Abstract
Zebrafish (Danio rerio) has recently emerged as a new animal model in neuroendocrinology and behavior (e.g., stress physiology and ecotoxicology studies). In these areas, the concentrations of steroid hormones in the blood are often used to study the endocrinological status of individuals. However, due to the small body size of zebrafish, blood sampling is difficult to perform and the amount of plasma obtained per sample for assaying hormones is very small (ca. 1–5 μL), and therefore most studies have been using whole-body hormone concentrations, which implies sacrificing the individuals and hampers sequential sampling of the same individual. Here a noninvasive method to assay steroid hormones from zebrafish holding-water, based on the fact that steroids are released into the fish holding-water through the gills by passive diffusion, is validated. Cortisol and the androgen 11-ketotestosterone (KT) were measured in water samples and compared to plasma levels in the same individuals. Cortisol released to holding-water correlates positively with plasma concentrations, but there was a lack of correlation between KT water and circulating levels. However, KT levels showed a highly significant sex difference that can be used to noninvasively sex individuals. An ACTH challenge test demonstrated that an induced increase in circulating cortisol concentration can be reliably detected in holding-water levels, hence attesting the responsiveness of holding-water levels to fluctuations in circulating levels.
Introduction
In recent years, the use of zebrafish as a model organism in ecotoxicology and in endocrinology,1–3 namely in stress research,4 raised the need for measuring circulating levels of steroid hormones. However, due to its small size, blood collection is difficult in zebrafish and implies sacrificing the animals, and the amount of plasma obtained is small (i.e., below 5 μL). As an alternative, researchers have been using whole-body homogenates to assay steroid hormones,5–9 but again, this method implies sacrificing the experimental animal, which imposes methodological constraints on these studies, such as making nonviable within-subject experimental designs where sequential sampling of the same individual is requested.
A noninvasive method for assaying steroids from fish holding-water, initially developed for studying pheromones, has since then been applied to measure steroid hormones in other contexts in a number of different species with promising results (see Scott and Ellis,10 for an historic perspective of the development of the method). Studies using holding-water steroid measurements have been successfully accomplished not only in groups of fish but also in single fish, and cover a wide range of research topics, from reproductive physiology,11–15 to stress research in aquaculture, fish welfare, and conservation contexts,16–22 to behavioural endocrinology.13,23–30 This method is based on the principle that different steroid fractions exit fish through different routes: free steroids are released into the water through the gills by passive diffusion, whereas sulfated and glucuronidated steroids come mainly from the urine and the feces (via the bile), respectively.31,32 Therefore, free steroids in the water must reflect circulating levels of the physiologically active fraction in the plasma.
In brief, the procedure for assaying holding-water steroids consists in extracting and concentrating the steroids present in the water sample using solid-phase extraction cartridges and organic solvents, such as diethyl ether or ethyl acetate. Steroids are then measured by specific immunoassays (e.g., RIA or ELISA). If levels from a single fish are to be obtained, that fish has to be housed individually in a small volume of water (e.g., 0.5 to 1 L, depending on size of the fish) for a given time period (e.g., 30 min to 1 h). Despite its simplicity, this method needs to be validated when it is first applied to a new species, because species differences in steroid metabolism may occur (e.g., variation in steroid biosynthetic pathways and release rates), and because different living conditions may also influence steroid release rates (e.g., cool vs. warm waters; freshwater vs. seawater; relatively clean vs. organically rich waters).10,33 This is particularly critical in zebrafish since the expression of a steroid-binding globulin in its gills has been described,34,35 which may interfere with the dynamics of steroid release.
In this article we aim to validate this method for assaying cortisol and the main teleost androgen, 11-ketotestosterone (KT). A number of validation procedures will be used, as proposed by a consensus article recently co-authored by majority of the active researchers in the field,33 namely to check:
(1) if the water provided to the fish has no background activity;
(2) that recovery rates of known amounts of steroids added to the water are acceptable (i.e., above 80%) and consistent;
(3) that there is parallelism between extracts and standards;
(4) for correlations between plasma and holding-water steroid levels;
(5) if holding-water steroid levels respond in a biologically meaningful manner to exogenous physiological challenges [e.g., if holding-water cortisol levels increase in response to an adrenocorticotrophic hormone (ACTH) injection].
The validation of this method in zebrafish would add another useful tool to the research tool kit already available for this species that makes it an increasingly popular model organism.
