Abstract
The syndrome of heart failure (HF) is a growing epidemic that causes a significant socio-economic burden. Despite considerable progress in the management of patients with HF, mortality and morbidity remain a major healthcare concern and frequent hospital admissions jeopardize daily life and social activities. Exercise training is an important adjunct nonpharmacological treatment modality for patients with HF that has proven positive effects on mortality, morbidity, exercise capacity and quality of life. Different training modalities are available to target the problems with which HF patients are faced. It is essential to tailor the prescribed exercise regimen, so that both efficiency and safety are guaranteed. Electrical implanted devices and mechanical support should not exclude patients from exercise training; however, particular precautions and a specialized approach are advised. At least 50% of patients with HF, older than 65 years of age, present with HF with preserved ejection fraction (HFPEF). Although the study populations included in studies evaluating the effect of exercise training in this population are small, the results are promising and seem to support the idea that exercise training is beneficial for HFPEF patients. Both the short- and especially long-term adherence to exercise training remain a major challenge that can only be tackled by a multidisciplinary approach. Efforts should be directed towards closing the gap between recommendations and the actual implementation of training programmes.
Keywords: exercise training, heart failure
Introduction
The syndrome of heart failure (HF) is a growing epidemic that causes a significant socio-economic burden. In developed countries, 1–2% of the adult population is diagnosed with HF, but prevalence reaches 10% among persons 70 years of age or older [Mosterd and Hoes, 2007]. Despite considerable progress in the management of patients with HF, mortality and morbidity remain a major healthcare concern [Corra et al. 2005], and frequent hospital admissions jeopardize daily life and social activities.
The recent 2012 European guidelines for the diagnosis and treatment of acute and chronic heart failure have incorporated a class IA recommendation for regular aerobic exercise in patients with HF to improve functional capacity and symptoms [McMurray et al. 2012]. The current therapeutic armamentarium, consisting of a titrated drug regimen, and carefully selected electrical implantable devices, still falls short when it comes to improving exercise tolerance. Exercise training specifically targets this shortcoming and is considered one of the most effective measures to improve patients’ wellbeing.
In contrast to HF with reduced ejection fraction (HFREF), the therapeutic approach of patients with preserved ejection fraction (HFPEF) is largely limited to symptomatic treatment. Since patients with HFPEF constitute around 50% of the current HF population [Kitzman et al. 2001], there is an urgent need for progress. With regard to the application of exercise training, a similar gap in evidence is seen. Nevertheless, positive results from recently conducted pilot trials [Alves et al. 2012; Haykowsky et al. 2012; Edelmann et al. 2011; Kitzman et al. 2010], will hopefully serve as an impetus to explore further this treatment modality for patients with HFPEF.
This review will briefly discuss the pathophysiological rationale for exercise training. The impact of exercise training on hard endpoints, as well as exercise capacity and quality of life (QoL), are addressed. Practical issues (i.e. evaluation of exercise capacity and prescription of exercise training) will be described, as well as the emerging role of exercise training for patients with HFPEF and HF patients with devices, such as the implantable cardioverter-defibrillator (ICD), cardiac resynchronization therapy (CRT) or assist devices. Endurance exercise at moderate intensity is still the most applied training modality for HF patients. Nevertheless, new training modes are tested and are progressively introduced into the clinical arena. For those readers who are interested in basic molecular changes as plausible explanations for exercise-derived benefits, we would like to refer to the recently published review by Gielen and colleagues [Gielen et al. 2010].
Targets for exercise training
The Fick equation explains the relationship between exercise capacity and cardiac performance: VO2 = Q (CaO2 – CvO2) (VO2: oxygen consumption; Q: cardiac output; CaO2: arterial oxygen content; CvO2: venous oxygen content) and clearly shows that exercise capacity depends on central cardiac, as well as peripheral mechanisms. The correlation between peak oxygen consumption (VO2peak) and resting left ventricular ejection fraction (LVEF) is poor in patients with chronic heart failure (CHF) [Franciosa et al. 1981]. Therefore, cardiac reserve during exercise, as well as peripheral factors implicated in oxygen transfer (peripheral vascular function), oxygen uptake and utilization (skeletal muscle), increased ergoreceptor activity and ventilatory inefficiency need to be taken into account.
Exercise training in CHF patients decreases circulating catecholamine levels [Passino et al. 2006], has anti-inflammatory [Gielen et al. 2003; Conraads et al. 2002] and antioxidative effects [Linke et al. 2005], reduces natriuretic peptide concentrations [Conraads et al. 2004], and increases shear stress and nitric oxide bioavailability [Ennezat et al. 2001], all leading to reduced peripheral vasoconstriction, improved endothelial function and enhanced endothelial repair [Hambrecht et al. 1998; Hornig et al. 1996]. Regular physical training also tackles muscle wasting and restores the anabolic/catabolic imbalance [Mann and Reid, 2003; Hambrecht et al. 1995], as well as hyperactive muscle ergoreflexes [Piepoli et al. 1996]. These changes parallel observed training-induced increases in VO2peak.
The effects of exercise on central haemodynamic function are less well established. Most studies show no significant effect on resting LVEF. However, Hambrecht and colleagues demonstrated that in patients with stable CHF, 6 months of aerobic exercise training led to a small, but significant improvement in stroke volume, with a concomitant reduction in peripheral resistance and left ventricular end-diastolic diameter [Hambrecht et al. 2000], suggesting training-induced reverse remodelling, compared with usual care. Belardinelli and colleagues demonstrated that aerobic exercise improves myocardial contractility and diastolic filling in patients with ischaemic cardiomyopathy and reduced LVEF [Belardinelli et al. 1996, 1998]. In 2007, a meta-analysis of 14 trials (including 812 patients) showed that aerobic exercise at moderate intensity led to a significant improvement of LVEF (weighted mean difference [WMD] = 2.59%, 95% confidence interval [CI] = 1.44–3.74%), left ventricular end-diastolic volume (LVEDV; WMD = -11.49 ml, 95% CI = -19.95 ml to -3.02 ml), and left ventricular end-systolic volume (WMD = -12.87 ml, 95% CI = -19.95 ml to -3.02 ml), compared with usual care [Haykowsky et al. 2007]. The combination of enhanced preload, improved myocardial contractility and augmented vascular reserve has been suggested as an explanation for the increase in LVEF that is seen after aerobic training. Therefore, based on this information, reverse remodelling with exercise training seems possible in clinically stable individuals with HF. Overall the magnitude of the improvement in LVEF was consistent with the magnitude of benefits seen with CRT and angiotensin-converting enzyme inhibitors [McAlister et al. 2004; Konstam et al. 1992].
