Abstract
Autoimmune liver disease spans three predominant processes, from the interface hepatitis of autoimmune hepatitis to the lymphocytic cholangitis of primary biliary cirrhosis, and finally the obstructive fibrosing sclerotic cholangiopathy of primary sclerosing cholangitis. Although all autoimmune in origin, they differ in their epidemiology, presentation and response to immunosuppressive therapy and bile acid based treatments. With an ongoing better appreciation of disease aetiology and pathogenesis, treatment is set ultimately to become more rational. We provide an overview of current and future therapies for patients with autoimmune liver disease, with an emphasis placed on some of the evidence that drives current practice.
Keywords: aetiology, autoimmune hepatitis, autoimmune liver disease, primary biliary cirrhosis, primary sclerosing cholangitis, pathogenesis, treatment
Introduction
Autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) represent three relatively uncommon autoimmune diseases affecting the hepatobiliary system. These conditions have a broad clinical spectrum and a variable, usually slow, natural history, making prospective trial design for therapies challenging. However, having made a clear diagnosis of autoimmune liver disease and having interpreted the laboratory testing correctly to identify the predominant active liver injury, clinicians should seek to treat patients in a way that is most in keeping with the evidence, be it from randomized controlled trials or observational studies. Prior to therapy, patients should understand the goals of intervention and the limitations of any surrogates that might be used to evaluate treatment success. In this article, we present a critical overview of the currently available treatment options for the major autoimmune liver diseases, before providing insight into new potential therapeutic agents. A complete approach to the management of autoimmune liver diseases should also include adequate symptom control, early recognition of extra-hepatic manifestations and surveillance for complications (Figure 1), aspects which are covered more fully elsewhere [Decock et al. 2009].
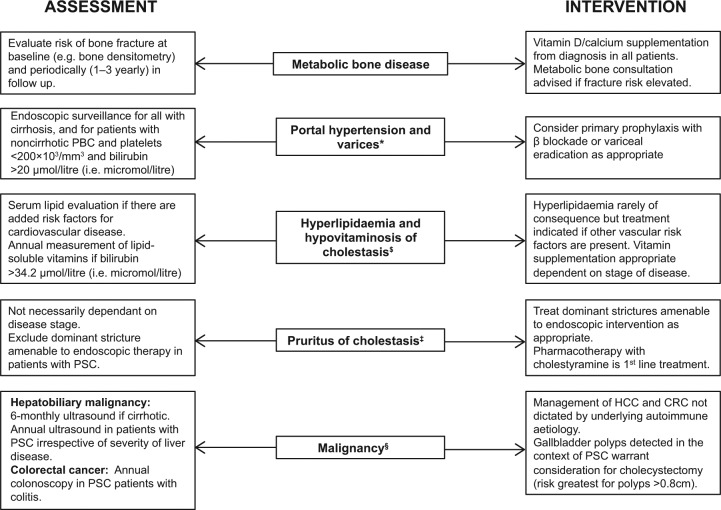
Figure 1.
Extrahepatic manifestations, complications and surveillance strategies in autoimmune liver disease. The complete management of autoimmune liver disease should encompass early symptom recognition and surveillance of complications, whether they are related to the side effects from drug therapy (e.g. osteoporosis and prednisolone) or the underlying disease itself (e.g. metabolic osteopathy of cholestasis). Some complications may be evident only in patients with advanced fibrosis/cirrhosis [e.g. hepatocellular carcinoma (HCC)] whereas others are unrelated to disease severity [e.g. colonic cancer and biliary malignancies in primary sclerosing cholangitis (PSC); the pruritus of cholestasis].
*Obstruction to portal venous flow may also occur at a presinusoidal level in primary biliary cirrhosis (PBC), and nodular regenerative hyperplasia is found in up to 43%. Thus, given the risk of noncirrhotic portal hypertension in patients with PBC, additional parameters for variceal surveillance have been adopted [Bressler et al. 2005; Levy et al. 2007].
$Largely applies to PBC.
‡Cholestyramine is ineffective in around 20% of patients. Other bile-acid sequestrants (e.g. colesevelam) have not been shown to improve symptoms. Rifampicin use is supported by meta-analyses, although side effects remain a concern. Opioid antagonists can be used as a third-line treatment although there may be a risk of withdrawal-like reactions. Pilot studies report a subjective amelioration of itch using ondansetron and sertraline although patient numbers were small. Those with refractory pruritus can be treated with drugs having anecdotal support, or referred to specialized centres where more invasive or experimental approaches (e.g. extracorporeal albumin dialysis, ultraviolet B therapy) may be considered [Kremer et al. 2011].
§Effective surveillance strategies for cholangiocarcinoma (CCA) are currently lacking, although proteomic analysis of bile and urine could provide helpful as a future prospective [Lankisch et al. 2011; Metzger et al. 2013]. The risk of gallbladder cancer in PSC appears greatest with polyps over 0.8 cm [Eaton et al. 2012].
Autoimmune hepatitis
The decision of which patients with AIH require therapy is founded on the results of prior placebo-controlled trials conducted over 30 years ago. These studies revealed that untreated, moderate–severe AIH [aspartate transaminase (AST) >5 × upper limit of normal (ULN), globulins >2 × ULN, liver biopsy showing confluent necrosis] has a very poor prognosis, with a 5- and 10-year survival of 50% and 10% respectively [Kirk et al. 1980; Murray-Lyon et al. 1973]. Such individuals should be offered treatment because of the clear survival benefit and patient outcome with appropriate immunosuppressive therapy is excellent, with 10-year survival rates exceeding 80% [Gleeson et al. 2011; Manns et al. 2010a; Hoeroldt et al. 2011].
Up to 30% of adults may have cirrhotic disease at diagnosis whereas de novo cirrhosis develops in around 12% after 10 years despite immunosuppression, in 49% if there are persistent mild–moderate laboratory abnormalities and in 82% when bridging necrosis or multilobular necrosis is present [Feld et al. 2005; Schalm et al. 1977; De Groote et al. 1978]. Cirrhosis development is associated with liver-related death/transplantation (hazard ratio 9.96) and reduced 10-year survival (67% versus 94% in patients without cirrhosis) [Gleeson et al. 2011; Feld et al. 2005; Verma et al. 2004]. However, 10-year survival of treated individuals in remission approaches 98% [Czaja, 2009a] and therapy should be considered in all patients having active inflammation and established (compensated) cirrhosis on biopsy, even those with milder biochemical abnormalities. The benefits of treatment in asymptomatic patients with mild interface hepatitis are less well established, as this group reportedly have a 10-year survival as high as 80% [Feld et al. 2005], whereas in another study the 10-year survival of eight untreated patients with mild disease was significantly lower than that of treated patients with severe disease (67% versus 98%) [Czaja, 2009a]. The true natural history of mild AIH is unknown, although some patients in this category can do well without immunosuppression. However, untreated mild AIH does not have a uniformly benign prognosis and asymptomatic patients may become symptomatic, a group of patients with a 10-year mortality that exceeds 10% [Feld et al. 2005]. Mild disease activity can be interspersed with phases of severe activity that can be aggressive, and the available data indicate that histological stage, including the frequency of patients with cirrhosis, is similar between symptomatic and symptom-free patients, although those with symptoms may have higher inflammatory scores [Feld et al. 2005]. Moreover, between 26% and 70% of asymptomatic patients may go on to develop symptoms during follow up, with fluctuating histological inflammatory activity [Feld et al. 2005; Kogan et al. 2002]. Spontaneous recovery of AIH may occur; however, resolution of inflammatory activity occurs much less frequently in untreated patients (12% versus 63%). Moreover, the rapidity at which disease resolution takes place rather than its occurrence is an important factor in preventing disease progression [Czaja, 2009b]. If left untreated, patients with mild AIH should be closely monitored and reviewed clinically on a regular basis for signs to suggest progressive disease worthy of treatment. Conversely, patients with decompensated liver disease or fulminant hepatic failure represent populations which may not always benefit from immunosuppression (Table 1), and management in this setting should be in the context of access to transplantation if appropriate [Ichai et al. 2007].
Table 1.
Autoimmune hepatitis – treatment considerations in special populations [Czaja, 2011].
| Acute, fulminant disease onset | Patients with acute, fulminant hepatitis at presentation have a less favourable response to corticosteroids and should be managed in the context of needing likely transplant |
| Patients with an acute but not fulminating disease onset may respond favourably to standard corticosteroid therapy. However, a failure of clinical and biochemical improvement after 1–2 weeks of therapy warrants careful review. A fall of less than two points in UKELD score after 7 days predicts treatment failure with 85% sensitivity [Yeoman et al. 2011] | |
| Pregnancy* | Pregnancy is generally uneventful in AIH, although can be associated with a lower live birth rate, maternal death, hepatic decompensation and need for transplant if the mother has cirrhosis |
| AIH flares are more common in the absence of immunosuppressive therapy although azathioprine is largely safe in pregnancy and no complications have been documented in the IBD literature in pregnant women. However, traces of azathioprine may be found in breast milk [Habal and Huang, 2012] | |
| Age >60 years | At presentation, disease is frequently indolent and aggressive with advanced liver fibrosis/cirrhosis and hepatic decompensation being common manifestations |
| Older patients respond very well to low doses of prednisolone (~10 mg) and improve more rapidly compared with individuals <40 years of age (24-month biochemical response 94% versus 64%); however, the risks of sepsis in decompensated patients require careful consideration | |
| Bone protection and maximizing the dose of azathioprine (to minimize corticosteroid use) are strongly encouraged | |
| Asymptomatic patients and mild disease$ | The decision whether to treat all in this group is controversial |
| 10-year survival of untreated patients is significantly lower than that of treated patients with severe disease, and taking the decision to refrain from treatment based on an assumption that mild disease does not progress may unnecessarily risk the development of adverse consequences | |
| Seronegative disease | 10–54% of patients with cryptogenic cirrhosis have AIH despite the absence of conventional autoantibodies |
| 19% of patients with AIH lack detectable autoantibodies at presentation | |
| Absence of autoantibodies should not delay the institution of immunosuppression in the patient with otherwise compatible features |
Liver disease may actually improve in pregnancy as the high oestrogen levels favour an anti-inflammatory cytokine shift. However, as blood oestrogen levels fall peri partum, AIH may be exacerbated.
Although the features of AIH may spontaneously resolve, rates are much less frequent in untreated individuals (12% versus 63%). The frequency of cirrhosis is similar between symptomatic and symptom-free patients, although those with symptoms may have higher inflammatory scores.
IBD, inflammatory bowel disease; UKELD, UK model for End-stage Liver Disease.
Inducing remission
Prednis(ol)one (20–30 mg/day) is the mainstay for inducing remission and in most cases is combined with azathioprine. Hepatotoxicity secondary to the latter is rare and in part dose dependent, being more common in those with decompensated liver disease [Lohse and Mieli-Vergani, 2011]. Therefore azathioprine can either be instituted from the outset (50 mg/day) or within a few weeks following steroid response at a dose of 1–2 mg/kg/day (Figure 2). Delaying introduction of azathioprine can be pragmatically helpful in managing and avoiding side effects of treatment. 6-Mercaptopurine as an alternative therapeutic option may be reserved for patients intolerant to azathioprine, although evidence for its efficacy in treating patients whose disease does not respond to azathioprine is lacking and largely anecdotal [Pratt et al. 1996].
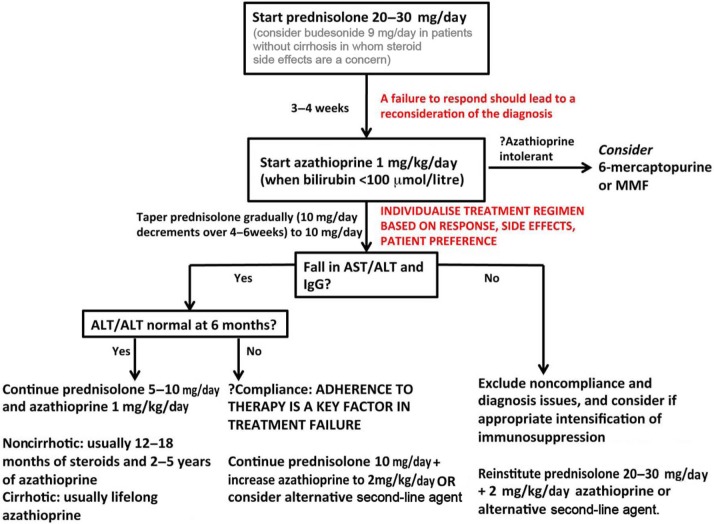
Figure 2.
