Abstract
Zebrafish (Danio rerio) are rapidly gaining popularity in translational neuroscience and behavioral research. Physiological similarity to mammals, ease of genetic manipulations, sensitivity to pharmacological and genetic factors, robust behavior, low cost, and potential for high-throughput screening contribute to the growing utility of zebrafish models in this field. Understanding zebrafish behavioral phenotypes provides important insights into neural pathways, physiological biomarkers, and genetic underpinnings of normal and pathological brain function. Novel zebrafish paradigms continue to appear with an encouraging pace, thus necessitating a consistent terminology and improved understanding of the behavioral repertoire. What can zebrafish ‘do’, and how does their altered brain function translate into behavioral actions? To help address these questions, we have developed a detailed catalog of zebrafish behaviors (Zebrafish Behavior Catalog, ZBC) that covers both larval and adult models. Representing a beginning of creating a more comprehensive ethogram of zebrafish behavior, this effort will improve interpretation of published findings, foster cross-species behavioral modeling, and encourage new groups to apply zebrafish neurobehavioral paradigms in their research. In addition, this glossary creates a framework for developing a zebrafish neurobehavioral ontology, ultimately to become part of a unified animal neurobehavioral ontology, which collectively will contribute to better integration of biological data within and across species.
Introduction
Expanding the spectrum of model organisms and creating a comprehensive catalog of behavioral phenotypes are important strategies in translational neuroscience research.1–3 Zebrafish (Danio rerio) are rapidly emerging as a useful species for studying normal or pathological behaviors4–10 and modeling complex brain disorders11–17 (Table 1). The zebrafish genome is fully characterized, and their physiology and neuroanatomy parallel those of humans.18–21 Zebrafish breed in large numbers, are inexpensive, small and easy to manipulate genetically or pharmacologically.22–27 Zebrafish behavioral responses are robust, appear to be evolutionarily conserved, and resemble those of mammalian species.28–33 Finally, they have significant potential for high-throughput screening due to powerful video-tracking tools developed for both larval and adult zebrafish.25,34–36
Table 1.
Major Behavioral Domains Presented in the Current Catalog of Zebrafish Phenotypes
| Behavioral domains | Selected references |
|---|---|
| Anxiety/fear-related behavior | 10, 29, 31, 36, 51, 68, 70, 71, 148 |
| Cognitive behavior | 59, 73, 127, 172, 173 |
| Social behavior | 11, 13, 67, 76, 174–176 |
| Reward-related behavior | 9, 13, 26, 135–139, 168, 177 |
| Sexual behavior | 42, 77, 133 |
| Pain-related behavior | 124, 128 |
| Sensory behavior | 73, 74, 120, 178–185 |
| Sleep behavior | 122, 144, 145 |
| Neurological phenotypes | 6, 149, 150 |
| General behavior, behavioral ecology and welfare | 14, 15, 25, 118, 186, 187 |
See also Zebrafish Neurophenome Project (ZNP) Database171 for details and specific endpoints.
However, as a novel model species, the zebrafish has its own challenges.17 For example, while rodent and primate behaviors have been comprehensively evaluated and defined,37–41 zebrafish behavioral terminology is far less developed and consistent (see further). As Edwin Land famously noted, “a problem well defined is half solved”. Recognizing the importance of detailed and standardized terminology in behavioral research, this article presents the first catalog of zebrafish behaviors, also outlining future strategic goals and potential of its application in biomedicine.
The current glossary (Zebrafish Behavioral Catalog, ZBC) is expected to help improve the interpretation of zebrafish literature, promote cross-species behavioral modeling, and encourage more groups to employ zebrafish neurobehavioral paradigms for their research. Most of the 190 ZBC terms listed here refer to a descriptive (phenotype-based) aspect of zebrafish behavior, while others briefly mention the function of the behavior as well. The terms for this catalog were selected to the best of our knowledge, aiming to be comprehensive, cover all major behavioral domains, and target (in a balanced manner) both adult and larval zebrafish models. Future work will be needed to include in this glossary higher-level terms that focus on behavioral processes and interpretation/function of zebrafish behavior (such as learning, memory, acquisition, recall, and a large spectrum of addiction-related behavioral phenomena). However, as the first attempt to consolidate zebrafish behaviors in a single organized resource, this catalog can already serve as a tool for zebrafish investigators. Representing the intellectual consensus of multiple active zebrafish laboratories worldwide, the terminology developed in the version 1.0 of this glossary can be recommended for use by other groups working with zebrafish models.
As our understanding of zebrafish behaviors continues to grow, the ZBC glossary may undergo regular revisions and updates, adding new behavioral phenotypes and related terms. Gradually becoming a universal reference guide for specialized zebrafish literature, such efforts will address the increasing demand for improved and standardized neurophenotyping in this rapidly expanding field.
Glossary
This section contains the alphabetized glossary of zebrafish behaviors (phenotypes) relevant to several key behavioral domains (Table 1) in adult and larval zebrafish. One of the existing challenges in the field is the nonstandardized terminology, when similar phenotypes have different meanings (e.g., circling as part of courtship42 vs. seizure-like behavior43). Providing necessary distinctions and contexts in such cases is important, better explaining phenomenological and semantic differences which otherwise may be unclear (e.g., to nonspecialists or non-native speakers). Another problem is using various terms in a laboratory-specific manner, when different terms describe the same phenomena or behaviors (e.g., jumping, leaping, or darting for erratic movements29,44,45). Therefore, detailed explanations of terms, relevant contexts and synonyms are also needed to develop the uniform terminology for zebrafish neurobehavioral research. Furthermore, several zebrafish behaviors may have similar or overlapping behavioral manifestations, with very fine (but essential and describable) differences. For example, vertical drift and sliding describe passive swimming, but in different directions (vertical vs. lateral, see glossary), whereas coasting and creeping both represent passive slow sliding, but with or without use of fins, respectively. Likewise, stress-related freezing (lack of body movements except for eyes, with increased opercular movements) can be differentiated from sleep-like/resting behavior (lack of body movement with reduced opercular activity, see glossary). Importantly, all phenotypes included here were generated by multiple active zebrafish laboratories working with both larval and adult models. While some other groups may use alternative working definitions and terms, this catalog represents a formalized consensus that can be used for standardizing terminology in this field. For this, references to published literature were provided to support the definitions and terms used in this glossary (note, however, that for some broader terms the relevant citations were provided for the individual entries, to avoid redundancy). In addition to synonyms, specific contexts (e.g., sexual, exploratory) were given for terms covering multiple distinct domains (e.g., approach, circling, swimming). Moreover, while many behaviors are relatively similar between adult and larval zebrafish models, there are some notable differences as well.10,32,46 which have been reflected in the glossary. Where appropriate, behavioral indices were also given as examples of measurable endpoints for the terms listed in this glossary (for more details on specific endpoints and protocols, see Refs. 47–49). For convenience, the terms provided here were numbered from 1.1 to 1.190, with 1.0 referring to the current, first version of the ZBC glossary. Citing specific behaviors in the text of research articles, referring to their respective ZBC numbers (e.g., “…recording freezing behavior (ZBC term 1.68), erratic movements (ZBC term 1.51), and vertical drifting (ZBC term 1.184)…”) may enable a better characterization and interpretation of behaviors assessed in various studies and across various laboratories. Subsequent modification and development of ZBC will provide ‘time-stamped’ references to future versions of this glossary (e.g., terms 2.68, 3.51 in ZBC 2.0 and 3.0, respectively), also see further for discussion on ontology development and additional solutions for creating unique IDs for each zebrafish behavioral term.
Finally, while most of these behaviors can be detected and quantified by manual observations, the development of reliable high-throughput IT-based techniques enables a more thorough and objective quantification of zebrafish phenotypes.27,31,50–58 For example, zebrafish swimming can be quantified using video-tracking software assessing velocity, distance traveled, and time spent moving during the trial.11,29,36,59–65 Recently developed software tools enable automated quantification of complex zebrafish group behavior, such as shoaling.11 Mounting evidence also supports the value of three-dimensional (3D) analysis of zebrafish behavior, demonstrating its high sensitivity to various experimental manipulations.36,56,59 Future advances in IT-based methodology will eventually enable fully automated quantification of zebrafish behaviors, thereby contributing to high-throughput, data-dense behavioral analyses best utilizing the potential of these aquatic models.
1.1. Abnormal body position: Contortion of the body (e.g., droopy tail and bending); typically has a long-term nature (unlike short-term twitches/spasms); caused by illness, genetic defects, toxic agents, or aging. In larval zebrafish, commonly associated with neurological phenotypes and/or neurodevelopmental abnormalities.66
1.2. Aggregation behavior: Seeking of conspecifics based on chemical, visual or other cues; exploratory approach that then leads to shoaling, schooling, and/or sexual aggregating behavior, where applicable.
1.3. Aggression: Complex behaviors (including approach, fin raise, undulating body movement, mouth opening behavior, body color change, biting, charging, chasing, and circling) directed at conspecifics (or other objects) in adult zebrafish; may appear in the context of defending the territory (territorial behavior), protecting resources (e.g., females) and establishing dominance (see Social interaction). Related to boldness phenotype; can be affected by different pharmacological manipulations.67
1.4. Akinesia: A slowness of swimming or loss of normal motor function; commonly observed in aged zebrafish or after exposure to selected compounds, such as dopamine-depleting drugs (e.g., reserpine), is often accompanied by droopy tail; can be assessed by a global reduction in distance traveled and/or swimming velocity (similar to hypolocomotion), also see Ataxia and Motor incoordination.
1.5. Alarm reaction: An adaptive escape reaction which serves as an anti-predatory response exhibited in the context of fear-inducing stimulation (e.g., chemical alarm cue or visual predator exposure).29,45,68 Typically characterized by increased speed of movement and rapid directional changes, a response set that is often referred to as erratic movement (also see Zig-zagging). Alarm reaction may also include freezing with frequent opercular movements, changes in shoaling (e.g., rapid 1–2 s decreases of shoal cohesion followed by longer-lasting, up to several minutes, increase of shoal cohesion) and diving.45,68,69
1.6. Anxiety (anxiety-like) behavior: Complex behavior evoked by dangerous or potentially dangerous environment/stimuli. Includes reduced exploration, and typically manifests in geotaxis (diving), thigmotaxis, scototaxis, freezing, opercular movements, body color change, and erratic movement (zig-zagging).31,51,60,61,70,71 Anxiolytic drugs generally reduce anxiety-like behaviors, while anxiogenic agents potentiate these responses. Zebrafish anxiety-like behavior frequently overlaps with fear-related behavior,33,51,72 and future studies are needed to better characterize these two domains.
1.7. Appetitive olfactory behavior (also see Olfactory response): Increased rate of swimming and distance traveled with frequent directional changes (>90o turns) that serve to sample appetitive odor plumes (e.g., L-alanine, food extract).73–75 Once visual contact with food is established, approach and nibbling behaviors are displayed.
1.8. Approach: Display of presence, movement towards an object. Sexual: Abrupt swimming movement (‘present’) expressed independently of any male courtship behaviors; performed by females during courtship.42 Exploratory: approach to the novel object (part of boldness phenotype), opposite of avoidance.76,77 Appetitive: can be part of attraction behavior (e.g., food seeking).
1.9. Ataxia: A general loss of normal body posture and/or coordination of movements (e.g., laying on a side, swimming on a side, corkscrew swimming); commonly observed as a result of neurotoxicity-induced motor incoordination, akinesia, seizure behavior, and/or paralysis.
1.10. Attack (attacking): Short bouts of fast swimming directed at an opponent, accompanied by the mouth opening behavior and biting;67 part of aggression-related behavior (differs from strike behavior by the presence of physical contact between fighting fish).
1.11. Attraction: Increased time spent nearby or movement towards an object (visual) or chemical stimulus (e.g., food extract); opposite to Avoidance.
1.12. Avoidance: Increased movement away and/or time spent away from an object or a stimulus (e.g., predator, bright light); opposite to Attraction.
1.13. Background adaptation: See Camouflage response, Body color change.
1.14. Backward swimming: Albeit rarely occurring in normal zebrafish, typically represents an aberrant motor behavior observed under some circumstances, such as following exposure to selected hallucinogenic drugs (e.g., lysergic acid diethylamide, LSD78, 79).