Impact of exercise training
Hard endpoints: mortality, morbidity and safety
Based on the analysis of 801 patients enrolled in nine randomized controlled clinical trials, the ExTraMATCH collaborative group calculated a 35% (p < 0.05) lower risk for mortality and a 28% (p < 0.05) lower risk for the composite endpoint of mortality or hospitalization in favour of exercise [Piepoli et al. 2004]. Smart and Marwick conducted a meta-analysis on 11 randomized clinical trials (including 729 patients) and found a 39% lower relative risk for mortality in the exercise group [Smart and Marwick, 2004].
However, none of the studies included in these meta-analyses had sufficient power to address hard endpoints. In addition, most of them were small single-centre trials. Therefore, the results of the HF-ACTION trial (Heart Failure – A Controlled Trial Investigating Outcomes of exercise TraiNing) were eagerly awaited. HF-ACTION is until now the largest multicentre, randomized controlled trial, designed to measure the effects of exercise training on clinical outcomes and safety in patients with stable systolic HF [O’Connor et al. 2009]. A total of 2331 patients (72% men, mean age 59 years) with LVEF ≤ 35%, in the New York Heart Association functional class II–IV, and under optimal medical treatment, were randomized 1:1 to either the training group (36 sessions of supervised, moderate-intensity training followed by home-based training) or the usual care group. After a median follow-up time of 30 months, and after adjustment for predefined prognostic predictors, the primary composite endpoint of all-cause mortality or all-cause hospital stay was significantly reduced (-11%, p = 0.03) in the training group.
Although HF-ACTION confirmed the safety of exercise training in HF patients – still considered at increased risk – the results did not actually meet the expectations of those active in the field. Several explanations for the observed gap between anticipation and results have been put forward; these include usual care crossover but also the high percentage of patients in both groups using evidence-based medical treatment. The major flaw, however, was the very low level of adherence to the prescribed exercise regimens, resulting in a smaller than expected improvement in aerobic capacity. The median improvement in VO2peak after 3 months was only 0.6 ml/kg/min (or 4%) in the training group, which is inferior to results reported in other, smaller studies: a mean increase in VO2peak of 2.16 ml/kg/min has been derived from the data of 848 randomized patients [Rees et al. 2004].
Only 30% of the patients reached the target number of 120 min training per week after 10–12 months. Optimization of short- and long-term adherence to exercise training is recognized as a major goal and appropriate strategies are needed [Conraads et al. 2012]. Findings of a recent post hoc analysis of the HF-ACTION data demonstrating a clear association between improvement in exercise capacity and volume of exercise, underscore the relevance of compliance (n = 959) [Keteyian et al. 2009]. Furthermore, a clear relation between a change in VO2peak and clinical outcomes was found. Swank and colleagues concluded that in the HF-ACTION population every 6% increase in VO2peak (adjusted for other significant predictors) was associated with a 5% lower risk of the primary endpoint (hazard ratio [HR] = 0.95; CI = 0.93–0.98; p < 0.001) and a 7% lower all-cause mortality (HR = 0.93; CI 0.90–0.97; p < 0.001) [Swank et al. 2012]. Supporting the relevance of exercise-induced increased aerobic capacity, Tabet and colleagues showed in a prospective study that absence of improvement in VO2peak following an exercise training programme is a strong and independent predictor for adverse cardiac events [Tabet et al. 2008].
In their systematic review Smart and Marwick reported no exercise-related deaths during over 60,000 h of exercise training by HF patients [Smart and Marwick, 2004]. However, none of the studies incorporated in this meta-analysis included safety as a primary objective. The HF-ACTION trial confirmed the earlier findings of the smaller, single-centre studies stating that regular aerobic-type exercise training is safe and generally well tolerated in patients with chronic, stable HF [Keteyian et al. 2010; McKelvie, 2008]. Overall, the adverse event rates during the entire study period did not differ between the exercise and control groups. The most frequent reported adverse events were worsening HF (26.1% in the exercise group versus 29.0% in the usual care group), ICD firing (22.2% versus 23.4%) and serious adverse arrhythmia (defined as sustained ventricular tachycardia lasting longer than 30 s, ventricular fibrillation, supraventricular tachycardia with rapid ventricular response lasting longer than 30 s, cardiac arrest or bradycardia, heart rate < 50/min, symptomatic, and not related to medication; 14.4% versus 14.0%). The relatively young age (59 years of age) of patients enrolled in the study compared with the general HF population needs to be taken into account and caution is necessary when generalizing these results to other populations.
‘Surrogate’ endpoints: exercise capacity and QoL
Maximal aerobic capacity is a strong and independent prognostic factor in patients with HF [Corra et al. 2012; Tabet et al. 2008] and determines the amount of activities of daily life a patient can perform independently. The latter translates directly into QoL. Since 1990, several single-centre clinical exercise trials have indicated that regular aerobic exercise has the potential to at least partly reverse exercise intolerance [Keteyian et al. 2010]. In 2004, a systematic review of randomized controlled trials on exercise training in CHF patients (848 patients) demonstrated a mean increase of 2.16 ml/kg/min in VO2peak, and an improvement in terms of health-related QoL in seven out of nine studies [Rees et al. 2004]. The HF-ACTION trial included the evaluation of the effects of exercise training on the self-reported health status among patients with HF. After 3 months, a modest, but significant, improvement in health status, using the Kansas City Cardiomyopathy Questionnaire (KCCQ), was seen in the exercise group (5.2 versus 3.3 points in usual care, p < 0.001). This improvement persisted over time and the effect was similar for the KCCQ subscales, that is, physical limitations, symptoms, QoL and social limitations.
Evaluation of exercise capacity: cardiopulmonary exercise testing with ventilatory gas analysis
Maximal or symptom-limited cardiopulmonary ergometer or treadmill testing, with ventilatory gas analysis (CPET), is considered the cornerstone for safe and efficient exercise prescription, particularly in HF patients. Whereas an in-depth description of the practicalities involved in conducting CPET, as well as its interpretation are beyond the scope of this paper, the relevance of the latter test for prognostication (i.e. VO2peak, VE/VCO2 slope, oscillatory breathing [Arena et al. 2007]), treatment adjustment (indication for heart transplantation [Corra et al. 2002]) and for a tailored exercise prescription [Conraads and Beckers, 2010], deserves to be emphasized. Such information cannot be derived from submaximal exercise testing, such as the 6-min walking test [Guazzi et al. 2009].