Suggested induction regimen for autoimmune hepatitis (AIH). A stratified, personalized approach to patient management is best achieved through good continuity of care, and it is rare that rapid decisions need to be made; guidelines exist purely as a framework from which to base care. Adherence to therapy is as important as anything clinical in AIH treatment. Budesonide in combination with azathioprine is emerging as an alternative frontline agent to prednisolone for patients without cirrhosis and ought to be considered for patients with comorbidities that might be exacerbated by prednisolone treatment. Patients who receive a lower maintenance dose of prednisolone (~10 mg/day) in combination with azathioprine (50 mg/day) respond similarly to those who receive prednisolone alone (≥20 mg/day) albeit with fewer side effects (10% versus 44%). Therefore, a combination maintenance regimen is preferred. Bone protection in those with prolonged steroid use is strongly recommended. Given the perceived hepatotoxicity of azathioprine, particularly in patients with pre-existing jaundice, individuals are normally initiated on steroids first, monitored, and azathioprine added when disease response (e.g. bilirubin <100 µmol/litre) has been confirmed.
ALT, alanine transaminase; AST, aspartate transaminase; IgG, immunoglobulin G; MMF, mycophenolate mofetil; ULN, upper limit of normal.
The American Association for the Study of Liver Diseases guidelines include an option for starting with prednisone at 1 mg/kg/day (maximum 60 mg/day) [Manns et al. 2010a] during the induction phase, although such high doses are rarely necessary and are associated with side effects. However, in patients without cirrhosis, this may result in a more rapid normalization of serum transaminases [Schramm et al. 2010].
Maintaining remission
Remission is defined as a complete normalization of all inflammatory parameters, including AST, alanine transaminase (ALT) and bilirubin (biochemical remission); immunoglobulin G (IgG) (immunological remission); and histologically active inflammation. Remission is achievable in around 75–80% of patients after 24 months of treatment (including those with cirrhosis) and associated with around 80% patient survival at 10 years [Montano-Loza et al. 2007a; Johnson et al. 1995]. Normalization of serum aminotransferase levels within 3 months of starting therapy is highly predictive for sustained biochemical remission at 2 years [Wang et al. 2011]. Remission is usually maintained with prednisolone at around 7.5–12.5 mg/day alongside azathioprine 1–2 mg/kg/day (2 mg/kg/day when using azathioprine monotherapy or if previous relapses). A thioguanine nucleotide (TGN) concentration greater than 220 pmol/ 8 × 108 erythrocytes is predictive of remission [odds ratio 7.7], although there does not appear to be an association between TGN or methylmercaptopurine nucleotide concentration, or thiopurine methyltransferase activity and the development of leucopenia [Dhaliwal et al. 2012]. The optimum duration of therapy is controversial, but for patients without cirrhosis with type I AIH, a finite therapy with steroids for 12–18 months and azathioprine for 2–5 years, prior to a single trial off therapy, assuming all inflammatory parameters remain normal, is quite reasonable. Maintenance treatment with prednisolone and azathioprine has not been proven to be superior to azathioprine alone; however, monotherapy with azathioprine has only been systematically evaluated following histological confirmation of disease remission [Stellon et al. 1988; Johnson et al. 1995]. Nevertheless, tapering of steroids is commonly performed in clinical practice without repeat liver biopsy, provided full biochemical and immunological remission has been attained. Follow-up biopsies should however be considered given that 5% of patients per year develop cirrhosis, a rate much higher in those with ongoing inflammation. Moreover, relapse and progression to fibrosis are almost universal when immunosuppression is stopped in the presence of residual interface hepatitis [Carpenter and Czaja, 2002; Montano-Loza et al. 2007b; Czaja and Carpenter, 2003]. The combination of normal IgG and transaminase levels together correlates with lower histological inflammatory scores (Knodell index <4) [Lüth et al. 2008] in around 90% of cases, although histological remission may lag 3–6 months behind biochemical remission. Treatment until normal liver AST, ALT, bilirubin and γ-globulin levels, and normal liver histology is ideal as this reduces the frequency of relapse after drug withdrawal from 86% to 60% [Montano-Loza et al. 2007c].
Disease relapse
Relapse is characterized by an increase in aminotransferase levels (>3 × ULN) while under therapy (poor drug compliance being the first consideration) [Sockalingam et al. 2012], following tapering of steroid doses or after a complete withdrawal of immunosuppression [van Gerven et al. 2013], and can still occur despite the prior attainment of complete remission, including a histologically inactive follow up biopsy. Most relapses (Table 2) occur within 12 weeks of stopping treatment and up to 80% of patients relapse around 3 years following treatment withdrawal. Disease relapses are associated with progression to cirrhosis (38%), liver failure (14–20%), an increasing frequency of subsequent relapse (86% after the third round of treatment), reduced transplant-free survival, hepatocellular carcinoma (HCC) and all-cause mortality [Hoeroldt et al. 2011; Montano-Loza et al. 2007b; Yoshizawa et al. 2012]. Relapse is treated by reinstituting corticosteroids (20–30 mg) and optimizing the dose of azathioprine (2 mg/kg/day). Patients who have relapsed once, or who have cirrhosis, must not be given an opportunity for future relapse and should receive lifelong therapy, usually the goal being monotherapy with azathioprine if tolerated.
Table 2.
Factors predictive of poor outcome in autoimmune hepatitis.
| At presentation | During the treatment phase | On treatment withdrawal | |
|---|---|---|---|
| Relapse when off treatment (50–90% of patients) | Prolonged symptomatic course Anti-LKM-1 positive* Anti-SLA/LP positive Nonconventional antibodies (anti-ASPGR, chromatin) Male sex HLA DRB1*03 |
Treatment nonadherence Short treatment duration Prolonged time to achieve remission Persistent portal inflammation or interface hepatitis Azathioprine-free maintenance regimens |
Increasing duration since stopping treatment (50% relapse at 6 months; 80% at 3 years) |
| Progressive fibrosis/cirrhosis (10–50%) | Hypoalbuminaemia$
Coagulopathy$ Confluent necrosis |
Treatment nonadherence Persistent inflammatory change on biopsy Increasing time to biochemical remission (>24 months) Persistently abnormal liver biochemistry Number of relapses per decade (>4) |
Multiple relapses (38% progress to cirrhosis) |
| Liver-related death or OLT (10–20%) | Fulminant presentation Confluent necrosis Cirrhosis Female sex Anti-LKM-1, LC-1 or SLA/LP positive |
Treatment nonadherence Poor responders (failure of transaminases to fall after 6 months; persistently raised ALT or AST) Frequent relapses Nontreatment with azathioprine |
Multiple relapses Development of cirrhosis |
Modified from Gleeson et al. [2011].
Relapse is near universal in type-II/anti-LKM-1 positive patients without corticosteroids, and these patients often require low-dose, maintenance prednisolone.
May reflect the presence of cirrhosis at baseline rather than risk of development.
ALT, alanine transaminase; ASPGR, asialoglycoprotein receptor; AST, aspartate transaminase; LC, liver cytosol; HLA, human leukocyte antigen; LKM, liver kidney microsomal; SLA/LP, soluble liver/pancreas antigen.
Treatment failure
Assuming compliance and a correct diagnosis, patients who do not show clinical or laboratory improvement within 6 months of starting therapy (13%) may be incompletely responsive, whereas those whose condition deteriorates by any clinical or laboratory parameter despite compliance (~9%) are considered treatment failures [Montano-Loza et al. 2007b]. Such situations justify the reinstitution of prednisolone (variable dose depending on clinical setting) alone or in conjunction with azathioprine (2 mg/kg/day). High-dose steroids, if chosen, are maintained for at least 1 month. Thereafter, the steroid dose may be reduced according to the clinical response. Continued deterioration despite the above measures may be an indication for alternative therapies or transplantation, but histological evidence of ongoing inflammatory disease should be considered to ensure accurate diagnosis and exclusion of confounders, such as drug-induced liver injury. The development of cholestasis can be a feature of azathioprine hepatotoxicity but should also prompt a search for overlapping PSC, particularly if the patient has coexistent inflammatory bowel disease (IBD) or a resistance to conventional therapy.
Alternative treatments
Alternative therapies are typically applied following the development of side effects/complications while on standard immunosuppression, or due to a failure of standard therapy to induce or maintain remission. Many early pharmacological trials defined biochemical remission as achieving serum transaminase levels up to 2 × ULN. It was not until the publication of more recent studies that the importance of complete normalization of aminotransferase levels became fully appreciated, albeit this is context specific (e.g. age and severity being relevant) [Manns et al. 2010a]. Further discussions in this section will therefore classify ALT or AST up to 2 × ULN as ‘biochemical response’ and normalization of ALT or AST as ‘biochemical remission.’
Budesonide
Budesonide has a higher affinity for the glucocorticoid receptor than prednisolone but undergoes over 90% hepatic first pass metabolism with the resulting catabolites being devoid of glucocorticoid activity, potentially limiting steroid-related side effects. Until recently, evidence supporting the use of budesonide in AIH was reliant on small case series, with some suggesting that budesonide was effective at achieving biochemical response in less than one-third of patients, whereas in others, figures for complete remission approached 58–83% [Danielsson and Prytz, 1994; Czaja and Lindor, 2000]. These preliminary results implied that, at best, budesonide was noninferior to prednisolone but with possible improved tolerability. More recently a multicentre double-blind study randomized 203 patients with AIH to receive either budesonide or prednisone (both in combination with azathioprine) and observed biochemical remission at 6 months in 60% of the budesonide group versus only 38.8% with prednisone [Manns et al. 2010b]. In the open-label extension, whereby patients in the prednisone arm were switched to budesonide, biochemical remission was again more frequently attained in the cohort originally randomized to budesonide (68.2% versus 50.6%). Although promising, the overall proportion achieving remission on prednisone was clearly below that reported in historical case series. Moreover, histological correlates were not provided due to the short follow-up period, and prospective evaluation of repeat liver biopsy specimens following the attainment of biochemical and immunological remission while on budesonide would be potentially informative.
Despite improved tolerability with budesonide, the presence of advanced liver disease or porto-systemic shunts poses a risk for corticosteroid-induced side effects as a result of altered hepatic clearance and increased systemic availability. For this reason budesonide is not suitable for patients with cirrhosis and concern also exists for its use in those with a severe presentation [Mederacke et al. 2012]. Furthermore, switching prednisolone to budesonide in individuals already established on the former can be problematic because of severe steroid withdrawal symptoms, and budesonide has proven ineffective as a salvage agent for steroid-refractory or steroid-dependent AIH [Czaja and Lindor, 2000]. Nevertheless budesonide in combination with azathioprine is emerging as a promising alternative for induction in treatment-naïve patients without cirrhosis with uncomplicated AIH and may be especially appropriate for those with obesity, metabolic bone disease, diabetes, hypertension or when concern exists over the cosmetic side effects of prednisolone.
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is a potent immunosuppressive agent, although side effects include diarrhoea and bone marrow suppression, which may necessitate discontinuation in some patients [Hlivko et al. 2008]. In one small study, five of seven patients with AIH with persistently active inflammation and either intolerance or resistance to azathioprine who were treated with MMF achieved normal transaminases, a fall in hepatic activity index and required steroid dose within 3–9 months [Richardson et al. 2000]. However, results from a multicentre European study (n = 37) were more sobering, with MMF inducing a biochemical response in only 43% of cases, any benefit largely being restricted to patients intolerant of azathioprine [Hennes et al. 2008]. More favourable reports from Greece (n = 59) and the Netherlands (n = 45) found that 67–88% of patients taking MMF as an alternative to azathioprine achieved biochemical remission [Zachou et al. 2011; Baven-Pronk et al. 2011]. Furthermore, 37% in the former study achieved complete remission off prednisolone. Data from the paediatric population have also been encouraging [Aw et al. 2009]. MMF represents a possible alternative, although its role has largely been reserved for instances when patients are intolerant of azathioprine/6-mercaptopurine as opposed to being nonresponders. Of practical concern is its reported teratogenicity, which precludes use in women planning pregnancy.
