Abstract
Although our understanding of the actions of cocaine in the brain has improved, an effective drug treatment for cocaine addiction has yet to be found. Methylphenidate binds the dopamine transporter and increases extracellular dopamine levels in mammalian central nervous systems similar to cocaine, but it is thought to elicit fewer addictive and reinforcing effects owing to slower pharmacokinetics for different routes of administration between the drugs. This study utilizes the fruit fly model system to quantify the effects of oral methylphenidate on dopamine uptake during direct cocaine exposure to the fly CNS. The effect of methylphenidate on the dopamine transporter has been explored by measuring the uptake of exogenously applied dopamine. The data suggest that oral consumption of methylphenidate inhibits the Drosophila dopamine transporter and the inhibition is concentration dependent. The peak height increased to 150% of control when cocaine was used to block the dopamine transporter for untreated flies but only to 110% for methylphenidate-treated flies. Thus, the dopamine transporter is mostly inhibited for the methylphenidate-fed flies before the addition of cocaine. The same is true for the rate of the clearance of dopamine measured by amperometry. For untreated flies the rate of clearance changes 40% when the dopamine transporter is inhibited with cocaine, and for treated flies the rate changes only 10%. The results were correlated to the in vivo concentration of methylphenidate determined by CE-MS. Our data suggest that oral consumption of methylphenidate inhibits the Drosophila dopamine transporter for cocaine uptake, and the inhibition is concentration dependent.
Keywords: In vivo, methylphenidate, voltammetry, mass spectrometry, dopamine, Drosophila
The molecular and cellular actions of cocaine in the brain are complex and affect the neurotransmission of several chemicals including dopamine, serotonin, and norepinephrine through alteration of their transporter function.1−4 Voltage-gated sodium channels are also blocked by cocaine,5 further supporting the idea that cocaine works as a nonselective drug in the central nervous system (CNS) and hindering the development of a suitable drug treatment to combat cocaine addiction.6,7 Methylphenidate (Ritalin), a commonly prescribed medication for the treatment of attention deficit hyperactivity disorder (ADHD),8 like cocaine, inhibits the human dopamine transporter with a binding affinity similar to that of cocaine and increases the extracellular dopamine concentration in the brain.1,9,10
Methylphenidate abuse is more limited than that of cocaine, which is considered addictive.11−13 The difference in the abuse potential of these two psychostimulants has been attributed to their pharmacokinetic properties for the different routes of administration between the drugs. The time for orally administered methylphenidate to reach maximal blockage of the human dopamine transporter is approximately eight times longer than that for intravenous administration of cocaine.14,15 The euphoric feeling experienced during cocaine use is associated with this rapid blockage of the dopamine transporter and the subsequent increase in extracellular dopamine.16 Indeed, the shorter the time interval between intake of a drug and its perceived effects lead to greater reinforcing properties and therefore addictive potential of that drug.17,18 The clearance rate of a drug is also a significant factor in abuse potential. The half-life of methylphenidate in the human brain, based on the duration of dopamine transporter blockage, is longer than that of cocaine (75–90 min vs 15–25 min, respectively).14 Since the clearance of the stimulant from the brain is necessary before it is possible for an individual to fully experience the reinforcing effects of the drug again, frequent repeated administration and overall abuse of methylphenidate is limited in comparison to cocaine.
Over the past decade, methylphenidate has been investigated as a potential agonist, or replacement medication, for intravenous cocaine addiction treatment,6,7 as a similar approach has been successful where methadone is used for treating opiate addiction.19 Several studies have investigated the effects of oral methylphenidate on cocaine users, and mixed results have been found.20−22 A better understanding of the chemical mechanisms in the CNS during coadministration of methylphenidate and cocaine might help to shed light on this potential treatment for cocaine addiction.
Animal models including rats, mice, baboons, and monkeys have been used to investigate neurochemical changes in the CNS associated with drug addiction.9,23−27 Techniques that use invertebrates, such as Drosophila melanogaster (fruit fly) and Apis mellifera (honey bee), for research involving drugs of abuse have been established as well.28−32 Recent methods utilizing fast-scan cyclic voltammetry (FSCV) coupled with carbon-fiber microelectrodes to quantify dopamine, an electroactive neurotransmitter, in the CNS of Drosophila have been developed.33−36 Here, we apply FSCV to study the efficacy of oral methylphenidate treatment on dopamine uptake in Drosophila and how it affects the actions of cocaine on the dopamine transporter in vivo. We also use capillary electrophoresis coupled to mass spectrometric analysis to determine the concentration of methylphenidate in the fly brain after feeding and use this in vivo concentration for our models.
Results and Discussion
Dopamine Clearance in the Drosophila CNS Following Cocaine Bath Treatment
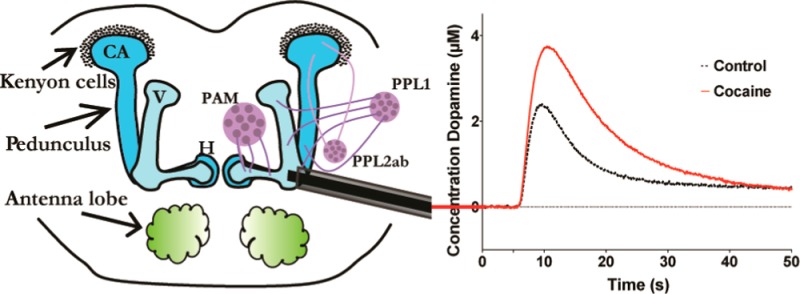
We have developed a procedure for in vivo electrochemical detection in adult Drosophila,33 and demonstrated its use to study the effects of cocaine and methylphenidate on the clearance of the redox-active neurotransmitter dopamine.35 The Drosophila brain contains dopaminergic neurons clustered together in several distinct locations with the largest neuronal cluster, located in the protocerebral anterior medial (PAM) region37 projecting into the mushroom body. By inserting a cylindrical carbon-fiber microelectrode into the mushroom body of a Drosophila brain, changes in the uptake of exogenously applied dopamine can be quantified. In this report, this method is used to monitor the effects of cocaine and methylphenidate on dopamine clearance in the Drosophila CNS.
Following fly microsurgery (see Methods), a carbon-fiber working electrode was placed at a 45° angle ∼60 μm deep inside the mushroom body, which was visualized with green fluorescent protein tagged tyrosine hydroxylase. Dopamine was exogenously applied just above the fly brain tissue with a micropipet injector, and background-subtracted FSCV was used to measure the current response in the extracellular fluid of the CNS over time. The micropipet injector was placed just above the brain, 50–60 μm from the electrode tip, and dopamine was injected with a time to initial signal of 0.5–1.2 s. Use of the peak dopamine concentration, [DA]max, to monitor changes in the clearance of extracellular dopamine in the CNS has been established,35,38 and this parameter is utilized here.
Initially, the in vivo baseline current response was recorded for 3 min after a 1.0 mM dopamine solution was exogenously applied to the PAM area for 1.0 s (∼150 pmol dopamine applied). The concentration of 1 mM dopamine equals a few μM after diffusion to the electrode tip area33 as can be seen in Figure 1. Following two stable baseline measurements after application of dopamine, the fly brain was bathed in 1.0 mM cocaine, which has been shown to inhibit dopamine uptake by the Drosophila dopamine transporter.35 A bath of 1.0 mM cocaine corresponds to a concentration of 12 μM in the brain,35 well above-reported IC50 concentrations for cocaine, which has been reported between 6.0 and 2.7 μM.39,40 After 5 min of cocaine exposure, dopamine was applied again while the current response was recorded. Dopamine injections were repeated every 5 min throughout the 20 min bath cocaine application.
Figure 1.