Materials and Methods
Animals and housing
Adult wild-type zebrafish (Danio rerio) from the stock held at Instituto Gulbenkian de Ciência (IGC, Oeiras, Portugal) were used. Fish were kept in a recirculating system (ZebraTec, Tecniplast), at 28°C with a 14L:10D photoperiod. Water was monitored for nitrites (<0.2 ppm), nitrates (<50 ppm), and ammonia (0.01–0.1 ppm), while pH and conductivity were maintained at 7 and 700 μSM, respectively. Fish were fed twice per day with Artemia salina and commercial flakes, except on the day of experiments.
Study 1: Correlation between plasma and holding-water steroid levels
Individuals [12 females and 27 males, body weight (average±S.E.M.): 0.36±0.11 g and 0.38±0.07 g, respectively] were removed from their home-tank and placed individually in a 2 L beaker filled with 500 mL of clean water (i.e., filtered through a reverse osmosis system) for 1 h. While in the beaker, individuals had no visual contact with other fish or with the researcher. After this period, individuals were anesthetized with Tricaine solution (MS222, Pharmaq; 160 mg/L in water, buffered with Tris base 1 M to pH 7), weighed, and blood was collected from the caudal vein using a 30G heparinized syringe. Collected blood was kept in microcentrifuge tubes on ice until centrifugation (800 g, 10 min, 4°C). The pellet was discarded and plasma was transferred to a new microcentrifuge tube and stored at −20°C until further processing. Experiments were always conducted in the morning, and blood sampling always took place between 11:00 and 12:00 am to avoid spurious effects of diurnal steroid variation. All glassware was washed with absolute ethanol and then distilled water to avoid possible contaminations. Water samples were processed immediately after sampling for 11-keto-testosterone and cortisol levels.
Study 2: ACTH challenge test
An ACTH challenge test, following the procedure described in Bshary and co-workers,29 was used. Fourteen females and 8 males (weight: 0.84±0.31 g and 0.67±0.05 g, respectively) were used. Fish were removed from their home tanks and placed individually in a 2 L beaker with 500 mL of clean water for 1 h. Experiments always started at the same time to account for diurnal steroid variations, and all material was washed with absolute ethanol and distilled water to avoid possible contamination. None of the subjects had visual contact with other animal or with the investigator. After this period, subjects were collected from the sampling container, anesthetized with Tricaine solution as mentioned above and weighted. An intraperitoneal injection, using a syringe with a 30G heparinized needle, of either ACTH solution (adrenocorticotrophic hormone porcine pituitary; Sigma A-6303; physiological challenge treatment) or Ringer solution (control treatment) was given and individuals were placed in a new beaker with clean water. Two different concentrations of ACTH were tested (0.046 and 0.23 IU/ 50 μL/g body weight), and fish were transferred to a new beaker at 15 min, 30 min, 1 h, 2 h, 3 h, and 4 h after the i.p. injection, making six sampling points. Sample sizes were: n=11 for the high-dose of ACTH, n=4 for the low-dose of ACTH, and n=7 for the Ringer treatment. All glassware was washed with absolute ethanol and then distilled water to avoid possible contamination. Water samples were immediately processed for cortisol analysis.
Determination of steroid extraction recoveries
Water samples were spiked with known quantities of steroids (5 ng/L of F, n=3; and 0.5 ng/L of KT, n=2). Samples were then processed, steroids quantified, and their respective rates of recovery determined.
Sexing
At the end of experiments 1 and 2, fish were sacrificed with an overdose of Tricaine solution (4 g/L in water, pH 7) and dissected in order to confirm sex, by direct inspection of the gonads under the microscope, using the aceto-carmine squash method.36
Extraction procedure
Water samples were filtered to remove particulates, and steroids were then extracted using a C18 solid-phase extraction cartridge (500 mg, #1.02023.0001, Merck), previously activated with 2×2 mL absolute ethanol followed by 2×2 mL of distilled water, with the help of a vacuum pump attached to a 12-port vacuum manifold. Columns were then stored at −20°C until further processing. Columns were later thawed at room temperature and steroids were eluted with 2×2 mL of ethanol, into glass tubes. Ethanol was then evaporated in a fume hood, eluent was resuspended in 100 μL of distilled water, 4 mL of diethyl ether were added, and the resuspension was agitated for 20 min. Samples were the centrifuged (163 g, 5 min, 4°C) for phase separation and kept at −80°C for 115–20 min in order to freeze the water phase. The organic layer was decanted into a glass vial and ether treatment repeated to the aqueous phase with addition of 3 mL of this solvent, in order to obtain a higher extraction efficiency.11 The final volume of ether was evaporated in a Speed-vac and the dried organic phase containing the free steroid fraction was reconstituted with 1 mL of the buffer solution supplied with the Enzyme Immunoassay (EIA) kit. Samples were stored at −20°C until analysis.