Calcineurin inhibitors
Cyclosporine A (CyA) has been shown to be beneficial in inducing and maintaining remission, particularly for children with AIH [Alvarez et al. 1999; Sciveres et al. 2004; Cuarterolo et al. 2006]. The rapid onset of induction is a theoretical advantage over azathioprine, although relapses occur on dose reduction [Sherman et al. 1994; Jackson and Song, 1995]. Tacrolimus has greater immunosuppressive potency than CyA, and in one study the average AST fell in 11 patients with steroid-refractory AIH treated with tacrolimus with no related adverse events [Aqel et al. 2004]. Larsen and colleagues also observed a marked improvement in ALT, IgG as well as hepatic necro-inflammatory score in nine patients taking low-dose tacrolimus for steroid-refractory AIH [Larsen et al. 2007], whereas high rates of biochemical remission were recently reported in 12 of 13 patients from Virginia treated with tacrolimus [Tannous et al. 2011]. These preliminary findings suggest that tacrolimus is probably safe and offers promise to those whose condition is nonresponsive to standard therapy. However, longer-term follow up and safety data from larger studies are needed.
Other agents
Ursodeoxycholic acid (UDCA) has not been systematically evaluated in AIH, although in early trials patients did demonstrate a clinical and biochemical improvement [Czaja et al. 1999] with a reduction in histological inflammation. However, the severity of fibrosis was unaffected and UDCA did not permit a reduction in corticosteroid dose. A group from Denmark recently treated seven patients with AIH with everolimus and after 3–5 months, all had ALT levels less than 1.2 × ULN [Ytting and Larsen, 2011]. Cyclophosphamide has been evaluated in small case series and may prove useful as a salvage therapy for problematic patients [Kanzler et al. 1997]. Isolated case reports exist reporting responsiveness of AIH to rituximab [Santos et al. 2006] and the anti-tumour necrosis factor α (TNFα) agent etanercept [Goldenberg and Jorizzo, 2005]; however, infliximab and adalimumab have been reported to induce and exacerbate AIH [Subramaniam et al. 2011; Doyle et al. 2011]. Nevertheless, a marked biochemical and immunological response was observed in 11 patients with AIH (six with azathioprine intolerance or side effects and five with AIH refractory to conventional therapy) treated with infliximab, although infectious complications developed in over 63% from this group [Weiler-Normann et al. 2012]. Methotrexate may be effective as a steroid sparing agent, although given its potential hepatotoxicity and the efficacy of azathioprine, a widespread role has never materialized.
Therapy with regulatory T cells
The putative role of regulatory T cells (Treg) in the pathogenesis of AIH [Longhi et al. 2004, 2007, 2011] represents an attractive target for immune intervention aimed at reconstituting self tolerance. However, treatment has the potential to fail if adoptively transferred Treg are inhibited or trans differentiate into an effector pathway, although drugs that maintain Treg differentiation may help facilitate functional stability [Goodyear et al. 2012]. Another important factor is whether Treg therapy needs to be antigen specific [Peiseler et al. 2012], in which case treatment would currently only be possible in type II AIH when the antigen is known (CYP450IID6). Nevertheless the advent of cell-based therapy for AIH is being actively pursued by a number of groups.
Primary biliary cirrhosis
The median survival of untreated patients with PBC is approximately 9–10 years from presentation, with 26% developing liver failure during this time [Prince et al. 2002]. In the absence of effective therapy the median time to develop extensive fibrosis is around 2 years, with a probability of remaining in early stage disease being 29% over 4 years [Christensen et al. 1980; Locke et al. 1996; Corpechot et al. 2000]. Over recent years, UDCA has demonstrated consistent evidence in improving liver biochemistry in PBC, with patients also showing improved transplant-free and overall survival [Lindor et al. 1996]. The 4-year risk of developing varices is lower for UDCA-treated patients (16% versus 58%) [Lindor et al. 1997], and UDCA therapy is associated with a fivefold lower progression rate (7%) from early stage disease to extensive fibrosis/cirrhosis compared with placebo (35%) [Corpechot et al. 2000].
When evaluating the UDCA trial data, note must be taken of the treatment dose used because some earlier studies applied lower than optimal treatment paradigms [Gong et al. 2008]. In PBC a dose of 13–15 mg/kg/day has been shown to be superior to 5–7 mg/kg/day or 23–25 mg/kg/day [Angulo et al. 1999]. The Barcelona study was able to demonstrate that patients with PBC achieving an alkaline phosphatase (ALP) reduction of at least 40% from baseline 1 year after being started on UDCA therapy had a survival akin to that of the control population, and of 192 patients treated in their cohort, 61% demonstrated a biochemical improvement falling within these parameters (‘UDCA responders’). Conversely, nonresponders were more likely to progress to liver transplantation (relative risk 7.47) [Parés et al. 2006]. The more recent ‘Paris’ criteria (ALP < 3 × ULN, AST< 2 × ULN and serum bilirubin<1 mg/dl after 1 year of UDCA therapy) have also been shown to predict a 10-year transplant-free survival of 90% (versus 51% in nonresponders), with patients being less well discriminated by the Barcelona criteria (79% versus 63%) [Corpechot et al. 2008]. Moreover, patients with stage I/II PBC achieving an AST and ALP less than 1.5 × ULN, and normal bilirubin 12 months after starting UDCA therapy had an outcome free of liver-related death, transplantation, progression to cirrhosis or liver failure [Corpechot et al. 2011]. These studies highlight that absence of a biochemical response to UDCA has clear prognostic implications, and additionally failure to respond biochemically is associated with histologic progression [Kumagi et al. 2010]. UDCA is widely used and shown to reduce mortality, adverse events and need for transplantation in PBC [Lee et al. 2007]. It is therefore advocated as first-line therapy [Lindor et al. 2009; Beuers et al. 2009].
Alternative and adjuvant therapies
Approximately one-third of patients with PBC may not respond to UDCA, remain at risk of progressive liver disease and are candidates for alternative therapy. The biochemical criteria provided by the Paris, Barcelona and related studies provide good correlation to long-term outcome for UDCA-treated patients and have been adopted by international bodies for assessing the efficacy of new treatments in PBC [Lindor et al. 2009; Beuers et al. 2009]. Although these criteria facilitate small-scale pilot studies of short duration, it remains unclear whether an improvement in liver biochemistry induced by agents other than UDCA has a true beneficial effect on PBC disease progression. An additional group to target are perhaps those with disease-specific antinuclear antibody (e.g. anti-gp210, anti-centromere) reactivity patterns [Bogdanos and Komorowski, 2011], individuals who are highly fatigued [Jones et al. 2010], patients with the rare premature ductopenic variant of PBC [Vleggaar et al. 2001] and those with interface hepatitis, as these also represent subgroups of patients with a poorer outcome, even when considering UDCA response.
Corticosteroids
In patients with PBC with ‘florid’ interface hepatitis on biopsy, there are anecdotal data demonstrating the efficacy of budesonide in improving liver histology and biochemistry when used in combination with UDCA [Chazouilleres et al. 2006; Poupon, 2011; Rautiainen et al. 2005; Rabahi et al. 2010; Leuschner et al. 1999]; the premise is in part a reflection of the association between AST and interface hepatitis with disease progression in PBC. Many of these studies are not yet supported by well controlled trials, and have been of too short duration to show any real survival benefit. However, it should be noted that transaminitis in PBC may in part be a feature of hepatocyte injury from the effects of cholestasis. Of note, portal vein thrombosis has been reported as a complication in up to 29% of patients with advanced liver fibrosis [Hempfling et al. 2003], precluding budesonide use in patients with cirrhosis. Due to concerns over side effects, evidence supporting the benefit of prednisolone is lacking, and although some restitution in serum and histological markers of liver inflammation has been documented [Mitchison et al. 1992], these findings have not translated into improved patient outcomes.
Colchicine
Colchicine has a historical role in the treatment of PBC and several double-blind prospective trials have found that colchicine decreased serum ALP, ALT and AST, although not as effectively as UDCA [Kaplan et al. 1986; Vuoristo et al. 1995]. The addition of colchicine to UDCA has also been reported to improve liver histological necroinflammatory score over a 10-year follow up but with little/no effect on histological stage or predicted patient survival [Almasio et al. 2000; Kaplan et al. 2004; Leung et al. 2011]. In many of these preliminary studies, patients were not receiving the optimum dose of UDCA, thus making it difficult to draw firm conclusions regarding the true efficacy of combination therapy.
Fibric acid derivates
The first studies to evaluate the use of fibrates for PBC appeared in Japanese journals in the late 1990s. Broadly speaking, a normalization of serum ALP has been observed in around 45% of UDCA nonresponders with the addition of a fibric acid derivative (versus ~18% taking placebo) [Kanda et al. 2003]. A recent prospective multicentre study (n = 66) demonstrated that bezafibrate monotherapy was at least as effective as UDCA in improving biochemical indices in PBC, whereas combination fibrate/UDCA therapy was effective in improving and maintaining normal biliary enzymes when the ineffectiveness of UDCA monotherapy was confirmed [Iwasaki et al. 2008]. These positive results have been replicated in numerous other pilot studies [Ohira et al. 2002; Levy et al. 2011]. Despite improving surrogate markers of long-term prognosis, the depth of evidence supporting fibrates in PBC remains small, with many trials employing undefined biochemical endpoints as a measure of treatment success, and only a few applying standardized criteria with relatively short duration (<1 year). The effects on histological progression are also unclear; some studies reporting improvement whereas others a deterioration [Yano et al. 2002; Kurihara et al. 2002]. Given that preliminary data have been promising, further studies should be encouraged. Of note, in the authors’ experience, use of fenofibrate in patients with advanced PBC (jaundice) was not associated with benefit.
Other drugs
A number of studies have evaluated methotrexate as an immunomodulatory treatment, although in most the number of patients assessed was too small and trial duration too short to draw any firm conclusions. The largest (n = 265) randomized controlled trial (methotrexate/UDCA versus UDCA alone) did not find any significant differences in the rates of transplantation, transplantation-free survival, development of hepatic decompensation, biochemical deterioration or histological progression over a median study time of 7.6 years [Combes et al. 2005]. Azathioprine has very little effect on improving serum liver biochemistry or hepatic histology in PBC [Heathcote et al. 1976; Wolfhagen et al. 1998] and although early trials suggested a tendency toward improved survival [Christensen et al. 1985], this failed to reach statistical significance. Randomized trials have shown that cyclosporine had some effect on slowing biochemical and histological progression [Wiesner et al. 1990; Lombard et al. 1993]. Due to an increased rate of side effects, there has been little enthusiasm for further study on this drug, although it may protect against recurrent PBC post transplant, a complex area some relate to its potential antiviral properties [Neuberger et al. 2004]. Studies of MMF in addition to UDCA have not yielded beneficial results, although 6 of 15 UDCA nonresponders treated with the addition of budesonide and MMF had normalized liver biochemistry with the remainder achieving partial resolution. Histological activity and fibrosis were markedly improved in all patients [Rabahi et al. 2010]. Antiretroviral therapy (combivir) has also been trialled, largely stemming from the proposed viral aetiology of PBC [Mason, 2011]. Preliminary results have been mixed, although some patients did demonstrate minor improvements in hepatic biochemistry, symptoms and histological features [Mason et al. 2004, 2008]. A recent pilot study (n = 6) demonstrated an improvement in IgM titre and an increase in intrahepatic regulatory T-cell number using the anti-CD20 antibody rituximab [Tsuda et al. 2012]. Copper chelation therapies (D-pencillamine, tetrahydromolybdate), silymarin, moexipril or atorvastatin have not been shown to be effective in PBC.