1.15. Beat-and-glide: An intermittent form of swimming characterized by tail beating followed by gliding; appears at ∼4 dpf in larvae.80
1.16. Bend (bending): Aberrant neurological phenotype involving swimming with the body in a laterally bent position; can be observed as part of seizure behavior (e.g., evoked by certain convulsant agents, such as caffeine). Short-lasting bouts of this behavior represent twitch/spasm behavior.43
1.17. Bite (biting): Quick movement towards target, with mouth opening and closing, with physical contact. Social: zebrafish will often bite/nip (nipping) each other around the gill region or fins during ‘fights’. Predatory/food: zebrafish can attempt to bite/consume any sufficiently small item moving through their field of vision at appropriate speeds; differs from non-aggressive nibbling.67,81
1.18. Boldness: Behavior characterized by bold personality trait, typically manifested in increased approach towards novel objects (also see Risk-taking behavior). Usually, bolder animals also present reduced anxiety-like behavior, body color change, and increased exploratory activity.76,77,82
1.19. Body color change (coloration response): A general change in body pigmentation resulting in a darker or lighter appearance; can be a sign of anxiety, a natural response to lighting/environmental conditions (camouflage response83,84), a part of social behavior (e.g., display, fight or courtship),42 as well as a result of stress/sickness, or drug-evoked dispersion (skin darkening) or aggregation (paling) of melanophores.85 Specific drugs (e.g., alcohol, ibogaine86,87) evoke robust skin darkening in adult zebrafish, while some factors (e.g., cold exposure, pathogens) can evoke paling (e.g., sickness behavior). Coloration response can be assessed manually (by visual inspection) or using automated (luminescence-based) tools.87
1.20. Breeding (reproductive) behavior: See Spawning.
1.21. Buoyancy dysregulation: Interference with the ability to control buoyancy. Characterized by an inability to remain at a constant elevation (sometimes in vertical or inclined/titles position) without exerting physical effort via swimming; most commonly caused by problems with the swim bladder or other peripheral systems;88,89 often manifests as surfacing, vertical drifting, Cartesian diver behavior, inclined swimming, and tilting.
1.22. Burst-and-coast behavior: Darting pattern specific to larval fish not yet able to perform continuous swimming.90 Fish move forward (burst) in a single motion and glide (coast) to a slow speed, or stop from which they burst forward again.
1.23. Burst swim (swimming): Fast forward swim with large bend angles, maximally at mid-body of larval zebrafish, appears at 2 dpf in larvae. Includes larger amplitude bending (large bend angles), faster speeds and greater yaw that during slow swimming; often associated with escape behaviors; pectoral fins are tucked against the body and not active80,91,92).
1.24. C-start (C-bend/turn, Mauthner reflex): Quick escape/startle response in which the fish body first curves to form a C-shape, and then the fish propels itself away at an angle from its previous position using a fast swim.93,94 Exhibited by both adult and larval fish, and is regulated by Mauthner cells (also see O-bend and S-start/bend). In larval zebrafish, head stimulation generally elicits C-starts, while tail stimulation evokes both C- and S-starts.94
1.25. Cannibalism: Eating of dead or alive conspecifics (also see Infanticide, including egg cannibalism95).
1.26. Camouflage response (background adaptation), also see: Body color change. A change in body pigmentation (resulting in a darker or lighter appearance) after being exposed to a darker or lighter background, respectively. Occurs due to melanophore dispersion (skin darkening) or aggregation (paling).83,84,86 Part of body color change response; in fish phenotyping literature represents ‘expanded melanophore phenotype’ (e.g., lack of body color change may indicate deficits in light perception, leaving the larva in a dark adapted pigmentation state).
1.27. Cartesian diver behavior: An aberrant phenotype that involves alternating between passive vertical drift and sinking; induced in fish by some neuroactive substances, such as LSD.79
1.28. Charge (charging): Movement towards a second fish, increasing acceleration, while second fish avoids the first. Establishes social dominance, and marks the resolution of a zebrafish ‘fight.’67
1.29. Chase (chasing): See Charge.
1.30. Chemotaxis (chemoattraction): Movement to/preference towards specific chemical cues serving as chemoattractants for zebrafish. Chemically-mediated attraction behaviors are diverse and include appetitive olfactory behavior (elicited by L-alanine, food extract and others), chemically mediated kin recognition and sexual aggregating behavior (elicited by sex pheromones).73–75,96,97 Usually characterized by higher speed as fish follow the increasing concentrations of chemoattractants and by slower speed when fish locate the signal source.
1.31. Chewing: While lacking an upper pharyngeal jaw, zebrafish can chew their food by grinding the teeth in their lower jaw against a chewing pad on the base of the skull.
1.32. Circling (cycling, rotation): Repetitive swimming in a circular direction (usually seen during seizures, neurological impairments, and following the selected drugs' action). Normal behaviors with circling include display67 (circling plays a part in lateral display behavior) and courtship (circling can be seen in sexual behavior);28 can be quantified manually or using automated video-tracking tools. Characteristic ‘tight’ circling evoked by some treatments (e.g., glutamatergic antagonists) can be defined by their diameter, expressed in body length (e.g., two body lengths/∼5 cm). Commonly used circling endpoints include the number of complete circles (360o) per trial, the number (%) of animals showing circles, and the direction of circling (left- or right-rotations);2,98 automated methods may also quantify turn angle and angular velocity.
1.33. Coast (coasting): Passive sliding without body/fin movements (i.e., after the fish stopped swimming actively),99 similar to drifting (also see Creeping, which involves slow swimming with only pectoral fin use).
1.34. Coil (coiling): Embryonic movement describing a full body contraction that brings the tip of the tail to the head (coils); can be spontaneous or evoked by touch.100,101 Involves single or alternating left-right bending of entire body; appears in embryo around 18 hpf, then gradually decreases in frequency.46,101
1.35. Color preference: A natural preference/bias towards specific colors. For example, zebrafish will remain near some ‘preferred’ colors and keep away from those that induce an innate aversion (e.g., preferring black (scototaxis), or yellow, green, or red vs. blue102), most likely associated with colors of natural threats, such as predators (this, however, may depend on context).46 The color of fish objects is also an important factor in social interaction (e.g., shoaling formation) in adult zebrafish.
1.36. Coloration response: See Body color change.
1.37. Corkscrew swimming (spiraling, whirling): Spiral swimming with an increased speed and in an uncoordinated direction; commonly observed as part of seizure phenotype.43,103
1.38. Courtship: Complex patterned behaviors that precede spawning.42 The male will follow or chase the female in a jerky swimming motion with his dorsal fin erect (fin raise), and attempt to tail-nose touch (this happens after the male makes visual contact, since first needs to chemically sense the readiness of female and display sexual aggregating behavior). If an immediate spawning attempt fails, the male may position himself just above the substrate with his body slightly angled downwards, and then will often display either circling (sometimes circling the female) or zig-zagging. The male will continually attempt to spawn with the female during this time. The female may approach, escort, present, and/or lead.42 If the male's advance is unwelcome, the female may chase the male away. Zebrafish courtship behavior can be quantified manually or using automated video-tracking systems, and characterized by the following endpoints: average distance between male and female; the number of contacts between male and female; time spent in spawning area by male and female; the number of entrances into spawning area by male and female; swimming distance and velocity inside and outside spawning area; total swimming activity and turning rate by male and female.
1.39. Creeping: Very slow swimming during which only the pectoral fins propel the fish forward;44 also see coasting/drifting (passive sliding without fin use).
1.40. Cycling: See Circling.
1.41. Dart (darting): A single fast acceleration in one direction (e.g., as part of escape behavior) with the use of caudal fin. May be part of dashing or erratic movement/zig-zagging (associated with multiple darts, representing fast acceleration bouts in rapid succession, in which the direction of movement also changes in a seemingly stochastic manner between the darts36). In some publications, darting was called leaping or jumping104 (which are presently defined in ZBC as separate, distinct behavior).
1.42. Dashing: A series of directed (propulsive) darting movements; commonly seen as an escape response.
1.43. Depth preference: Natural tendency to prefer depth over shallow water60 (note, however, that shallow water can trigger breeding behavior).
1.44. Dispersion: Rapid escape-like behaviors of multiple fish moving away from each other, before reuniting with the group; typically caused by the sudden exposure to a large (potentially dangerous) moving object, such as predator (also see Shoaling).
1.45. Display: Agonistic social behavior used to establish dominance/hierarchy, plays a role in fighting behavior.28 Lateral display: Two fish line up parallel to each other head to tail, raise their dorsal fins (fin raise), extend their caudal fins, darken in color (body color change), and swim in circles (circling), often ascending. Frontal display: Two fish approach each other from the front with the attempt of nippling/biting.81
1.46. Dive (diving, geotaxis): Movement to/preference towards the bottom of the tank, often in response to threat.4,52,59,61 Generally, a very sensitive measure of anxiety/avoidance behavior; can be quantified by latency to bottom, time in bottom, frequency of visits to the bottom, distance traveled in bottom, and also expressed as respective top:bottom ratios. Is commonly reduced during habituation or anxiolytic treatments, increased by sedative/anxiogenic drugs, and can be atypically reversed by some hallucinogenic drugs (e.g., ibogaine87). Diving is an active, fast and directed zebrafish behavior (with body heading towards the bottom head first); differs from passive, more slow and undirected behaviors, such as sinking or Cartesian diver behavior (typically occurring in horizontal body position), or resting behavior.
1.47. Dorsal light reflex (DLR): A tilting of the body axis toward a light source, commonly observed in teleost fishes.105,106 Briefly, illuminated horizontally, the fish inclines its dorsoventral axis and turns dorsal surface toward the light source, with its body tilt corrected antagonistically by the vestibular righting reflex (i.e., the body inclination increases with the illumination intensity but decreases with the gravity).106
1.48. Drift (drifting): See Sliding, Coasting.
1.49. Droopy (drooping) tail: Motor phenotype associated with neurological deficits, akinesia, and global hypolocomotion. Can be evoked by aging, motor impairments or genetic and pharmacological modulations (e.g., exposure to monoamine-depleting agent reserpine; Kalueff et al., 2011–2012, unpublished observations); extreme phenotypes may result in inclined swimming. Droopy tail is a long-lasting phenotype, and differs from tail dip (a short episode of submissive behavior); can also be part of a normal resting/sleep behavior.107
1.50. Epilepsy-like behavior: See Seizure behavior.
1.51. Erratic movement (erratic swimming/locomotion): Complex behavior characterized by sharp changes in direction or velocity and repeated rapid darting; Commonly observed in adult zebrafish,29,108 erratic movement is associated with multiple darts (fast acceleration bouts in rapid succession in which the direction of movement also changes in a seemingly stochastic manner between the rapid darts; also see Zig-zagging). Usually evoked by acute stressors (predator exposure, alarm cue release) or reflects a general baseline anxiety/fear state; commonly seen immediately before or after freezing bouts; part of the alarm reaction. Larval zebrafish can also display erratic movements, for example, in response to sudden change in the light.46
1.52. Escape (startle response, tail thrash/ing): A large body angular acceleration and displacement in response to a startling stimulus.24 The first stage is a bodily ‘C-bend’ (C-start), followed by a contralateral bend and tail beat(s). The initial acceleration is often followed by rapid zig-zagging near the bottom of the tank; in some cases, escape can lead to jumping behavior. In larval fish, involves fast turning followed by burst swimming.80,91,92
1.53. Escort (escorting): Swimming alongside a male or remaining still while being courted; performed by females during courtship.42
1.54. Exploratory activity: A complex group of behaviors directed at exploration of novel environments. Related to, but not dependent on, locomotor activity and anxiety-related parameters (for example, the exploratory profile of zebrafish can be measured by quantifying the ratio of their activity in different horizontal sections and vertical areas of a tank).
1.55. Fast turn (turning): Escape-like turns in larval zebrafish, characterized by fast, large-angle turns that involve bending of the entire body with high angular velocity; takes 12 ms to turn head 180°, followed by a C-shaped counter-bend and vigorous swimming episode, as larvae swim away at a 90–180° angle. Associated with escape responses (e.g., in response to a stimulus), typically last 6–14 ms in larvae.91
1.56. Fear-like behavior: See Anxiety-like behavior for details. Traditional clinical view of anxiety is that it is a state or response induced by potential (but not currently present) aversive stimuli, whereas fear is in direct response to the appearance or perception of such stimuli. Therefore, anxiety is more diffuse, and fear is more cue-oriented.14,33,45,68,72 Currently, it is unclear how exactly the two conditions translate into zebrafish behavior, although certain conditions (e.g., alarm reaction) are more relevant to fear, while others (e.g., withdrawal) seem to represent pathological anxiety-like state.