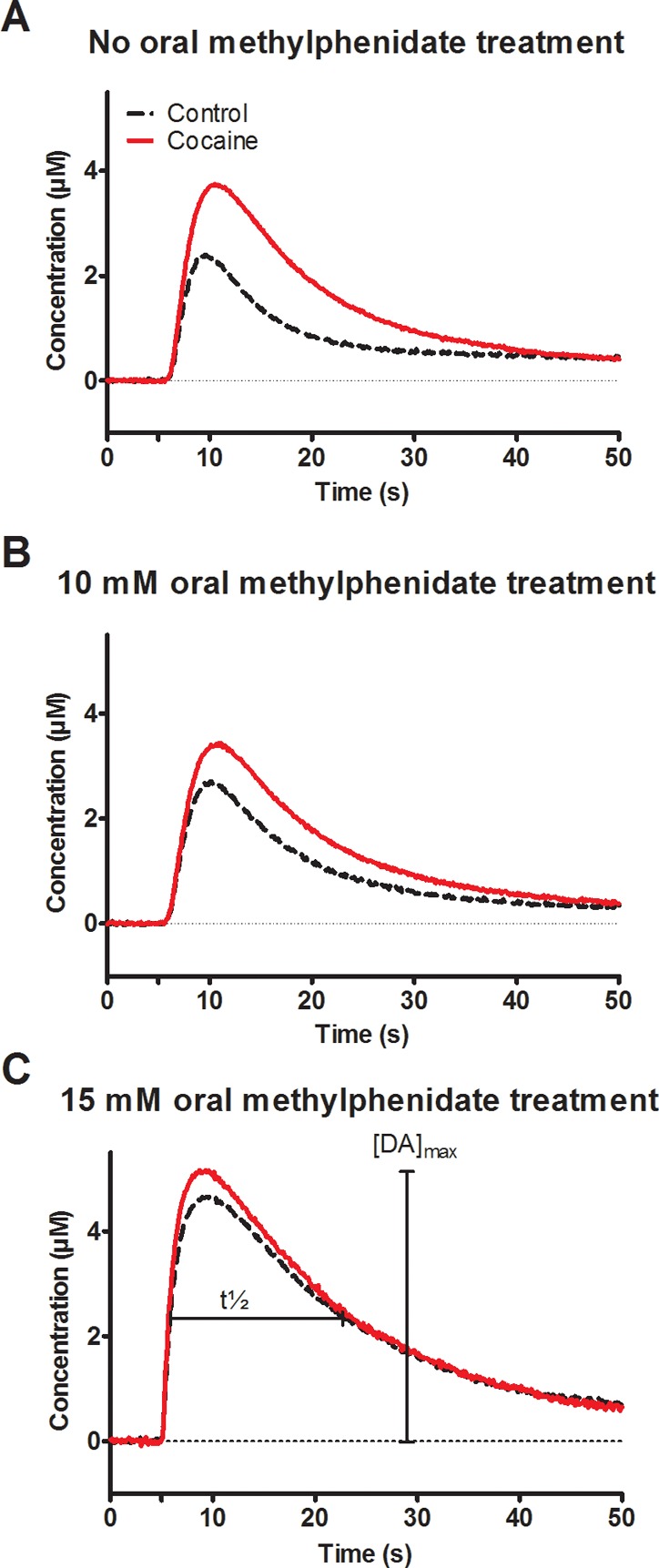
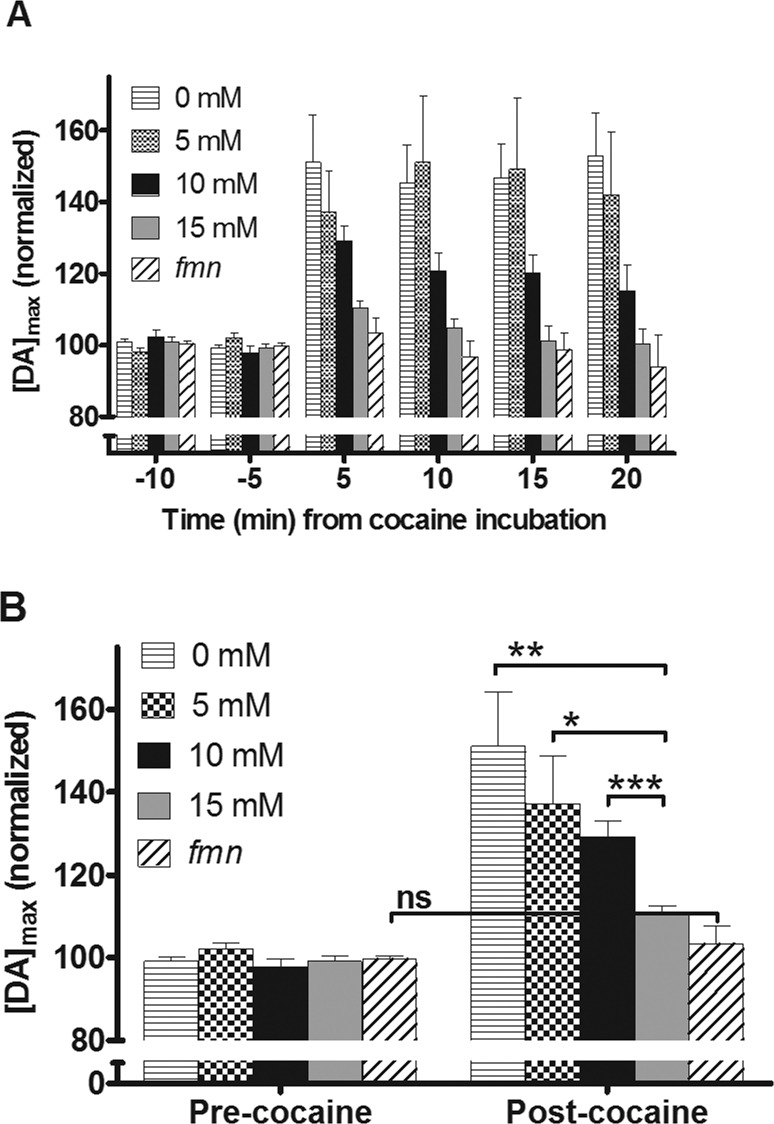
Effect of oral methylphenidate treatment on cocaine inhibition of the dopamine transporter in the adult Drosophila brain. (A) Representative concentration traces (taken from the maximum anodic peak potential) of exogenously applied 1.0 mM dopamine in a TH-type fly that did not receive oral methylphenidate treatment before the experiment. The two traces show dopamine before (dotted line) compared to after (smooth line) 1.0 mM cocaine bath incubation. A significant increase in dopamine peak max concentration was observed following cocaine application as well as a longer clearance time. Dopamine concentration was determined from conversion of the measured current using in vitro electrode calibration. The tic at 5 s corresponds to a 1.0 s dopamine application. (B) Representative concentration traces of exogenously applied 1.0 mM dopamine in a TH-type fly that received 10 mM oral methylphenidate treatment before exogenously dopamine (precocaine, dotted line) and after 1.0 mM cocaine bath application (smooth line). The peak max increased but not to the same extent as without methylphenidate treatment. (C) Representative concentration traces of exogenously applied 1.0 mM dopamine in a TH-type fly that received the highest concentration, 15 mM, oral methylphenidate treatment before exogenously dopamine (precocaine, dotted line) and after 1.0 mM cocaine bath application (smooth line). No significant change in dopamine concentration or clearance rate between before and after cocaine was observed following cocaine application. [DA]max, the amplitude peak maximum of dopamine signal, and t1/2, the full width of time at half-maximum, are two parameters used to compare dopamine clearance.
A 1.0 mM bath application of cocaine inhibits the dopamine transporter in the fly, and the decrease in clearance of dopamine by the transporter can be quantified by examining [DA]max.35 Clearance can also be quantified by t1/2 (Figure 1C) and by fitting the clearance rate to that predicted with an exponential decay rate constant. Both methods are discussed later. Figure 1A compares one concentration trace of dopamine before cocaine incubation (dotted line) with one concentration trace obtained after cocaine incubation (solid line). The representative traces demonstrate the effectiveness of a bath application of cocaine in inhibiting dopamine uptake via the dopamine transporter.
