Abstract
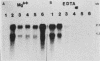
Mouse testis contains two size classes of actin mRNAs of 2.1 and 1.5 kilobases (kb). The 2.1-kb actin mRNA codes for cytoplasmic beta- and gamma-actin and is found throughout spermatogenesis, while the 1.5-kb actin mRNA is first detected in postmeiotic cells. Here we identify the testicular postmeiotic actin encoded by the 1.5-kb mRNA as a smooth-muscle gamma-actin (SMGA) and present its cDNA sequence. The amino acid sequence deduced from the postmeiotic actin cDNA sequence was nearly identical to that of a chicken gizzard SMGA, with one amino acid replacement at amino acid 359, where glutamine was substituted for proline. The nucleotide sequence of the untranslated region of the SMGA differed substantially from those of other isotypes of mammalian actins. By using the 3' untranslated region of the testicular SMGA, a highly specific probe was obtained. The 1.5-kb mRNA was detected in RNA from mouse aorta, small intestine, and uterus, but not in RNA isolated from mouse brain, heart, and spleen. Testicular SMGA mRNA was first detected and increased substantially in amount during spermiogenesis in the germ cells, in contrast to the decrease of the cytoplasmic beta- and gamma-actin mRNAs towards the end of spermatogenesis. Testicular SMGA mRNA was present in the polysome fractions, indicating that it was translated. These studies demonstrate the existence of an SMGA in male haploid germ cells. The implications of the existence of an SMGA in male germ cells are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camatini M., Casale A., Cifarelli M. Immunocytochemical identification of actin in rabbit spermiogenesis and spermatozoa. Eur J Cell Biol. 1988 Feb;45(2):274–281. [PubMed] [Google Scholar]
- Casale A., Camatini M., Skalli O., Gabbiani G. Characterization of actin isoforms in ejaculated boar spermatozoa. Gamete Res. 1988 Jun;20(2):133–144. doi: 10.1002/mrd.1120200204. [DOI] [PubMed] [Google Scholar]
- Chang K. S., Rothblum K. N., Schwartz R. J. The complete sequence of the chicken alpha-cardiac actin gene: a highly conserved vertebrate gene. Nucleic Acids Res. 1985 Feb 25;13(4):1223–1237. doi: 10.1093/nar/13.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. D., Kistler W. S. Nuclear transition protein 2 (TP2) of mammalian spermatids has a very basic carboxyl terminal domain. Biochem Biophys Res Commun. 1987 Aug 31;147(1):437–442. doi: 10.1016/s0006-291x(87)80140-7. [DOI] [PubMed] [Google Scholar]
- Cooper A. D., Crain W. R., Jr Complete nucleotide sequence of a sea urchin actin gene. Nucleic Acids Res. 1982 Jul 10;10(13):4081–4092. doi: 10.1093/nar/10.13.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePonti-Zilli L., Seiler-Tuyns A., Paterson B. M. A 40-base-pair sequence in the 3' end of the beta-actin gene regulates beta-actin mRNA transcription during myogenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1389–1393. doi: 10.1073/pnas.85.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel R. J., Kleene K. C., Hecht N. B. Haploid expression of a mouse testis alpha-tubulin gene. Science. 1984 Apr 6;224(4644):68–70. doi: 10.1126/science.6701535. [DOI] [PubMed] [Google Scholar]
- Erba H. P., Gunning P., Kedes L. Nucleotide sequence of the human gamma cytoskeletal actin mRNA: anomalous evolution of vertebrate non-muscle actin genes. Nucleic Acids Res. 1986 Jul 11;14(13):5275–5294. doi: 10.1093/nar/14.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files J. G., Carr S., Hirsh D. Actin gene family of Caenorhabditis elegans. J Mol Biol. 1983 Mar 5;164(3):355–375. doi: 10.1016/0022-2836(83)90056-6. [DOI] [PubMed] [Google Scholar]
- Flaherty S. P., Winfrey V. P., Olson G. E. Localization of actin in mammalian spermatozoa: a comparison of eight species. Anat Rec. 1986 Dec;216(4):504–515. doi: 10.1002/ar.1092160407. [DOI] [PubMed] [Google Scholar]