1.57. Feeding: Behaviors related to consumption of food (see Biting, Chewing, Nibbling); can include some specific types of food (e.g., cannibalism, prey capture).
1.58. Fight (fighting): Agonistic confrontation between two individuals often used to establish social dominance;67 comprises two distinct phases: the fish first assess each other by exhibiting display, biting/nipping, flicking, and circling behaviors, which continues until the first chase/flee occurs. Next, the ‘winner’ (chaser) initiates all agonistic behaviors, while the ‘loser’ displays fleeing, submission behavior, or freezing.67
1.59. ‘Figure eight’ swimming: A specific swimming pattern observed in zebrafish following selected drug treatments (e.g., nicotine or ketamine; Kalueff et al., 2010–2012, unpublished observations); can also be part of natural courtship behavior (when male fish swims around female with raised fins28).
1.60. Fin raise (fin extension/erection): Raising the dorsal fin and/or extending the caudal fins; common in zebrafish during aggression and courtship.109
1.61. Flee (fleeing), flight behavior: Accelerating movement away from another fish or stimulus.67
1.62. Flick (flicking): A specific agonistic behavior observed when two zebrafish swim towards each other, briefly touch mouths, and then simultaneously flick away in opposite directions; can be repeatedly displayed during agonistic interactions (fight).
1.63. Flight behavior: See Flee.
1.64. Floating: Passive swimming (typically near the water surface), differs from surfacing (typically a more active locomotion at the water), drifting (typically in the middle of the water layer) or sleep/resting (typically near/at the bottom); can be related to neurological impairments or buoyancy dysregulation.
1.65. Food seeking: A common form of zebrafish foraging behavior. Is triggered by hunger and can be suppressed by pathogenic conditions (e.g., sickness behavior) or by selected psychotropic drugs acting as appetite suppressants.
1.66. Follow (following): Behavior similar to chase, typically a nonaggressive movement towards (after) another fish; common during courtship and social interaction.42
1.67. Foraging: Searching and/or probing movements typically in response to sensory cues (e.g., food seeking). Chemically induced foraging is characterized by frequent displays of directional turns as animal samples turbid chemical plumes (see Appetitive olfactory behavior).
1.68. Freeze (freezing): A complete cessation of movement (except for gills and eyes) by the fish while at the bottom of the tank.29–31,61,70 Generally, a result of high stress/anxiety or part of the submissive behavior (e.g., submissive immobile postures67); can be quantified by assessing the latency, frequency, duration and location of freezing. Opercular movements (respiration/gill movements) are usually very frequent during stress-induced freezing. Freezing behavior differs from immobility, which is typically not associated with increased opercular movements, and usually caused by toxic/sedative agents (e.g., high ethanol concentrations), during which the animals also present hypolocomotion and akinesia; can also result in sinking.67
1.69. Fright: See Escape.
1.70. Geotaxis: See Dive/diving.
1.71. Jaw movements: Stereotypic non-foraging mouth opening behavior observed following treatment with some drugs (e.g., hallucinogenic phencyclidine, Kalueff, 2012, unpublished observations; or convulsant agents, such as domoic acid110).
1.72. Habituation: Tendency to show a robustly decreased response upon repeated exposure to a novel stimulus/environment. Includes inter-trial (inter-session) and intra-trial (intra-session) habituation. Over time, typically includes increased top exploration, reduced diving, and unaltered erratic movements. Zebrafish habituation can be quantified by calculating the ratios of behavioral activity during the initial vs. latest trials, or by assessing the behavioral profile of fish across the trial(s).4,111
1.73. Head-butting: Single or repeated pushing head against the vertical surface (e.g., glass, rock); commonly observed during mirror stimulation response, during thrashing behavior, or as the result of action of selected psychotropic/hallucinogenic drugs (e.g., LSD or ibogaine87).
1.74. Head shake movements: A type of seizure/tremor-like behavior, in larval zebrafish often coupled with convulsions (typical for some convulsant drugs, e.g., domoic acid110).
1.75. Hide (hiding): Attempt by the fish to conceal itself (e.g., under the stationary object/shelter).112
1.76. Homebase formation/behavior: The tendency to establish a key ‘safe’ location which the fish spends more time in and repeatedly returns to after exploring a novel environment.113 A natural form of place preference behavior; can be assessed by time spent, number of visits and distance traveled in homebase (vs. non-homebase) areas. May be sensitive to some pharmacological manipulations.87
1.77. Hyperactivity: See Hyperlocomotion, Hyperactivity burst.
1.78. Hyperactivity burst: Episode of darting-like erratic movements with rapid turning and high velocity locomotion within a single behavioral bout; can be seen in both adult and larval fish, e.g., during high anxiety states51 or as seizure behavior.114
1.79. Hyperlocomotion (hyperactivity): Abnormally fast swimming endured for an extended period of time; typically related to psychostimulant/convulsant action or anxiety-like behavior.
1.80. Hypoactivity: See Hypolocomotion.
1.81. Hypolocomotion (hypoactivity): Abnormally slow swimming for an extended period of time; typically related to sedation, neuromotor deficits, and akinesia.
1.82. Immobility: A complete cessation of movement (except for gills and eyes) at the bottom of the tank;60 differs from freezing and resting as not always associated with altered (respectively, increased or reduced) opercular movements (note, however, that immobility and freezing are often used as synonyms in zebrafish literature87). Can be caused by sedative agents (such as high ethanol concentrations), during which the animals may also present hypolocomotion, akinesia, or paralysis.
1.83. Inclined swimming: An aberrant phenotype (swimming with an angle relative to the water surface; tilting), commonly induced by neuroactive/neurotoxic substances (also see vertical swimming and swimming upside down). Can be related to droopy tail, buoyancy dysregulation, and triggered by motor deficits or aging.
1.84. Infanticide: Cannibalizing eggs (egg cannibalism95) or larvae/fry.
1.85. J-bend (J-turn): Fine reorientation tuning in which the larva body slightly curves (∼30°), with a characteristic bend at tail.115
1.86. ‘Jittery’ swimming: A specific pattern of swimming characterized by multiple short ‘jerky’ movements with reduced smoothness of swimming trajectories; common for some seizure behavior, can be induced by selected consultants (e.g., RDX, strychnine).116
1.87. Jump (jumping): A specific zebrafish behavior involving jumping out of water/tank (similar to leaping); usually caused by anxiogenic factors, as part of escape behavior or alarm reaction (e.g., can be triggered by predator or alarm cue exposure)117; also see Terrestrial jump (note, however, that in some publications, terms ‘jumping’ or ‘leaping’ are used to describe darting behavior104).
1.88. Kin preference: The preference for kin vs. unrelated zebrafish, absent in larvae but particularly robust in juvenile 21-dpf zebrafish; based on chemical and visual cues (see Kin recognition).28
1.89. Kin recognition: The ability to recognize kin (from unrelated zebrafish) and seek kin based on chemical and visual cues; involves approach and attraction and will ultimately lead to increased time spent near kin (kin preference; also see Social preference/recognition).28
1.90. Laying on a side: Loss of normal body posture due to ataxia; commonly observed as a result of sedation and/or neurotoxicity-induced motor incoordination.104
1.91. Lead (leading): Returning at least three times to one location in the tank; performed by females during courtship.42
1.92. Leap (leaping): See Jumping.
1.93. Lethargy: Behavioral state indicative of chronic distress and/or illness (similar to a broader term sickness behavior) in adult zebrafish that includes decreased locomotor activity, reduced escape response, atypical body coloration and staying close to the bottom, with fins (especially dorsal) typically held close to the body.118 Differs from social submissive behavior by the chronic nature and independence of social context.
1.94. Loop (looping): Distinct circular swimming behavior in larvae around a virtual point outside of the larva's body (differs from circling); occurs as early as 5 dpf, common in mutants with visual feedback defects.119,120
1.95. Magnetic behavior: Behavioral responsivity of zebrafish (e.g., preferred spatial orientation) to the magnetic fields.121
1.96. Mauthner reflex: See C-start/bend.
1.97. Meander (meandering): Movement without a fixed direction or path;36,61 assessed as o/m; can be increased during periods of high anxiety, especially during erratic movement.
1.98. Mirror stimulation response: Complex behaviors evoked in fish by mirror exposure; most likely linked to aggression; typically includes approach, head-butting, biting (the mirror), and chasing (own reflection).67,87
1.99. Motor incoordination: A general loss of normal coordination of body movements (e.g., swimming on a side, corkscrew swimming); commonly observed as a result of neurotoxicity or other neurological defects (also see Akinesia, ataxia).
1.100. Mouth opening behavior: Frequent mouth opening (different from chewing or biting behavior) which can be part of aggression/attack, snapping (e.g., when exposed to food odors) or a specific stereotypic behavior (e.g., jaw movements) observed following treatment with some drugs.110 Mouth opening rate is significantly reduced during resting/sleep states.122
1.101. Neophobia: Avoidance of novel objects or food (e.g., food neophobia);4,123 can be assessed by measuring the latency and time spent/frequency of contacting the novel object.
1.102. Nip (nipping): See Bite (biting).
1.103. Nibble (nibbling): Nonaggressive biting on an object (usually, in food seeking or as part of object exploration).
1.104. Nocifensive (pain-related) behavior: Pain response to noxious stimuli, often can be experimentally induced in fish by chemical, thermal, or electrical stimulation;124,125 characterized by increased swimming, escape, and tail-beating responses (phasic stimuli induce tail beating and escape, while tonic stimuli induce rubbing, tail-beating, and increased opercular movements). While early views questioned pain responses in fish,126 mounting evidence indicates the presence of pain and pain-related behavior in zebrafish.124,125,127,128
1.105. O-bend: Orientation movement in which the larval zebrafish body curves to change the orientation (∼180°) of swimming. In contrast to C-bend, this response is slower and independent of Mauthner cells; commonly elicited by dark flashes.129
1.106. Olfactory response (olfactory behavior): Complex, odorant-evoked behavioral activity (also see Chemotaxis). Common changes in behavior include altered swimming speed and distance traveled (appetitive olfactory behavior), avoidance (e.g., alarm response) and attraction (i.e., during foraging, spawning or kin recognition).28,74
1.107. Opercular movements: Respiration/gill movements of zebrafish, can be visualized using slow-mode video-recording; bi-directionally modulated by various psychotropic drugs, are markedly increased during distress130 (e.g., during stress-induced freezing, where opercular beat rate can serve as an additional index of anxiety) and reduced during resting/sleep.122
1.108. Optokinetic response/reflex (OKR): Stereotyped tracking eye movements triggered by moving objects across the visual field. Has two components: a smooth pursuit movement following the object, and a fast saccadic movement resetting the eyes after the object has left the visual field.131
1.109. Optomotor response/reflex (OMR): Locomotion induced by a repetitive moving stimulus presentation (e.g., rotating drum), as zebrafish will generally swim in the same direction as the moving pattern.132
1.110. Oscillations of locomotor activity: Sinusoidal aspect of zebrafish locomotion/swimming, with alternating high- and lower-velocity phases (usually, with the frequency of 4–5 min) when exposed to novel environments.62
1.111. Oviposition: Release of eggs by the female during spawning.28,133
1.112. Pain-related behavior: See Nocifensive behavior.
1.113. Parallel (paralleling): Behavior during spawning, when the male swims alongside the female, in contact but slightly behind it, with head approximately leveling the female's operculum.133
1.114. Paralysis: A complete cessation of all movement, including eyes, gills/operculum and fins (similar to ataxia, but with more severe/global motor impairment, often with an abnormal posture, such as laying on the side, floating upside down or standing vertically104). Usually caused by selected neuroparalyzing agents80 or genetic neurological mutations.134
1.115. Photokinesis (phototaxis): General movement in response to light, including positive (light seeking/dark avoidance, scotophobia) and negative (light avoidance, dark preference, scototaxis). Zebrafish display sensitivity to visible light (positive phototaxis in larval fish; light avoidance in adult fish), and negative phototaxis to ultraviolet (UV) light (UV avoidance).