Although the effect of different administration routes of methylphenidate has been studied in mammalian systems, to our knowledge, no reports have been published on the efficacy of oral methylphenidate treatment in Drosophila. To investigate this, flies were orally fed a paste41 consisting of a 10 mM methylphenidate solution mixed with yeast for 3–5 days to ensure the delivery of an effective drug concentration42 prior to the cocaine bath application experiment described above. The 1.0 mM cocaine bath incubation did affect the [DA]max measured after the dopamine injection for the flies that orally consumed the methylphenidate paste to some degree but not to the same extent as for the yeast fed flies (Figure 1B). Since the feeding with 10 mM methylphenidate did not completely inhibit the effect of cocaine, the flies were subsequently fed a higher concentration of 15 mM. The 1.0 mM cocaine bath application did not affect the [DA]max measured after the dopamine injection for the flies that orally consumed the high concentration methylphenidate paste (Figure 1C). Although the maximum change in DA varies for controls between flies, in the relative measurements, the 1.0 mM cocaine bath application does not significantly affect the [DA]max measured after the dopamine injection for the flies that orally consume the high concentration methylphenidate paste (Figure 1C). The width of the peak, t1/2, is also affected by the oral methylphenidate dose as observed when the black traces in Figure 1A and C are compared. In Figure 1A, t1/2 without cocaine addition is 10 ± 0.4 s. For the highest methylphenidate feeding concentration (15 mM), t1/2 without cocaine is 13.0 ± 1.2 s. This shows the peak width of the clearance of dopamine changes for the methylphenidate treated flies. The peak width t1/2 is discussed more in more detail below.
Effect of Repeated Methylphenidate Treatment on Dopamine Clearance in Drosophila Following Acute Methylphenidate Bath Treatment in Drosophila
A 1.0 mM bath application of methylphenidate has been shown to effectively inhibit dopamine uptake occurring via the dopamine transporter in Drosophila wild-type flies.35 Here, we compare the results to wild-type flies that have orally consumed methylphenidate prior to the application of a 1.0 mM bath of methylphenidate to determine if repeated oral administration of methylphenidate is capable to inhibit the Drosophila dopamine transporter in vivo to a similar degree as the bath administration.
Flies that consumed a paste consisting of a 10 mM methylphenidate solution mixed with yeast for 3–5 days prior to the methylphenidate bath application were compared to flies that consumed only control yeast paste. This feeding is semichronic as it represents 25% of the adult fly lifetime. To date, no data has been published concerning an effective concentration of methylphenidate for oral consumption by flies; however, it has been demonstrated that an ∼5 mM cocaine solution mixed with yeast causes physiological effects when orally consumed by adult Drosophila.43 To eliminate systematic effects, such as slight differences in dopamine injector positioning between flies, the peak height, [DA]max, was normalized. For normalization, the [DA]max from the two dopamine baseline measurements were averaged together for each fly, and calculated as a percentage of the average baseline measurement (i.e., [DA]max normalized). The normalized [DA]max for the two groups of flies, those that consumed the 10 mM oral methylphenidate paste and those that did not, were compared.
The 1.0 mM methylphenidate bath application treatment had no effect on the peak current response following dopamine injection for the flies that consumed methylphenidate (Figure 2). The flies that did not receive the oral methylphenidate treatment (control) displayed a significant increase in peak height [DA]max following the acute exposure of 1.0 mM bath application of methylphenidate compared to the flies that received the oral methylphenidate treatment (two-way analysis of variance (ANOVA), p = 0.05 for interaction, p < 0.0001 for the two fly groups, with and without methylphenidate feeding, p = 0.03 for the time from methylphenidate bath treatment, n = 5–6). Thus, the acute bath application of methylphenidate does not appear to affect uptake by the dopamine transporter of flies that have previously consumed methylphenidate. For the yeast-fed flies, the methylphenidate bath does not totally inhibit the transporter function instantaneously after application; it takes up to 20 min before maximum inhibition is reached. This suggests oral consumption of methylphenidate inhibits the Drosophila dopamine transporter in a manner similar to that of orally consumed methylphenidate in humans.44
Figure 2.
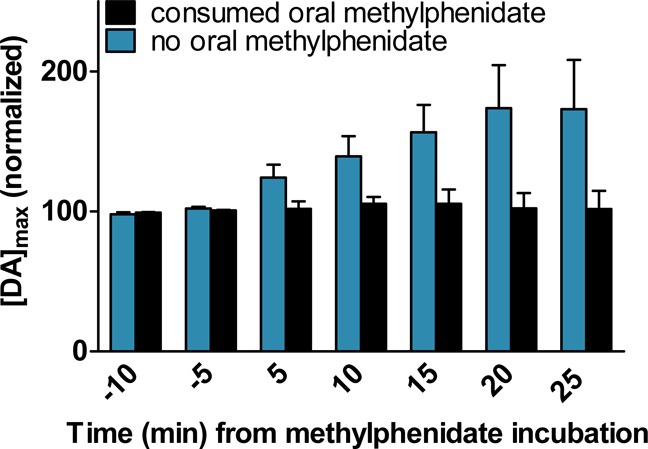
Effect of acute methylphenidate treatment on the uptake of dopamine for untreated and oral methylphenidate treated wild-type flies. The uptake of exogenously applied 1.0 mM dopamine by flies that orally consumed 10 mM methylphenidate paste (black) was compared with flies that did not orally consume methylphenidate (blue). Following baseline dopamine measurements, both groups of flies were treated with bath-applied 1.0 mM methylphenidate for 25 min. There was a significant increase in normalized [DA]max for the flies that did not receive oral methylphenidate treatment prior to the bath methylphenidate treatment (mean ± SEM; two-way ANOVA, p = 0.05 for interaction, p < 0.0001 for two fly groups, p = 0.03 for bath treatment, n = 5–6).
Cocaine Effects Are Diminished Following Repeated Oral Consumption of Methylphenidate
Bath application of 1.0 mM cocaine has been reported to effectively inhibit the Drosophila dopamine transporter with an effective cocaine concentration of 12 μM at the electrode tip.35 To investigate if methylphenidate consumption is able to affect the action of cocaine on the dopamine transporter, flies were fed three concentrations, 5, 10, and 15 mM, of methylphenidate paste for 3–5 days. Also, control groups of the TH-flies used for all experiments below and mutant fmn flies, with a nonfunctional dopamine transporter, were fed yeast paste without drug before testing. Electrochemistry was used to monitor exogenously applied dopamine clearance before (baseline) and after application of a 1.0 mM cocaine bath. Voltammograms were obtained for exogenously applied dopamine every 5 min for 20 min. Figure 3A is a comparison of the normalized maximum dopamine peak [DA]max for the groups of flies. The flies that were not fed oral methylphenidate experienced a significant increase in the normalized [DA]max following the cocaine bath application, which inhibits the dopamine transporter. Flies that had orally consumed methylphenidate (15 mM) only exhibited a small change in dopamine uptake after cocaine incubation (Figure 3B), since the dopamine transporter was already blocked by methylphenidate. The [DA]max observed for the flies fed the highest methylphenidate concentration, 15 mM, was not significantly different (Student’s t test, p = 0.1) from [DA]max for the fmn mutant flies and the [DA]max for the fmn flies was not significantly different from baseline before cocaine incubation (Student’s t test, p = 0.4, n = 5). All the methylphenidate fed fly groups, though, had significantly different [DA]max from those before application. The [DA]max for flies treated with 15 mM oral methylphenidate (n = 8) was significantly different from that observed following feeding with 10 mM methylphenidate (p = 0.008, n = 8), as well as from those fed 5 mM methylphenidate (p = 0.030, n = 7) and 0 mM methylphenidate (p = 0.006, n = 7), showing a concentration dependence of the methylphenidate effect on cocaine inhibition of the dopamine transporter. Thus, this indicates that orally consumed methylphenidate effectively inhibit or desensitizes the Drosophila dopamine transporter function in vivo, so the effect of cocaine is diminished in a concentration dependent manner. This result is comparable to the mechanism of action observed in baboons that were given methylphenidate prior to cocaine administration.14 In addition, the regional distribution patterns of methylphenidate and cocaine within the human brain have been found to be almost identical using positron emission tomography with similar in vivo potencies at the dopamine transporter.10,14
Figure 3.