- Fornwald J. A., Kuncio G., Peng I., Ordahl C. P. The complete nucleotide sequence of the chick a-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res. 1982 Jul 10;10(13):3861–3876. doi: 10.1093/nar/10.13.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Garner I., Minty A. J., Alonso S., Barton P. J., Buckingham M. E. A 5' duplication of the alpha-cardiac actin gene in BALB/c mice is associated with abnormal levels of alpha-cardiac and alpha-skeletal actin mRNAs in adult cardiac tissue. EMBO J. 1986 Oct;5(10):2559–2567. doi: 10.1002/j.1460-2075.1986.tb04535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. S., Kiessling A. A., Millette C. F., Cooper G. M. Expression of c-mos RNA in germ cells of male and female mice. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4509–4513. doi: 10.1073/pnas.84.13.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. Molecular structure and evolutionary origin of human cardiac muscle actin gene. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5901–5905. doi: 10.1073/pnas.79.19.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer A., Levin M., Heilig R., Daegelen D., Kahn A., Mandel J. L. Isolation and characterization of cDNA clones for human skeletal muscle alpha actin. Nucleic Acids Res. 1983 Jun 11;11(11):3503–3516. doi: 10.1093/nar/11.11.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidaran M. A., Kistler W. S. Isolation of a cDNA clone for transition protein 1 (TP1), a major chromosomal protein of mammalian spermatids. Gene. 1987;54(2-3):281–284. doi: 10.1016/0378-1119(87)90498-7. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hu M. C., Sharp S. B., Davidson N. The complete sequence of the mouse skeletal alpha-actin gene reveals several conserved and inverted repeat sequences outside of the protein-coding region. Mol Cell Biol. 1986 Jan;6(1):15–25. doi: 10.1128/mcb.6.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene K. C., Borzorgzadeh A., Flynn J. F., Yelick P. C., Hecht N. B. Nucleotide sequence of a cDNA clone encoding mouse transition protein 1. Biochim Biophys Acta. 1988 Jul 13;950(2):215–220. doi: 10.1016/0167-4781(88)90013-9. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984 Sep;105(1):71–79. doi: 10.1016/0012-1606(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. cDNA clones encoding cytoplasmic poly(A)+ RNAs which first appear at detectable levels in haploid phases of spermatogenesis in the mouse. Dev Biol. 1983 Aug;98(2):455–464. doi: 10.1016/0012-1606(83)90375-5. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Flynn J. F. Characterization of a cDNA clone encoding a basic protein, TP2, involved in chromatin condensation during spermiogenesis in the mouse. J Biol Chem. 1987 Dec 25;262(36):17272–17277. [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Analysis of alpha-smooth-muscle actin mRNA expression in rat aortic smooth-muscle cells using a specific cDNA probe. Differentiation. 1987;34(3):201–209. doi: 10.1111/j.1432-0436.1987.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Cerutti D., Gabbiani F., Gabbiani G. Cytoskeletal features of rat aortic cells during development. An electron microscopic, immunohistochemical, and biochemical study. Circ Res. 1985 Jun;56(6):829–838. doi: 10.1161/01.res.56.6.829. [DOI] [PubMed] [Google Scholar]
- Kost T. A., Theodorakis N., Hughes S. H. The nucleotide sequence of the chick cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Dec 10;11(23):8287–8301. doi: 10.1093/nar/11.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. P., Gall I., Campbell P., Frischauf A. M. Isolation and characterization of cDNA clones from mouse skeletal muscle actin mRNA. DNA. 1986 Jun;5(3):235–238. doi: 10.1089/dna.1986.5.235. [DOI] [PubMed] [Google Scholar]