With regard to safety, again the HF-ACTION trial provides a wealth of information. Out of 4411 exercise tests, 0 deaths per 1000 exercise tests and 0.45 nonfatal major cardiovascular events per 1000 exercise tests (95% CI = 0.11–1.81) were reported. There were no exercise test-related ICD discharges requiring hospitalization [Keteyian et al. 2009]. More detailed information on the principles of CPET and the assessment of functional capacity can be found in a recent extensive review [Balady et al. 2010].
Prescription of a training programme: types of exercise
Apart from lifestyle changes, including the promotion of common daily activities, both American and European guidelines on HF advise the participation by stable HF patients in a structured exercise training programme [McMurray et al. 2012; Bonow et al. 2012]. Contraindications for participation in an exercise training programme are listed in Table 1.
Table 1.
Contraindications for participation in an exercise training programme.
| Cardiac | Decompensated or unstable heart failure, New York Heart Association functional class IV Exercise training-induced myocardial ischaemia, hypotension, nonsustained or sustained ventricular tachycardia, atrial fibrillation (until resolved) Severe valvular dysfunction (regurgitation or stenosis) |
| Extracardiac | Active inflammatory disease, including peri- or myocarditis Cerebrovascular disease preventing exercise testing or training Musculoskeletal disease preventing exercise testing or training Severe obstructive lung disease Uncontrolled diabetes mellitus, thyroid dysfunction Hypo- or hyperkalaemia, hypovolaemia Pulmonary embolism Deep venous thrombosis/thrombophlebitis |
Up until now, there are no clearly delineated practical guidelines for exercise prescription in the setting of HF, resulting in a variety of centre-specific approaches for these patients. The programmes differ in a number of characteristics: type (endurance, resistance and strength), intensity (aerobic versus anaerobic); method (continuous versus intermittent/interval); setting (hospital/centre-based versus home-based); application (systemic, regional and respiratory muscle) and control (supervised versus nonsupervised) [Piepoli et al. 2011]. In order to optimize the benefits of exercise training for patients with HF, an individualized programme addressing both peak aerobic capacity (prognosis), as well the ability to continue submaximal exercise during prolonged time (QoL and independent functionality) should be designed.
Three different training modalities have been proposed in different combinations: (a) endurance or aerobic exercise training (continuous and interval); (b) strength/resistance training; (c) inspiratory muscle training.
Endurance or aerobic exercise training
Aerobic or endurance training (i.e. cycling, walking, rowing) is the most investigated training modality in CHF patients, and is recommended as baseline activity [Vanhees et al. 2012; Davies et al. 2010; Flynn et al. 2009; O’Connor et al. 2009]; cycling is usually preferred because of the reproducible power output, the possible low workloads and reduced injury rate. Traditionally, the first ventilatory anaerobic threshold (VAT) (50–60% of VO2peak) was identified as the maximum training intensity for HF patients, avoiding exercise-related risks and adverse events [Myers, 2008; Meyer et al. 2005a, 2005b].
However, since CHF patients need a higher percentage of their VO2peak (compared with normal individuals) to perform daily life activities [Kervio et al. 2004; Riley et al. 1992], and since one of the main targets of exercise training is to allow these patients to perform daily tasks with less effort, training intensities above the VAT have progressively been tested and introduced. The respiratory compensation point (RCP) (65–90% of VO2peak [Mezzani et al. 2010]), which is strictly related to the so-called ‘critical power’ or the limit between high intensity and severe intensity of effort, is now accepted as the limit for prolonged aerobic exercise without additional risk for patients with HF [Carvalho and Mezzani, 2011; Mezzani et al. 2009]. Figure 1 illustrates the VAT and RCP during an incremental exercise test. Nowadays, exercise intensities between 70% and 80% of VO2peak are prescribed [Roveda et al. 2003; Dubach et al. 1997]. Nevertheless, in HF patients with significantly reduced pretraining VO2peak and/or high exercise-related risks, aerobic training intensities as low as 40% of VO2peak have proven to be effective [Demopoulos et al. 1997; Belardinelli et al. 1995].
Figure 1.
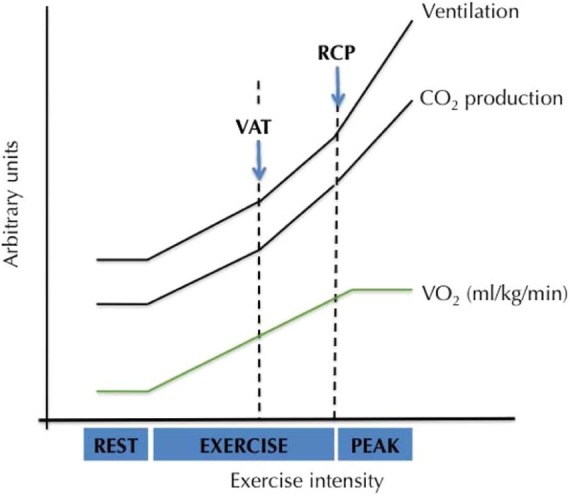
VAT and RCP during an incremental exercise test. RCP, respiratory compensation point; VAT, ventilatory anaerobic threshold.
Session duration should be progressed according to patients’ tolerance, with a minimum goal of 30 min/session, at least three times a week [Flynn et al. 2009; O’Connor et al. 2009]. However, patients with recent haemodynamic instability, lower exercise capacity or fatigue should start with shorter exercise bouts (i.e. 10 min), which can be repeated several times a day.
During training sessions, the rate of perceived exertion (RPE) (Borg scale 6–20) should be used as an adjunctive measure of exercise intensity. Carvalho and colleagues evaluated the reliability of the RPE Borg scale for exercise training monitoring in HF patients on beta-blockers, showing that values of 11–13 (i.e. perceived exertion ranging between ‘light’ and ‘somewhat hard’) corresponded with an energy expenditure between the VAT and RCP [Carvalho et al. 2009]. Noteworthy is the fact that indoor workloads cannot reliably be extrapolated to outdoor exercise, since environmental conditions can aggravate the exercise.
In 2007, Wisloff and colleagues compared the effect of aerobic interval training (AIT), consisting of 4-min training intervals at high intensity (90–95% of peak heart rate), separated by 3-min active pauses (walking at 50–70% of peak heart rate), total exercise time 38 min, three times weekly, with moderate continuous training (MCT), which consisted of walking continuously at 70–75% of peak heart rate, for 47 min (to compare isocaloric sessions) [Wisloff et al. 2007]. The study population consisted of 27 postinfarction HF patients (aged 75.5 ± 11.1 years) (LVEF 29%). The investigators demonstrated that interval training led to greater improvements in aerobic capacity (improvement in VO2peak 46% versus 14%, p < 0.001), reverse left ventricular remodelling, endothelial function and QoL. Recently, the same group of investigators published results on the safety of AIT in cardiac patients (however not exclusively HF patients) [Rognmo et al. 2012]. It was concluded that the risk of a cardiovascular event is low after both high-intensity exercise and moderate-intensity exercise in a cardiovascular rehabilitation setting. A randomized multicentre trial (SMARTEX-HF study) is currently enrolling HF patients to compare the efficacy and safety of AIT versus MCT [Stoylen et al. 2012]. This trial is expected to provide a more solid basis for future recommendations on training modes.