1.116. Photomotor response (PMR): A stereotypic series of motor behaviors in embryonic zebrafish in response to light stimulation, as zebrafish show motor excitation (lasting 5–7 s) with vigorous shaking, followed by a refractory phase, during which basal locomotion is suppressed and animals do not respond to another light pulse.57,58
1.117. Phototaxis: See positive and negative photokinesis (scotophobia and scototaxis).
1.118. Piping: Gulping air at the water surface; can be indicative of distress (e.g., hypoxia or toxicity),130 but may also be seen during depth-related adjustment of swim bladder volume (during diving).88,89
1.119. Place preference: The tendency to establish a preferred location in which the fish spends more time. Can be induced by drugs (e.g., in conditioned place preference paradigms, CPP9,123,135–139), repeated administration of food/food odors,73 social reward,13 or be based on natural behaviors (e.g., homebase formation113) or preferences (e.g., depth preference, scototaxis, thigmotaxis31,59,60).
1.120. Polarization: Behavioral characteristic of adult zebrafish reflecting the degree to which members of the group are moving in the same direction; is high in zebrafish schools (see Schooling) and reduced in shoals (see Shoaling).140
1.121. Prey capture (capturing): A complex behavior of larval zebrafish; consists of identifying the prey (e.g., paramecium) visually or using chemosensation, tracking it with a series of routine turns, forward slow swim and/or J-turns, followed by capture and ingestion (feeding behavior).141 The initial bends have low amplitude and are prominent at far-caudal locations; later bends originate more rostrally, have higher amplitude and are accompanied with increased tail-beat frequency.91,142
1.122. Predator inspection: An exploratory/boldness-related risk-taking behavior in fish associated with either increased or decreased tendency to approach a predator, potentially to gather information about the identity, precise location and/or current motivational state of the predator.143 Commonly observed in fish when shoaling, as they leave the shoal, swim towards the predator, and then return to the group.
1.123. Predatory attack: Adult and larval zebrafish may attempt to bite/consume any sufficiently small item moving through their field of vision at appropriate speeds (see Biting); in larval zebrafish, develops at ∼4 dpf, and manifests as prey capture behavior.91,141,142
1.124. Present (presenting): Halting and exposing side in front of a male or swimming up and down in front of male; performed by females during courtship.42
1.125. Quiver (quivering): High frequency, low amplitude tail oscillation by a male while aligned against the side of a female; occurs during spawning.28,42
1.126. Reflection chase (chasing): A behavior that includes chasing own reflection (e.g., in the observation tank or as part of the mirror stimulation response);67 can also be triggered by selected psychoactive (e.g., hallucinogenic) drugs.87
1.127. Reproductive (breeding) behavior: see Spawning.
1.128. Rest behavior (resting): Sleep-related behavior in adult and larval zebrafish. Typical rest behavior in larval zebrafish includes floating with head down, or staying horizontal close to the bottom of the tank (immobility, hypolocomotion).144,145
1.129. Retreat (retreating): A social behavior relevant to dominance in zebrafish, generally involves a submissive fish swimming rapidly away from the opponent (e.g., from a dominant fish) in response to an attack (e.g., after a strike, bite, chase, or charge), part of fleeing behavior.67
1.130. Rheotaxis: A common behavior in aquatic species, includes turning towards a current and a tendency to swim upstream; displayed by both larval and adult zebrafish. In experimental setting, manifests in avoidance by zebrafish of the sucking source (e.g., sucking pump or standpipe).146,147
1.131. Risk-taking behavior: Propensity of zebrafish to engage in dangerous situations (part of their boldness phenotype);143 commonly occurs when a prey fish approaches/inspects a predator (see Approach, Predator inspection), and/or when a shoal member leaves the group (see Shoaling).
1.132. Rotation behavior: See Circling, Cycling.
1.133. Routine turn(ing); R-turn(ing): A slow spontaneous turn (20–30 ms) with a large bend angle (∼60°) resulting in reorientation of the larva before forward swimming, prey capture; lacks the large counter-bend (shown in escape turns), with only a small portion of the tail bending; has a slow angular velocity with relatively slow turning angles.91
1.134. Rub (rubbing): A characteristic aberrant zebrafish behavior involving rubbing body sides on the sides of the tank (or the surface of other objects); typically caused by pathogenic conditions (e.g., skin disease and/or parasitic infection).118
1.135. Scoot swim (swimming): See Slow swim.
1.136. Scotophobia: A natural preference for light (or avoidance of dark) lighting/environment, commonly observed in larval fish; usually is replaced with scototaxis in adult fish (also see Photokinesis).46
1.137. Scototaxis: A natural preference for dark (or avoidance of bright) lighting/environment in adult zebrafish.70,71,148 Generally, a measure of anxiety (reduced by anxiolytic drugs and increased by anxiogenic agents). Note that larval zebrafish display opposite behavior (scotophobia). Can be quantified in the light-dark box tests (by assessing the latency, time spent in light or dark, distance traveled, the number of visits, the average duration of a visit; and by the respective behaviors' light:dark ratios); also see Photokinesis.
1.138. School (schooling): Formation of a relatively polarized group (school) in which multiple fish swim together, in a coordinated/synchronous fashion; part of aggregation behavior that increases foraging efficiency as well as the ability to detect and/or avoid predators. Schools may disperse into shoals (see Shoaling) which show reduced polarization.140
1.139. Seizure (seizure-like/epilepsy-like) behavior: Involuntary, rapid movements of body (usually, as a result of pathology, such as epilepsy) observed in both larval and adult zebrafish; include ataxia, corkscrew (spiral) swimming, hyperactivity, circling, spasms, weavering, head shake movements, tremor, and/or jittery locomotion. Severe cases include death. Can be quantified manually (using seizure scale) or applying automated video-tracking tools (by assessing the velocity and distance traveled).110,114,149,150
1.140. Sexual aggregating behavior: Instinctive response to chemical cues released from females during ovulation; males display attraction, approach and courtship behaviors once female is identified.42
1.141. Shoal (shoaling): Formation of a relatively nonpolarized group (shoal) of adult zebrafish, held together by social pressures (i.e., not by individual attraction to an external stimulus); part of aggregation behavior. Anxiety/fear causes the shoal to ‘tighten’ (the fish swim closer together) and potentially form a school (see Schooling).64,140,151 Hunger/habituation causes the shoal to become looser and less organized. Zebrafish shoaling has an oscillating dynamic, and this behavior can be quantified manually or using automated video-tracking systems, assessing several endpoints, including the average inter-fish distance; shoal area size; proximity (time each member of the shoal spent within a specified distance from each other); nearest and farthest neighbor distances; time spent in shoal; time spent away from shoal; number of animals leaving the shoal (also relevant to risk-taking behavior) and polarization (reflecting the uniformity of heading).
1.142. Shyness: A reduced exploratory activity, reduced general activity in a novel environment and/or in response to stimuli, or reduced risk-taking behavior (opposite to boldness).82
1.143. Sink (sinking): Freezing behavior during which the fish remains immobile (except for the eyes and gills) but changes its position in the water column (moving from top to bottom) without moving any of its fins44 (also see Cartesian diver behavior).
1.144. Sickness behavior: A broad cluster of behaviors indicative of illness (or pain) that include hypoactivity, inhibited exploration, feeding or food seeking, pale body color, and lethargy (with fins typically held close to the body).152
1.145. Sleep (sleep-like behavior): Activity characterized in zebrafish by rest behavior, including reversible immobility/hypolocomotion, elevated arousal threshold, reduced respiratory rate (e.g., opercular movements) and mouth opening frequency, and a compensatory rebound in response to sleep deprivation.107,122,144,145,153–156 In adult zebrafish, includes brief periods of inactivity, often with a drooping caudal fin (see Droopy tail), alternated with active periods of swimming; can be easily reversed by startling stimuli, such as tapping, sound, or weak electric field.111,112,119–124
1.146. Slide (sliding): See Coasting.
1.147. Slow swim (slow/scoot swimming): Larval zebrafish slow swimming (scoots) characterized by small bend angles with bend location near the tail. Maximal bending occurs close to the tail; low degree of bending and tail beat frequency; yaw angles are <3°; pectoral fins are active and alternate right to left between adduction and abduction.91,92
1.148. Snap (snapping): Reflexive opening and closing of mouth during exposure to high concentrations of appetitive stimuli (e.g., L-alanine, food extract); signals initiation of ingestive phase during feeding.157
1.149. Spasm (twitch, twitching): Spontaneous, rapid movements of body (usually, as a result of neurological/neurotoxic impairment, such as seizure116).
1.150. Spatiotemporal stability: The ability to withstand changes in environmental characteristics during exploration of a novel environment, primarily by scaling locomotor activity (e.g., distance traveled) to the size of the environment, but retaining the temporal budgeting of the activity; can be evaluated by temporal distribution of locomotion and position in a test tank (e.g., distance traveled, transitions and time spent in each area).62
1.151. Social interaction: Normal social behavior of zebrafish, represents a reciprocal change in zebrafish behavior influenced by the presence or actions of other conspecifics.13,67,151 Some examples include fighting/aggression, shoaling/schooling, courtship and spawning; can also manifest in approach/boldness (social investigation), social recognition, and social preference.
1.152. Social preference: A natural tendency to spend time close to conspecifics; can be observed as part of shoaling behavior, kin recognition, social recognition, or preference of the ‘conspecific’ vs. ‘empty’ compartments of the tank.13,28
1.153. Social recognition: The ability of zebrafish to recognize familiar from unfamiliar zebrafish.28
1.154. Spiraling: See Corkscrew swimming, whirling.
1.155. Spawning (breeding/reproductive behavior): During breeding, the male zebrafish approaches the female and curves body around, positioning his genital pore next to hers (also see Parallel). The male then quivers in an attempt to trigger oviposition in the female; sperm is released simultaneously to fertilize the newly released eggs. Spawning behavior can be promoted by exposure of zebrafish to shallow water.28
1.156. S-start/bend: Quick escape/startle response in which the fish body curves to form an S-shaped body bend with simultaneous activity rostrally on one side, and caudally on the other (also see O-bend and C-start/bend). In larval zebrafish, head stimulation generally elicits C-starts, while tail stimulation evokes both C- and S-starts.94
1.157. Startle response: An evolutionarily conserved, adaptive behavior in response to sudden, usually aversive, stimuli (see Escape), such as vibration, light, sound, or touching (e.g., touch response);24,94 may involve ‘C-start’ behavior, during which coiling and dashing may be observed.94
1.158. Stereotypic behaviors: A pattern of rigid, repetitive behaviors other than swimming (see Stereotypic locomotion/swimming), evoked in zebrafish under some conditions, e.g., stereotypic mouth opening behavior following treatment with some hallucinogenic drugs (e.g., phencyclidine; Kalueff et al., 2011–2012, unpublished observations).
1.159. Stereotypic locomotion (stereotypic swimming): A pattern of rigid, repetitive behaviors (e.g., swimming from corner to corner) evoked in zebrafish under some conditions (e.g., treatment with psychostimulants like nicotine and caffeine, or hallucinogens like ibogaine or phencyclidine87,98).
1.160. Strike (striking): An aggression-related behavior, observed in zebrafish when the fish swims rapidly toward the opponent, but without physical contacts between them. Differs from approach by a generally much higher velocity and its aggressive (rather than investigatory) nature (also see Attack, which occurs with physical contact).67
1.161. Struggle (struggling): A behavior observed in larval zebrafish, characterized by longer alternating motor bursts at lower frequencies than swimming. During struggling, motor bursts propagate in inverse directions along the body, compared with swimming behavior.158
1.162. Submissive behavior: A social behavior following aggressive confrontations between zebrafish. Submissive fish stays immobile (with fins retracted), typically near the bottom or near the surface of the aquaria (also see Freezing), with the caudal part of the body oriented downward (also see Tail dip, representing the initial form of submissive behavior).67
1.163. Surface touching: See Surfacing.
1.164. Surfacing (surface touching): Dwelling of fish at the surface of the water; is usually evoked by selected neuroactive drugs, mainly serotonergic agonists and glutamatergic antagonists,79,159 may also be related to buoyancy dysregulation.
1.165. Swim (swimming): Simple zebrafish locomotion; can be categorized by its duration as ‘prolonged,’ which may be maintained for minutes, or as ‘sustained,’ which may be maintained for hours. In larvae, includes slow swim (point of maximal body bending occurs close to the tail) and burst swim (maximal body bending occurs near the mid-body; maintained over seconds or less, includes larger-amplitude bending, faster speed, and greater yaw (vs. slow swim), also see Beat-and-glide). Selected forms of aberrant swimming include swimming on a side, upside down, vertical swimming, Cartesian diver behavior.
1.166. Swimming on a side: Loss of normal body posture due to ataxia; commonly observed as a result of sedation and/or neurotoxicity-induced motor incoordination.