Effect of various concentrations of oral methylphenidate treatment on cocaine uptake inhibition measured as maximum peak concentration normalized against the average of precocaine measurements. (A) The clearance of exogenously applied 1.0 mM dopamine by TH-flies that orally consumed 5, 10, and 15 mM methylphenidate paste was compared with flies that did not orally consume methylphenidate and fmn flies that lacked the dopamine transporter. Following pre-cocaine dopamine measurements, all groups of flies were incubated with bath-applied 1.0 mM cocaine for 20 min. Error bars are mean ± SEM and n = 6–8 for the TH-flies and n = 5 for fmn flies. (B) Zoom in view of the data from the 5 min before and after cocaine bath. All post-cocaine groups except for the fmn flies were significantly different from before cocaine bath application. The change in cocaine effect on uptake following oral methylphenidate treatment with 15 mM concentration is significantly different from 10 mM (p = 0.008), from 5 mM (p = 0.030), and from 0 mM (p = 0.006) but not to fmn. The fmn group is significantly different from the other groups.
Methylphenidate Concentrations in Vivo
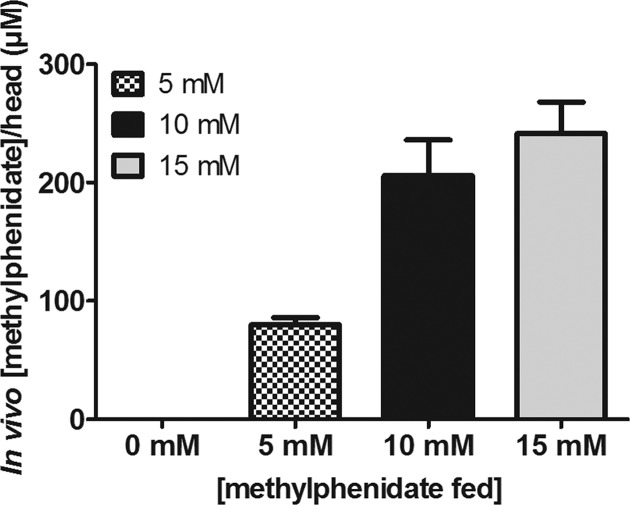
For studies of drugs of abuse by oral treatment, drug dose are usually reported by the level of drug administered, as shown in Figure 3. It is valuable, however, to know the concentration at the site of action, within the fly brain, following oral feeding. Capillary electrophoresis coupled to a mass spectrometry (CE-MS) was used to determine the concentration of methylphenidate in the fly head following repeated drug administration in the fly food. Figure 4 shows the in vivo concentrations found when flies were fed with methylphenidate in doses of 5, 10, and 15 mM. The results show that the methylphenidate concentration in vivo was 80, 206, and 242 μM in the brain, respectively. Therapeutic doses of oral MP (0.25–1 mg/kg) induced significant DAT blockade (50–75%) in the human brain.44 The in vivo doses to the methylphenidate fed flies corresponds to doses of 1.9, 4.8, and 5.6 mg/kg (Supporting Information Figure 4) which is within the range of the doses (0.75–10 mg/kg) used for pharmacokinetics and bioavailability of oral methylphenidate examined in rats.45,46 The methylphenidate concentration in the brain significantly increases from 5 to 10 mM, and then increases less significantly with the 15 mM feeding dose. This may indicate that another mechanism for methylphenidate clearance is activated at high concentrations in the fly brain. Alternatively, these doses of methylphenidate might affect the feeding rate. A one-way ANOVA analysis showed that the concentration observed at 5 mM feeding is significantly different from that for the other doses; the concentration observed at 15 mM feeding, however, is not significantly different from that at 10 mM (p ≤ 0.05). These in vivo concentrations of methylphenidate were used for the kinetic analysis of the effects of methylphenidate on cocaine uptake (vide infra).
Figure 4.
Capillary electrophoresis mass spectrometry quantification of methylphenidate in a fly head corresponding to various oral methylphenidate administration at 0, 5, 10, and 15 mM. Error bars are mean ± SEM of n = 9–12 samples for each level of administration and ranged from 5 to 29 μM. The analyses were carried out using 30 fly heads by CE-MS. Separation was performed in citric acid buffer (50 mM) at voltage 20 kV, positive mode. MS conditions: ESI voltage 4500 V, positive mode, sheath liquid isopropanol/water (70:30, v/v) with flow rate 3 μL mL–1.
Clearance Rate of Exogenous Dopamine in Methylphenidate Fed Flies after Cocaine
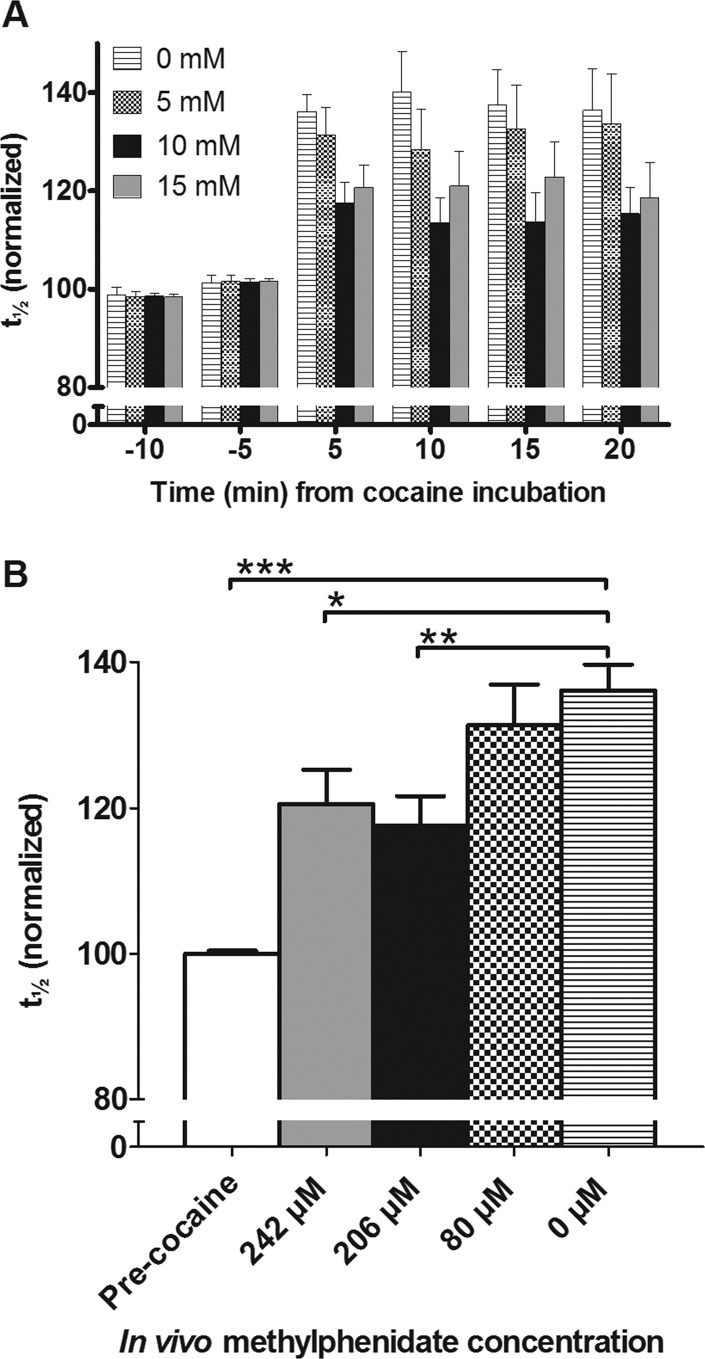
Both the rise and fall times during amperometric detection of dopamine in the extracellular fluid have been used previously to evaluate the kinetics of clearance by reuptake.38,47−49 This work has been carried out predominantly in rats. Here, we use the full width at half-maximum, t1/2, to monitor the effects of cocaine on both the rise and fall of the response (shown in Figure 1C). Figure 5A shows the normalized t1/2 values observed at 5 min intervals for 20 min of cocaine application. The value of t1/2 is maximal after 5 min and statistically does not vary over the 20 min experiment. In Figure 5B, t1/2 for the first 5 min after cocaine application is plotted against the in vivo concentrations of methylphenidate obtained with CE-MS. Previously, this change in t1/2 has not been observed in the adult Drosophila following cocaine exposure,35 and might be attributed to diffusional effects in the earlier experiments. With practice, these experiments have become more precise.
