubaidi MR. Protein tyrosine-o-sulfation in bovine ocular tissues. Adv.Exp.Med.Biol. 2012;723:835–841. doi: 10.1007/978-1-4614-0631-0_107. [DOI] [PubMed] [Google Scholar]
- Moore KL. The biology and enzymology of protein tyrosine O-sulfation. J.Biol.Chem. 2003;278:24243–24246. doi: 10.1074/jbc.R300008200. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Murray AR, Kanan Y, Arbogast KL, Hamilton RA, Fliesler SJ, Burns ME, Moore KL, Al-Ubaidi MR. Lack of protein-tyrosine sulfation disrupts photoreceptor outer segment morphogenesis, retinal function and retinal anatomy. Eur.J.Neurosci. 2010;32:1461–1472. doi: 10.1111/j.1460-9568.2010.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]