Future therapeutic prospects
Farnesoid-X-receptor agonists
Farnesoid X receptor (FXR) is a nuclear receptor expressed at high levels in the liver and intestine. One of the primary functions of FXR activation is the suppression of cholesterol 7α hydroxylase-1 (CYP7A1), the rate-limiting enzyme in the synthesis of bile acids from cholesterol. Obeticholic acid (OCA) is a primary agonist for FXR and evidence from animal models supports the role of such agents in protecting the liver from cholestatic injury [Fiorucci et al. 2005; Modica et al. 2012]. Phase II clinical studies in PBC have recently reported that OCA significantly lowered ALP by around 40% compared with placebo (21–24.7% versus 7%) in UDCA nonresponders; however, some patients had to stop therapy due to severe pruritus [Mason et al. 2010; Kowdley et al. 2011]. A multicentre phase III trial is currently underway. Given the promising results thus far, exploring the therapeutic potential of manipulating other nuclear receptors involved in bile salt metabolism (e.g. liver X receptor, vitamin D receptor) may also prove beneficial in PBC.
Molecular therapies
A number of novel molecular therapies are also currently undergoing clinical trials in PBC (Figure 3), having arisen as our understanding of disease aetiopathogenesis improves [Trivedi and Cullen, 2012].
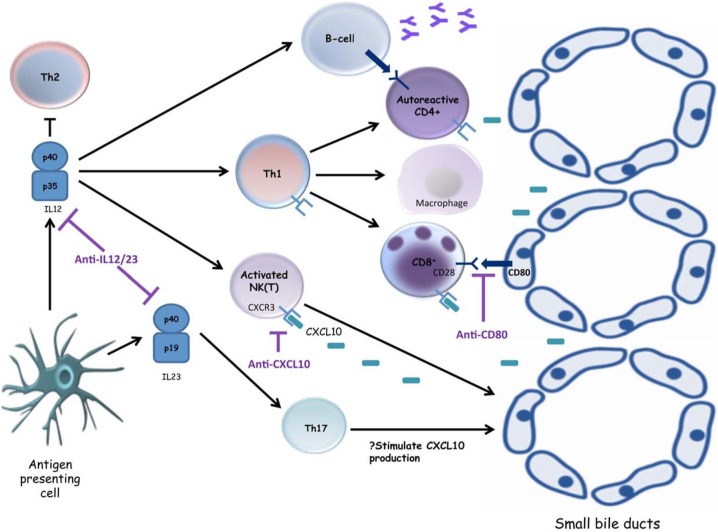
Figure 3.
Emerging small-molecule inhibitors in primary biliary cirrhosis. Interleukin (IL)-12 secretion by an activated antigen presenting cell leads to natural killer (NK) cytotoxicity, macrophage activation and T helper 1 (Th1) differentiation, with subsequent activation of cytotoxic CD8+ T cells as well as antibody-secreting B cells, all of which potentiate the destruction of cholangiocytes. The p40 subunit of IL12 is also a component of IL23, an essential cytokine for the differentiation of Th17 cells. Targeting the p40 subunit has been shown to ameliorate experimental immune-mediated cholangiopathy, and it is hoped these promising results can be translated into clinical practice. The immune-mediated destruction of small-sized bile ducts in primary biliary cirrhosis (PBC) is predominantly cell mediated, characterized by Th1 cells, CD8+ T cells, NK cells and NKT cells which express CXCR3. Neutralizing antibodies to CXCL10, a ligand for CXCR3, may offer the possibility to interfere with one of the key inflammatory processes contributing to immune-mediated biliary destruction in PBC. Blockade of costimulatory signals between T cells expressing CD28 and antigen-presenting cells expressing CD80 (e.g. cholangiocytes, antibody-secreting B cells) could also represent an important approach for the treatment of autoimmune diseases.
CXCL10 monoclonal antibodies
In PBC, liver-infiltrating CD8+ T cells, T helper 1 cells and natural killer cells express the chemokine receptor CXCR3, and murine models have demonstrated that knockout of CXCR3 results in amelioration of hepatic disease [Zhang et al. 2011]. One of the natural ligands for CXCR3 is the chemokine CXCL10, which is induced in damaged bile ducts and elevated in the serum of patients with PBC, with levels increasing as disease progresses [Chuang et al. 2005]. Anti-CXCL10 antibodies have also been shown to ameliorate lymphocyte-mediated damage and dramatically reduce liver fibrosis [Hintermann et al. 2010] thus may offer the possibility to interfere with one of the key inflammatory processes contributing to disease pathogenesis in PBC. A multicentre phase II trial using an anti-CXCL10 monoclonal antibody in PBC is currently in progress.
Anti-interleukin 12/interleukin 23
Interleukin (IL)-12 and IL23 are heterodimeric cytokines, sharing the p40 subunit, and play critical roles in linking innate and adaptive immune responses [Lleo et al. 2012]. Genome-wide association studies (GWAS) have identified components of the IL12 signalling pathway as potential players in the effector mechanisms leading to biliary destruction in PBC [Hirschfield et al. 2009], and deletion of the p40 subunit of IL12 in mouse models has been shown to modify murine autoimmune cholangiopathy [Yoshida et al. 2009]. A human monoclonal antibody directed against IL12/IL23 has shown success in the treatment of psoriasis and Crohn’s disease [Sandborn et al. 2008], and a phase II study in PBC has begun recruiting.
Anti-CD80
Polymorphisms in CD80 have been identified as conferring an increased susceptibility to PBC in a recent UK GWAS [Mells et al. 2011]. CD80 and CD86 represent potent costimulatory signals for T-cell activation and are, for example, expressed on small-duct cholangiocytes in PBC. This interaction confers an increased susceptibility to apoptosis and is also involved in activation of the nuclear factor κB pathway, which modulates the balance of survival and apoptosis in activated hepatic stellate cells. Blockade of costimulation between T cells and antigen-presenting cells through CD80 could represent an important therapeutic approach for the treatment of PBC and a phase II trial of an oral anti-CD80 agent has been proposed.
Primary sclerosing cholangitis
Up to 44% of patients with PSC may be asymptomatic at presentation, being diagnosed following the detection of cholestatic liver biochemistry in the context of pre-existing IBD [Broomé et al. 1996]. In some patients with previously stable disease can rapidly progress following the development of septic biliary complications (recurrent ascending cholangitis) due to impeded biliary drainage. Dominant strictures (DSt) are the principal cause of such complications, with 11.7–35.9% of patients with PSC presenting with DSt at diagnosis [Gotthardt et al. 2010]. Improved transplant-free survival may be achieved when endoscopic biliary or surgical intervention is performed [Gotthardt et al. 2010; Tsai and Pawlik, 2009]; however, given a paucity of randomized controlled trials in the area, the optimal approach for treating DSt remains unclear and the mainstay of treatment in PSC remains largely pharmacological. However, as the pathogenesis remains largely undefined, a causal therapeutic approach has yet to be developed and no currently identified medical treatment has been consistently shown to improve outcome free of liver transplantation in PSC.
The mean time from diagnosis to death/liver transplantation ranges from 9.6 to 12 years, whereas there also lies a 10–15% lifetime risk of cholangiocarcinoma (CCA) [Broomé et al. 1996; Ponsioen et al. 2002], the risk being greatest in patients with a dominant stricture (>25%) [Chapman et al. 2012]. Furthermore, up to 5% of patients develop CCA within 12 months of PSC diagnosis [Bergquist et al. 1998, 2002; Tischendorf et al. 2007]. ‘Small-duct PSC’ has a considerably better outcome than the classical form, with a longer transplant-free and overall survival (29 years versus 17 years) [Björnsson et al. 2002, 2008; Broomé et al. 2002; Angulo et al. 2002]. Although these patients do not develop CCA, approximately 28% progress to the large duct variant over a median of 7.4 years.
Patients with PSC with IBD (>75%) appear to have a shorter transplant-free and overall survival, as well as an increased risk of CCA compared with patients without IBD [Trivedi and Chapman 2012]. IBD is diagnosed at least 1 year before PSC, more often among patients with CCA (90%) compared with those without (65%), and the duration of IBD before diagnosis of PSC is also significantly longer in patients who develop CCA (17.4 years versus 9.0 years) [Boberg et al. 2002]. In a 20-year prospective follow up of 171 patients with PSC, all who developed CCA in the context of DSt had evidence of concurrent IBD, whereas no CCA was found in patients with PSC without IBD. The 18-year transplant-free survival for those with IBD was also considerably lower than those without (23% versus77.8%) [Rudolph et al. 2010].
Identifying endpoints of therapy
Adverse endpoints like malignancy, death or transplantation are impractical for evaluating new therapies in PSC given the long duration of disease. Moreover, the heterogeneity of PSC makes accurate patient selection and stratification for clinical trials difficult, particularly with regard to cholangiographic pattern of disease, the presence and influence of coexisting IBD, and in those having overlapping features with AIH/IgG4-associated cholangitis.
Despite there being some evidence that patients who achieve (and sustain) a completely normal serum ALP have a good prognosis [Stanich et al. 2011], there has historically been little support to the notion that merely improving liver biochemistry translates into better long-term clinical outcome in PSC. However, this paradigm has recently been challenged following the publication of a study by Al-Mamari and colleagues (n = 139) in which patients who attained a reduction in serum ALP up to 1.5 × ULN over 24 months were observed to have a significantly better outcome than individuals with a persistently elevated ALP [Al-Mamari et al. 2012]. In this observational study, biochemical improvement within these parameters was achieved by 40% of patients over a median of 2 years, of which only 6% (n = 3) of individuals developed a clinical endpoint (hepatic decompensation or liver transplantation) versus 38% in the group without an ALP reduction (p < 0.0001). The end point free survival was also significantly longer in patients with an ALP improvement (p < 0.0001). Moreover, no patient in the group achieving an ALP less than 1.5 × ULN developed CCA or liver-related death versus 15% and 23% (p = 0.002) respectively in the group with persistently raised ALP. Similar results were observed in a population of 198 patients from Sweden in which a reduction in serum ALP by 40% from baseline was found to correlate with better long-term patient survival [Lindström et al. 2012a]. In both of these studies, improvement in serum ALP within the aforementioned parameters identified patients more likely to experience a benign clinical outcome irrespective of whether ALP reduction was achieved whilst taking UDCA or off therapy. Taken together, these findings may help in the design of future clinical trials, provided the results can be validated by other centres.
Ursodeoxycholic acid
Numerous studies have endeavoured to address the efficacy of UDCA in PSC; however, due to relatively small patient numbers, suboptimal doses and short follow-up periods, results have been inconsistent. Most demonstrated some improvement in liver biochemistry [Poropat et al. 2011; Harnois et al. 2001; O’Brien et al. 1991; Lindor 1997; van Hoogstraten et al. 1998], but few suggest an improvement in histological features [Mitchell et al. 2001; Beuers et al. 1992; Stiehl et al. 1994; Cullen et al. 2008]. Some data also point to an improvement in the cholangiographic appearances of PSC [Mitchell et al. 2001], degree of liver fibrosis [Mitchell et al. 2001; Cullen et al. 2008] and survival [Cullen et al. 2008; Olsson et al. 2005]. Enrichment of bile with UDCA is dose dependant, reaching a plateau between 22 and 25 mg/kg/day. Higher doses (28–30 mg/kg/day), although associated with improvements in liver biochemistry, appear to confer harm to patients, with greater numbers developing endpoints including death and transplantation [Lindor et al. 2009; Imam et al. 2011]. This may be due to larger quantities of unabsorbed UDCA entering the colon, being subsequently modified to hepatotoxic bile acids or perhaps the antiapoptotic activity of UDCA preventing apoptosis of activated stellate cells, thereby promoting fibrogenesis [Lindor et al. 2009; Benedetti et al. 1997; Fickert et al. 2002]. In sum, the available data suggest that UDCA at doses of 17–20 mg/kg/day improves liver biochemistry, although transplant-free survival does not appear to be affected [Triantos et al. 2011], with higher doses leading to adverse consequences. Therefore, it is quite reasonable to not prescribe UDCA to patients with PSC.
Ursodeoxycholic acid and chemoprevention
Nontreatment with UDCA has previously been identified as an independent predictor of hepatobiliary malignancy in PSC [Brandsaeter et al. 2004]; however, actual benefit is difficult to demonstrate as around 50% of CCA occur within the first year after diagnosis of PSC. One randomized controlled trial did not find any significant difference comparing UDCA with placebo over a 5-year period [Olsson et al. 2005], although conflicting results were obtained from a German study in which no cases of CCA were detectable in 150 patients with PSC taking UDCA for longer than 8 years [Rudolph et al. 2007].