Strength/resistance training
To complete daily life tasks, HF patients are often hindered by skeletal muscle weakness, particularly at the level of the upper limbs. Although there is only limited information available on the effect and safety of dynamic resistive exercises in HF patients, experience is growing and results are encouraging. It has been shown that dynamic resistance training has anti-inflammatory effects, improves insulin resistance and counteracts loss of skeletal muscle mass and strength, thereby improving QoL [Bjarnason-Wehrens et al. 2004; Cheetham et al. 2002; Conraads et al. 2002; Singh et al. 1999]. Standard exercise training protocols usually involve a combination of aerobic and resistance training. Beckers and colleagues demonstrated in HF patients that combined exercise training had a more pronounced effect on submaximal exercise capacity, muscle strength and QoL than pure endurance training, without unfavourable effects on left ventricular remodelling and outcome parameters [Beckers et al. 2008].
HF patients are advised to train smaller muscle groups (including upper and lower body muscle groups) in a dynamic way, avoiding Valsalva manoeuvres, and at low-to-moderate intensity. Training intensity should be determined on the basis of the one repetition maximum (1-RM), the highest weight that one can lift once with correct form, throughout a complete range of motion. Usually, exercise is implemented at 50–70% of the 1-RM [Williams et al. 2007; Bjarnason-Wehrens et al. 2004]. Sustained maximal isometric exercise (i.e. weight lifting) is contraindicated in these patients, because of the excessive rise in blood pressure and the lowering of the stroke volume.
Inspiratory muscle training
Respiratory muscle dysfunction, characterized by respiratory muscle fibre atrophy, deoxygenation and impaired mitochondrial oxidative capacity has been predominantly observed in patients with advanced HF [Wong et al. 2011; Meyer et al. 2001]. Winkelmann and colleagues demonstrated in 2009 that adding inspiratory muscle training (IMT), using respiratory muscle-specific training devices, to aerobic training in 24 patients with HF and inspiratory muscle weakness resulted in an additional improvement in inspiratory muscle performance, VO2peak and functional status compared with aerobic training without IMT [Winkelmann et al. 2009]. Laoutaris and colleagues recently showed that combined aerobic/resistance/inspiratory training in 27 patients with HF is safe, and resulted in incremental benefits in peripheral and respiratory muscle weakness, cardiopulmonary function and QoL, compared with the effects of aerobic training alone [Laoutaris et al. 2012]. Although these results are promising, the study populations are small and the outcomes need to be addressed in larger randomized studies.
HFPEF
At least 50% of HF patients older than 65 years present with HFPEF [Kitzman et al. 1991]. The prevalence of HFPEF increases rapidly, and morbidity and mortality rates are almost comparable to HFREF [Bhatia et al. 2006; Owan et al. 2006].
The clinical manifestations of both patient groups are largely similar and characterized by exercise-related complaints of dyspnoea and fatigue, with a major impact on QoL [Kitzman et al. 1991, 2002].
The pathophysiological background of exercise intolerance in patients with HFPEF is less well understood and seems to differ substantially from HFREF. Tan and colleagues demonstrated that patients with HFPEF present a combination of systolic and diastolic abnormalities [Tan et al. 2009]; this combination is more obvious during exercise than at rest and includes reduced myocardial systolic strain, rotation, left ventricular suction, longitudinal function and delayed untwisting. Kitzman and colleagues provided evidence to suggest that the observed reduction in VO2peak in patients with HFPEF is primarily due to reduced cardiac output, secondary to an inability to increase the end-diastolic and stroke volume via the Frank–Starling mechanism [Kitzman et al. 2010]. Other investigators have shown that, similar to patients with HFREF, peripheral factors, such as abnormal blood flow distribution [Esposito et al. 2010], impaired vascular reserve [Borlaug et al. 2010], and skeletal muscle dysfunction [Wilson et al. 1993], also contribute to exercise intolerance in HFPEF patients.
Haykowsky and colleagues demonstrated recently that both reduced arteriovenous oxygen difference and reduced cardiac output contribute significantly to the exercise intolerance observed in elderly HFPEF patients [Haykowsky et al. 2011]. Moreover, the arteriovenous oxygen reserve was an independent predictor of VO2peak, which strongly suggests that peripheral factors at least partly determine the limited exercise tolerance in these patients. Interventions that increase heart rate, skeletal muscle perfusion and/or oxygen extraction by active working muscles could improve peak exercise performance in elderly HFPEF patients. The same group of researchers was able to demonstrate in a group of 40 stable, compensated HFPEF patients (mean age 69 ± 6 years), that a 4-month endurance training programme consisting of walking and cycling significantly improved VO2peak compared with a controlled usual care group (16.3 ± 2.6 versus 13.1 ± 3.4, respectively, p = 0.002). The peak arteriovenous oxygen difference was higher after the training programme and was the main contributor of the observed improvement in VO2peak [Haykowsky et al. 2012]. These findings were confirmed by the Ex-DHF study (a multicentre, prospective randomized control trial in 64 patients with HFPEF). After a 3-month training programme, consisting of supervised endurance/resistance training, there was an improved functional capacity and QoL. This benefit was associated with an improved left ventricular diastolic function and reversed left atrial remodelling. The exercise training programme was safe and the positive effect on exercise capacity was at least as large as reported in patients with HFREF [Edelmann et al. 2011]. Although the study populations included in these studies are small, the results are promising and seem to support the idea that exercise training is beneficial for HFPEF patients. However, large randomized controlled studies are still needed to evaluate outcomes, effectiveness and safety of various training modalities.