Figure 5.
Effect of various concentrations of oral methylphenidate treatment on inhibition of uptake by cocaine measured as t1/2, full width of time at half-maximum, normalized against average of precocaine. (A) Values of t1/2 for the rise and clearance of exogenously applied 1.0 mM dopamine by TH-flies that orally consumed 5, 10, and 15 mM methylphenidate paste were compared with flies that did not orally consume methylphenidate and fmn flies that lack the dopamine transporter. Following pre-cocaine dopamine measurements, all groups of flies were incubated with bath-applied 1.0 mM cocaine for 20 min. Error bars are mean ± SEM and n = 6–8 for the TH-flies and n = 5 for fmn. (B) Values of t1/2 after 5 min cocaine bath application compared to t1/2 before cocaine application (white bar) plotted against the methylphenidate concentration found in vivo. For the flies not fed with methylphenidate (0 mM), the t1/2 value changed significantly (p < 0.0001) from before cocaine addition and this change was also significant from the flies with an in vivo concentration of 206 μM (p = 0.006) and 242 μM (p = 0.027).
Following cocaine exposure, there is a significant change in t1/2 (p < 0.0001) relative to baseline measurements thus showing that cocaine application indeed inhibits the rate of clearance of dopamine here. For the control group, t1/2 increases following cocaine exposure by ∼35% and t1/2 for those fed methylphenidate increased by only 20% (206 μM (p = 0.006) and 242 μM (p = 0.03) methylphenidate). Flies fed methylphenidate to give an in vivo concentration of only 80 μM (after 5 mM feeding) and then exposed to cocaine did not show a significant difference in t1/2 from controls.
These data show that the rate of clearance of dopamine by uptake is indeed decreased when cocaine is added to the fly brain, as has been reported before in rat striatum by Gerhardt and co-workers.38 This also shows that repeated methylphenidate exposure in vivo decreases the ability of cocaine to inhibit dopamine uptake and reduces the effect of this drug.50 The lack of a clear dose response with varied concentrations is probably the result of variations in pipet placement leading to small changes in peak amplitude and the previous reported diffusion effects being predominant in the earlier experiments.
Kinetic Analysis of the Clearance of Exogenous Applied Dopamine
We analyzed the fall time from [DA]max to the end of the recording for the amperometric response to dopamine following cocaine with and without repeated exposure to methylphenidate. The data (Supporting Information Figure 1A) appear to consist of two first order reactions for all the data collected and were therefore fitted to a double-exponential decay equation:
where Amax is the maximum dopamine concentration, which was constrained, Ainf is the inflection point in the curve with transition to the slow phase of decay, t is time, and t0 is the start time. From these fitted data, the decay rate constant of the two components (kfast and kslow) could be extracted and used as parameters of the efficiency of dopamine clearance. Attempts to fit the data to a single exponential function resulted in a poor fit (Supporting Information Figure 1B). The first term in the equation dominates the late part of the fall time. This is given the rate constant kslow. The second term in the equation dominates the early part of the fall time (fast decay from max). This is given the rate constant kfast. A term y0 is used to compensate for variations in background levels. When the rate constant for the first part of the equation, kslow, is plotted against time from cocaine application, no correlation is found between them. In fact, only random scatter is observed between all data ranging from 0.020 to 0.005 s–1, even after normalization (Supporting Information Figure 2A) and is probably dominated by diffusion.
The faster rate constant, kfast, is almost 10 times as high as kslow and varies from 0.19 s–1 for the control flies down to 0.05 s–1 for flies lacking the dopamine transporter (fmn flies) (Supporting Information Figure 2B). For the fmn flies, the lower value of kfast appears to indicate it is affected more by diffusion than kfast for the control flies which is dominated by the clearing effect by the dopamine transporter. As shown in Figure 6A, the rate constant is dependent on the concentration of methylphenidate. The increase of the rate constant in fmn flies after cocaine application is not significant, and likely due to normalization of small signals for those flies. By comparing the rate constants following the 5 min exposure of cocaine to the baseline, all groups (p = 0.0002 and lower) except for fmn (p = 0.2) and 15 mM methylphenidate fed flies (p = 0.06) are observed to significantly decrease when cocaine is added. In Figure 6B, the rate constant after 5 min of cocaine exposure is plotted against the in vivo concentration of the drug measured by CE-MS. There is a 12% change in rate constant between the feeding concentrations of 15 and 10 mM even though this increase in feeding does not result in as high a fractional change in the in vivo concentration as observed for the 5–10 mM dose change. The smaller fractional increase in the measured in vivo concentration going from 10 to 15 mM feeding dose relative to that for 5–10 mM feeding might be caused by less consumption of the fly food compared to the lower concentrations or perhaps activation of an unknown system for clearing out methylphenidate at higher concentrations. It is clear, however, that the effect of cocaine on the rate constant is diminished by repeated methylphenidate in a dose dependent manner.
Figure 6.
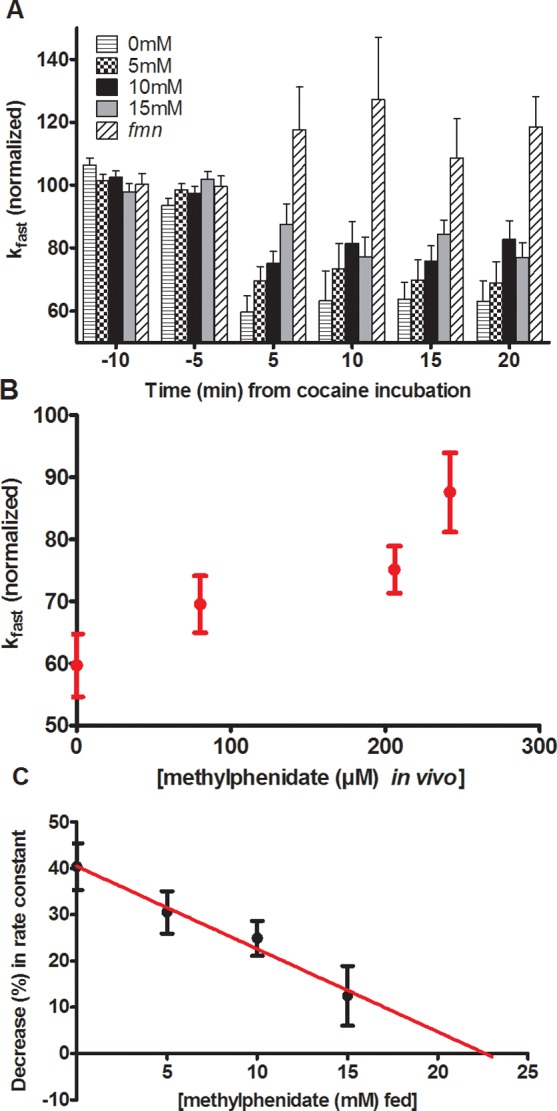
Oral methylphenidate treatment effect on cocaine uptake inhibition after fitting the clearance of exogenously applied 1.0 mM dopamine to a double exponential decay equation. Fitting the clearance rate for dopamine to the equation gave the rate constant kfast (s–1), a measure of cocaine’s inhibition effect on the dopamine transporter. (A) The rate constant normalized against the average precocaine uptake measured every fifth minute up to 20 min of cocaine application. Cocaine bath application decreases the rate constant significantly for the untreated flies after the first 5 min of inhibition, but for the methylphenidate treated flies the cocaine application did not have the same effect. For the untreated flies, cocaine decreased the rate constant by 40%, but for flies treated with 15 mM methylphenidate orally the decrease was only ∼10%. (B) Plot of the rate constant after 5 min of cocaine application against concentration methylphenidate found in vivo. (C) The decrease in rate constant after 5 min of cocaine exposure plotted against feeding concentration of methylphenidate. By fitting a line to the data (r2 value of 0.98), the intercept gives the theoretical concentration for total inhibition of cocaine uptake, 23 mM methylphenidate.