The estimated risk of colorectal cancer in patients with PSC with ulcerative colitis is approximately 30% over 20 years, despite colitis activity being quiescent in most [Trivedi and Chapman 2012]. Several studies have found a significantly reduced prevalence of colonic dysplasia in patients taking ‘standard-dose’ UDCA [Pardi et al. 2003]. Although some describe this chemoprotective effect as being relatively modest [Wolf et al. 2005], the cumulative mortality did appear better in those taking UDCA. However, a recently published follow-up study to a clinical trial of UDCA (17–23 mg/kg/day) in PSC reported no difference in the frequency of colorectal dysplasia, cancer or dysplasia/cancer-free survival over a 5-year period [Lindström et al. 2012b]. Moreover a study from the Mayo Clinic found a greater association of colonic dysplasia in patients with PSC/IBD taking UDCA compared with those receiving placebo (hazard ratio 4.44) [Eaton et al. 2011], although these patients were receiving high doses (28–30 mg/kg/day).
Immunosuppression
A host of immunomodulatory agents have been evaluated in PSC. Although they may induce an improvement in biochemical indices, these therapies have proven largely disappointing and fail to slow disease progression or improve prognosis, with the possible exception of carefully selected patients having overlapping features with AIH [Trivedi and Hirschfield, 2012]. Moreover, in individuals with suspected PSC and markedly elevated serum IgG4 levels, or an IgG4-positive plasma cell infiltrate evident histologically, treatment with corticosteroids may be considered [Webster et al. 2009]. In such a situation, the diagnosis of PSC should be questioned since an autoimmune cholangiopathy might be present. A form of sclerosing cholangitis associated with florid autoimmune features has also been described in children [Gregorio et al. 2001], where despite the parenchymal liver damage responding well to immunosuppression, biliary disease progresses in around 50% of patients.
Antibiotics
In advanced stages of PSC, patients have recurrent episodes of bacterial cholangiosepsis which itself can lead to disease progression. Such patients may benefit from prophylactic antibiotics whereas other approaches have sought to identify their efficacy longer term. Metronidazole together with UDCA (compared with UDCA alone) was shown to significantly improve serum ALP in PSC; however, an impact on liver histology or cholangiography was not found to be statistically significant in a randomized controlled study of 80 patients conducted over 36 months [Farkkila et al. 2004]. Similar results have been found using minocycline [Silveira et al. 2009]. A paediatric study (n = 14) evaluating the efficacy of vancomycin also found an improvement in biochemical parameters as well as symptom profile after 2 months of therapy [Davies et al. 2008]. This was particularly marked in patients without cirrhosis.
Emerging therapeutic candidates
24-norUrsodeoxycholic acid
24-norUrsodeoxycholic acid (norUDCA) is the C23-homologue of UDCA, largely being secreted in an unchanged or glucuronidated form. Because of cholehepatic shunting, norUDCA increases the flow of a bicarbonate-rich choleresis. In animal models of PSC, norUDCA has been shown to exhibit anti-inflammatory, antifibrotic and antiproliferative effects, increasing the hydrophilicity of biliary bile acids whilst stimulating bile flow [Fickert et al. 2006]. These encouraging results can hopefully be extrapolated to human PSC and clinical trials are currently being planned.
Adhesion molecule/chemokine manipulation
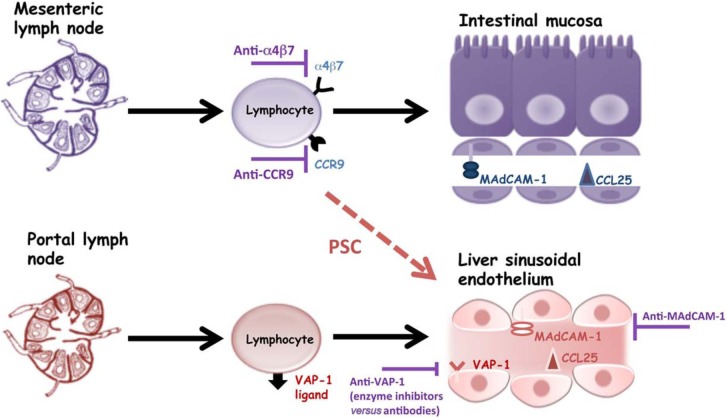
In adults, the liver and gut have distinct endothelial phenotypes whereby vascular adhesion protein 1 (VAP-1) expressed on hepatic sinusoids is implicated in lymphocyte recruitment to the liver, and similarly mucosal addressin cell adhesion molecule 1 (MAdCAM-1) and the chemokine CCL25 are expressed on intestinal mucosal vessels where they are involved in the recruitment of lymphocytes expressing the integrin α4β7 and chemokine receptor CCR9. However, VAP-1 expression becomes induced on mesenteric vessels in IBD, whereas MAdCAM-1 and CCL25 are increased on hepatic endothelium in PSC where they support the recruitment and adhesion of α4β7hiCCR9+ intestinal mucosal lymphocytes [Adams et al. 2008]. As well as being an adhesion molecule, VAP-1 is an enzyme possessing amine oxidase activity, and deamination of the dietary constituent methylamine by VAP-1 has been recently shown to induce the expression of functional MAdCAM-1 on hepatic endothelium in the presence of TNFα [Liaskou et al. 2011]. Although trials of anti-TNFα therapy did not show benefit in treating patients with PSC, the availability of enzyme inhibitors and neutralizing antibodies against VAP-1 are potentially attractive as future therapeutic agents. Monoclonal antibodies targeting α4β7, MAdCAM-1 and CCR9, which are currently undergoing clinical trials in IBD, may also represent potential for future treatment in PSC (Figure 4).
Figure 4.
Therapeutic manipulation of adhesion molecules in primary sclerosing cholangitis (PSC). In PSC, hepatic vascular adhesion protein 1 (VAP-1) expression is upregulated and its inherent enzymatic properties enable the generation of mucosal addressin cell adhesion molecule 1 (MAdCAM-1) on liver sinusoidal endothelium. Hepatic CCL25 expression is also upregulated in the PSC liver, although the reasons for this are unclear. The end result is that lymphocytes that have been generated to recognize gut antigens in the setting of inflammatory bowel disease are now misdirected to the liver where they contribute to inflammation and biliary destruction. There are now several available agents targeting these adhesion molecule and chemokine interactions which may represent viable therapeutic options for investigation in PSC.
Other agents
The antiproliferative effects of sirolimus were recently shown to improve liver fibrosis and inflammation in bile duct ligated rats [Patsenker et al. 2011] and represent an avenue to be explored further in human studies. In cftr−/− knockout mice, supplementation of the fatty acid docosahexaenoic acid (DHA) was shown to reverse the development of bile duct injury. In a recent pilot study (n = 23) the mean ALP significantly improved after 12 months of DHA treatment [Martin et al. 2012]. Fibric acid derivates stimulate biliary phospholipid secretion via the peroxisome proliferator-activated receptor α–multi-drug resistance protein 3 (MDR3) pathway and may also represent putative therapeutic agents in carefully selected patients [Kita et al. 2002].
Conclusion
When managing patients with autoimmune liver disease, a long-term, longitudinal approach to care must be adopted that focuses on clarity, accuracy and rationale for intervention. In the majority of patients with AIH and PBC, remission can be obtained with currently available pharmacotherapy yielding a favourable patient outcome. However, in one-third of patients with PBC and the majority of those with PSC there exists a shortfall of available medical therapies. This void may hopefully be overcome with the advent of molecular-targeted agents. However, the slow natural history exhibited by these diseases and the fact that patients are being diagnosed at a much earlier stage represents a clinical hurdle when choosing surrogate endpoints for clinical studies.
Footnotes
Funding: Dr Palak Trivedi received research funding from the Wellcome Trust Clinical Research Fellowship Program.
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Palak J. Trivedi, Centre for Liver Research and NIHR Biomedical Research Unit, University of Birmingham, Birmingham, UK
Gideon M. Hirschfield, Centre for Liver Research and NIHR Biomedical Research Unit, 5th Floor, Institute of Biomedical Research, The Medical School, University of Birmingham, Birmingham B15 2TT, UK
References
- Adams D., Eksteen B., Curbishley S. (2008) Immunology of the gut and liver: a love/hate relationship. Gut 57: 838–848 [DOI] [PubMed] [Google Scholar]
- Al Mamari S., Djordjevic J., Halliday J., Chapman R. (2012) Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol 58: 329–334 [DOI] [PubMed] [Google Scholar]
- Almasio P., Floreani A., Chiaramonte M., Provenzano G., Battezzati P., Crosignani A., et al. (2000) Multicentre randomized placebo-controlled trial of ursodeoxycholic acid with or without colchicine in symptomatic primary biliary cirrhosis. Aliment Pharmacol Ther 14: 1645–1652 [DOI] [PubMed] [Google Scholar]
- Alvarez F., Ciocca M., Canero-Velasco C., Ramonet M., de Davila M., Cuarterolo M., et al. (1999) Short-term cyclosporine induces a remission of autoimmune hepatitis in children. J Hepatol 30: 222–227 [DOI] [PubMed] [Google Scholar]
- Angulo P., Dickson E., Therneau T., Jorgensen R., Smith C., DeSotel C., et al. (1999) Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomized trial. J Hepatol 30: 830–835 [DOI] [PubMed] [Google Scholar]
- Angulo P., Maor-Kendler Y., Lindor K. (2002) Small-duct primary sclerosing cholangitis: prevalence and natural history. Hepatology 35: 1494–1500 [DOI] [PubMed] [Google Scholar]
- Aqel B., Machicao V., Rosser B., Satyanarayana R., Harnois D., Dickson R. (2004) Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol 2004; 38: 805–809 [DOI] [PubMed] [Google Scholar]
- Aw M., Dhawan A., Samyn M., Bargiota A., Mieli-Vergani G. (2009) Mycophenolate mofetil as rescue treatment for autoimmune liver disease in children: a 5-year follow-up. J Hepatol 51: 156–160 [DOI] [PubMed] [Google Scholar]
- Baven-Pronk A., Coenraad M., van Buuren H., de Man R., van Erpecum K., Lamers M., et al. (2011) The role of mycophenolate mofetil in the management of autoimmune hepatitis and overlap syndromes. Aliment Pharmacol Ther 34: 335–343 [DOI] [PubMed] [Google Scholar]
- Benedetti A., Alvaro D., Bassotti C., Gigliozzi A., Ferretti G., La Rosa T., et al. (1997) Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductule fragments and isolated perfused rat liver. Hepatology 26: 9–21 [DOI] [PubMed] [Google Scholar]
- Bergquist A., Ekbom A., Olsson R., Kornfeldt D., Lööf L., Danielsson A., et al. (2002) Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 36: 321–327 [DOI] [PubMed] [Google Scholar]
- Bergquist A., Glaumann H., Persson B., Broome U. (1998) Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case–control study. Hepatology 27: 311–316 [DOI] [PubMed] [Google Scholar]
- Beuers U., Boberg K., Chapman R., Chazouillères O., Invernizzi P., Jones D., et al. (2009) EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol 51: 237–267 [DOI] [PubMed] [Google Scholar]
- Beuers U., Spengler U., Kruis W., Aydemir U., Wiebecke B., Heldwein W., et al. (1992) Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo controlled trial. Hepatology 16: 707–714 [DOI] [PubMed] [Google Scholar]
- Björnsson E., Boberg K., Cullen S., Fleming K., Clausen O., Fausa O., et al. (2002) Patients with small duct primary sclerosing cholangitis have a favorable long-term prognosis. Gut 51: 731–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsson E., Olsson R., Berquist A., Lindgren S., Braden B., Chapman R., et al. (2008) The natural history of small-duct primary sclerosing cholangitis. Gastroenterology 134: 975–980 [DOI] [PubMed] [Google Scholar]
- Boberg K., Bergquist A., Mitchell S., Pares A., Rosina F., Broomé U., et al. (2002) Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol 37: 1205–1211 [DOI] [PubMed] [Google Scholar]