Patients with implanted devices
The number of patients implanted with an electrical device (i.e. ICD or CRT) is steadily increasing. Exercise training in patients with an ICD and/or CRT can substantially increase QoL and exercise capacity and seems to be safe [Vanhees et al. 2004]. Vanhees and colleagues evaluated the effect of a 3-month training programme in 92 ICD patients, compared with a control group of 473 patients [Vanhees et al. 2001]. A total of 23 out of 34 ICD patients had a LVEF below 40%, compared with 41 out of 320 patients of the control group (68% versus 13%, p < 0,001). The training programme resulted in a 21% increase in VO2peak in the ICD group, which was comparable with earlier study results [Vanhees et al. 2004]. Only one inappropriate ICD shock was reported. Conraads and colleagues studied the effect of endurance training after CRT implantation in 17 patients (mean age 59 ± 9 years) with HF and dyssynchrony [Conraads et al. 2007]. Patients were randomized to CRT with (n = 8) or without (n = 9) exercise training. The observed increase in VO2peak was significantly greater in the trained versus the untrained CRT patients (40% versus 16%, p = 0.005), thus demonstrating an additive effect of CRT and exercise training. Apart from the favourable effect on exercise capacity, exercise training can also have a positive effect on anxiety in ICD patients as well [Belardinelli et al. 2006; Fitchet et al. 2003]. The importance of this effect is underscored by a prospective study of van den Broeck and colleagues who demonstrated that anxiety predicted a 70% increase in the risk of arrhythmias in type D (or distressed) patients with an ICD [van den Broeck et al. 2009].
We think there is a strong rationale to provide exercise programmes for ICD and CRT patients. However, some precautions have to be taken into account. Inappropriate shocks should be avoided at all time. Therefore, the maximum training heart rate should be at least 20 bpm below the ICD-intervention heart rate. Activities with pronounced arm–shoulder movements should be avoided, especially during the first 2 months after implantation. Before starting an exercise training programme, a CPET is indispensable in patients with an ICD and/or CRT. Besides definition of a tailored training heart rate, the heart rate response to exercise and the occurrence of exercise-induced arrhythmias must be assessed. Patients will feel safer and more comfortable if such reassurance is provided. In patients with an implanted CRT-D, adaptation of the tracking rate and rate response during maximal or near maximal effort may be necessary to avoid loss of biventricular pacing at high intrinsic heart rates.
Despite technical progress in the development of ventricular assist devices (VAD), patients who need mechanical support are often severely debilitated and suffer from skeletal muscle wasting and pronounced physical deconditioning. Up until now, scientific information on the safety and effect of exercise training in patients with VADs is limited. Laoutaris and colleagues examined the effect of exercise training (45 min of moderate-intensity aerobic exercise, 3–5 times/week) + high-intensity inspiratory muscle training (2–3 times/week) on top of 30–45 min daily walking in 10 patients with a left-ventricular assist device (LVAD) or a biventricular assist device (BiVAD) [Laoutaris et al. 2011]. The control group consisted of five patients with an LVAD or BiVAD (30–45 min daily walking). Exercise training appeared safe. VO2peak (19.3 ± 4.5 versus 16.8 ± 3.7 ml/kg/min, p = 0.008), QoL and inspiratory lung capacity (2.4 ± 0.9 versus 1.7 ± 0.7, p = 0.008) improved compared with baseline measurements; in the control group no significant improvement was seen. Early initiation of exercise training was possible, but the beneficial effects of training were also determined at a later stage after implantation. Up until now, no standard exercise training programme for VAD patients has been established. At the time of writing, it seems prudent to organize exercise training for VAD patients in specialized centres, supervised by a skilled rehabilitation team. Patients should be closely monitored for signs of exercise intolerance or assist device dysfunction. Exercises that irritate the driveline outlet(s) must be avoided, as well as shaking movements and strong vibrations.
Adherence
Despite the evidence on the benefits of exercise training in patients with HF and the importance (class I level of recommendation) that is attributed to cardiac rehabilitation programmes in all major evidence-based guidelines [Komajda et al. 2005], this nonpharmacological treatment stays largely underutilized. A recent inventory study on the use of cardiac rehabilitation in Europe demonstrated that only 14% of HF patients eligible for inclusion in a cardiac rehabilitation programme actually were enrolled. This issue is not typical for the HF population, but it is also recognized in the cardiovascular patient population as a whole throughout Europe [Bjarnason-Wehrens et al. 2010]. A low level of adherence is one of the most plausible explanations for the lower than expected increments in terms of VO2peak observed in the HF-ACTION trial. Only 31.5% of the patients enrolled in the study completed 36 supervised training sessions (median completion time was 3.9 months, interquartile range 3.4–4.8 months), and only approximately 40% of patients in the exercise group reported weekly training volumes at or above the recommended 90 min/week at month 3, or 120 min/week from month 3 to month 12 [Keteyian et al. 2009].
Explanations for low adherence and compliance to strategies that introduce lifestyle changes can be found at three levels [Conraads et al. 2012]: the system level (i.e. socio-economic factors, limited availability of cardiac rehabilitation programmes), the physician level (i.e. low referral and lack of education on the importance of exercise training), and the patient level (i.e. low level of education or inadequate social support [Beckie and Beckstead, 2010]).
Several strategies have been proposed, but up until now, there is little evidence for interventions to improve adherence [Tierney et al. 2012]. Education alone is not enough; interventions could benefit from cognitive behavioural therapy and strategies that improve patients’ ownership. For the interested reader we would like to refer to a recently published paper of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology, which includes several recommendations and strategies to optimize implementation and adherence to exercise training in HF [Conraads et al. 2012].
Conclusion
Exercise training is an important adjunct nonpharmacological treatment modality for patients with HF that has proven positive effects on mortality, morbidity, exercise capacity and QoL. Different training modalities are available to target the problems with which HF patients are faced. It is essential to tailor the prescribed exercise regimen, so that both efficiency and safety are guaranteed. Electrical implanted devices and mechanical support should not exclude patients from exercise training; however, particular precautions and a specialized approach are advised. Future research should aim at the development of more effective training modalities and the assessment of higher intensity regimens. In addition, efforts should be directed towards closing the gap between recommendations and the actual implementation of training programmes. One of the major challenges lies within optimizing both short- and especially long-term adherence to exercise training.
Footnotes
Funding: Viviane Conraads is a Senior Clinical Investigator of the Fund for Scientific Research, Flanders, Belgium.
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Catherine De Maeyer, Department of Cardiology, Antwerp University Hospital, Wilrijkstraat 10, 2650 Edegem, Belgium.
Paul Beckers, Department of Cardiology, Antwerp University Hospital, Edegem, Belgium and University of Antwerp, Antwerp, Belgium.