For the control flies, the average change in rate constant from before cocaine exposure compared to after cocaine decreases 40% as expected from uptake inhibition. Following repeated methylphenidate consumption, the change in rate constant following administration of cocaine is 31%, 24%, and 12% for 5, 10, and 15 mM methylphenidate, respectively. Plotting these data results in a straight line (r2 value of 0.98), and the intercept of the plot (Figure 6C) can be used to calculate the theoretical concentration for total inhibition of cocaine uptake to 23 mM methylphenidate.
These results reinforce the validity of using Drosophila as a model system for studying mechanisms of oral methylphenidate and direct application of cocaine onto the CNS, which can be applied to the study of addiction in humans. We have used in vivo voltammetry to monitor dopamine clearance rates with cocaine exposure to flies previously fed methylphenidate in different doses. In addition, we have used capillary electrophoresis with mass spectrometry detection to determine the concentration of drug in the fly brain after repeated feeding. The data from these experiments support the conclusion that oral consumption of methylphenidate is capable of inhibiting the Drosophila dopamine transporter and thereby inhibiting the actions of directly applied cocaine. Methylphenidate, in the doses administered here, appears to effectively inhibit the dopamine transporter. Thus, further inhibition of the transporter by cocaine applied directly to the CNS appears to be prevented.
Methods
Chemicals
All chemicals were used as received and purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Methylphenidate-D9 was purchased from LGC Standards (Teddington, West London, UK). Adult hemolymph-like (AHL) saline (108 mM NaCl, 5 mM KCl, 2 mM CaCl2 (Merck, Whitehouse Station, NJ) 8.2 mM MgCl2, 4 mM NaHCO3, 1 mM NaH2PO4, 5 mM trehalose, 10 mM sucrose, 20 mM Trizma base, pH 7.5) was made using ultrapure (18 MΩ·cm) water (ELGA Labwater system, VWS UK) and filtered through a 0.2 μm filter.51 All collagenase, dopamine, (+)cocaine, and methylphenidate hydrochloride bath treatment solutions were prepared in AHL saline.
Drosophila Rearing and in Vivo Preparation
Male flies, 4–8 days old, of two mutant strains of Drosophila melanogaster and wild type flies were used. For experiments where the dopamine transporter was intact, TH-GFP flies were used. TH-GPF are transgenic flies carrying tyrosine hydroxylase (TH)-GAL4 and UAS-mCD::GFP (membrane tethered green fluorescent protein) and were used for the ability to visualize the dopamine neurons. For the experiments where the dopamine transporter was knocked out, the fumin (fmn) mutant52 that has a genetic lesion abolishing the dopamine transporter function was used. The genetic background of the w;fmn mutant was replaced with the Canton-S background. Wild-type Canton-S flies were used for experiments where methylphenidate was used instead of cocaine for acute treatment. All flies were cultured on standard potato meal/agar medium at 25 °C. Some flies received an additional food supplement consisting of a yeast paste containing 0, 5, 10, or 15 mM methylphenidate aqueous solution that was prepared fresh daily. The flies were reared on the methylphenidate yeast paste for 3–5 days prior to experimentation. All flies were prepared for in vivo voltammetry as previously described.33,35 Briefly, flies were mounted in a homemade collar (38.1 mm diameter concave plexiglass disk with a 1.0 mm hole in the center) with low melting agarose following immobilization with ice. Under a stereoscope (Olympus SZX10, Melville, NY), the cuticle was removed from the top portion of the head using dissection forceps and scissors (World Precision Instruments, Sarasota, FL) to expose the brain. Following microsurgery, 0.1% collagenase solution was applied to the head for 15 min to relax the extracellular matrix in the brain. The immobilized fly head was then rinsed and bathed with AHL saline, allowing the preparation to remain viable for 1.5–2.5 h.
Drosophila Rearing for Capillary Electrophoresis Mass Spectrometry (CE-MS) Preparation
Seven to eight day old male flies (TH-GFP mutant) were used for quantification of methylphenidate by CE-MS. Prior to experiments, the flies were fed with methylphenidate for three consecutive days and then quickly frozen in liquid nitrogen and vortexed to detach the heads from the bodies. Thirty fly heads were collected and homogenized in 60 μL of concentrated formic acid with methylphenidate-D9 as internal standard and subsequently centrifuged at 14 000 rpm for 20 min. The supernatant was transferred into a new vial, gently dried, reconstituted in 10 μL ammonium formate (10 mM), and centrifuged again followed by injection into CE-MS for analysis.
Electrochemical Measurements
The fabrication of the cylindrical carbon-fiber microelectrodes used for this study has been described in detail previously.33,53 The exposed carbon fiber portion of the cylindrical electrodes was 40–50 μm in length. In all experiments, the Ag/AgCl reference electrode used was made by chloridizing a silver wire (0.25 mm diameter, 99.999% purity, Alfa Aesar, Ward Hill, MA) by immersing the wire connected to the positive side of a 9 V battery and a platinum wire connected to the negative side in 5 M HCl for ∼1 min. Electrodes were positioned 50–60 μm into the brain at an angle of 45° using micromanipulators purchased from Newport (421 series, Irvine, CA). Glass capillaries (B120-69-10, Sutter Instruments, Novato, CA) were pulled using a glass capillary puller (P-1000, Sutter Instruments) and cut to an opening of ∼5 μm to form the micropipet injectors. The injectors were used to exogenously apply 1.0 mM dopamine solution on top of the brain at a maximum distance of 50–60 μm from the electrode, by coupling them to a microinjection system (Picospritzer III, General Valve Corporation, Fairfield, NJ).
A Dagan Chem-Clamp potentiostat (Dagan Corporation, Minneapolis, MN) and two data acquisition boards (PCI-6221, National Instruments, Austin, TX) run by the TH 1.0 CV program (ESA, Chelmsford, MA) were used to collect all electrochemical data.54 Cyclic voltammograms were obtained by applying a triangular waveform potential (−0.4 to +1.0 V vs Ag/AgCl) repeated every 100 ms at a scan rate of 200 V/s (low pass Bessel filter at 3 kHz). Each cyclic voltammogram was a background-subtracted average of 10 successive cyclic voltammograms taken at the maximum oxidation peak current. All electrodes were allowed to cycle for at least 15 min prior to recording to stabilize the background current. The recorded current response was converted to dopamine concentration via in vitro electrode calibration with standard dopamine solution after each experiment. Statistical analysis was accomplished using Prism 5.0 (GraphPad Software, La Jolla, CA).
CE-MS Measurements
A capillary electrophoresis (CE) system from Waters was interfaced with a Micro-ToF-Q II mass spectrometer (BrukerDaltonics, Germany). Separations were carried out in 80 cm long and 50 μm id fused silica capillaries (Polymicro Technologies, Phoenix, AZ) in 50 mM citric acid (pH ∼ 2.1). The samples were introduced into the CE system using hydrodynamic injection with 30 s sampling time. After injection, a separation voltage of +20 kV was used. The mass spectrometer was operated in positive mode with electrospray ionization (4.5 kV) sample introduction and an isopropanol/water sheath liquid (70:30, v/v) at 3 μLmin–1. The drying gas was heated at 180 °C with a flow rate of 4 L min–1. Nebulizer gas was kept at 0.4 bar pressure. Methylphenidate and methylphenidate-D9 were detected and quantified using selected ions m/z 234 and m/z 243, respectively. A CE-MS electropherogram with selected ions for methylphenidate and methylphenidate-D9 can be found in Supporting Information Figure 3.