Plasma samples
Due to the reduced quantity of blood and consequently of plasma obtained for each subject (ca. 1–5 μL), plasma samples were not extracted. Instead, they were diluted in EIA buffer to 1:50–1:200 dilution. Samples were stored at −20°C until assayed. To confirm the reliability of nonextracted plasma samples, these were serially diluted and compared to a standard curve. Equality of slopes between the serial dilution and the standard curves indicates that there were no interferences of other immunoreactive substances in the nonextracted plasma samples (ANCOVA: cortisol, F1,14=0.087, p=0.773; KT, F1,8=2.326, p=0,166).
Hormone analysis
Cortisol and KT levels, both from water and from plasma, were assayed using enzyme immunassay (EIA) kits from Cayman Chemical Company (#500360 and #582751, respectively) following the manufacturer's instructions. For each individual, water samples were analyzed within the same assay, and it was used a 1:5 dilution for the analysis of cortisol and 1:5 and 1:20 dilutions for the analysis of KT in females and males, respectively. Both kits were validated for plasma and water samples by assessing parallelism of a serial dilution curve, run in duplicate, with the standard curve. The serial dilution curve was obtained, by diluting in EIA buffer, a pool of plasma or water samples from several individuals, to make the following dilutions: 1:2, 1:4, 1:8, 1:16; 1:32, and 1:64. Intra-assay coefficients of variation were 6.49% for cortisol and 4.41% for KT; inter-assay coefficients of variation were 12.6% and 6.2% for cortisol and KT, respectively.
Statistical analysis
In the first experiment, parametric statistics were used, since data followed the assumptions of normality and homogeneity of variance. Correlations between hormone levels in plasma and water were obtained with Pearson correlation coefficients. A Student's t-test was used to assess sex differences in hormone values both in plasma and in water. In the ACTH challenge experiment, cortisol fold-change (i.e., final cortisol value – initial cortisol value) was used as dependent variable to account for variation between individuals in baseline values. The effect of the different injection treatments on cortisol fold-change was analyzed using a repeated measures ANOVA [repeated factor=sampling time; independent factor=treatment (ringer: low ACTH dose: high ACTH dose), followed post-hoc tests and by planned comparisons of least squares means between the control (Ringer) and each ACTH dosage for each sampling point. Even though data did not follow the assumptions of normality, this parametric test was still undertaken due to the lack of an equivalent nonparametric test and since the F-statistic is known to be remarkably robust to deviations of normality.37 An ANCOVA was used to compare the slopes between the serial dilution and standard curves. A value of p<0.05 was used for statistical significance in all tests. All statistical tests were run on the STATISTICA data analysis software system, version 10 (StatSoft, Inc. 2011, www.statsoft.com).
Ethical note
All experiments were conducted in compliance with the regulations on animal experimentation in Portugal.
Results
There is little background (i.e., water without fish) steroid immune-reactivity in the water (cortisol: n=6, mean±SEM=0.404±0.052 ng/L; KT: n=2, mean±SEM=0.084±0.002 ng/L) so that the presence of the fish is associated with a 16-fold increase in water cortisol and a 3-fold increase in water KT concentrations (Table 1).
Table 1.