1.167. Swimming upside down: An aberrant phenotype (swimming in an upside down position), commonly induced by neuroactive/neurotoxic substances;104 also see Inclined and Vertical swimming.
1.168. Tail dip (tail dipping): An agonistic behavior during fighting, when one fish slightly drops its tail for a short period of time, to signal its submission and end the confrontation160 (differs from droopy tail, which a long-lasting behavior; see Submissive behavior).
1.169. Tail beat (beating, slapping): Characterized by repeating episodes of rhythmic, rostro-caudally progressing peripheral nerve discharges that are alternated between the two sides of the body. Viewed from above, tail beating is physically apparent in side-to-side sweeping of the tail (that can be measured as tail beat amplitude).
1.170. Tail-nose touch(ing): Touching the side or tail of another fish with the nose or head. Performed by males during social interaction, especially courtship.42
1.171. Terrestrial jump(ing): A coordinated leap (using tail-flip) in response to placement on a damp surface (e.g., after jumping out of water); can be quantified by amplitude (height) and frequency of the leaps.161
1.172. Territorial behavior: Monopolization and aggressive defense of a defined area in a habitat/tank162 (e.g., spawning sites are a commonly defended territory in zebrafish). Trespassers into the territory may be chased or bitten by the dominant fish, or can ‘fight’ to challenge its dominance.
1.173. Thigmotaxis: A preference for staying in close proximity to the edge/side (and avoiding the central open areas); generally, can serve as a measure of zebrafish anxiety.31,51,60
1.174. Thrashing: Forceful swimming with the use of the caudal fin while physically in contact with the side or bottom wall of the tank; swimming against the glass wall of the tank that appears as if the fish is trying to swim through the glass barrier.44 This behavior can be thrashing towards an appetitive cue (e.g., food) or thrashing away from an aversive stimulus (e.g., representing an escape); can be similar to head-butting behavior (e.g., during the mirror stimulation test).
1.175. Tilt (tilting): Deviation from the horizontal position;44 is often seen during inclined swimming.
1.176. Top dwelling: Dwelling in the top half of the tank; can include aberrant swimming very close to water surface (surfacing).
1.177. Touch response: Startle behavior-related phenotype evoked by the touch in an embryo, which responds with fast coiling of the trunk bending over the head; appears around 21 hpf.100
1.178. Trance-like swim(ming): A slow swimming motion induced in fish by specific psychotropic drugs (e.g., chronic fluoxetine or hallucinogens, such as LSD79 or salvinorin A139); often includes a swimming pattern characterized by a slow (albeit active) bout of horizontal swimming for 1–2 s, followed by a similarly short passive gliding motion.
1.179. Tremor behavior: Specific shivering-like behavior, most typically evoked in adult or larval zebrafish by selected neurotoxic/convulsant drugs, such as domoic acid (also see Seizures and Weavering);110 particularly visible in the tail area.
1.180. Turn (turning): A simple change in swimming direction observed in both adult36,61 or larval91 zebrafish. Larval zebrafish show several specific forms of turning behavior, including slow routine turns (lacking the large counterbend of escape turns) and fast turns (fast, large angle turns characteristic of escape responses).91
1.181. Twitch (twitching): See Spasm.
1.182. Undulating body movement: A wave-like or snake-like motion; part of aggression-related behaviors,109 and occurs mainly at the beginning of the fight, especially between two equal opponents. This behavior is common in fish species, and is likely related to the use of lateral line by fish to size-up the opponent by the waves generated by its movement. Although less visible in zebrafish (compared to other fishes), their undulating body movement can be observed using high-resolution videos (sometimes this behavior is followed by forceful tail beats).109
1.183. UV avoidance: Avoidance of UV light (negative photokinesis/phototaxis) reported in zebrafish larvae as early as 4 dpf.163
1.184. Vertical drift (drifting): An aberrant phenotype that involves passive floating vertically (i.e., passive vertical motion from bottom to top);164 commonly induced by neuroactive/neurotoxic substances or agents related to buoyancy dysregulation (also see Vertical swimming); opposite to sinking (also see Cartesian diver behavior).
1.185. Vertical swim (swimming): An aberrant fish phenotype that involves swimming vertically (typically heads up at the surface);79 commonly induced by neuroactive (e.g., LSD) or neurotoxic substances, or agents related to buoyancy dysregulation (also see Inclined swimming and Swimming upside down).
1.186. Vestibulo-ocular reflex (VOR): Compensatory eye movements in zebrafish in response to linear/angular acceleration as well as changes in head position with respect to gravity, in order to stabilize the retinal image.165,166 These movements are evoked through the semicircular canals of the vestibular apparatus in zebrafish.
1.187. Weaver (weavering): Aberrant tremor/shaking-like phenotype, typically evoked by selected neurotoxic/convulsant agents (e.g., strychnine; Kalueff et al., 2011–2012, unpublished observations); similar to tremor and head shake movements (also see Seizure behavior).43
1.188. Whirl (whirling): See Corkscrew swimming, spiraling.
1.189. Withdrawal-related behavior: A characteristic behavioral syndrome observed in zebrafish following discontinuation of drugs of abuse (e.g., ethanol, morphine); typically characterized by elevated anxiety/fear-like behavior.167,168
1.190. Zig-zagging (zigzagging): Sexual: a tail sweep and circle along a female's body resembling a ‘figure eight’; typically performed by males during courtship.42 Stress-induced: erratic movement with multiple darts, during which the direction of movement changes in a seemingly alternating (zig-zag-like) manner between the darts.45,51
Conclusion
Overall, the terminology developed here represents an updated and standardized catalog of multiple zebrafish behavioral phenotypes. Its primary goal is to enable a better understanding of fish behavior, thereby enhancing zebrafish-based neuroscience research. There are several additional potential applications of this catalog, including fostering further translational cross-species behavioral modeling; developing novel models using zebrafish (and other fish species); providing a starting point for training investigators and students working with zebrafish behavioral tests; and encouraging new groups to actively adopt zebrafish neurobehavioral paradigms for their research.
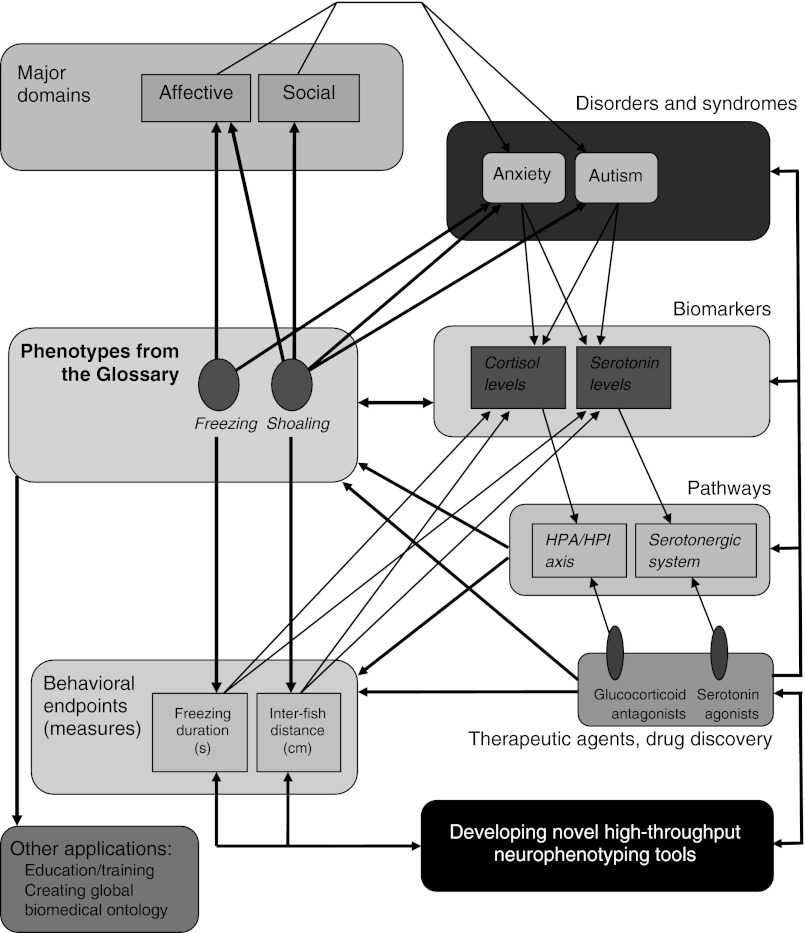
Representing a community-driven neurophenotyping effort initiated by the International Zebrafish Neuroscience Research Consortium (ZNRC) and the Zebrafish Neurophenome Project (ZNP),169 this catalog can eventually lead to the development of comprehensive ethogram of zebrafish behaviors. It can also contribute to the development of a zebrafish neurobehavioral ontology (ZNBO), in which the outlined behaviors (phenotypes) will be integrated with other biological categories and phenomena. As shown in Figure 1, individual zebrafish behaviors presented here (e.g., freezing, shoaling) can be further connected into a large-scale dynamic network, which can include ‘vertical links’ to larger semantic units, such as ‘behavioral domains’ (e.g., affective, social; Table 1), brain disorders or syndromes associated with them (e.g., anxiety/fear, autism), or developmental stages (e.g., adult vs. larval zebrafish). This network may also include multiple relevant ‘horizontal’ links, such as physiological biomarkers/correlates (e.g., specific endocrine or neurochemical responses), environmental modifiers (e.g., genetic mutations or pharmacological treatments), as well as top-down links to smaller semantic units, such as individual behavioral endpoints/indices (e.g., no-mobility duration during freezing or average inter-fish distance in fish shoals; Fig. 1) used to quantify various zebrafish phenotypes.
FIG. 1.
Potential for further integration of zebrafish behavioral phenotypes with other biological phenomena and related processes.
Although an important initiative per se, the proposed ZNBO can be joined with other biological ontologies. For example, it can contribute to the existing zebrafish ontologies within the Zebrafish Information Network (ZFIN) Project,170 which currently does not contain behavioral data. Collaborative efforts are currently underway to achieve this goal. Likewise, ZNBO can be a valuable addition to the growing Animal Behavior Ontology (ABO) and Neurobehavior Ontology (NBO), eventually becoming part of the global biological ontology. NBO currently consists of two main components (an ontology of behavioral phenotypes and an ontology of behavioral processes), but lacks zebrafish data. Therefore, the present catalog of zebrafish phenotypes may become a useful addition to the first component of NBO, while future efforts (expanding this glossary to include behavioral processes) can be federated with the second component of NBO. Furthermore, while references to the ZBC term ID numbers (e.g., ZBC term 1.28 for charging, ZBC term 1.67 for foraging) offers one way of improved characterization and presenting of specific behaviors assessed in various studies from different laboratories, ZNRC is currently establishing collaborations with expert ontologists, to assign each catalogue term a unique ‘bio-ontology’ ID (which will then be easily searchable and compatible across ZBC as well as ZFIN, NBO, and multiple other existing biological ontologies). Collectively, these efforts will foster better and more global integration of behavioral data within and across species, including human behavior-related disease phenotypes.
Contributor Information
Collaborators: the Zebrafish Neuroscience Research Consortium (ZNRC)
Acknowledgments
This project is supported in part by the NIH/NIDA Grant (DA030900-2) to AVK, as well as by the International Zebrafish Neuroscience Research Consortium (ZNRC), the Zebrafish Neurophenome Project (ZNP), and ZENEREI Institute (New Orleans, LA). MB and JSC were supported by the 2012 NIH/NIDA Summer Research Program and the LSU Genetics Summer Research Program. JMC is supported by the ZENEREI Institute's graduate fellowship fund. The authors thank ZNRC members and other zebrafish experts for their valuable discussion and feedback during this project. Colleagues from active zebrafish laboratories worldwide are invited to contribute zebrafish phenotypes and related terminology to the forthcoming revisions of this catalog by contacting the ZNRC Secretariat (e-mail: info@zenerei.com).
Disclosure Statement
No competing financial interests exist.