Kinetic Analysis of Dopamine Clearance
The rate of clearance has previously been calculated directly from the slope of the pseudolinear segment of the signal decay,38,49,50,55 but this method is dependent on the amplitude of the max peak. It has been difficult to compare the max amplitude between flies because of systematic effects, such as slight differences in dopamine injector positioning between flies. The rate of decay of the dopamine signal followed a curve with two separate sections appearing to be first-order reaction kinetics. Therefore, instead of using the slope of the pseudolinear segment or fitting the signal to a single-exponential decay equation as has been done before,56 the clearance signals were fitted to a double-exponential decay function:
where [DA] was the amplitude in μM at any time t in s. For the first part of the equation Ainf represented the start of the slow first-order reaction (inflection point) and kslow its first-order rate constant. The second part of the equation Amax was the peak amplitude, and kfast the first-order rate constant for that decay. The time t0 was set to approximately 80% of Amax decay. The last term y0 was used to compensate for the background level.
Nonlinear regression analysis was preformed on Igor Pro 6.22A (WaveMetrics Portland, OR).
Acknowledgments
The TH-GFP and Canton-S flies were kindly provided by Professor K.A. Han (The University of Texas at El Paso). The fumin mutant in the w genetic background was kindly provided by F.R. Jackson (Tufts University).
Supporting Information Available
Additional rate plots, representative CE-MS data, and more detail of the brain concentrations of drug after different doses. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
E.C.B. contributed to the final experimental design, collected and analyzed data, interpreted the data and models and wrote and edited the manuscript. M.A.P. contributed to the original experimental design, collected, analyzed and interpreted data for Figure 2 and fmn data and writing of the manuscript. J.D.K. provided expertise in discussions about uptake kinetics, helped to interpret the kinetic data and edited the manuscript. N.P. collected, analyzed and interpreted the mass spectrometry data and helped in writing of the manuscript. M.L.H. aided in experimental design, interpretation of data, and writing the manuscript. A.G.E. participated in design and interpretation of the experiments, writing of the manuscript, and acquired the resources and environment for the work to be done.
This work was supported by the European Research Council (ERC), Knut and Alice Wallenberg Foundation, and the Swedish Research Council (VR). A.G.E. is co-PI on a National Institutes of Health grant.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Ritz M. C.; Lamb R. J.; Goldberg S. R.; Kuhar M. J. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237, 1219–1223. [DOI] [PubMed] [Google Scholar]
- Rocha B. A.; Fumagalli F.; Gainetdinov R. R.; Jones S. R.; Ator R.; Giros B.; Miller G. W.; Caron M. G. (1998) Cocaine self-administration in dopamine-transporter knockout mice. Nat. Neurosci. 1, 132–137. [DOI] [PubMed] [Google Scholar]
- Sora I.; Wichems C.; Takahashi N.; Li X.-F.; Zeng Z.; Revay R.; Lesch K.-P.; Murphy D. L.; Uhl G. R. (1998) Cocaine reward models: Conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc. Natl. Acad. Sci. U.S.A. 95, 7699–7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.; Gainetdinov R. R.; Wetsel W. C.; Jones S. R.; Bohn L. M.; Miller G. W.; Wang Y.-M.; Caron M. G. (2000) Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat. Neurosci. 3, 465–471. [DOI] [PubMed] [Google Scholar]
- Weidmann S. (1955) Effects of calcium ions and local anaesthetics on electrical properties of Purkinje fibres. J. Physiol. 129, 568–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick D. A.; Gardner E. L.; Xi Z.-X. (2004) Agents in Development for the Management of Cocaine Abuse. Drugs 64, 1547–1573. [DOI] [PubMed] [Google Scholar]
- Karila L.; Gorelick D.; Weinstein A.; Noble F.; Benyamina A.; Coscas S.; Blecha L.; Lowenstein W.; Martinot J. L.; Reynaud M.; Lépine J. P. (2008) New treatments for cocaine dependence: a focused review. Int. J. Neuropsychopharmacol. 11, 425–438. [DOI] [PubMed] [Google Scholar]
- Carrey N. J.; Wiggins D. M.; Milin R. P. (1996) Pharmacological treatment of psychiatric disorders in children and adolescents - Focus on guidelines for the primary care practitioner. Drugs 51, 750–759. [DOI] [PubMed] [Google Scholar]
- Di Chiara G.; Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U.S.A. 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D.; Wang G. J.; Fowler J. S.; Fischman M.; Foltin R.; Abumrad N. N.; Gatley S. J.; Logan J.; Wong C.; Gifford A.; Ding Y.-S.; Hitzemann R.; Pappas N. (1999) Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 65, PL7–PL12. [DOI] [PubMed] [Google Scholar]
- Johanson C. E.; Fischman M. W. (1989) The pharmacology of cocaine related to its abuse. Pharmacol. Rev. 41, 3–52. [PubMed] [Google Scholar]
- Kuhar M. J.; Ritz M. C.; Boja J. W. (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 14, 299–302. [DOI] [PubMed] [Google Scholar]
- Kollins S. H.; MacDonald E. K.; Rush C. R. (2001) Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol., Biochem. Behav. 68, 611–627. [DOI] [PubMed] [Google Scholar]
- Volkow N. D.; Ding Y.-S.; Fowler J. S.; Wang G.-J.; Logan J.; Gatley J. S.; Dewey S.; Ashby C.; Liebermann J.; Hitzemann R.; Wolf A. P. (1995) Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch. Gen. Psychiatry 52, 456–463. [DOI] [PubMed] [Google Scholar]
- Volkow N. D.; Fowler J. S.; Wang G. J.; Ding Y. S.; Gatley S. J. (2002) Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur. Neuropsychopharmacol. 12, 557–566. [DOI] [PubMed] [Google Scholar]
- Volkow N. D.; Wang G. J.; Fischman M. W.; Foltin R. W.; Fowler J. S.; Abumrad N. N.; Vitkun S.; Logan J.; Gatley S. J.; Pappas N.; Hitzemann R.; Shea C. E. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386, 827–830. [DOI] [PubMed] [Google Scholar]
- Balster R. L.; Schuster C. R. (1973) Fixed-interval schedule of cocaine reinforcement - effect of dose and infusion duration. J. Exp. Anal. Behav. 20, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf W. H. (1992) Some relationships between addiction and drug delivery to the brain. NIDA Res. Monogr. 120, 13–25. [PubMed] [Google Scholar]
- Dole V. P.; Nyswande. M. (1965) A medical treatment for diacetylmorphine (heroin) addition - a clinical trial with methadone hydrochloride. JAMA, J. Am. Med. Assoc. 193, 646–650. [DOI] [PubMed] [Google Scholar]
- Grabowski J.; Roache J. D.; Schmitz J. M.; Rhoades H.; Creson D.; Korszun A. M. (1997) Replacement medication for cocaine dependence: methylphenidate. J. Clin. Psychopharmacol. 17, 485–488. [DOI] [PubMed] [Google Scholar]
- Roache J. D.; Grabowski J.; Schmitz J. M.; Creson D. L.; Rhoades H. M. (2000) Laboratory measures of methylphenidate effects in cocaine-dependent patients receiving treatment. J. Clin. Psychopharmacol. 20, 61–68. [DOI] [PubMed] [Google Scholar]