Sex Differences in Cortisol and 11-Ketotestosterone (KT) Parameters in Zebrafish
| Variable | Males Mean±S.E.M. (N) | Females Mean±S.E.M. (N) | T-test |
|---|---|---|---|
| Body mass (g) | 0.38±0.01 (41) | 0.36±0.02 (20) | T=1.09, p=0.28 |
| Plasma cortisol (ng/mL) | 57.47±8.28 (30) | 45.85±8.04 (12) | T=0.82, p=0.41 |
| Water cortisol (ng/L) | 7.47±0.42 (31) | 4.35±0.37 (12) | T=4.37, p<0.0001 |
| Cortisol release rate (ng/g/h) | 20.16±1.28 (31) | 13.17±1.74 (12) | T=3.00, p<0.01 |
| Plasma KT (ng/mL) | 2.39±0.28 (21) | 0.15±0.03 (16) | T=6.92, p<0.0001 |
| Water KT (ng/L) | 0.37±0.03 (21) | 0.09±0.01 (17) | T=8.39, p<0.0001 |
| KT release rate (ng/g/h) | 0.97±0.07 (21) | 0.27±.03 (17) | T=8.26, p<0.0001 |
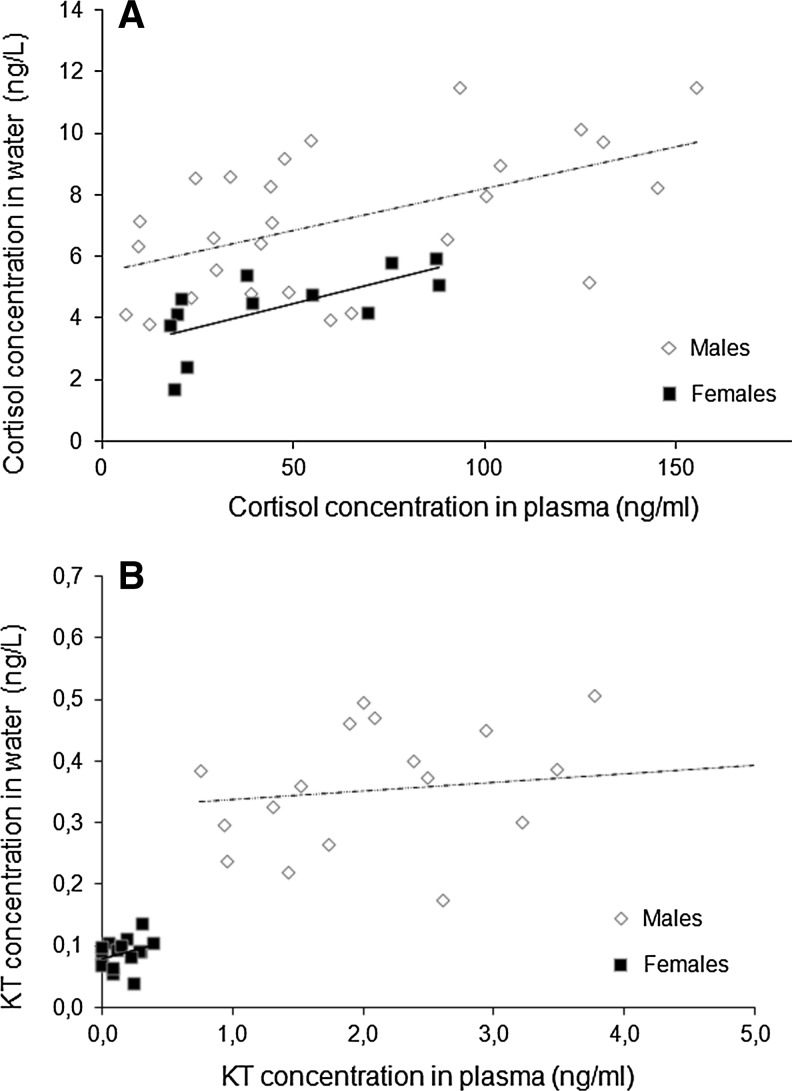
Although there are no significant sex differences in circulating cortisol levels, males have higher cortisol release rates into the water than females (Table 1). There is a positive significant correlation between holding-water and plasma cortisol levels both for males and females (males: n=27, r=0.53, P<0.01; females: n=12, r=0.68, p<0.05, Fig. 1A). Both KT circulating levels and KT release rates are significantly higher in males than in females (Table 1). Water and plasma KT levels are not correlated in any of the sexes (males: n=19, r=0.18, p=0.46; females: n=16, r=0.33, p=0.21, Fig 1B).
FIG. 1.
Correlation between plasma and holding-water cortisol (A) and 11-ketotestosterone (B) concentrations for male and female zebrafish.
