Abstract
Background
Pre-analytical conditions are key factors in maintaining the high quality of biospecimens. They are necessary for accurate reproducibility of experiments in the field of biomarker discovery as well as achieving optimal specificity of laboratory tests for clinical diagnosis. In research at the National Biobank of Korea, we evaluated the impact of pre-analytical conditions on the stability of biobanked blood samples by measuring biochemical analytes commonly used in clinical laboratory tests.
Methods
We measured 10 routine laboratory analytes in serum and plasma samples from healthy donors (n=50) with a chemistry autoanalyzer (Hitachi 7600-110). The analyte measurements were made at different time courses based on delay of blood fractionation, freezing delay of fractionated serum and plasma samples, and at different cycles (0, 1, 3, 6, 9) of freeze-thawing. Statistically significant changes from the reference sample mean were determined using the repeated-measures ANOVA and the significant change limit (SCL).
Results
The serum levels of GGT and LDH were changed significantly depending on both the time interval between blood collection and fractionation and the time interval between fractionation and freezing of serum and plasma samples. The glucose level was most sensitive only to the elapsed time between blood collection and centrifugation for blood fractionation. Based on these findings, a simple formula (glucose decrease by 1.387 mg/dL per hour) was derived to estimate the length of time delay after blood collection. In addition, AST, BUN, GGT, and LDH showed sensitive responses to repeated freeze-thaw cycles of serum and plasma samples.
Conclusion
These results suggest that GGT and LDH measurements can be used as quality control markers for certain pre-analytical conditions (eg, delayed processing or repeated freeze-thawing) of blood samples which are either directly used in the laboratory tests or stored for future research in the biobank.
Introduction
Blood is a biofluid that can be readily obtained from patients or health examinees, and analyzed to assess the current physiological state of the body. Consequently, serum and plasma samples have long been used for identification of disease-related biomarkers and for clinical diagnosis.1–5 Detection technologies, such as mass spectrometry and immuno-hybridization technologies used in many omics fields, have dramatically improved in terms of accuracy and sensitivity, with significant increases in the possibility of detection and identification of trace amounts of molecules in the circulatory system. However, despite use of the same analytic platform to profile the plasma proteome of identical samples, different research groups have reported profiling results so disparate that only a small portion of the identified proteins overlapped.2 These types of discrepancy can be caused by high sample complexity and a wide dynamic range of plasma proteins. Further, incomplete sampling, false-positive matches, and integration of diverse datasets have been implicated as contributing to the disparate results obtained in analyses of plasma and serum proteins. These observations reinforce the critical importance of maintaining high quality serum and plasma samples throughout the analysis of circulating protein biomarkers.6–8 Analysis of laboratory errors showed that at least 40% of errors are made in the pre-analytical phase.9–12 However, researchers may be less meticulous in the pre-analytical phase than in the experimental or analytical phases of the process.13 Therefore, quality control of pre-analytical variables can be considered a key factor in generating consistent experimental data and providing accurate and sensitive diagnostic tools. It is important to maintain and monitor sample quality, as well as to handle and control samples properly.
To date, numerous reports have provided information about the stability of blood samples, but few have investigated analytes and conditions relevant to generalized research. Most studies have focused on one or a few analytes or sets of proteins, such as panels of specific diagnostic markers.14 In previous studies of the effects of delayed processing of whole blood, conditions such as storage time at high temperature vs. room temperature have been analyzed.15–17 Although it is generally recognized that repeated freeze-thaw cycles can influence biomarker measurements,18,19 a single specimen may be subjected to multiple freeze-thaw cycles in the course of blood processing in multiple laboratories.
We therefore examined the effect of some pre-analytical conditions, including time intervals before and after blood sample processing and the number of freeze-thaw cycles on blood sample quality. We measured the concentrations of 10 commonly assayed analytes of serum and plasma under previously unstudied conditions that simulate extreme conditions. These common biochemical markers can be easily tested in basic lab tests, and the results can be managed in biobank databases. Our goal was also to suggest potentially robust and sensitive biomarkers for blood sample quality.
Materials and Methods
Participants
All volunteers were informed of the rationale for the study, and consent was obtained. The subjects included 25 males and 25 females ranging from 31 to 59 years of age, without disease in their medical histories, and currently not on medicine. The study was approved by the local Ethics Committee at Ewha Women's University Mogdong Hospital.
Blood collection and processing
Venous blood was collected from each participant into ten sterile 5-mL Vacutainer Serum separator tubes with clot activator (SST; Becton Dickinson, NJ) and two sterile Vacutainer Plasma separator tubes containing EDTA (K2 EDTA; Becton Dickinson). To study the effects of delayed processing time on whole blood, we allowed four 5-mL SST tubes to stand at room temperature for 0.5, 1, 2, 4, and 24 h. At the end of the treatment period, the tubes were centrifuged at 3000 g for 10 min and the serum samples were transferred into several cryovials (Nalgene: Fisher Scientific) for analyte measurements. Next, to study the effects of delayed freezing on serum samples, we allowed four 5-mL SSTs from each volunteer to stand for 0.5 h at room temperature, after which those tubes were centrifuged (3000 g for 10 min). We mixed the serum samples (four) from each volunteer in a 50-mL tube and transferred aliquots into cryovials. The cryovialed serum samples were stored at room temperature for 0.5, 2, and 4 h.