References
- 1.Kalueff AV. Wheaton M. Murphy DL. What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Riehl R. Kyzar E. Allain A, et al. Behavioral and physiological effects of acute ketamine exposure in adult zebrafish. Neurotoxicol Teratol. 2011;33:658–667. doi: 10.1016/j.ntt.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Manger PR. Cort J. Ebrahim N, et al. Is 21st century neuroscience too focussed on the rat/mouse model of brain function and dysfunction? Front Neuroanat. 2008;2:5. doi: 10.3389/neuro.05.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart A. Kadri F. DiLeo J, et al. The developing utility of zebrafish in modeling neurobehavioral disorders. Int J Comp Psychol. 2010;23:104–121. [Google Scholar]
- 5.Stewart A. Wong K. Cachat J, et al. Zebrafish models to study drug abuse-related phenotypes. Revs Neurosci. 2010;22:95–105. doi: 10.1515/RNS.2011.011. [DOI] [PubMed] [Google Scholar]
- 6.Best JD. Alderton WK. Zebrafish: An in vivo model for the study of neurological diseases. Neuropsychiatr Dis Treat. 2008;4:567–576. doi: 10.2147/ndt.s2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton WH. Webb K. Harris M, et al. Approaches to Analyse Mood Disorders in Zebrafish. In: Spink AJ, editor; Ballintijn MR, editor; Bogers ND, editor; Grieco F, editor; Loijens LWS, editor; Noldus LPJJ, et al., editors. Proc Meas Behavior, Maastricht; The Netherlands: 2008. [Google Scholar]
- 8.Jesuthasan S. Zebrafish in the Spotlight. Science. 2002;297:1484–1485. doi: 10.1126/science.1076115. [DOI] [PubMed] [Google Scholar]
- 9.Webb KJ. Norton WH. Trumbach D, et al. Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biol. 2009;10:R81. doi: 10.1186/gb-2009-10-7-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norton W. Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller N. Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Gerlai R. Ahmad F. Prajapati S. Differences in acute alcohol-induced behavioral responses among zebrafish populations. Alcohol Clin Exp Res. 2008;32:1763–1773. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Imari L. Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio) Behav Brain Res. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Gerlai R. Zebrafish antipredatory responses: A future for translational research? Behav Brain Res. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlai R. A small fish with a big future: Zebrafish in behavioral neuroscience. Rev Neurosci. 2011;22:3–4. doi: 10.1515/RNS.2011.002. [DOI] [PubMed] [Google Scholar]
- 16.Stewart A. Wu N. Cachat J, et al. Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1421–1431. doi: 10.1016/j.pnpbp.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Kalueff A. Stewart A. Kyzar E, et al. Time to recognize zebrafish ‘affective’ behavior. Behaviour. 2012;149:1019–1036. [Google Scholar]
- 18.Panula P. Sallinen V. Sundvik M, et al. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 2006;3:235–247. doi: 10.1089/zeb.2006.3.235. [DOI] [PubMed] [Google Scholar]
- 19.Peitsaro N. Sundvik M. Anichtchik OV. Kaslin J. Panula P. Identification of zebrafish histamine H1, H2 and H3 receptors and effects of histaminergic ligands on behavior. Biochem Pharmacol. 2007;73:1205–1214. doi: 10.1016/j.bcp.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Chen YC. Priyadarshini M. Panula P. Complementary developmental expression of the two tyrosine hydroxylase transcripts in zebrafish. Histochem Cell Biol. 2009;132:375–381. doi: 10.1007/s00418-009-0619-8. [DOI] [PubMed] [Google Scholar]
- 21.Panula P. Chen YC. Priyadarshini M, et al. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Burne T. Scott E. van Swinderen B, et al. Big ideas for small brains: What can psychiatry learn from worms, flies, bees and fish? Mol Psychiatry. 2011;16:7–16. doi: 10.1038/mp.2010.35. [DOI] [PubMed] [Google Scholar]
- 23.Bilotta J. Saszik S. DeLorenzo AS. Hardesty HR. Establishing and maintaining a low-cost zebrafish breeding and behavioral research facility. Behav Res Methods Instrum Comput. 1999;31:178–184. doi: 10.3758/bf03207707. [DOI] [PubMed] [Google Scholar]
- 24.Dlugos CA. Rabin RA. Ethanol effects on three strains of zebrafish: Model system for genetic investigations. Pharmacol Biochem Behav. 2003;74:471–480. doi: 10.1016/s0091-3057(02)01026-2. [DOI] [PubMed] [Google Scholar]
- 25.Gerlai R. Event recording and video-tracking: Towards the development of high throughput zebrafish screens. Proc 5th Conf Methods Behav Res, Wageningen; The Netherlands. 2005. [Google Scholar]
- 26.Ninkovic J. Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 2006;39:262–274. doi: 10.1016/j.ymeth.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Kokel D. Peterson RT. Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Brief Funct Genom Proteom. 2008;7:483–490. doi: 10.1093/bfgp/eln040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spence R. Gerlach G. Lawrence C. Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 29.Egan RJ. Bergner CL. Hart PC, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong K. Elegante M. Bartels B, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Blaser RE. Chadwick L. McGinnis GC. Behavioral measures of anxiety in zebrafish (Danio rerio) Behav Brain Res. 2010;208:56–62. doi: 10.1016/j.bbr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Champagne DL. Hoefnagels CC. de Kloet RE. Richardson MK. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav Brain Res. 2010;214:332–342. doi: 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Jesuthasan S. Fear, anxiety and control in the zebrafish. Dev Neurobiol. 2012;72:395–403. doi: 10.1002/dneu.20873. [DOI] [PubMed] [Google Scholar]
- 34.Stewart A. Wu N. Cachat J, et al. Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1421–1431. doi: 10.1016/j.pnpbp.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 35.Mathur P. Guo S. Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis. 2010;40:66–72. doi: 10.1016/j.nbd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cachat J. Stewart A. Utterback E, et al. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE. 2011;6:e17597. doi: 10.1371/journal.pone.0017597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McInnes LA. Freimer NB. Mapping genes for psychiatric disorders and behavioral traits. Curr Opin Genet Dev. 1995;5:376–381. doi: 10.1016/0959-437x(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 38.Xu F. Xie L. Li X, et al. Construction and validation of a systematic ethogram of Macaca fascicularis in a free enclosure. PLoS One. 2012;7:e37486. doi: 10.1371/journal.pone.0037486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sade DS. An ethogram for Rhesus monkeys. I. Antithetical contrasts in posture and movement. Am J Phys Anthropol. 1973;38:537–542. doi: 10.1002/ajpa.1330380263. [DOI] [PubMed] [Google Scholar]
- 40.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: Experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 41.Shettleworth SJ. Cognition, Evolution, and Behavior. 2nd. Oxford ; New York: Oxford University Press; 2010. [Google Scholar]
- 42.Darrow KO. Harris WA. Characterization and development of courtship in zebrafish, Danio rerio. Zebrafish. 2004;1:40–45. doi: 10.1089/154585404774101662. [DOI] [PubMed] [Google Scholar]
- 43.Stewart AM. Desmond D. Kyzar E, et al. Perspectives of zebrafish models of epilepsy: What, how and where next? Brain Res Bull. 2012;87:135–143. doi: 10.1016/j.brainresbull.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Sison M. Gerlai R. Behavioral performance altering effects of MK-801 in zebrafish (Danio rerio) Behav Brain Res. 2011;220:331–337. doi: 10.1016/j.bbr.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Speedie N. Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad F. Noldus LPJJ. Tegelenbosch R. Richardson M. Zebrafish embryos and larvae in behavioural assays. Behaviour. 2012;149:1241–1281. [Google Scholar]
- 47.Kalueff A. Cachat J. Zebrafish Neurobehavioral Protocols. Humana Press; 2010. [Google Scholar]
- 48.Kalueff A. Cachat J. Zebrafish Models for Neurobehavioral Research. Humana Press; 2010. [Google Scholar]
- 49.Kalueff A. Stewart A. Zebrafish Protocols for Neurobehavioral Research. Humana Press; 2012. [Google Scholar]
- 50.Stewart A. Gaikwad S. Kyzar E. Green J. Roth A. Kalueff AV. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology. 2012;62:135–143. doi: 10.1016/j.neuropharm.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maximino C. de Brito TM. da Silva Batista AW. Herculano AM. Morato S. Gouveia A., Jr. Measuring anxiety in zebrafish: A critical review. Behav Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 52.Sackerman J. Donegan JJ. Cunningham CS, et al. Zebrafish behavior in novel environments: Effects of acute exposure to anxiolytic compounds and choice of Danio rerio Line. Int J Comp Psychol. 2010;23:43–61. [PMC free article] [PubMed] [Google Scholar]
- 53.Piato AL. Capiotti KM. Tamborski AR, et al. Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:561–567. doi: 10.1016/j.pnpbp.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 54.Guo S. Wagle M. Mathur P. Toward molecular genetic dissection of neural circuits for emotional and motivational behaviors. Dev Neurobiol. 2012;72:358–365. doi: 10.1002/dneu.20927. [DOI] [PubMed] [Google Scholar]
- 55.Okamoto H. Agetsuma M. Aizawa H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev Neurobiol. 2012;72:386–394. doi: 10.1002/dneu.20913. [DOI] [PubMed] [Google Scholar]
- 56.Zhu L. Weng W. Catadioptric stereo-vision system for the real-time monitoring of 3D behavior in aquatic animals. Physiol Behav. 2007;91:106–119. doi: 10.1016/j.physbeh.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 57.Kokel D. Bryan J. Laggner C, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6:231–237. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kokel D. Peterson RT. Using the zebrafish photomotor response for psychotropic drug screening. Methods Cell Biol. 2011;105:517–524. doi: 10.1016/B978-0-12-381320-6.00022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosemberg DB. Rico EP. Mussulini BH, et al. Differences in spatio-temporal behavior of zebrafish in the open tank paradigm after a short-period confinement into dark and bright environments. PLoS ONE. 2011;6:e19397. doi: 10.1371/journal.pone.0019397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blaser RE. Rosemberg DB. Measures of anxiety in zebrafish (Danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE. 2012;7:e36931. doi: 10.1371/journal.pone.0036931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cachat JM. Canavello PR. Elkhayat SI, et al. Zebrafish Neurobehavioral Protocols. Humana Press; 2010. Video-aided analysis of zebrafish locomotion and anxiety-related behavioral responses; pp. 191–201. [Google Scholar]
- 62.Stewart AM. Gaikwad S. Kyzar E. Kalueff AV. Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Res. 2012;1451:44–52. doi: 10.1016/j.brainres.2012.02.064. [DOI] [PubMed] [Google Scholar]
- 63.Maaswinkel H. Zhu L. Weng W. The immediate and the delayed effects of buspirone on zebrafish (Danio rerio) in an open field test: A 3-D approach. Behav Brain Res. 2012;234:365–374. doi: 10.1016/j.bbr.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Miller N. Gerlai R. Automated tracking of zebrafish shoals and the Analysis of shoaling behavior. Zebrafish Protocols for Neurobehavioral Research. 2012:217–230. [Google Scholar]
- 65.Kato S. Nakagawa T. Ohkawa M, et al. A computer image processing system for quantification of zebrafish behavior. J Neurosci Methods. 2004;134:1–7. doi: 10.1016/j.jneumeth.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 66.Aspatwar A. Tolvanen ME. Jokitalo E, et al. Abnormal cerebellar development and ataxia in CARP VIII morphant zebrafish. Human Mol Genet. 2013;22:417–432. doi: 10.1093/hmg/dds438. [DOI] [PubMed] [Google Scholar]
- 67.Oliveira RF. Silva JF. Simoes JM. Fighting zebrafish: Characterization of aggressive behavior and winner-loser effects. Zebrafish. 2011;8:73–81. doi: 10.1089/zeb.2011.0690. [DOI] [PubMed] [Google Scholar]
- 68.Jesuthasan SJ. Mathuru AS. The alarm response in zebrafish: Innate fear in a vertebrate genetic model. J Neurogenet. 2008;22:211–228. doi: 10.1080/01677060802298475. [DOI] [PubMed] [Google Scholar]