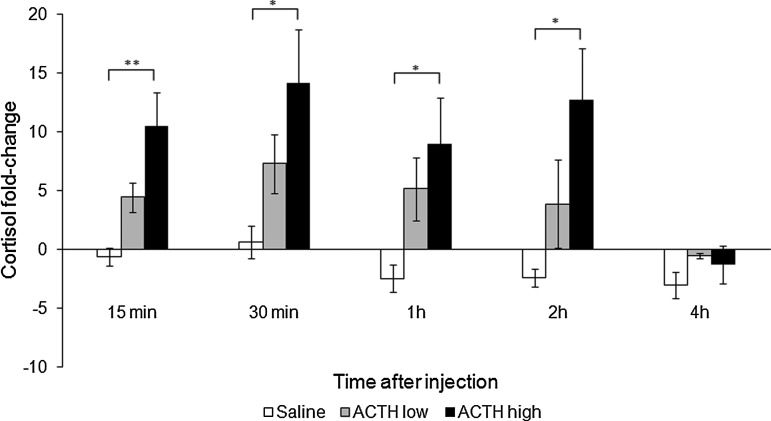
In the ACTH challenge test, there was a main effect of treatment (F2,19=5.06, p<0.05; post-hoc Tukey unequal N HSD test: ACTH high dose>saline=ACTH low dose, p<0.05) and of time after injection (F4,76=4.51, p<0.01; post-hoc Tukey unequal N HSD test: 15 min=30 min=1 h=2 h>4 h, p<0.05 or higher), and here was no significant interaction between treatment and time post-injection (F8,76=1.41, p=0.21), indicating a similar time course of the cortisol response to the 2 ACTH dosages. Planned comparisons between the saline treatment and each ACTH dosage for each sampling point revealed that cortisol levels elicited by the high ACTH dose are significantly higher than those of control treatment 15 min (F1,19=10.41, p<0.01), 30 min (F1,19=6.06, p<0.05), 1 h (F1,19=5.67, p<0.05), and 2 h (F1,19=8.03, p<0.05) after injection, but not at 4 h post-injection (F1,19=0.69, p=0.42), whereas cortisol levels induced by the low ACTH dose were not significantly higher than control levels at any of the sampling points (all p values>0.20, Fig. 2).
FIG. 2.
Response of holding-water cortisol to an ACTH challenge. Bars=Mean+SEM; *p<0.05; **p<0.01.
The dilution curves (slopes) for extracted cortisol and for extracted KT from holding-water did not differ from their respective standard curves (ANCOVA: cortisol, F1,7=0.28, p=0.61; KT, F1,8=0.01, p=0.93). Average steroid recoveries were 82.60±0.03 % (n=3) for cortisol and 82.50±0.09 % (n=2) for KT.
Discussion
The results presented here validate the use of holding-water as a noninvasive measure of circulating cortisol levels: there is residual background cortisol immunoreactivity, cortisol recovery is considerably high (82.6%) and within the scope of recovery values reported in other studies,17,26 and the cortisol values for the extracts dilution curve parallels those of the standard curve, indicating an absence of interference by other immunoreactive substances in the medium. Furthermore, plasma and holding-water cortisol levels show positive significant correlations for both sexes despite males having higher cortisol baseline levels than females, and the ACTH challenge elicited a significant increase in holding-water cortisol levels. The response of cortisol immunoreactivity in the water to the high ACTH challenge was already detected in the first sampling point (15 min post-injection), reached a peak 30 min after the injection, and remained until 2 h post-injection. The saline control (Ringer injection) of this experiment produced similar cortisol values (4.81±1.14 ng/L) to those obtained in Study 1 (4.99±1.33 ng/L), suggesting that, despite being handled several times, fish in the control treatment exhibit baseline cortisol levels. When compared to other species, zebrafish cortisol release rates are one order of magnitude higher (13–20 vs. 0.13–2.5 ng g−1 h−1).17,19,20 This higher cortisol release rates in zebrafish have also been independently documented by another laboratory that also assayed cortisol from holding-water in zebrafish (ca. 10 ng g−1 h−1, M. Pavlidis, personal communication, September 2011). It is not clear at the moment which factor contributes to this difference. It is known that temperature may influence cortisol release rates in the scope of one order of magnitude within the same species (e.g,. 0.13 ng g−1 h−1 at 25°C vs. 0.01 ng g−1 h−1 at 19°C in the dusky grouper, Epinephelus marginatus),20 and in comparison to most other species for which cortisol release rates into the water have been reported, zebrafish was kept at much higher temperatures (28°C for zebrafish and 12–22°C for the other species.17,19,20 However, other warm water species still have lower cortisol release rates than zebrafish (e.g., 0.150–300 pg g−1 h−1 at 26°C in the convict cichlid Amatitlania nigrofasciata38), suggesting also an effect of species differences. Finally, the fact that plasma levels of cortisol are similar between the sexes but males have higher cortisol release rates into the water than females may suggest a sex difference in the ratio of free to protein-bound (to a putative cortisol binding globulin) circulating cortisol in zebrafish. In other teleost species, it has been shown that females have a lower percentage of free cortisol than males (ca. 50% less).39 If that is also the case in zebrafish, since the cortisol present in the holding-water in principle reflects only the free fraction and not the protein-bound, it would explain the higher cortisol holding-water levels for males.