- Winhusen T.; Somoza E.; Singal B. M.; Haffer J.; Apparaju S.; Mezinskis J.; Desai P.; Elkashef A.; Chiang C. N.; Horn P. (2006) Methylphenidate and cocaine: A placebo-controlled drug interaction study. Pharmacol., Biochem. Behav. 85, 29–38. [DOI] [PubMed] [Google Scholar]
- Fowler J. S.; Volkow N. D.; Wolf A. P.; Dewey S. L.; Schlyer D. J.; MacGregor R. R.; Hitzemann R.; Logan J.; Bendriem B.; Gatley S. J.; Christman D. (1989) Mapping cocaine binding sites in human and baboon brain in vivo. Synapse 4, 371–377. [DOI] [PubMed] [Google Scholar]
- Bergman J.; Madras B. K.; Johnson S. E.; Spealman R. D. (1989) Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J. Pharmacol. Exp. Ther. 251, 150–155. [PubMed] [Google Scholar]
- Giros B.; Jaber M.; Jones S. R.; Wightman R. M.; Caron M. G. (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612. [DOI] [PubMed] [Google Scholar]
- Calipari E. S.; Ferris M. J.; Melchior J. R.; Bermejo K.; Salahpour A.; Roberts D. C.; Jones S. R. (2012) Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict. Biol. 10.1111/j.1369-1600.2012.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris M. J.; Calipari E. S.; Mateo Y.; Melchior J. R.; Roberts D. C.; Jones S. R. (2012) Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology 37, 1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C.; Hirsh J. (1998) Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr. Biol. 8, 109–112. [DOI] [PubMed] [Google Scholar]
- Bainton R. J.; Tsai L. T. Y.; Singh C. M.; Moore M. S.; Neckameyer W. S.; Heberlein U. (2000) Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 10, 187–194. [DOI] [PubMed] [Google Scholar]
- Lee H.-G.; Kim Y.-C.; Dunning J. S.; Han K.-A. (2008) Recurring ethanol exposure induces disinhibited courtship in Drosophila. PLoS ONE 3, e1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A. B.; Maleszka R.; Helliwell P. G.; Robinson G. E. (2009) Effects of cocaine on honey bee dance behaviour. J. Exp. Biol. 212, 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makos M. A.; Kuklinski N. J.; Berglund E. C.; Heien M. L.; Ewing A. G. (2009) Chemical measurements in Drosophila. TrAC, Trends Anal. Chem. 28, 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makos M. A.; Kim Y.-C.; Han K.-A.; Heien M. L.; Ewing A. G. (2009) In Vivo Electrochemical Measurements of Exogenously Applied Dopamine in Drosophila melanogaster. Anal. Chem. 81, 1848–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey T. L.; Condron B.; Venton B. J. (2009) Detection of endogenous dopamine changes in Drosophila melanogaster using fast-scan cyclic voltammetry. Anal. Chem. 81, 9306–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makos M. A.; Han K.-A.; Heien M. L.; Ewing A. G. (2010) Using in vivo electrochemistry to study the physiological effects of cocaine and other stimulants on the Drosophila melanogaster dopamine transporter. ACS Chem. Neurosci. 1, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey T. L.; Venton B. J. (2011) Drosophila Dopamine2-like receptors function as autoreceptors. ACS Chem. Neurosci. 2, 723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel D. R.; Elekes K. (1992) Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 267, 147–167. [DOI] [PubMed] [Google Scholar]
- Zahniser N. R.; Larson G. A.; Gerhardt G. A. (1999) In Vivo Dopamine Clearance Rate in Rat Striatum: Regulation by Extracellular Dopamine Concentration and Dopamine Transporter Inhibitors. J. Pharmacol. Exp. Ther. 289, 266–277. [PubMed] [Google Scholar]
- Chen R.; Wei H.; Hill E. R.; Chen L.; Jiang L.; Han D. D.; Gu H. H. (2007) Direct evidence that two cysteines in the dopamine transporter form a disulfide bond. Mol. Cell. Biochem. 298, 41–48. [DOI] [PubMed] [Google Scholar]
- Porzgen P.; Park S. K.; Hirsh J.; Sonders M. S.; Amara S. G. (2001) The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: A primordial carrier for catecholamines. Mol. Pharmacol. 59, 83–95. [DOI] [PubMed] [Google Scholar]
- Manev H.; Dimitrijevic N.; Dzitoyeva S. (2003) Techniques: Fruit flies as models for neuropharmacological research. Trends Pharmacol. Sci. 24, 41–43. [DOI] [PubMed] [Google Scholar]
- Leal S. M.; Neckameyer W. S. (2002) Pharmacological evidence for GABAergic regulation of specific behaviors in Drosophila melanogaster. J. Neurobiol. 50, 245–261. [DOI] [PubMed] [Google Scholar]
- Willard S. S.; Koss C. M.; Cronmiller C. (2006) Chronic cocaine exposure in Drosophila: Life, cell death and oogenesis. Dev. Biol. 296, 150–163. [DOI] [PubMed] [Google Scholar]
- Volkow N. D.; Wang G.-J.; Fowler J. S.; Gatley S. J.; Logan J.; Ding Y.-S.; Hitzemann R.; Pappas N. (1998) Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am. J. Psychiatry 155, 1325–1331. [DOI] [PubMed] [Google Scholar]
- Wargin W.; Patrick K.; Kilts C.; Gualtieri C. T.; Ellington K.; Mueller R. A.; Kraemer G.; Breese G. R. (1983) Pharmacokinetics of methylphenidate in man, rat and monkey. J. Pharmacol. Exp. Ther. 226, 382–386. [PubMed] [Google Scholar]
- Kuczenski R.; Segal D. S. (2002) Exposure of adolescent rats to oral methylphenidate: Preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J. Neurosci. 22, 7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G.; Gerhardt G.; Stromberg I.; Olson L.; Hoffer B. (1985) Monoamine release from dopamine-depleted rat caudate nucleus reinnervated by substantia nigra transplants: an in vivo electrochemical study. Brain Res. 341, 92–100. [DOI] [PubMed] [Google Scholar]
- Cass W. A.; Gerhardt G. A.; Mayfield R. D.; Curella P.; Zahniser N. R. (1992) Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J. Neurochem. 59, 259–266. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny M. F.; Dugast C.; Chergui K.; Msghina M.; Gonon F. (1995) Uptake of dopamine released by impulse flow in the rat mesolimbic and striatal systems in vivo. J. Neurochem. 65, 2603–2611. [DOI] [PubMed] [Google Scholar]
- Stamford J. A.; Kruk Z. L.; Millar J. (1986) In vivo voltammetric characterization of low affinity striatal dopamine uptake: drug inhibition profile and relation to dopaminergic innervation density. Brain Res. 373, 85–91. [DOI] [PubMed] [Google Scholar]
- Wang J. W.; Wong A. M.; Flores J.; Vosshall L. B.; Axel R. (2003) Two-Photon Calcium Imaging Reveals an Odor-Evoked Map of Activity in the Fly Brain. Cell 112, 271–282. [DOI] [PubMed] [Google Scholar]
- Kume K.; Kume S.; Park S. K.; Hirsh J.; Jackson F. R. (2005) Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25, 7377–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton M. A.; Brown J. C.; Stutts K. J.; Wightman R. M. (1980) Faradaic Electrochemistry at Micro-Voltammetric Electrodes. Anal. Chem. 52, 946–950. [Google Scholar]
- Heien M. L. A. V.; Phillips P. E. M.; Stuber G. D.; Seipel A. T.; Wightman R. M. (2003) Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 128, 1413–1419. [DOI] [PubMed] [Google Scholar]
- Wightman R. M.; Amatore C.; Engstrom R. C.; Hale P. D.; Kristensen E. W.; Kuhr W. G.; May L. J. (1988) Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 25, 513–523. [DOI] [PubMed] [Google Scholar]
- Sabeti J.; Adams C. E.; Burmeister J.; Gerhardt G. A.; Zahniser N. R. (2002) Kinetic analysis of striatal clearance of exogenous dopamine recorded by chronoamperometry in freely-moving rats. J. Neurosci. Methods 121, 41–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.