- 69.Egan RJ. Bergner CL. Hart PC, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maximino C. de Brito TM. Colmanetti R, et al. Parametric analyses of anxiety in zebrafish scototaxis. Behav Brain Res. 2010;210:1–7. doi: 10.1016/j.bbr.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 71.Maximino C. Marques de Brito T. Dias CA. Gouveia A., Jr. Morato S. Scototaxis as anxiety-like behavior in fish. Nat Protoc. 2010;5:209–216. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- 72.Okamoto H. Agetsuma M. Aizawa H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev Neurobiol. 2012;72:386–394. doi: 10.1002/dneu.20913. [DOI] [PubMed] [Google Scholar]
- 73.Braubach OR. Wood HD. Gadbois S. Fine A. Croll RP. Olfactory conditioning in the zebrafish (Danio rerio) Behav Brain Res. 2009;198:190–198. doi: 10.1016/j.bbr.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 74.Bhinder G. Tierney KB. Olfactory evoked activity assay for larval zebrafish. In: Kalueff AV, editor; Stewart AM, editor. Zebrafish Neurobehavioral Protocols. II. Humana Press; 2012. pp. 71–84. [Google Scholar]
- 75.Koide T. Miyasaka N. Morimoto K, et al. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc Natl Acad Sci USA. 2009;106:9884–9889. doi: 10.1073/pnas.0900470106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wright D. Rimmer LB. Pritchard VL. Krause J. Butlin RK. Inter and intra-population variation in shoaling and boldness in the zebrafish (Danio rerio) Naturwissenschaften. 2003;90:374–377. doi: 10.1007/s00114-003-0443-2. [DOI] [PubMed] [Google Scholar]
- 77.Dahlbom SJ. Lagman D. Lundstedt-Enkel K. Sundstrom LF. Winberg S. Boldness predicts social status in zebrafish (Danio rerio) PLoS One. 2011;6:e23565. doi: 10.1371/journal.pone.0023565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arbit J. Effects of LSD-25 upon Betta splendens: Reliability of a bioassay technique. J Appl Physiol. 1957;10:317–318. doi: 10.1152/jappl.1957.10.2.317. [DOI] [PubMed] [Google Scholar]
- 79.Abramson HA. Evans LT. Lysergic acid diethylamide (LSD 25). II. Psychobiological effects on the Siamese fighting fish. Science. 1954;120:990–991. doi: 10.1126/science.120.3128.990. [DOI] [PubMed] [Google Scholar]
- 80.Buss RR. Drapeau P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J Neurophysiol. 2001;86:197–210. doi: 10.1152/jn.2001.86.1.197. [DOI] [PubMed] [Google Scholar]
- 81.Schneider H. Measuring agonistic behavior in zebrafish. Zebrafish Neurobehav Protocol Neuromethods. 2011;51:125–134. [Google Scholar]
- 82.Oswald ME. Drew RE. Racine M. Murdoch GK. Robison BD. Is behavioral variation along the bold-shy continuum associated with variation in the stress axis in zebrafish? Physiol Biochem Zool. 2012;85:718–728. doi: 10.1086/668203. [DOI] [PubMed] [Google Scholar]
- 83.Zhang C. Song Y. Thompson DA, et al. Pineal-specific agouti protein regulates teleost background adaptation. Proc Natl Acad Sci USA. 2010;107:20164–20171. doi: 10.1073/pnas.1014941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wagle M. Mathur P. Guo S. Corticotropin-releasing factor critical for zebrafish camouflage behavior is regulated by light and sensitive to ethanol. J Neurosci. 2011;31:214–224. doi: 10.1523/JNEUROSCI.3339-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salim S. Ali SA. Vertebrate melanophores as potential model for drug discovery and development: A review. Cell Mol Biol Lett. 2011;16:162–200. doi: 10.2478/s11658-010-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peng J. Wagle M. Mueller T, et al. Ethanol-modulated camouflage response screen in zebrafish uncovers a novel role for cAMP and extracellular signal-regulated kinase signaling in behavioral sensitivity to ethanol. J Neurosci. 2009;29:8408–8418. doi: 10.1523/JNEUROSCI.0714-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cachat J. Kyzar EJ. Collins C, et al. Unique and potent effects of acute ibogaine on zebrafish: The developing utility of novel aquatic models for hallucinogenic drug research. Behav Brain Res. 2013;236:258–269. doi: 10.1016/j.bbr.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 88.Finney JL. Robertson GN. McGee CA. Smith FM. Croll RP. Structure and autonomic innervation of the swim bladder in the zebrafish (Danio rerio) J Comp Neurol. 2006;495:587–606. doi: 10.1002/cne.20948. [DOI] [PubMed] [Google Scholar]
- 89.Robertson GN. Lindsey BW. Dumbarton TC. Croll RP. Smith FM. The contribution of the swimbladder to buoyancy in the adult zebrafish (Danio rerio): a morphometric analysis. J morphol. 2008;269:666–673. doi: 10.1002/jmor.10610. [DOI] [PubMed] [Google Scholar]
- 90.Muller UK. van Leeuwen JL. Swimming of larval zebrafish: Ontogeny of body waves and implications for locomotory development. J Exp Biol. 2004;207:853–868. doi: 10.1242/jeb.00821. [DOI] [PubMed] [Google Scholar]
- 91.Budick SA. O'Malley DM. Locomotor repertoire of the larval zebrafish: Swimming, turning and prey capture. J Exp Biol. 2000;203:2565–2579. doi: 10.1242/jeb.203.17.2565. [DOI] [PubMed] [Google Scholar]
- 92.Thorsen DH. Hale ME. Neural development of the zebrafish (Danio rerio) pectoral fin. J Comp Neurol. 2007;504:168–184. doi: 10.1002/cne.21425. [DOI] [PubMed] [Google Scholar]
- 93.Roberts AC. Reichl J. Song MY, et al. Habituation of the C-start response in larval zebrafish exhibits several distinct phases and sensitivity to NMDA receptor blockade. PLoS One. 2011;6:e29132. doi: 10.1371/journal.pone.0029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu YC. Bailey I. Hale ME. Alternative startle motor patterns and behaviors in the larval zebrafish (Danio rerio) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198:11–24. doi: 10.1007/s00359-011-0682-1. [DOI] [PubMed] [Google Scholar]
- 95.Uusi-Heikkila S. Bockenhoff L. Wolter C. Arlinghaus R. Differential allocation by female zebrafish (Danio rerio) to different-sized males. An example in a fish species lacking parental care. PLoS One. 2012;7:e48317. doi: 10.1371/journal.pone.0048317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gerlach G. Hodgins-Davis A. Avolio C. Schunter C. Kin recognition in zebrafish: A 24-hour window for olfactory imprinting. Proc Biol Sci/Royal Soc. 2008;275:21652170. doi: 10.1098/rspb.2008.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harden MV. Newton LA. Lloyd RC. Whitlock KE. Olfactory imprinting is correlated with changes in gene expression in the olfactory epithelia of the zebrafish. J Neurobiol. 2006;66:1452–1466. doi: 10.1002/neu.20328. [DOI] [PubMed] [Google Scholar]
- 98.Kyzar EJ. Collins C. Gaikwad S. Green J. Roth A. Monnig L, et al. Effects of hallucinogenic agents mescaline and phencyclidine on zebrafish behavior and physiology. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:194–202. doi: 10.1016/j.pnpbp.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McHenry MJ. Lauder GV. The mechanical scaling of coasting in zebrafish (Danio rerio) J Exp Biol. 2005;208:2289–2301. doi: 10.1242/jeb.01642. [DOI] [PubMed] [Google Scholar]
- 100.Saint-Amant L. Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 101.Drapeau P. Saint-Amant L. Buss RR. Chong M. McDearmid JR. Brustein E. Development of the locomotor network in zebrafish. Prog Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 102.Avdesh A. Martin-Iverson MT. Mondal A. Chen M. Verdile G. Martins RN. Natural colour preference in the zebrafish (Danio rerio) Proc Meas Behav. 2010;2010:155–157. [Google Scholar]
- 103.Wong K. Stewart A. Gilder T, et al. Modeling seizure-related behavioral and endocrine phenotypes in adult zebrafish. Brain Res. 2010;1348:209–215. doi: 10.1016/j.brainres.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 104.Gerlai R. Lee V. Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yanagihara D. Watanabe S. Mitarai G. Neuroanatomical substrate for the dorsal light response. I. Differential afferent connections of the lateral lobe of the valvula cerebelli in goldfish (Carassius auratus) Neurosci Res. 1993;16:25–32. doi: 10.1016/0168-0102(93)90005-b. [DOI] [PubMed] [Google Scholar]
- 106.Yanagihara D. Watanabe S. Takagi S. Mitarai G. Neuroanatomical substrate for the dorsal light response. II. Effects of kainic acid-induced lesions of the valvula cerebelli on the goldfish dorsal light response. Neurosci Res. 1993;16:33–37. doi: 10.1016/0168-0102(93)90006-c. [DOI] [PubMed] [Google Scholar]
- 107.Yokogawa T. Marin W. Faraco J, et al. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:e277. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cachat J. Stewart A. Utterback E, et al. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS One. 2011;6:e17597. doi: 10.1371/journal.pone.0017597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gerlai R. Zebra fish: An uncharted behavior genetic model. Behav Genet. 2003;33:461–468. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- 110.Tiedeken JA. Ramsdell JS. DDT exposure of zebrafish embryos enhances seizure susceptibility: Relationship to fetal p,p'-DDE burden and domoic acid exposure of California sea lions. Environ Health Perspect. 2009;117:68–73. doi: 10.1289/ehp.11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stewart AM. Cachat J. Green J, et al. Constructing the habituome for phenotype-driven zebrafish research. Behav Brain Res. 2013;236:110–117. doi: 10.1016/j.bbr.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 112.Marks C. Kaut KP. Moore FB. Bagatto B. Ontogenetic oxygen changes alter zebra fish size, behavior, and blood glucose. Physiol Biochem Zool. 2012;85:635–644. doi: 10.1086/666508. [DOI] [PubMed] [Google Scholar]
- 113.Stewart A. Cachat J. Wong K, et al. Homebase behavior of zebrafish in novelty-based paradigms. Behav Proc. 2010;85:198–203. doi: 10.1016/j.beproc.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 114.Stewart AM. Roth A. Gaikwad S, et al. Constructing habituome for neurobehavioral research. ISBS Stress Behav Proc. 2012;18:18. [Google Scholar]
- 115.Bianco IH. Kampff AR. Engert F. Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front Syst Neurosci. 2011;5:101. doi: 10.3389/fnsys.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Williams LR. Wong K. Stewart A, et al. Behavioral and physiological effects of RDX on adult zebrafish. Comp Biochem Physiol C Toxicol Pharmacol. 2012;155:33–38. doi: 10.1016/j.cbpc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 117.Cachat J. Stewart A. Grossman L, et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc. 2010;5:1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- 118.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio) Eugene: Westerfield M; 2007. [Google Scholar]
- 119.Huang YY. Tschopp M. Straumann D. Neuhauss SC. Vestibular deficits do not underlie looping behavior in achiasmatic fish. Commun Integ Biol. 2010;3:379–381. doi: 10.4161/cib.3.4.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang YY. Tschopp M. Neuhauss SC. Illusionary self-motion perception in zebrafish. PLoS One. 2009;4:e6550. doi: 10.1371/journal.pone.0006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takebe A. Furutani T. Wada T, et al. Zebrafish respond to the geomagnetic field by bimodal and group-dependent orientation. Sci Rep. 2012;2:727. doi: 10.1038/srep00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhdanova IV. Sleep in zebrafish. Zebrafish. 2006;3:215–226. doi: 10.1089/zeb.2006.3.215. [DOI] [PubMed] [Google Scholar]
- 123.Yu L. Tucci V. Kishi S. Zhdanova IV. Cognitive aging in zebrafish. PLoS One. 2006;1:e14. doi: 10.1371/journal.pone.0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gonzalez-Nunez V. Rodriguez RE. The zebrafish: A model to study the endogenous mechanisms of pain. ILAR J. 2009;50:373–386. doi: 10.1093/ilar.50.4.373. [DOI] [PubMed] [Google Scholar]
- 125.Sneddon LU. Pain perception in fish: Indicators and endpoints. ILAR J. 2009;50:338–342. doi: 10.1093/ilar.50.4.338. [DOI] [PubMed] [Google Scholar]
- 126.Rose JD. Anthropomorphism and 'mental welfare' of fishes. Dis Aquatic Org. 2007;75:139–154. doi: 10.3354/dao075139. [DOI] [PubMed] [Google Scholar]