The validation of the cortisol holding-water assay for zebrafish is a significant breakthrough for stress research, since zebrafish is emerging as a model organism for translational stress research both in biomedicine and in aquaculture, due to the similarity of its neuroendocrine stress axis to that of mammals,40 and to the available genetic resources and logistic advantages when compared to commercial species.41,42 Furthermore, this method presents several advantages such as: (1) the measured water concentrations represent steroid integration over time, reducing the problem of short-term fluctuations in hormone levels that may occur in plasma due to the pulsatile nature of their release; and (2) enables the measurement of sequential hormone levels, which cannot be achievable either by blood collection or by whole-body homogenates.
Although the results for KT also show little background immunoreactivity, a relatively high recovery (82.5%), there was a lack of correlations between KT levels in holding-water and in plasma for both sexes. This fact can be explained by the presence of a steroid hormone binding globulin (SHBG) in zebrafish gills, which has high binding for androgens and almost no affinity for cortisol.43 Despite the lack of correlation between plasma and water concentrations, the KT sex difference detected in plasma is also detectable in holding-water. Males release higher concentrations of androgen KT to water than females, with the minimum concentration obtained for males being above the maximum value reported for females. Therefore, although KT water levels cannot be used to study changes in KT circulating levels, they can be used as a tool to sex zebrafish with high reliability. Since sex dimorphism is reduced in zebrafish, leading to common mistakes in sexing fish for experiments, using KT can prove to be a valuable research tool that can give researchers a priori sex identification using a noninvasive method.
In summary, the results presented here validate the use of zebrafish holding-water to measure circulating levels of cortisol and to noninvasively sex individuals with high reliability.
Acknowledgments
This work was funded by the research grants PEst-OE/MAR/UI0331/2011 and PTDC/PSI/71811/2006 from Fundação para a Ciência e a Tecnologia (FCT, Portugal) and the European Union Framework Program 7 project COPEWELL (#265957). AIF is supported by a FCT Ph.D. Fellowship (SFRH/BD/79087/2011).
Disclosure Statement
No competing financial interests exist.
References
- 1.Scholz S. Fischer S. Gundel U. Kuster E. Luckenbach T. Voelker D. The zebrafish embryo model in environmental risk assessment—Applications beyond acute toxicity testing. Environ Sci Poll Res Intl. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- 2.McGonnell IM. Fowkes RC. Fishing for gene function—Endocrine modelling in the zebrafish. J Endocrinol. 2006;189:425–439. doi: 10.1677/joe.1.06683. [DOI] [PubMed] [Google Scholar]
- 3.Lieschke GJ. Currie PD. Animal models of human disease: Zebrafish swim into view. Nature Rev Gen. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 4.Steenbergen PJ. Richardson MK. Champagne DL. The use of the zebrafish model in stress research. Prog Neuro-Psychopharmacol Biol Psych. 2011;35:1432–1451. doi: 10.1016/j.pnpbp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Cachat J. Stewart A. Grossman L. Gaikwad S. Kadri F. Chung KM, et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Prot. 2010;5:1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- 6.Ramsay JM. Feist GW. Varga ZM. Westerfield M. Kent ML. Schreck CB. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture. 2006;258:565–574. [Google Scholar]
- 7.Barcellos LJG. Ritter F. Kreutz LC. Quevedo RM. da Silva LB. Bedin AC, et al. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture. 2007;272:774–778. [Google Scholar]