- Luerssen H., Hoyer-Fender S., Engel W. The nucleotide sequence of human transition protein 1 cDNA. Nucleic Acids Res. 1988 Aug 11;16(15):7723–7723. doi: 10.1093/nar/16.15.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh K. M., Lessard J. L. The development expression of the rat alpha-vascular and gamma-enteric smooth muscle isoactins: isolation and characterization of a rat gamma-enteric actin cDNA. Mol Cell Biol. 1988 Dec;8(12):5224–5231. doi: 10.1128/mcb.8.12.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer D., Hermans A., von Lindern M., van Agthoven T., de Klein A., Mackenbach P., Grootegoed A., Talarico D., Della Valle G., Grosveld G. Molecular characterization of the testis specific c-abl mRNA in mouse. EMBO J. 1987 Dec 20;6(13):4041–4048. doi: 10.1002/j.1460-2075.1987.tb02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistrich M. L., Longtin J., Brock W. A., Grimes S. R., Jr, Mace M. L. Purification of rat spermatogenic cells and preliminary biochemical analysis of these cells. Biol Reprod. 1981 Dec;25(5):1065–1077. doi: 10.1095/biolreprod25.5.1065. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Guénet J. L., Buckingham M. E. Number and organization of actin-related sequences in the mouse genome. J Mol Biol. 1983 Jun 15;167(1):77–101. doi: 10.1016/s0022-2836(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. K., Thompson M. M. Developmental changes in isoactin expression in rat aortic smooth muscle cells in vivo. Relationship between growth and cytodifferentiation. J Biol Chem. 1986 Oct 5;261(28):13373–13380. [PubMed] [Google Scholar]
- Ponzetto C., Wolgemuth D. J. Haploid expression of a unique c-abl transcript in the mouse male germ line. Mol Cell Biol. 1985 Jul;5(7):1791–1794. doi: 10.1128/mcb.5.7.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romrell L. J., Bellvé A. R., Fawcett D. W. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976 Mar;49(1):119–131. doi: 10.1016/0012-1606(76)90262-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M. A., McOsker P., Keller E. B. DNA sequence of two linked actin genes of sea urchin. Mol Cell Biol. 1983 Mar;3(3):448–456. doi: 10.1128/mcb.3.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987 Jul 3;50(1):89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- Slaughter G. R., Needleman D. S., Means A. R. Developmental regulation of calmodulin, actin, and tubulin RNAs during rat testis differentiation. Biol Reprod. 1987 Dec;37(5):1259–1270. doi: 10.1095/biolreprod37.5.1259. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A. S., Mahbubani H., Williams J. G. Cell-type-specific actin mRNA populations in dictyostelium discoideum. Cell. 1982 Dec;31(2 Pt 1):375–382. doi: 10.1016/0092-8674(82)90131-3. [DOI] [PubMed] [Google Scholar]
- Ueyama H., Hamada H., Battula N., Kakunaga T. Structure of a human smooth muscle actin gene (aortic type) with a unique intron site. Mol Cell Biol. 1984 Jun;4(6):1073–1078. doi: 10.1128/mcb.4.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama H., Kurokawa K., Sasaki I., Ueda K. Characterization of acidic actin in mouse sarcoma 180 cells. Cell Struct Funct. 1987 Oct;12(5):463–470. doi: 10.1247/csf.12.463. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Villasante A., Wang D., Dobner P., Dolph P., Lewis S. A., Cowan N. J. Six mouse alpha-tubulin mRNAs encode five distinct isotypes: testis-specific expression of two sister genes. Mol Cell Biol. 1986 Jul;6(7):2409–2419. doi: 10.1128/mcb.6.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. H., Distel R. J., Hecht N. B. Mouse testes contain two size classes of actin mRNA that are differentially expressed during spermatogenesis. Mol Cell Biol. 1985 Jul;5(7):1649–1654. doi: 10.1128/mcb.5.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch J. E., O'Rand M. G. Identification and distribution of actin in spermatogenic cells and spermatozoa of the rabbit. Dev Biol. 1985 Jun;109(2):411–417. doi: 10.1016/0012-1606(85)90467-1. [DOI] [PubMed] [Google Scholar]
- Zakut R., Shani M., Givol D., Neuman S., Yaffe D., Nudel U. Nucleotide sequence of the rat skeletal muscle actin gene. Nature. 1982 Aug 26;298(5877):857–859. doi: 10.1038/298857a0. [DOI] [PubMed] [Google Scholar]