Christiaan J. Vrints, Department of Cardiology, Antwerp University Hospital, Edegem, Belgium and University of Antwerp, Antwerp, Belgium
Viviane M. Conraads, Department of Cardiology, Antwerp University Hospital, Edegem, Belgium and University of Antwerp, Antwerp, Belgium
References
- Alves A., Ribeiro F., Goldhammer E., Rivlin Y., Rosenschein U., Viana J., et al. (2012) Exercise training improves diastolic function in heart failure patients. Med Sci Sports Exerc 44: 776–785 [DOI] [PubMed] [Google Scholar]
- Arena R., Myers J., Abella J., Peberdy M., Bensimhon D., Chase P., et al. (2007) Development of a ventilatory classification system in patients with heart failure. Circulation 115: 2410–2417 [DOI] [PubMed] [Google Scholar]
- Balady G., Arena R., Sietsema K., Myers J., Coke L., Fletcher G., et al. (2010) Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122: 191–225 [DOI] [PubMed] [Google Scholar]
- Beckers P., Denollet J., Possemiers N., Wuyts F., Vrints C., Conraads V. (2008) Combined endurance-resistance training versus endurance training in patients with chronic heart failure: a prospective randomized study. Eur Heart J 29: 1858–1866 [DOI] [PubMed] [Google Scholar]
- Beckie T., Beckstead J. (2010) Predicting cardiac rehabilitation attendance in a gender-tailored randomized clinical trial. J Cardiopulm Rehabil Prev 30: 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli R., Capestro F., Misiani A., Scipione P., Georgiou D. (2006) Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil 13: 818–825 [DOI] [PubMed] [Google Scholar]
- Belardinelli R., Georgiou D., Cianci G., Purcaro A. (1996) Effects of exercise training on left ventricular filling at rest and during exercise in patients with ischemic cardiomyopathy and severe left ventricular systolic dysfunction. Am Heart J 132: 61–70 [DOI] [PubMed] [Google Scholar]
- Belardinelli R., Georgiou D., Ginzton L., Cianci G., Purcaro A. (1998) Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 97: 553–561 [DOI] [PubMed] [Google Scholar]
- Belardinelli R., Georgiou D., Scocco V., Barstow T., Purcaro A. (1995) Low intensity exercise training in patients with chronic heart failure. J Am Coll Cardiol 26: 975–982 [DOI] [PubMed] [Google Scholar]
- Bhatia R., Tu J., Lee D., Austin P., Fang J., Haouzi A., et al. (2006) Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355: 260–269 [DOI] [PubMed] [Google Scholar]
- Bjarnason-Wehrens B., Mayer-Berger W., Meister E., Baum K., Hambrecht R., Gielen S. (2004) Recommendations for resistance exercise in cardiac rehabilitation. Recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 11: 352–361 [DOI] [PubMed] [Google Scholar]
- Bjarnason-Wehrens B., McGee H., Zwisler A., Piepoli M., Benzer W., Schmid J., et al. (2010) Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil 17: 410–418 [DOI] [PubMed] [Google Scholar]
- Bonow R., Ganiats T., Beam C., Blake K., Casey D., Goodlin S., et al. (2012) ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association–Physician Consortium for Performance Improvement. J Am Coll Cardiol 59: 1812–1832 [DOI] [PubMed] [Google Scholar]
- Borlaug B., Olson T., Lam C., Flood K., Lerman A., Johnson B., et al. (2010) Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56: 845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho V., Bocchi E., Guimaraes G. (2009) The Borg scale as an important tool of self-monitoring and self-regulation of exercise prescription in heart failure patients during hydrotherapy. A randomized blinded controlled trial. Circ J 73: 1871–1876 [DOI] [PubMed] [Google Scholar]
- Carvalho V., Mezzani A. (2011) Aerobic exercise training intensity in patients with chronic heart failure: principles of assessment and prescription. Eur J Cardiovasc Prev Rehabil 18: 5–14 [DOI] [PubMed] [Google Scholar]
- Cheetham C., Green D., Collis J., Dembo L., O’Driscoll G. (2002) Effect of aerobic and resistance exercise on central hemodynamic responses in severe chronic heart failure. J Appl Physiol 93: 175–180 [DOI] [PubMed] [Google Scholar]
- Conraads V., Beckers P. (2010) Exercise training in heart failure: practical guidance. Heart 96: 2025–2031 [DOI] [PubMed] [Google Scholar]
- Conraads V., Beckers P., Bosmans J., De Clerck L., Stevens W., Vrints C., et al. (2002) Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J 23: 1854–1860 [DOI] [PubMed] [Google Scholar]
- Conraads V., Beckers P., Vaes J., Martin M., Van Hoof V., De Maeyer C., et al. (2004) Combined endurance/resistance training reduces NT-proBNP levels in patients with chronic heart failure. Eur Heart J 25: 1797–1805 [DOI] [PubMed] [Google Scholar]
- Conraads V., Deaton C., Piotrowicz E., Santaularia N., Tierney S., Piepoli M., et al. (2012) Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 14: 451–458 [DOI] [PubMed] [Google Scholar]
- Conraads V., Vanderheyden M., Paelinck B., Verstreken S., Blankoff I., Miljoen H., et al. (2007) The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: a pilot trial. Eur J Cardiovasc Prev Rehabil 14: 99–106 [DOI] [PubMed] [Google Scholar]
- Corra U., Giannuzzi P., Adamopoulos S., Bjornstad H., Bjarnason-Weherns B., Cohen-Solal A., et al. (2005) Executive summary of the position paper of the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology (ESC): core components of cardiac rehabilitation in chronic heart failure. Eur J Cardiovasc Prev Rehabil 12: 321–325 [DOI] [PubMed] [Google Scholar]
- Corra U., Giordano A., Bosimini E., Mezzani A., Piepoli M., Coats A., et al. (2002) Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest 121: 1572–1580 [DOI] [PubMed] [Google Scholar]
- Corra U., Giordano A., Mezzani A., Gnemmi M., Pistono M., Caruso R., et al. (2012) Cardiopulmonary exercise testing and prognosis in heart failure due to systolic left ventricular dysfunction: a validation study of the European Society of Cardiology Guidelines and Recommendations (2008) and further developments. Eur J Prev Cardiol 19: 32–40 [DOI] [PubMed] [Google Scholar]
- Davies E., Moxham T., Rees K., Singh S., Coats A., Ebrahim S. (2010) Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev 4: CD003331. [DOI] [PubMed] [Google Scholar]
- Demopoulos L., Bijou R., Fergus I., Jones M., Strom J., LeJemtel T. (1997) Exercise training in patients with severe congestive heart failure: enhancing peak aerobic capacity while minimizing the increase in ventricular wall stress. J Am Coll Cardiol 29: 597–603 [DOI] [PubMed] [Google Scholar]
- Dubach P., Myers J., Dziekan G., Goebbels U., Reinhart W., Muller P., et al. (1997) Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. J Am Coll Cardiol 29: 1591–1598 [DOI] [PubMed] [Google Scholar]