- Bogdanos D., Komorowski L. (2011) Disease-specific autoantibodies in primary biliary cirrhosis. Clin Chim Acta 412: 502–512 [DOI] [PubMed] [Google Scholar]
- Brandsaeter B., Isoniemi H., Broome U., Olausson M., Bäckman L., Hansen B., et al. (2004) Liver transplantation for primary sclerosing cholangitis: predictors and consequences of hepatobiliary malignancy. J Hepatol 40: 815–822 [DOI] [PubMed] [Google Scholar]
- Bressler B., Pinto R., El-Ashry D., Heathcote E. (2005) Which patients with primary biliary cirrhosis or primary sclerosing cholangitis should undergo endoscopic screening for oesophageal varices detection? Gut 54: 407–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomé U., Glaumann H., Lindstöm E., Lööf L., Almer S., Prytz H., et al. (2002) Natural history and outcome in 32 Swedish patients with small duct primary sclerosing cholangitis (PSC). J Hepatol 36: 586–589 [DOI] [PubMed] [Google Scholar]
- Broomé U., Olsson R., Lööf L., Bodemar G., Hultcrantz R., Danielsson A., et al. (1996) Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 38: 610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter H., Czaja A. (2002) The role of histologic evaluation in the diagnosis and management of autoimmune hepatitis and its variants. Clin Liver Dis 6: 685–705 [DOI] [PubMed] [Google Scholar]
- Chapman M., Webster G., Bannoo S., Johnson G., Wittmann J., Pereira S. (2012) Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol 24: 1051–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazouilleres O., Wendum D., Serfaty L., Rosmorduc O., Poupon R. (2006) Long term outcome and response to therapy of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. J Hepatol 44: 400–406 [DOI] [PubMed] [Google Scholar]
- Christensen E., Crowe J., Doniach D., Popper H., Ranek L., Rodés J., et al. (1980) Clinical pattern and course of disease in primary biliary cirrhosis based on an analysis of 236 patients. Gastroenterology 78: 236–246 [PubMed] [Google Scholar]
- Christensen E., Neuberger J., Crowe J., Altman D., Popper H., Portmann B., et al. (1985) Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology 89: 1084–1091 [DOI] [PubMed] [Google Scholar]
- Chuang Y., Lian Z., Cheng C., Lan R., Yang G., Moritoki Y., et al. (2005) Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J Autoimmun 25: 126–132 [DOI] [PubMed] [Google Scholar]
- Combes B., Emerson S., Flye N., Munoz S., Luketic V., Mayo M., et al. (2005) Methotrexate (MTX) plus ursodeoxycholic acid (UDCA) in the treatment of primary biliary cirrhosis. Hepatology 42: 1184–1193 [DOI] [PubMed] [Google Scholar]
- Corpechot C., Abenavoli L., Rabahi N., Chrétien Y., Andréani T., Johanet C., et al. (2008) Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 48: 871–877 [DOI] [PubMed] [Google Scholar]
- Corpechot C., Carrat F., Bonnand A., Poupon R., Poupon R. (2000) The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology 32: 1196–1199 [DOI] [PubMed] [Google Scholar]
- Corpechot C., Chazouillères O., Poupon R. (2011) Early primary biliary cirrhosis: Biochemical response to treatment and prediction of long-term outcome. J Hepatol 55: 1361–1367 [DOI] [PubMed] [Google Scholar]
- Cuarterolo M., Ciocca M., Velasco C., Ramonet M., González T., López S., et al. (2006) Follow-up of children with autoimmune hepatitis treated with cyclosporine. J Pediatr Gastroenterol Nutr 43: 635–639 [DOI] [PubMed] [Google Scholar]
- Cullen S., Rust C., Fleming K., Edward C., Beuers U., Chapman R. (2008) High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol 48: 792–800 [DOI] [PubMed] [Google Scholar]
- Czaja A. (2009a) Features and consequences of untreated type 1 autoimmune hepatitis. Liver Int 29: 816–823 [DOI] [PubMed] [Google Scholar]
- Czaja A. (2009b) Rapidity of treatment response and outcome in type 1 autoimmune hepatitis. J Hepatol 51: 161–167 [DOI] [PubMed] [Google Scholar]
- Czaja A. (2011) Autoimmune hepatitis in special patient populations. Best Pract Res Clin Gastroenterol 25: 689–700 [DOI] [PubMed] [Google Scholar]
- Czaja A., Carpenter H. (2003) Histological features associated with relapse after corticosteroid withdrawal in type 1 autoimmune hepatitis. Liver Int 23: 116–123 [DOI] [PubMed] [Google Scholar]
- Czaja A., Carpenter H., Lindor K. (1999) Ursodeoxycholic acid as adjunctive therapy for problematic type 1 autoimmune hepatitis: a randomized placebo-controlled treatment trial. Hepatology 30: 1381–1386 [DOI] [PubMed] [Google Scholar]
- Czaja A., Lindor K. (2000) Failure of budesonide in a pilot study of treatment-dependent autoimmune hepatitis. Gastroenterology 119: 1312–1316 [DOI] [PubMed] [Google Scholar]
- Danielsson A., Prytz H. (1994) Oral budesonide for treatment of autoimmune chronic active hepatitis. Aliment Pharmacol Ther 8: 585–590 [DOI] [PubMed] [Google Scholar]
- Davies Y., Cox K., Abdullah B., Safta A., Terry A., Cox K. (2008) Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. J Pediatr Gastroenterol Nutr 47: 61–67 [DOI] [PubMed] [Google Scholar]
- Decock S., McGee P., Hirschfield G. (2009) Autoimmune liver disease for the non-specialist. BMJ 339: b3305. [DOI] [PubMed] [Google Scholar]
- De Groote J., Fevery J., Lepoutre L. (1978) Long-term follow-up of chronic active hepatitis of moderate severity. Gut 19: 510–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal H., Anderson R., Thornhill E., Schneider S., McFarlane E., Gleeson D., et al. (2012) Clinical significance of azathioprine metabolites for the maintenance of remission in autoimmune hepatitis. Hepatology 56: 1401–1408 [DOI] [PubMed] [Google Scholar]
- Doyle A., Forbes G., Kontorinis N. (2011) Autoimmune hepatitis during infliximab therapy for Crohn’s disease: a case report. J Crohns Colitis 5: 253–255 [DOI] [PubMed] [Google Scholar]
- Eaton J., Silveira M., Pardi D., Sinakos E., Kowdley K., Luketic V., et al. (2011) High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol 106: 1638–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J., Thackeray E., Lindor K. (2012) Likelihood of malignancy in gallbladder polyps and outcomes following cholecystectomy in primary sclerosing cholangitis. Am J Gastroenterol 107: 431–439 [DOI] [PubMed] [Google Scholar]
- Farkkila M., Karvonen A., Nurmi H., Nuutinen H., Taavitsainen M., Pikkarainen P., et al. (2004) Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology 40: 1379–1386 [DOI] [PubMed] [Google Scholar]
- Feld J., Dinh H., Arenovich T., Marcus V., Wanless I., Heathcote E. (2005) Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology 42: 53–62 [DOI] [PubMed] [Google Scholar]
- Fickert P., Wagner M., Marschall H., Fuchsbichler A., Zollner G., Tsybrovskyy O., et al. (2006) 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 130: 465–481 [DOI] [PubMed] [Google Scholar]
- Fickert P., Zollner G., Fuchsbichler A., Stumptner C., Weiglein A., Lammert F., et al. (2002) Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 123: 1238–1251 [DOI] [PubMed] [Google Scholar]
- Fiorucci S., Clerici C., Antonelli E., Orlandi S., Goodwin B., Sadeghpour B., et al. (2005) Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther 313: 604–612 [DOI] [PubMed] [Google Scholar]
- Gleeson D., Heneghan M.; British Society of Gastroenterology (2011) British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut 60: 1611–1629 [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Jorizzo J. (2005) Use of etanercept in treatment of pyoderma gangrenosum in a patient with autoimmune hepatitis. J Dermatol Treat 16: 347–349 [DOI] [PubMed] [Google Scholar]
- Gong Y., Huang Z., Christensen E., Gluud C. (2008) Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev (3): CD000551. [DOI] [PubMed] [Google Scholar]
- Goodyear O., Dennis M., Jilani N., Loke J., Siddique S., Ryan G., et al. (2012) Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 119: 3361–3369 [DOI] [PubMed] [Google Scholar]
- Gotthardt D., Rudolph G., Kloters-Plachky P., Kulaksiz H., Stiehl A. (2010) Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc 71: 527–534 [DOI] [PubMed] [Google Scholar]
- Gregorio G., Portmann B., Karani J., Harrison P., Donaldson P., Vergani D., et al. (2001) Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology 33: 544–553 [DOI] [PubMed] [Google Scholar]
- Habal F., Huang V. (2012) Review article: a decision-making algorithm for the management of pregnancy in the inflammatory bowel disease patient. Aliment Pharmacol Ther 35: 501–515 [DOI] [PubMed] [Google Scholar]
- Harnois D., Angulo P., Jorgensen R., Larusso N., Lindor K. (2001) High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol 96: 1558–1562 [DOI] [PubMed] [Google Scholar]
- Heathcote J., Ross A., Sherlock S. (1976) A prospective controlled trial of azathioprine in primary biliary cirrhosis. Gastroenterology 70: 656–660 [PubMed] [Google Scholar]
- Hempfling W., Grunhage F., Dilger K., Reichel C., Beuers U., Sauerbruch T. (2003) Pharmacokinetics and pharmacodynamic action of budesonide in early- and late-stage primary biliary cirrhosis. Hepatology 38: 196–202 [DOI] [PubMed] [Google Scholar]
- Hennes E., Oo Y., Schramm C., Denzer U., Buggisch P., Wiegard C., et al. (2008) Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol 103: 3063–3070 [DOI] [PubMed] [Google Scholar]
- Hintermann E., Bayer M., Pfeilschifter J., Luster A., Christen U. (2010) CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun 35: 424–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfield G., Liu X., Xu C., Lu Y., Xie G., Lu Y., et al. (2009) Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med 360: 2544–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlivko J., Shiffman M., Stravitz R., Luketic V., Sanyal A., Fuchs M., et al. (2008) A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol 6: 1036–1040 [DOI] [PubMed] [Google Scholar]
- Hoeroldt B., McFarlane E., Dube A., Basumani P., Karajeh M., Campbell M., et al. (2011) Long-term outcomes of patients with autoimmune hepatitis managed at a nontransplant center. Gastroenterology 140: 1980–1989 [DOI] [PubMed] [Google Scholar]
- Ichai P., Duclos-Vallee J., Guettier C., Hamida S., Antonini T., Delvart V. (2007) Usefulness of corticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transpl 13: 996–1003 [DOI] [PubMed] [Google Scholar]
- Imam M., Sinakos E., Gossard A., Kowdley K., Luketic V., Edwyn Harrison M., et al. (2011) High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis. Aliment Pharmacol Ther 34: 1185–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S., Ohira H., Nishiguchi S., Zeniya M., Kaneko S., Onji M., et al. (2008) The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: a prospective, multicenter study. Hepatol Res 38: 557–564 [DOI] [PubMed] [Google Scholar]
- Jackson L., Song E. (1995) Cyclosporin in the treatment of corticosteroid resistant autoimmune chronic active hepatitis. Gut 36: 459–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., McFarlane I., Williams R. (1995) Azathioprine for long-term maintenance of remission in autoimmune hepatitis. N Engl J Med 333: 958–963 [DOI] [PubMed] [Google Scholar]
- Jones D., Al-Rifai A., Frith J., Patanwala I., Newton J. (2010) The independent effects of fatigue and UDCA therapy on mortality in primary biliary cirrhosis: results of a 9 year follow-up. J Hepatol 53: 911–917 [DOI] [PubMed] [Google Scholar]
- Kanda T., Yokosuka O., Imazeki F. (2003) Saisho, H. Bezafibrate treatment: a new medical approach for PBC patients? J Gastroenterol 38: 573–578 [DOI] [PubMed] [Google Scholar]