- 8.Ramsay JM. Feist GW. Varga ZM. Westerfield M. Kent ML. Schreck CB. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture. 2009;297:157–162. doi: 10.1016/j.aquaculture.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuzzen ML. Van Der Kraak G. Bernier NJ. Stirring up new ideas about the regulation of the hypothalamic-pituitary-interrenal axis in zebrafish (Danio rerio) Zebrafish. 2010;7:349–358. doi: 10.1089/zeb.2010.0662. [DOI] [PubMed] [Google Scholar]
- 10.Scott AP. Ellis T. Measurement of fish steroids in water—A review. Gen Comp Endocrinol. 2007;153:392–400. doi: 10.1016/j.ygcen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood LN. Scott AP. Vermeirssen ELM. Mylonas CC. Pavlidis M. Plasma steroids in mature common dentex (Dentex dentex) stimulated with a gonadotropin-releasing hormone agonist. Gen Comp Endocrinol. 2001;123:1–12. doi: 10.1006/gcen.2000.7519. [DOI] [PubMed] [Google Scholar]
- 12.Pavlidis M. Greenwood L. Scott AP. The role of sex ratio on spawning performance and on the free and conjugated sex steroids released into the water by common dentex (Dentex dentex) broodstock. Gen Comp Endocrinol. 2004;138:255–262. doi: 10.1016/j.ygcen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Hirschenhauser K. Taborsky M. Oliveira T. Canario AVM. Oliveira RF. A test of the 'challenge hypothesis' in cichlid fish: Simulated partner and territory intruder experiments. Anim Behav. 2004;68:741–750. [Google Scholar]
- 14.Huertas M. Scott AP. Hubbard PC. Canário AVM. Cerdà J. Sexually mature European eels (Anguilla anguilla L.) stimulate gonadal development of neighbouring males: Possible involvement of chemical communication. Gen Comp Endocrinol. 2006;147:304–313. doi: 10.1016/j.ygcen.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Sebire M. Katsiadaki I. Scott AP. Noninvasive measurement of 11-ketotestosterone, cortisol and androstenedione in male three-spined stickleback (Gasterosteus aculeatus) Gen Comp Endocrinol. 2007;152:30–38. doi: 10.1016/j.ygcen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Ruane NM. Komen H. Measuring cortisol in the water as an indicator of stress caused by increased loading density in common carp (Cyprinus carpio) Aquaculture. 2003;218:685–693. [Google Scholar]
- 17.Ellis T. James J. Stewart C. Scott A. A noninvasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. J Fish Biol. 2004;65:1233–1252. [Google Scholar]
- 18.Lower N. Moore A. Scott A. Ellis T. James J. Russell I. A noninvasive method to assess the impact of electronic tag insertion on stress levels in fishes. J Fish Biol. 2005;67:1202–1212. [Google Scholar]
- 19.Wysocki LE. Dittami JP. Ladich F. Ship noise and cortisol secretion in European freshwater fishes. Biol Cons. 2006;128:501–508. [Google Scholar]
- 20.Fanouraki E. Mylonas CC. Papandroulakis N. Pavlidis M. Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. Gen Comp Endocrinol. 2011;173:313–322. doi: 10.1016/j.ygcen.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Fanouraki E. Papandroulakis N. Ellis T. Mylonas C. Scott A. Pavlidis M. Water cortisol is a reliable indicator of stress in European sea bass, Dicentrarchus labrax. Behaviour. 2008;145:1267–1281. [Google Scholar]
- 22.Kittilsen S. Ellis T. Schjolden J. Braastad BO. Øverli Ø. Determining stress-responsiveness in family groups of Atlantic salmon (Salmo salar) using noninvasive measures. Aquaculture. 2009;298:146–152. [Google Scholar]
- 23.Oliveira R. Ros A. Hirschenhauser K. Canario A. Androgens and mating systems in fish: Intra-and interspecific analyses. In: Goos HJ, editor; Rastogi RK, editor; Vaudry H, editor; Pierantoni R, editor. Perspectives in Comparative Endocrinology: Unity and Diversity. 2001. pp. 203–215. [Google Scholar]
- 24.Oliveira RF. Hirschenhauser K. Canario AVM. Taborsky M. Androgen levels of reproductive competitors in a co-operatively breeding cichlid. J Fish Biol. 2003;63:1615–1620. [Google Scholar]