- Edelmann F., Gelbrich G., Dungen H., Frohling S., Wachter R., Stahrenberg R., et al. (2011) Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction. Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol 58: 1780–1791 [DOI] [PubMed] [Google Scholar]
- Ennezat P., Malendowicz S., Testa M., Colombo P., Cohen-Solal A., Evans T., et al. (2001) Physical training in patients with chronic heart failure enhances the expression of genes encoding antioxidative enzymes. J Am Coll Cardiol 38: 194–198 [DOI] [PubMed] [Google Scholar]
- Esposito F., Mathieu-Costello O., Shabetai R., Wagner P., Richardson R. (2010) Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 55: 1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchet A., Doherty P., Bundy C., Bell W., Fitzpatrick A., Garratt C. (2003) Comprehensive cardiac rehabilitation programme for implantable cardioverter-defibrillator patients: a randomised controlled trial. Heart 89: 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn K., Pina I., Whellan D., Lin L., Blumenthal J., Ellis S., et al. (2009) Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1451–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciosa J., Park M., Levine T. (1981) Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol 47: 33–39 [DOI] [PubMed] [Google Scholar]
- Gielen S., Adams V., Mobius-Winkler S., Linke A., Erbs S., Yu J., et al. (2003) Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 42: 861–868 [DOI] [PubMed] [Google Scholar]
- Gielen S., Schuler G., Adams V. (2010) Cardiovascular effects of exercise training: molecular mechanisms. Circulation 122: 1221–1238 [DOI] [PubMed] [Google Scholar]
- Guazzi M., Dickstein K., Vicenzi M., Arena R. (2009) Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail 2: 549–555 [DOI] [PubMed] [Google Scholar]
- Hambrecht R., Fiehn E., Weigl C., Gielen S., Hamann C., Kaiser R., et al. (1998) Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 98: 2709–2715 [DOI] [PubMed] [Google Scholar]
- Hambrecht R., Gielen S., Linke A., Fiehn H., Yu J., Walther C., et al. (2000) Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 283: 3095–3101 [DOI] [PubMed] [Google Scholar]
- Hambrecht R., Niebauer J., Fiehn E., Kalberer B., Offner B., Hauer K., et al. (1995) Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol 25: 1239–1249 [DOI] [PubMed] [Google Scholar]
- Haykowsky M., Brubaker P., John J., Stewart K., Morgan T., Kitzman D. (2011) Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 58: 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykowsky M., Brubaker P., Stewart K., Morgan T., Eggebeen J., Kitzman D. (2012) Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol 60: 120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykowsky M., Liang Y., Pechter D., Jones L., McAlister F., Clark A. (2007) A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol 49: 2329–2336 [DOI] [PubMed] [Google Scholar]
- Hornig B., Maier V., Drexler H. (1996) Physical training improves endothelial function in patients with chronic heart failure. Circulation 93: 210–214 [DOI] [PubMed] [Google Scholar]
- Kervio G., Ville N., Leclercq C., Daubert J., Carre F. (2004) Cardiorespiratory adaptations during the six-minute walk test in chronic heart failure patients. Eur J Cardiovasc Prev Rehabil 11: 171–177 [DOI] [PubMed] [Google Scholar]
- Keteyian S., Ellis S., Houston M., O’Connor C.M., Whellan D., Cooper L. (2009) A dose-response analysis of patients with heart failure enrolled in a controlled trial investigating ouctomes of exercise training (HF-ACTION). American College of Cardiology 58th Annual Scientific Sessions. Late Breaking Clinical Trial Oral Abstract Presentation. [Google Scholar]
- Keteyian S., Isaac D., Thadani U., Roy B., Bensimhon D., McKelvie R., et al. (2009) Safety of symptom-limited cardiopulmonary exercise testing in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Am Heart J 158: S72–S77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keteyian S., Pina I., Hibner B., Fleg J. (2010) Clinical role of exercise training in the management of patients with chronic heart failure. J Cardiopulm Rehabil Prev 30: 67–76 [DOI] [PubMed] [Google Scholar]
- Kitzman D., Brubaker P., Morgan T., Stewart K., Little W. (2010) Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 3: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman D., Gardin J., Gottdiener J., Arnold A., Boineau R., Aurigemma G., et al. (2001) Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 87: 413–419 [DOI] [PubMed] [Google Scholar]
- Kitzman D., Higginbotham M., Cobb F., Sheikh K., Sullivan M. (1991) Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank–Starling mechanism. J Am Coll Cardiol 17: 1065–1072 [DOI] [PubMed] [Google Scholar]
- Kitzman D., Little W., Brubaker P., Anderson R., Hundley W., Marburger C., et al. (2002) Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288: 2144–2150 [DOI] [PubMed] [Google Scholar]
- Komajda M., Lapuerta P., Hermans N., Gonzalez-Juanatey J., van Veldhuisen D., Erdmann E., et al. (2005) Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J 26: 1653–1659 [DOI] [PubMed] [Google Scholar]
- Konstam M., Rousseau M., Kronenberg M., Udelson J., Melin J., Stewart D., et al. (1992) Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation 86: 431–438 [DOI] [PubMed] [Google Scholar]
- Laoutaris I., Adamopoulos S., Manginas A., Panagiotakos D., Kallistratos M., Doulaptsis C., et al. (2012) Benefits of combined aerobic/resistance/inspiratory training in patients with chronic heart failure. A complete exercise model? A prospective randomised study. Int J Cardiol (Epub ahead of print on 31 May, 2012). [DOI] [PubMed] [Google Scholar]
- Laoutaris I., Dritsas A., Adamopoulos S., Manginas A., Gouziouta A., Kallistratos M., et al. (2011) Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long-term postimplantation. Eur J Cardiovasc Prev Rehabil 18: 33–40 [DOI] [PubMed] [Google Scholar]
- Linke A., Adams V., Schulze P., Erbs S., Gielen S., Fiehn E., et al. (2005) Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 111: 1763–1770 [DOI] [PubMed] [Google Scholar]
- Mann D., Reid M. (2003) Exercise training and skeletal muscle inflammation in chronic heart failure: feeling better about fatigue. J Am Coll Cardiol 42: 869–872 [DOI] [PubMed] [Google Scholar]
- McAlister F., Ezekowitz J., Wiebe N., Rowe B., Spooner C., Crumley E., et al. (2004) Systematic review: cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med 141: 381–390 [DOI] [PubMed] [Google Scholar]
- McKelvie R. (2008) Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev 13: 3–11 [DOI] [PubMed] [Google Scholar]