- Kanzler S., Gerken G., Dienes H., Meyer zum Buschenfelde K., Lohse A. (1997) Cyclophosphamide as alternative immunosuppressive therapy for autoimmune hepatitis – report of three cases. Z Gastroenterol 35: 571–578 [PubMed] [Google Scholar]
- Kaplan M., Alling D., Zimmerman H., Wolfe H., Sepersky R., Hirsch G., et al. (1986) A prospective trial of colchicine for primary biliary cirrhosis. N Engl J Med 315: 1448–1454 [DOI] [PubMed] [Google Scholar]
- Kaplan M., Cheng S., Price L., Bonis P. (2004) A randomized controlled trial of colchicine plus ursodiol versus methotrexate plus ursodiol in primary biliary cirrhosis: ten-year results. Hepatology 39: 915–923 [DOI] [PubMed] [Google Scholar]
- Kirk A., Jain S., Pocock S., Thomas H., Sherlock S. (1980) Late results of the Royal Free Hospital prospective controlled trial of prednisolone therapy in hepatitis B surface antigen negative chronic active hepatitis. Gut 21: 78–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita R., Kita-Sasai Y., Hanaoka I., Kimura T., Kokuryu H., Takamatsu S., et al. (2002) Beneficial effect of bezafibrate on primary sclerosing cholangitis (three case reports). Am J Gastroenterol 97: 1849–1851 [DOI] [PubMed] [Google Scholar]
- Kogan J., Safadi R., Ashur Y., Shouval D., Ilan Y. (2002) Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J Clin Gastroenterol 35: 75–81 [DOI] [PubMed] [Google Scholar]
- Kowdley K., Jones D., Luketic V., Chapman R., Burroughs A., Hirschfield G., et al. (2011) An international study evaluating the farnesoid X receptor agonist obeticholic acid as monotherapy in PBC [abstract]. J Hepatol 54(Suppl.): S13 [Google Scholar]
- Kremer A., Oude Elferink R., Beuers U. (2011) Pathophysiology and current management of pruritus in liver disease. Clin Res Hepatol Gastroenterol 35: 89–97 [DOI] [PubMed] [Google Scholar]
- Kumagi T., Guindi M., Fischer S., Arenovich T., Abdalian R., Coltescu C., et al. (2010) Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol 105: 2186–2194 [DOI] [PubMed] [Google Scholar]
- Kurihara T., Maeda A., Shigemoto M., Yamashita K., Hashimoto E. (2002) Investigation into the efficacy of bezafibrate against primary biliary cirrhosis, with histological references from cases receiving long term monotherapy. Am J Gastroenterol 97: 212–214 [DOI] [PubMed] [Google Scholar]
- Lankisch T., Metzger J., Negm A., Vosskuhl K., Schiffer E., Siwy J., et al. (2011) Bile proteomic profiles differentiate cholangiocarcinoma from primary sclerosing cholangitis and choledocholithiasis. Hepatology 53: 875–884 [DOI] [PubMed] [Google Scholar]
- Larsen F., Vainer B., Eefsen M., Bjerring P., Hansen B. (2007) Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J Gastroenterol 13: 3232–3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Belanger A., Joucette J., Stanca C., Friedman S., Bach N. (2007) Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol 5: 1313–1315 [DOI] [PubMed] [Google Scholar]
- Leung J., Bonis P., Kaplan M. (2011) Colchicine or methotrexate, with ursodiol, are effective after 20 years in a subset of patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol 9: 776–780 [DOI] [PubMed] [Google Scholar]
- Leuschner M., Maier K., Schlichting J., Strahl S., Herrmann G., Dahm H., et al. (1999) Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology 117: 918–925 [DOI] [PubMed] [Google Scholar]
- Levy C., Peter J., Nelson D., Keach J., Petz J., Cabrera R., et al. (2011) Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther 33: 235–242 [DOI] [PubMed] [Google Scholar]
- Levy C., Zein C., Gomez J., Soldevila-Pico C., Firpi R., Morelli G., et al. (2007) Prevalence and predictors of esophageal varices in patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol 5: 803–808 [DOI] [PubMed] [Google Scholar]
- Liaskou E., Karikoski M., Reynolds G., Lalor P., Weston C., Pullen N., et al. (2011) Regulation of mucosal addressin cell adhesion molecule 1 expression in human and mice by vascular adhesion protein 1 amine oxidase activity. Hepatology 53: 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor K. (1997) Ursodiol for primary sclerosing cholangitis: Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med 336: 691–695 [DOI] [PubMed] [Google Scholar]
- Lindor K., Gershwin E., Poupon R., Kaplan M., Bergasa N., Heathcote J. (2009) AASLD practice guidelines: primary biliary cirrhosis. Hepatology 50: 291–308 [DOI] [PubMed] [Google Scholar]
- Lindor K., Jorgensen R., Therneau T., Malinchoc M., Dickson E. (1997) Ursodeoxycholic acid delays the onset of esophageal varices in primary biliary cirrhosis. Mayo Clin Proc 72: 1137–1140 [DOI] [PubMed] [Google Scholar]
- Lindor K., Kowdley K., Luketic V., Harrison M., McCashland T., Befeler A., et al. (2009) High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 50: 808–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor K., Therneau T., Jorgensen R., Malinchoc M., Dickson E. (1996) Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology 110: 1515–1518 [DOI] [PubMed] [Google Scholar]
- Lindström L., Boberg K., Ingalill Friis-Liby I., Hultcrantz R., Bergquist A. (2012a) A reduction in alkaline phosphatase levels is associated to improved prognosis in primary sclerosing cholangitis: a 14 year follow up of the Scandinavian ursodeoxycholic acid trial [abstract]. Hepatology 56(Suppl. 1): A110 [Google Scholar]
- Lindström L., Boberg K., Wikman O., Friis-Liby I., Hultcrantz R., Prytz H., et al. (2012b) High dose ursodeoxycholic acid in primary sclerosing cholangitis does not prevent colorectal neoplasia. Aliment Pharmacol Ther 35: 451–457 [DOI] [PubMed] [Google Scholar]
- Lleo A., Gershwin M., Mantovani A., Invernizzi P. (2012) Towards common denominators in primary biliary cirrhosis: the role of IL-12. J Hepatol 256:731–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke G., Therneau T., Ludwig J., Dickson E., Lindor K. (1996) Time course of histological progression in primary biliary cirrhosis. Hepatology 23: 52–56 [DOI] [PubMed] [Google Scholar]
- Lohse A., Mieli-Vergani G. (2011) Autoimmune hepatitis. J Hepatol 55: 171–182 [DOI] [PubMed] [Google Scholar]
- Lombard M., Portmann B., Neuberger J., Williams R., Tygstrup N., Ranek L., et al. (1993) Cyclosporin A treatment in primary biliary cirrhosis: results of a long-term placebo controlled trial. Gastroenterology 104: 519–526 [DOI] [PubMed] [Google Scholar]
- Longhi M., Hussain M., Bogdanos D., Quaglia A., Mieli-Vergani G., Ma Y., et al. (2007) Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology 46: 472–484 [DOI] [PubMed] [Google Scholar]
- Longhi M., Hussain M., Kwok W., Mieli-Vergani G., Ma Y., Vergani D. (2011) Autoantigen-specific regulatory T cells, a potential tool for immune-tolerance reconstitution in type-2 autoimmune hepatitis. Hepatology 53: 536–547 [DOI] [PubMed] [Google Scholar]
- Longhi M., Ma Y., Bogdanos D., Cheeseman P., Mieli-Vergani G., Vergani D. (2004) Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol 41: 31–37 [DOI] [PubMed] [Google Scholar]
- Lüth S., Herkel J., Kanzler S., Frenzel C., Galle P., Dienes H., et al. (2008) Serologic markers compared with liver biopsy for monitoring disease activity in autoimmune hepatitis. J Clin Gastroenterol 42: 926–930 [DOI] [PubMed] [Google Scholar]
- Manns M., Czaja A., Gorham J., Krawitt E., Mieli-Vergani G., Vergani D., et al. (2010a) Diagnosis and management of autoimmune hepatitis. Hepatology 51: 2193–2213 [DOI] [PubMed] [Google Scholar]
- Manns M., Woynarowski M., Kreisel W., Lurie Y., Rust C., Zuckerman E., et al. (2010b) Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology 139: 1198–1206 [DOI] [PubMed] [Google Scholar]
- Martin C., Blanco P., Keach J., Petz J., Zaman M., Bhaskar K., et al. (2012) The safety and efficacy of oral docosahexaenoic acid supplementation for the treatment of primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther 35: 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A. (2011) The evidence supports a viral aetiology for primary biliary cirrhosis. J Hepatol 54: 1312–1314 [DOI] [PubMed] [Google Scholar]
- Mason A., Farr G., Xu L., Hubscher S., Neuberger J. (2004) Pilot studies of single and combination antiretroviral therapy in patients with primary biliary cirrhosis. Am J Gastroenterol 99: 2348–2355 [DOI] [PubMed] [Google Scholar]
- Mason A., Lindor K., Bacon B., Vincent C., Neuberger J., Wasilenko S. (2008) Clinical trial: randomized controlled trial of zidovudine and lamivudine for patients with primary biliary cirrhosis stabilized on ursodiol. Aliment Pharmacol Ther 28: 886–894 [DOI] [PubMed] [Google Scholar]
- Mason A., Luketic V., Lindor K., Hirschfield G., Gordon S., Mayo M., et al. (2010) Farnesoid-X receptor agonists: a new class of drugs for the treatment of PBC? An international study evaluating the addition of INT-747 to ursodeoxycholic acid [abstract]. J Hepatol 4(Suppl.): S1–S2 [Google Scholar]
- Mederacke I., Helfritz F., Puls F., Ringe K., Klempnauer J., Manns M., et al. (2012) Budd-Chiari syndrome after treatment with budesonide in a cirrhotic patient with autoimmune hepatitis. Ann Hepatol 11: 143–144 [PubMed] [Google Scholar]
- Mells G., Floyd J., Morley K., Cordell H., Franklin C., Shin S., et al. (2011) Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet 43: 329–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J., Negm A., Plentz R., Weismüller T., Wedemeyer J., Karlsen T., et al. (2013) Urine proteomic analysis differentiates cholangiocarcinoma from primary sclerosing cholangitis and other benign biliary disorders. Gut 62: 122–130 [DOI] [PubMed] [Google Scholar]
- Mitchell S., Bansi D., Hunt N., Von Bergmann K., Fleming K., Chapman R. (2001) A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology 121: 900–907 [DOI] [PubMed] [Google Scholar]
- Mitchison H., Palmer J., Bassendine M., Watson A., Record C., James O. (1992) A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three-year results. J Hepatol 15: 336–344 [DOI] [PubMed] [Google Scholar]
- Modica S., Petruzzelli M., Bellafante E., Murzilli S., Salvatore L., Celli N., et al. (2012) Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 142: 355–365 [DOI] [PubMed] [Google Scholar]
- Montano-Loza A., Carpenter H., Czaja A. (2007a) Consequences of treatment withdrawal in type 1 autoimmune hepatitis. Liver Int 27: 507–515 [DOI] [PubMed] [Google Scholar]
- Montano-Loza A., Carpenter H., Czaja A. (2007b) Features associated with treatment failure in type 1 autoimmune hepatitis and predictive value of the model of end-stage liver disease. Hepatology 46: 1138–1145 [DOI] [PubMed] [Google Scholar]
- Montano-Loza A., Carpenter H., Czaja A. (2007c) Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gastrol 102: 1005–1012 [DOI] [PubMed] [Google Scholar]
- Murray-Lyon I., Stern R., Williams R. (1973) Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet 1: 735–737 [DOI] [PubMed] [Google Scholar]
- Neuberger J., Gunson B., Hubscher S., Nightingale P. (2004) Immunosuppression affects the rate of recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl 10: 488–491 [DOI] [PubMed] [Google Scholar]
- O‘Brien C., Senior J., Arora-Mirchandani R., Batta A., Salen G. (1991) Ursodeoxycholic acid for the treatment of primary sclerosing cholangitis: a 30-month pilot study. Hepatology 14: 838–847 [DOI] [PubMed] [Google Scholar]
- Ohira H., Sato Y., Ueno T., Sata M. (2002) Fenofibrate treatment in patients with primary biliary cirrhosis. Am J Gastroenterol 97: 2147–2149 [DOI] [PubMed] [Google Scholar]