Finally, to examine the effects of repeated freeze-thawing cycles on sample quality, we allowed two 5-mL SSTs and two EDTA tubes from each volunteer to stand for 0.5 h at room temperature, after which tubes were centrifuged (3000 g for 10 min). The serum and plasma were subsequently aliquoted and then one serum and one plasma aliquot were immediately analyzed on the Hitachi 7600-110 (Hitachi Co., Tokyo, Japan) to use as a reference for subsequent measurements for freeze-thaw cycles. The remaining aliquots were stored at −196°C. One day after the remaining aliquots were stored at −196°C, the frozen samples were thawed completely at room temperature. These freeze-thaw cycles were further repeated 3, 6, or 9 times. The sample collection, handling, and storage methods are summarized in Supplementary Table ST1 (supplementary data are available online at www.liebertpub.com/bio).
Biochemical analysis
The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, gamma-glutamyltransferase (GGT), triglycerides, lactate dehydrogenase (LDH), C-reactive protein, creatinine, glucose, and blood urea nitrogen (BUN) in serum and plasma were measured using an automated chemistry analyzer, Hitachi 7600-110 (Hitachi Co.) in routine chemistry workflow of the diagnostics. A detailed description of the assay methods is shown in Supplementary Table ST2. We carried out all measurements in triplicate to assess the reproducibility of the analytic methods.
Statistical analysis
Statistical analysis was performed with the SPSS 17.0 (SPSS Inc., Chicago, IL) software package. The level of each biochemical in the different pre-analytical samples was expressed as a relative concentration by dividing each result by the control measurement. Statistically significant changes were determined for each of the analytes by repeated-measures ANOVA. Clinically significant changes were determined using the significant change limit (SCL) approach,20 defined as: SCL=initial value±2.8 usual standard deviation (USD) and was based on the assumption that the USD would be representative of the inherent batch-to-batch variability of the method. In the present study, the calculated mean for each of the analytes at 0.5 h represented the initial value. The USD was obtained by averaging the SD of the initial values and the SCL was computed by establishing the range (±2.8 USD) from the mean reference value.
To estimate the effects of pre-analytic conditions retroactively, such as the duration of delay before centrifugation, we applied linear regression analysis using biomarker concentration as a dependent variable and pre-analytic condition as an independent variable. An estimate was considered significant if the multiple correlation coefficient (R) was higher than 0.2 and the p value was less than 0.05.
Results
Effect of delayed processing between blood collection and fractionation
We assayed 10 analytes widely used as diagnostic markers to examine the stability of serum samples kept at room temperature for up to 24 h prior to fractionation/separation. The levels of eight analytes tended to increase with increasing time prior to separation; the exceptions were TG and CRP (Table 1). Based on analysis using repeated measures ANOVA, the serum concentrations of AST, GGT, LDH, creatinine, and glucose showed significant (p<0.05) changes within 1 h of delay prior to separation compared with the reference. Of these analytes, the glucose concentrations of samples incubated for 24 h prior to separation showed significant (p<0.0001) decreases of approximately 1.6-fold compared to glucose concentrations of sera separated immediately after collection. In contrast, no statistically significant differences were observed in TG and CRP concentrations, even after a 24 h delay in sample processing, compared to the reference serum samples. Using the SCL approach to determine relevant clinical changes in serum and plasma components, we found that the concentrations of GGT, LDH, and glucose changed significantly even after short delays of 2 h, although the levels of AST, ALT, TG, TC, and CRP were unchanged (Table 1).
Table 1.