- McMurray J., Adamopoulos S., Anker S., Auricchio A., Bohm M., Dickstein K., et al. (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33: 1787–1847 [DOI] [PubMed] [Google Scholar]
- Meyer F., Borst M., Zugck C., Kirschke A., Schellberg D., Kubler W., et al. (2001) Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 103: 2153–2158 [DOI] [PubMed] [Google Scholar]
- Meyer T., Görge G., Schwaab B., Hildebrandt K., Walldorf J., Schäfer C. (2005a) An alternative approach for exercise prescription and efficacy testing in patients with chronic heart failure – a randomized controlled training study. Am Heart J 149: e1–e7 [DOI] [PubMed] [Google Scholar]
- Meyer T., Lucia A., Earnest C., Kindermann W. (2005b) A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters – theory and application. Int J Sports Med 26(Suppl 1): S38–S48 [DOI] [PubMed] [Google Scholar]
- Mezzani A., Agostoni P., Cohen-Solal A., Corra U., Jegier A., Kouidi E., et al. (2009) Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 16: 249–267 [DOI] [PubMed] [Google Scholar]
- Mezzani A., Corra U., Giordano A., Colombo S., Psaroudaki M., Giannuzzi P. (2010) Upper intensity limit for prolonged aerobic exercise in chronic heart failure. Med Sci Sports Exerc 42: 633–639 [DOI] [PubMed] [Google Scholar]
- Mosterd A., Hoes A. (2007) Clinical epidemiology of heart failure. Heart 93: 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. (2008) Principles of exercise prescription for patients with chronic heart failure. Heart Fail Rev 13: 61–68 [DOI] [PubMed] [Google Scholar]
- O’Connor C., Whellan D., Lee K., Keteyian S., Cooper L., Ellis S., et al. (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owan T., Hodge D., Herges R., Jacobsen S., Roger V., Redfield M. (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259 [DOI] [PubMed] [Google Scholar]
- Passino C., Severino S., Poletti R., Piepoli M., Mammini C., Clerico A., et al. (2006) Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol 47: 1835–1839 [DOI] [PubMed] [Google Scholar]
- Piepoli M., Clark A., Volterrani M., Adamopoulos S., Sleight P., Coats A. (1996) Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93: 940–952 [DOI] [PubMed] [Google Scholar]
- Piepoli M., Conraads V., Corra U., Dickstein K., Francis D., Jaarsma T., et al. (2011) Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 13: 347–357 [DOI] [PubMed] [Google Scholar]
- Piepoli M., Davos C., Francis D., Coats A. (2004) Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 328: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees K., Taylor R., Singh S., Coats A., Ebrahim S. (2004) Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev: CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., McParland J., Stanford C., Nicholls D. (1992) Oxygen consumption during corridor walk testing in chronic cardiac failure. Eur Heart J 13: 789–793 [DOI] [PubMed] [Google Scholar]
- Rognmo O., Moholdt T., Bakken H., Hole T., Molstad P., Myhr N., et al. (2012) Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 126: 1436–1440 [DOI] [PubMed] [Google Scholar]
- Roveda F., Middlekauff H., Rondon M., Reis S., Souza M., Nastari L., et al. (2003) The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860 [DOI] [PubMed] [Google Scholar]
- Singh M., Ding W., Manfredi T., Solares G., O’Neill E.F., Clements K., et al. (1999) Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol 277: E135–E143 [DOI] [PubMed] [Google Scholar]
- Smart N., Marwick T. (2004) Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med 116: 693–706 [DOI] [PubMed] [Google Scholar]
- Stoylen A., Conraads V., Halle M., Linke A., Prescott E., Ellingsen O. (2012) Controlled study of myocardial recovery after interval training in heart failure: SMARTEX-HF-rationale and design. Eur J Prev Cardiol 19: 813–821 [DOI] [PubMed] [Google Scholar]
- Swank A., Horton J., Fleg J., Fonarow G., Keteyian S., Goldberg L., et al. (2012) Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 5: 579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet J., Meurin P., Beauvais F., Weber H., Renaud N., Thabut G., et al. (2008) Absence of exercise capacity improvement after exercise training program: a strong prognostic factor in patients with chronic heart failure. Circ Heart Fail 1: 220–226 [DOI] [PubMed] [Google Scholar]
- Tan Y., Wenzelburger F., Lee E., Heatlie G., Leyva F., Patel K., et al. (2009) The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 54: 36–46 [DOI] [PubMed] [Google Scholar]
- Tierney S., Mamas M., Woods S., Rutter M., Gibson M., Neyses L., et al. (2012) What strategies are effective for exercise adherence in heart failure? A systematic review of controlled studies. Heart Fail Rev 17: 107–115 [DOI] [PubMed] [Google Scholar]
- van den Broek K., Nyklicek I., van der Voort P., Alings M., Meijer A., Denollet J. (2009) Risk of ventricular arrhythmia after implantable defibrillator treatment in anxious type D patients. J Am Coll Cardiol 54: 531–537 [DOI] [PubMed] [Google Scholar]
- Vanhees L., Kornaat M., Defoor J., Aufdemkampe G., Schepers D., Stevens A., et al. (2004) Effect of exercise training in patients with an implantable cardioverter defibrillator. Eur Heart J 25: 1120–1126 [DOI] [PubMed] [Google Scholar]
- Vanhees L., Rauch B., Piepoli M., van Buuren F., Takken T., Borjesson M., et al. (2012) Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular disease (Part III). Eur J Prev Cardiol 19: 1333–1356 [DOI] [PubMed] [Google Scholar]
- Vanhees L., Schepers D., Heidbuchel H., Defoor J., Fagard R. (2001) Exercise performance and training in patients with implantable cardioverter-defibrillators and coronary heart disease. Am J Cardiol 87: 712–715 [DOI] [PubMed] [Google Scholar]
- Williams M., Haskell W., Ades P., Amsterdam E., Bittner V., Franklin B., et al. (2007) Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 116: 572–584 [DOI] [PubMed] [Google Scholar]
- Wilson J., Mancini D., Dunkman W. (1993) Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation 87: 470–475 [DOI] [PubMed] [Google Scholar]
- Winkelmann E., Chiappa G., Lima C., Viecili P., Stein R., Ribeiro J. (2009) Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am Heart J 158: 768.e1–768.e7 [DOI] [PubMed] [Google Scholar]
- Wisloff U., Stoylen A., Loennechen J., Bruvold M., Rognmo O., Haram P., et al. (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094 [DOI] [PubMed] [Google Scholar]
- Wong E., Selig S., Hare D. (2011) Respiratory muscle dysfunction and training in chronic heart failure. Heart Lung Circ 20: 289–294 [DOI] [PubMed] [Google Scholar]