- Olsson R., Boberg K., de Muckadell O., Lindgren S., Hultcrantz R., Folvik G., et al. (2005) High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology 129: 1464–1472 [DOI] [PubMed] [Google Scholar]
- Pardi D., Loftus, Loftus E., Jr, Kremers W., Keach J., Lindor K. (2003) Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology 124: 889–893 [DOI] [PubMed] [Google Scholar]
- Parés A., Caballería L., Rodés J. (2006) Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 130: 715–720 [DOI] [PubMed] [Google Scholar]
- Patsenker E., Schneider V., Ledermann M., Saegesser H., Dorn C., Hellerbrand C., et al. (2011) Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine A and tacrolimus in experimental liver fibrosis. J Hepatol 55: 388–398 [DOI] [PubMed] [Google Scholar]
- Peiseler M., Sebode M., Franke B., Wortmann F., Schwinge D., Quaas A., et al. (2012) FOXP3+ regulatory T cells in autoimmune hepatitis are fully functional and not reduced in frequency. J Hepatol 57: 125–132 [DOI] [PubMed] [Google Scholar]
- Ponsioen C., Vrouenraets M., Prawirodirdjo W., Rajaram R., Rauws E., Mulder C., et al. (2002) Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut 51: 562–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poropat G., Giljaca V., Stimac D., Gluud C. (2011) Bile acids for primary sclerosing cholangitis. Cochrane Database Syst Rev (1): CD003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupon R. (2011) Treatment of primary biliary cirrhosis with ursodeoxycholic acid, budesonide and fibrates. Dig Dis 29: 85–88 [DOI] [PubMed] [Google Scholar]
- Pratt D., Flavin D., Kaplan M. (1996) The successful treatment of autoimmune hepatitis with 6-mercaptopurine after failure with azathioprine. Gastroenterology 110: 271–274 [DOI] [PubMed] [Google Scholar]
- Prince M., Chetwynd A., Newman W., Metcalf J., James O. (2002) Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology 123: 1044–1051 [DOI] [PubMed] [Google Scholar]
- Rabahi N., Chrétien Y., Gaouar F., Wendum D., Serfaty L., Chazouillères O., et al. (2010) Triple therapy with ursodeoxycholic acid, budesonide and mycophenolate mofetil in patients with features of severe primary biliary cirrhosis not responding to ursodeoxycholic acid alone. Gastroenterol Clin Biol 34: 283–287 [DOI] [PubMed] [Google Scholar]
- Rautiainen H., Kärkkäinen P., Karvonen A., Nurmi H., Pikkarainen P., Nuutinen H., et al. (2005) Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology 41: 747–752 [DOI] [PubMed] [Google Scholar]
- Richardson P., James P., Ryder S. (2000) Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol 33: 371–375 [DOI] [PubMed] [Google Scholar]
- Rudolph G., Gotthardt D., Kloeters-Plachky P., Rost D., Kulaksiz H., Stiehl A. (2010) In PSC with dominant bile duct stenosis, IBD is associated with an increase of carcinomas and reduced survival. J Hepatol 53: 313–317 [DOI] [PubMed] [Google Scholar]
- Rudolph G., Kloeters-Plachky P., Rost D., Steihl A. (2007) The incidence of cholangiocarcinoma in primary sclerosing cholangitis after long-time treatment with ursodeoxycholic acid. Eur J Gastroenterol Hepatol 19: 487–491 [DOI] [PubMed] [Google Scholar]
- Sandborn W., Feagan B., Fedorak R., Scherl E., Fleisher M., Katz S., et al. (2008) A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 135: 1130–1141 [DOI] [PubMed] [Google Scholar]
- Santos E., Arosemena L., Raez L., O’Brien C., Regev A. (2006) Successful treatment of autoimmune hepatitis and idiopathic thrombocytopenic purpura with the monoclonal antibody, rituximab: case report and review of literature. Liver Int 26: 625–629 [DOI] [PubMed] [Google Scholar]
- Schalm S., Korman M., Summerskill W., Czaja A., Baggenstoss A. (1977) Severe chronic active liver disease. Prognostic significance of initial morphologic patterns. Am J Dig Dis 22: 973–980 [DOI] [PubMed] [Google Scholar]
- Schramm C., Weiler-Normann C., Wiegard C., Hellweg S., Müller S., Lohse A. (2010) Treatment response in patients with autoimmune hepatitis. Hepatology 52: 2247–2248 [DOI] [PubMed] [Google Scholar]
- Sciveres M., Caprai S., Palla G., Ughi C., Maggiore G. (2004) Effectiveness and safety of ciclosporin as therapy for autoimmune diseases of the liver in children and adolescents. Aliment Pharmacol Ther 19: 209–217 [DOI] [PubMed] [Google Scholar]
- Sherman K., Narkewicz M., Pinto P. (1994) Cyclosporine in the management of corticosteroid-resistant type I autoimmune chronic active hepatitis. J Hepatol 21: 1040–1047 [DOI] [PubMed] [Google Scholar]
- Silveira M., Torok N., Gossard A., Keach J., Jorgensen R., Petz J., et al. (2009) Minocycline in the treatment of patients with primary sclerosing cholangitis: results of a pilot study. Am J Gastroenterol 104: 83–88 [DOI] [PubMed] [Google Scholar]
- Sockalingam S., Blank D., Abdelhamid N., Abbey S., Hirschfield G. (2012) Identifying opportunities to improve management of autoimmune hepatitis: evaluation of drug adherence and psychosocial factors. J Hepatol 57: 1299–1304 [DOI] [PubMed] [Google Scholar]
- Stanich P., Björnsson E., Gossard A., Enders F., Jorgensen R., Lindor K. (2011) Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis 43: 309–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellon A.J., Keating J.J., Johnson P.J., McFarlane I.G., Williams R. (1988) Maintenance of remission in autoimmune chronic active hepatitis with azathioprine after corticosteroid withdrawal. Hepatology 8(4): 781–784 [DOI] [PubMed] [Google Scholar]
- Stiehl A., Walker S., Stiehl L., Rudolph G., Hofmann W., Theilmann L. (1994) Effect of ursodeoxycholic acid on liver and bile duct disease in primary sclerosing cholangitis. A 3-year pilot study with a placebo-controlled study period. J Hepatol 20: 57–64 [DOI] [PubMed] [Google Scholar]
- Subramaniam K., Chitturi S., Brown M., Pavli P. (2011) Infliximab-induced autoimmune hepatitis in Crohn’s disease treated with budesonide and mycophenolate. Inflamm Bowel Dis 17: E149–E150 [DOI] [PubMed] [Google Scholar]
- Tannous M., Cheng J., Muniyappa K., Farooq I., Bharara A., Kappus M., et al. (2011) Use of tacrolimus in the treatment of autoimmune hepatitis: a single centre experience. Aliment Pharmacol Ther 34: 405–407 [DOI] [PubMed] [Google Scholar]
- Tischendorf J., Hecker H., Kruger M., Manns M., Meier P. (2007) Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol 102: 107–114 [DOI] [PubMed] [Google Scholar]
- Triantos C., Koukias N., Nikolopoulou V., Burroughs A. (2011) Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther 34: 901–910 [DOI] [PubMed] [Google Scholar]
- Trivedi P., Chapman R. (2012) PSC, AIH and overlap syndrome in inflammatory bowel disease. Clin Res Hepatol Gastroenterol 36: 420–436 [DOI] [PubMed] [Google Scholar]
- Trivedi P., Cullen S. (2012) Etiopathogenesis of primary biliary cirrhosis: an overview of recent developments. Hepatol Int [Epub ahead of print]. doi: 10.1007/s12072-012-9362-7 [DOI] [PubMed] [Google Scholar]
- Trivedi P., Hirschfield G. (2012) Review article: Overlap syndromes associated with autoimmune liver disease. Alim Pharm Ther 36: 517–533 [DOI] [PubMed] [Google Scholar]
- Tsai S., Pawlik T. (2009) Primary sclerosing cholangitis: the role of extrahepatic biliary resection. Adv Surg 43: 175–188 [DOI] [PubMed] [Google Scholar]
- Tsuda M., Moritoki Y., Lian Z., Zhang W., Yoshida K., Wakabayashi K., et al. (2012) Biochemical and immunologic effects of rituximab in primary biliary cirrhosis patients with an incomplete response to ursodeoxycholic acid. Hepatology 55: 512–521 [DOI] [PubMed] [Google Scholar]
- van Gerven N., Verwer B., Witte B., van Hoek B., Coenraad M., van Erpecum K., et al. (2013) Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol 58: 141–147 [DOI] [PubMed] [Google Scholar]
- van Hoogstraten H., Wolfhagen F., van de Meeberg P., Kuiper H., Nix G., Becx M., et al. (1998) Ursodeoxycholic acid therapy for primary sclerosing cholangitis: results of a 2-year randomized controlled trial to evaluate single vs. multiple daily doses. J Hepatol 29: 417–423 [DOI] [PubMed] [Google Scholar]
- Verma S., Gunuwan B., Mendler M., Govindrajan S., Redeker A. (2004) Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol 99: 1510–1516 [DOI] [PubMed] [Google Scholar]
- Vleggaar F., van Buuren H., Zondervan P., ten Kate F., Hop W. (2001) Jaundice in non-cirrhotic primary biliary cirrhosis: the premature ductopenic variant. Gut 49: 276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoristo M., Farkkila M., Karvonen A., Leino R., Lehtola J., Mäkinen J., et al. (1995) A placebo-controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid. Gastroenterology 108: 1470–1478 [DOI] [PubMed] [Google Scholar]
- Wang Q., Qiu D., Ma X. (2011) Early normalisation of aminotransferase predicts complete biochemical remission in autoimmune hepatitis patients. Aliment Pharmacol Ther 34: 107–109 [DOI] [PubMed] [Google Scholar]
- Webster G., Pereira S., Chapman R. (2009) Autoimmune pancreatitis/IgG4-associated cholangitis and primary sclerosing cholangitis – overlapping or separate diseases? J Hepatol 51: 398–402 [DOI] [PubMed] [Google Scholar]
- Weiler-Normann C., Schramm C., Quaas A., Wiegard C., Glaubke C., Pannicke N., et al. (2012) Infliximab as a rescue-treatment in difficult-to-treat autoimmune hepatitis. J Hepatol 21 November [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wiesner R., Ludwig J., Lindor K., Jorgensen R., Baldus W., Homburger H., et al. (1990) A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. N Engl J Med 322: 1419–1424 [DOI] [PubMed] [Google Scholar]
- Wolf J., Rybicki L., Lashner B. (2005) The impact of ursodeoxycholic acid on cancer, dysplasia and mortality in ulcerative colitis patients with primary sclerosing cholangitis. Aliment Pharmacol Ther 22: 783–788 [DOI] [PubMed] [Google Scholar]
- Wolfhagen F., van Hoogstraten H., van Buuren H., van Berge-Henegouwen G., ten Kate F., Hop W., et al. (1998) Triple therapy with ursodeoxycholic acid, prednisone and azathioprine in primary biliary cirrhosis: a 1-year randomized, placebo-controlled study. J Hepatol 29: 736–742 [DOI] [PubMed] [Google Scholar]
- Yano K., Kato H., Morita S., Takahara O., Ishibashi H., Furukawa R. (2002) Is bezafibrate Histologically effective for primary biliary cirrhosis? Am J Gastroenterol 97: 1075–1077 [DOI] [PubMed] [Google Scholar]
- Yeoman A., Westbrook R., Zen Y., Maninchedda P., Portmann B., Devlin J., et al. (2011) Early predictors of corticosteroid treatment failure in icteric presentations of autoimmune hepatitis. Hepatology 53: 926–934 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Yang G., Zhang W., Tsuda M., Tsuneyama K., Moritoki Y., et al. (2009) Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology 50: 1494–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa K., Matsumoto A., Ichijo T., Umemura T., Joshita S., Komatsu M., et al. (2012) Long-term outcome of Japanese patients with type 1 autoimmune hepatitis. Hepatology 56: 668–676 [DOI] [PubMed] [Google Scholar]
- Ytting H., Larsen F. (2011) Everolimus treatment of severe autoimmune hepatitis [abstract]. Hepatology 54(Suppl. 1): 229A21503947 [Google Scholar]
- Zachou K., Gatselis N., Papadamou G., Rigopoulou E., Dalekos G. (2011) Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naïve patients. J Hepatol 55: 636–646 [DOI] [PubMed] [Google Scholar]
- Zhang W., Fei Y., Gao J., Liu B., Zhang F. (2011) The role of CXCR3 in the induction of primary biliary cirrhosis. Clin Dev Immunol 2011: 564062. [DOI] [PMC free article] [PubMed] [Google Scholar]