- 127.Gomez-Laplaza LM. Gerlai R. Latent learning in zebrafish (Danio rerio) Behav Brain Res. 2010;208:509–515. doi: 10.1016/j.bbr.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Macho Sanchez-Simon F. Rodriguez RE. Expression of the nociceptin receptor during zebrafish development: Influence of morphine and nociceptin. Int J Dev Neurosci. 2009;27:315–320. doi: 10.1016/j.ijdevneu.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 129.Burgess HA. Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol. 2007;210:2526–2539. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- 130.Wilson JM. Bunte RM. Carty AJ. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio) J Am Assoc Lab Animal Sci. 2009;48:785–789. [PMC free article] [PubMed] [Google Scholar]
- 131.Easter SS. Nicola GN. The development of eye movements in the zebrafish (Danio rerio) Dev Psychobiol. 1997;31:267–276. doi: 10.1002/(sici)1098-2302(199712)31:4<267::aid-dev4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 132.Maaswinkel H. Ti L. Spatio-temporal frequency characteristics of the optomotor response in zebrafish. Vision Res. 2003;43:21–30. doi: 10.1016/s0042-6989(02)00395-4. [DOI] [PubMed] [Google Scholar]
- 133.Spence R. Smith C. Male territoriality mediates density and sex ratio effects on oviposition in the zebrafish (Danio rerio) Animal Behav. 2005;69:1317–1323. [Google Scholar]
- 134.Ono F. Higashijima S. Shcherbatko A. Fetcho JR. Brehm P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J Neurosci. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bretaud S. Li Q. Lockwood BL. Kobayashi K. Lin E. Guo S. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience. 2007;146:1109–1116. doi: 10.1016/j.neuroscience.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 136.Lau B. Bretaud S. Huang Y. Lin E. Guo S. Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes Brain Behav. 2006;5:497–505. doi: 10.1111/j.1601-183X.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 137.Kily LJ. Cowe YC. Hussain O, et al. Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J Exp Biol. 2008;211:1623–1634. doi: 10.1242/jeb.014399. [DOI] [PubMed] [Google Scholar]
- 138.Ninkovic J. Folchert A. Makhankov YV, et al. Genetic identification of AChE as a positive modulator of addiction to the psychostimulant D-amphetamine in zebrafish. J Neurobiol. 2006;66:463–475. doi: 10.1002/neu.20231. [DOI] [PubMed] [Google Scholar]
- 139.Braida D. Limonta V. Pegorini S, et al. Hallucinatory and rewarding effect of salvinorin A in zebrafish: Kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology (Berl) 2007;190:441–448. doi: 10.1007/s00213-006-0639-1. [DOI] [PubMed] [Google Scholar]
- 140.Miller N. Gerlai R. From schooling to shoaling: Patterns of collective motion in zebrafish (Danio rerio) PLoS One. 2012;7:e48865. doi: 10.1371/journal.pone.0048865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McClenahan P. Troup M. Scott EK. Fin-tail coordination during escape and predatory behavior in larval zebrafish. PLoS One. 2012;7:e32295. doi: 10.1371/journal.pone.0032295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Borla MA. Palecek B. Budick S. O'Malley DM. Prey capture by larval zebrafish: evidence for fine axial motor control. Brain Behav Evol. 2002;60:207–229. doi: 10.1159/000066699. [DOI] [PubMed] [Google Scholar]
- 143.Dugatkin LA. McCall MA. Gregg RG. Cavanaugh A. Christensen C. Unseld M. Zebrafish (Danio rerio) exhibit individual differences in risk-taking behavior during predator inspection. Ethol Ecol Evol. 2005;17:77–81. [Google Scholar]
- 144.Zhdanova IV. Wang SY. Leclair OU. Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- 145.Zhdanova IV. Sleep and its regulation in zebrafish. Rev Neurosci. 2011;22:27–36. doi: 10.1515/RNS.2011.005. [DOI] [PubMed] [Google Scholar]
- 146.Olszewski J. Haehnel M. Taguchi M. Liao JC. Zebrafish larvae exhibit rheotaxis and can escape a continuous suction source using their lateral line. PLoS One. 2012;7:e36661. doi: 10.1371/journal.pone.0036661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suli A. Watson GM. Rubel EW. Raible DW. Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells. PLoS One. 2012;7:e29727. doi: 10.1371/journal.pone.0029727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maximino C. da Silva AW. Gouveia A., Jr. Herculano AM. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:624–631. doi: 10.1016/j.pnpbp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 149.Hortopan GA. Baraban SC. Aberrant expression of genes necessary for neuronal development and notch signaling in an epileptic mind bomb zebrafish. Dev Dyn. 2011;240:1964–1976. doi: 10.1002/dvdy.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hortopan GA. Dinday MT. Baraban SC. Spontaneous seizures and altered gene expression in GABA signaling pathways in a mind bomb mutant zebrafish. J Neurosci. 2010;30:13718–13728. doi: 10.1523/JNEUROSCI.1887-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miller NY. Gerlai R. Shoaling in zebrafish: What we don't know. Rev Neurosci. 2011;22:17–25. doi: 10.1515/RNS.2011.004. [DOI] [PubMed] [Google Scholar]
- 152.Ewald HS. UMI Dissertation Publishers; 2008. A zebrafish model of schizophrenia and sickness behavior: MK-801 and endogenous NMDAR antagonism. [Google Scholar]
- 153.Jones R. Let sleeping zebrafish lie: A new model for sleep studies. PLoS Biol. 2007;5:e281. doi: 10.1371/journal.pbio.0050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhdanova IV. Yu L. Lopez-Patino M. Shang E. Kishi S. Guelin E. Aging of the circadian system in zebrafish and the effects of melatonin on sleep and cognitive performance. Brain Res Bull. 2008;75:433–441. doi: 10.1016/j.brainresbull.2007.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Appelbaum L. Wang GX. Maro GS, et al. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc Natl Acad Sci USA. 2009;106:21942–21947. doi: 10.1073/pnas.906637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rihel J. Prober DA. Schier AF. Monitoring sleep and arousal in zebrafish. Methods Cell Biol. 2010;100:281–294. doi: 10.1016/B978-0-12-384892-5.00011-6. [DOI] [PubMed] [Google Scholar]
- 157.Baier H. Korsching S. Olfactory glomeruli in the zebrafish form an invariant pattern and are identifiable across animals. J Neurosci. 1994;14:219–230. doi: 10.1523/JNEUROSCI.14-01-00219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liao JC. Fetcho JR. Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. J Neurosci. 2008;28:12982–12992. doi: 10.1523/JNEUROSCI.3330-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abramson HA. Gettner HH. Hewitt MP. Dean G. Effect of lysergic acid diethylamide on the surfacing behaviour of large carp. Nature. 1962;193:320–321. doi: 10.1038/193320a0. [DOI] [PubMed] [Google Scholar]
- 160.De Froment AJ. Ann Arbor: UMI Dissertation Publisher; 2010. Fighting for information: Decision-making, animal contests and the emergence of social hierarchy. [Google Scholar]
- 161.Gibb A. Ashley-Ross MA. Pace CM. Long JH. Fish out of water: Terrestrial jumping by fully aquatic fishes. J Exp Zool. 2011;313A:1–5. doi: 10.1002/jez.711. [DOI] [PubMed] [Google Scholar]
- 162.Spence R. Jordan WC. Smith C. Genetic analysis of male reproductive success in relation to density in the zebrafish, Danio rerio. Front Zool. 2006;3:5. doi: 10.1186/1742-9994-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nava SS. An S. Hamil T. Visual detection of UV cues by adult zebrafish (Danio rerio) J Vis. 2011;11:2. doi: 10.1167/11.6.2. [DOI] [PubMed] [Google Scholar]
- 164.Grossman L. Utterback E. Stewart A, et al. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res. 2010;214:277–284. doi: 10.1016/j.bbr.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 165.Beck JC. Gilland E. Tank DW. Baker R. Quantifying the ontogeny of optokinetic and vestibuloocular behaviors in zebrafish, medaka, and goldfish. J Neurophysiol. 2004;92:3546–3561. doi: 10.1152/jn.00311.2004. [DOI] [PubMed] [Google Scholar]
- 166.Mo W. Chen F. Nechiporuk A. Nicolson T. Quantification of vestibular-induced eye movements in zebrafish larvae. BMC Neurosci. 2010;11:110. doi: 10.1186/1471-2202-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cachat J. Canavello P. Elegante M, et al. Modeling withdrawal syndrome in zebrafish. Behav Brain Res. 2010;208:371–376. doi: 10.1016/j.bbr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 168.Stewart A. Wong K. Cachat J, et al. Zebrafish models to study drug abuse-related phenotypes. Rev Neurosci. 2011;22:95–105. doi: 10.1515/RNS.2011.011. [DOI] [PubMed] [Google Scholar]
- 169.Kyzar E. Zapolsky I. Green J, et al. The Zebrafish Neurophenome Database (ZND): A dynamic open-access resource for zebrafish neurophenotypic data. Zebrafish. 2012;9:8–14. doi: 10.1089/zeb.2011.0725. [DOI] [PubMed] [Google Scholar]
- 170.Sprague J. Doerry E. Douglas S. Westerfield M. The Zebrafish Information Network (ZFIN): A resource for genetic, genomic and developmental research. Nucleic Acids Res. 2001;29:87–90. doi: 10.1093/nar/29.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kyzar E. Zapolsky I. Green J, et al. The Zebrafish Neurophenome Database (ZND): A dynamic open-access resource for zebrafish neurophenotypic data. Zebrafish. 2012;9:8–14. doi: 10.1089/zeb.2011.0725. [DOI] [PubMed] [Google Scholar]
- 172.Best JD. Berghmans S. Hunt JJ, et al. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- 173.Grossman L. Stewart A. Gaikwad S. Utterback E. Wu N. Dileo J, et al. Effects of piracetam on behavior and learning in adult zebrafish. Brain Res Bull. 2011;85:58–63. doi: 10.1016/j.brainresbull.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 174.Wright D. Krause J. Repeated measures of shoaling tendency in zebrafish (Danio rerio) and other small teleost fishes. Nat Protoc. 2006;1:1828–1831. doi: 10.1038/nprot.2006.287. [DOI] [PubMed] [Google Scholar]
- 175.Wright D. Nakamichi R. Krause J. Butlin RK. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio) Behav Genet. 2006;36:271–284. doi: 10.1007/s10519-005-9029-4. [DOI] [PubMed] [Google Scholar]
- 176.Filby AL. Paull GC. Hickmore TF. Tyler CR. Unravelling the neurophysiological basis of aggression in a fish model. BMC Genomics. 2010;11:498. doi: 10.1186/1471-2164-11-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cadet JL. Amphetamine recapitulates developmental programs in the zebrafish. Genome Biol. 2009;10:231. doi: 10.1186/gb-2009-10-7-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shamchuk AL. Tierney KB. Phenotyping stimulus evoked responses in larval zebrafish. Behavior. 2012;149:1177–1207. [Google Scholar]
- 179.Tierney KB. Sekela MA. Cobbler CE, et al. Evidence for behavioral preference towards environmental concentrations of urban-use herbicides in a model adult fish. Environ Toxicol Chem. 2011;30:2046–2054. doi: 10.1002/etc.588. [DOI] [PubMed] [Google Scholar]
- 180.Tierney KB. Review: Behavioural assessments of neurotoxic effects and neurodegeneration in zebrafish. Biochim Biophys Acta Mol Basis Dis. 2011;1812:381–389. doi: 10.1016/j.bbadis.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 181.Tierney KB. Ren X. Alyasha'e Z. Zielinski B. Towards a mechanistic understanding of food odor driven motion using zebrafish (Danio rerio) Chemical Senses. 2008;33:S156–S157. [Google Scholar]
- 182.Tierney KB. Baldwin DH. Hara TJ. Ross PS. Scholz NL. Kennedy CJ. Review: Olfactory toxicity in fishes. Aquatic Toxicol. 2010;96:2–26. doi: 10.1016/j.aquatox.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 183.Fleisch VC. Neuhauss SC. Visual behavior in zebrafish. Zebrafish. 2006;3:191–201. doi: 10.1089/zeb.2006.3.191. [DOI] [PubMed] [Google Scholar]
- 184.Hodel C. Neuhauss SC. Computer-based analysis of the optokinetic response in zebrafish larvae. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4961. pdb prot4961. [DOI] [PubMed] [Google Scholar]
- 185.Huang YY. Neuhauss SC. The optokinetic response in zebrafish and its applications. Front Biosci. 2008;13:1899–1916. doi: 10.2741/2810. [DOI] [PubMed] [Google Scholar]
- 186.Spence R. Zebrafish Ecology and Behaviour. In: Kalueff AV, editor; Cachat JM, editor. Zebrafish Models in Neurobehavioral Research. Humana Press; NY: 2010. pp. 1–39. [Google Scholar]
- 187.Gerlai R. High-throughput behavioral screens: The first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules. 2010;15:2609–2622. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]