- 25.Bender N. Heg D. Hamilton IM. Bachar Z. Taborsky M. Oliveira RF. The relationship between social status, behaviour, growth and steroids in male helpers and breeders of a cooperatively breeding cichlid. Horm Behav. 2006;50:173–182. doi: 10.1016/j.yhbeh.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Dzieweczynski TL. Eklund AC. Rowland WJ. Male 11-ketotestosterone levels change as a result of being watched in Siamese fighting fish, Betta splendens. Gen Comp Endocrinol. 2006;147:184–189. doi: 10.1016/j.ygcen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Earley RL. Edwards JT. Aseem O. Felton K. Blumer LS. Karom M, et al. Social interactions tune aggression and stress responsiveness in a territorial cichlid fish (Archocentrus nigrofasciatus) Physiol Behav. 2006;88:353–363. doi: 10.1016/j.physbeh.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers EW. Earley RL. Grober MS. Elevated 11-ketotestosterone during paternal behavior in the Bluebanded goby (Lythrypnus dalli) Horm Behav. 2006;49:610–614. doi: 10.1016/j.yhbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Bshary R. Oliveira RF. Oliveira TS. Canario AV. Do cleaning organisms reduce the stress response of client reef fish? Front Zool. 2007;4:21. doi: 10.1186/1742-9994-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soares MC. Bshary R. Cardoso SC. Côté IM. Oliveira RF. Face your fears: Cleaning gobies inspect predators despite being stressed by them. PloS one. 2012;7:e39781. doi: 10.1371/journal.pone.0039781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermeirssen ELM. Scott AP. Excretion of free and conjugated steroids in rainbow trout (Oncorhynchus mykiss): Evidence for branchial excretion of the maturation-inducing steroid, 17, 20-beta-dihydroxy-4-pregnen-3-one. Gen Comp Endocrinol. 1996;101:180–194. doi: 10.1006/gcen.1996.0020. [DOI] [PubMed] [Google Scholar]
- 32.Ellis T. James J. Scott A. Branchial release of free cortisol and melatonin by rainbow trout. J Fish Biol. 2005;67:535–540. [Google Scholar]
- 33.Scott AP. Hirschenhauser K. Bender N. Oliveira R. Earley RL. Sebire M, et al. Non-invasive measurement of steroids in fish-holding-water: Important considerations when applying the procedure to behaviour studies. Behaviour. 2008;145:1307–1328. [Google Scholar]
- 34.Miguel-Queralt S. Hammond GL. Sex hormone-binding globulin in fish gills is a portal for sex steroids breached by xenobiotics. Endocrinology. 2008;149:4269–4275. doi: 10.1210/en.2008-0384. [DOI] [PubMed] [Google Scholar]
- 35.Miguel-Queralt S. Knowlton M. Avvakumov GV. Al-Nouno R. Kelly GM. Hammond GL. Molecular and functional characterization of sex hormone binding globulin in zebrafish. Endocrinology. 2004;145:5221–5230. doi: 10.1210/en.2004-0678. [DOI] [PubMed] [Google Scholar]
- 36.Guerrero RD., III Shelton WL. An aceto-carmine squash method for sexing juvenile fishes. Progr Fish-Cult. 1974;36:56. [Google Scholar]
- 37.Lindman HR. Analysis of Variance in Complex Experimental Designs. WH Freeman & Co; 1974. [Google Scholar]
- 38.Wong SC. Dykstra M. Campbell JM. Earley RL. Measuring water-borne cortisol in convict cichlids (Amatitlania nigrofasciata): Is the procedure a stressor? Behaviour. 2008;145:1283–1305. [Google Scholar]
- 39.Caldwell CA. Kattesh HG. Strange RJ. Distribution of cortisol among its free and protein-bound fractions in rainbow trout (Oncorhynchus mykiss): Evidence of control by sexual maturation. Comp Biochem Physiol A Comp Physiol. 1991;99:593–595. doi: 10.1016/0300-9629(91)90135-y. [DOI] [PubMed] [Google Scholar]
- 40.Alsop D. Vijayan M. The zebrafish stress axis: Molecular fallout from the teleost-specific genome duplication event. Gen Comp Endocrinol. 2009;161:62–66. doi: 10.1016/j.ygcen.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Alestrom P. Holter JL. Nourizadeh-Lillabadi R. Zebrafish in functional genomics and aquatic biomedicine. Trends Biotech. 2006;24:15–21. doi: 10.1016/j.tibtech.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Dahm R. Geisler R. Learning from small fry: The zebrafish as a genetic model organism for aquaculture fish species. Mar Biotechnol. 2006;8:329–345. doi: 10.1007/s10126-006-5139-0. [DOI] [PubMed] [Google Scholar]
- 43.Miguel-Queralt S. Knowlton M. Avvakumov GV. Al-Nuono R. Kelly GM. Hammond GL. Molecular and functional characterization of sex hormone binding globulin in zebrafish. Endocrinology. 2004;145:5221–5230. doi: 10.1210/en.2004-0678. [DOI] [PubMed] [Google Scholar]