Concentration of 10 Analytes in Serum According to Pre-analytical Processing
| |
|
AST |
ALT |
GGT |
LDH |
TG |
TC |
CRP |
BUN |
creatinine |
glucose |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (U/L) | (U/L) | (U/L) | (U/L) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | ||
| Ref | 25.73 | 26.64 | 28.67 | 334.04 | 115.52 | 189.67 | 0.21 | 13.15 | 0.93 | 90.6 | |
| USD | 0.33 | 0.35 | 0.24 | 1.84 | 1.20 | 3.07 | 0.00 | 0.10 | 0.03 | 1.64 | |
| SCL range* | 24.79–26.66 | 25.65–27.63 | 27.99–29.34 | 328.70–339.38 | 112.16–118.88 | 181.08–198.27 | 0.20–0.23 | 12.86–13.44 | 0.80–1.00 | 86.02–95.18 | |
| Duration of delay before centrifugation | 1 | 26.15‡±0.27 | 26.55±0.37 | 29.03‡±0.50 | 343.43†‡±2.36 | 114.87±0.93 | 189.75±3.42 | 0.22±0.00 | 13.19±0.10 | 0.95‡±0.03 | 86.57‡±1.48 |
| 2 | 26.23‡±0.39 | 26.57±0.38 | 29.41†‡±0.44 | 352.60†‡±2.73 | 114.77±0.82 | 189.89±3.31 | 0.22±0.00 | 13.25±0.12 | 0.95‡±0.04 | 83.45†‡±1.34 | |
| 4 | 25.99±0.34 | 26.41‡±0.44 | 29.63†‡±0.36 | 358.44†‡±2.46 | 114.11±0.85 | 188.12‡±2.85 | 0.23±0.00 | 13.31‡±0.13 | 0.97‡±0.04 | 77.54†‡±1.20 | |
| 24 | 26.49‡±0.36 | 27.04‡±0.37 | 29.95†‡±0.56 | 363.85†‡±2.76 | 115.39±1.31 | 194.38‡±3.78 | 0.23±0.00 | 14.34†‡±0.20 | 1.13†‡±0.06 | 54.59†‡±1.60 | |
| Duration of delay after centrifugation | 2 | 26.49‡±0.51 | 27.13‡±0.44 | 29.72†‡±0.77 | 341.77†‡±3.34 | 117.33‡±1.88 | 191.97‡±4.03 | 0.23±0.00 | 13.55†‡±0.32 | 0.98‡±0.04 | 91.5‡±1.30 |
| 4 | 26.97b‡±0.50 | 27.10‡±0.62 | 29.96†‡±0.72 | 352.94†‡±3.31 | 117.73‡±1.77 | 195.68‡±3.62 | 0.24†±0.03 | 13.67†‡±0.33 | 1.00‡±0.04 | 92.01‡±1.40 | |
| Numbers of freeze/thaw cycles | 1 | 26.98†‡±0.62 | 27.39‡±0.20 | 29.71†‡±0.43 | 345.08†‡±2.98 | 114.16±0.96 | 189.85±2.02 | 0.20±0.00 | 14.59†‡±0.36 | 0.96‡±0.04 | 93.09‡±1.25 |
| 3 | 27.34†‡±0.58 | 26.89‡±0.38 | 29.90†‡±0.64 | 377.13†‡±3.25 | 113.6‡±1.45 | 189.17±2.50 | 0.20±0.00 | 14.76†‡±0.28 | 0.97‡±0.03 | 93.44‡±1.49 | |
| 6 | 26.99†‡±0.65 | 24.96†‡±0.28 | 29.46†‡±0.47 | 374.11†‡±4.65 | 112.01†‡±1.39 | 187.29‡±2.79 | 0.20±0.00 | 14.71†‡±0.28 | 0.96‡±0.03 | 92.67‡±1.61 | |
| 9 | 27.13†‡±0.52 | 24.69†‡±0.46 | 29.43†‡±0.43 | 378.21†‡±4.29 | 110.54†‡±5.66 | 189.93±1.35 | 0.20±0.01 | 14.75†‡±0.20 | 0.96‡±0.02 | 94.37‡±1.19 | |
Mean value of reference±(2.8×USD); †Excess over the SCL range; ‡Statistically significant difference from reference (repeated-measure ANOVA, p<0.05).
Effect of elapsed time between blood fractionation and freezing of serum
The same analytes were measured in serum samples maintained at room temperature for up to 4 h after fractionation and before storage. The concentration of nine analytes (AST, ALT, GGT, LDH, TG, TC, BUN, creatinine, and glucose) showed a tendency to increase statistically with elapsed time (Table 1). Of these analytes, the concentrations of GGT, LDH, and BUN were significantly changed, as determined by SCL, after a 2 h delay at room temperature prior to storage, compared to the reference.
Effect of repeated freeze-thaw cycles
To assess the influence of repeated freeze-thaw cycles on blood sample composition, serum and plasma samples were subjected to multiple freeze-thaw cycles. The concentrations of the 10 analytes were measured and compared to the reference samples (Table 1). The concentrations of seven analytes (ALT, AST, GGT, LDH, creatinine, glucose, and BUN) were statistically different after a single freeze-thaw cycle except for TG, TC, and CRP. Particularly following SCL analysis, the concentrations of four analytes (AST, GGT, LDH, and BUN) appeared susceptible to freeze-thaw cycles; however, TC and CRP were unaffected (Table 1), and TG was affected only after the first freeze-thaw cycle.
The levels of seven analytes (ALT, GGT, LDH, TG, BUN, creatinine, and glucose) were changed significantly (p<0.05) after three freeze-thaw cycles, as shown by repeated-measures ANOVA (Supplementary Table ST3). Among these analytes, LDH showed the greatest response to freeze-thaw cycles.
In summary, changes in concentrations of GGT and LDH reflected the delays in time of blood processing and storage, as well as in the repeated freeze-thaw cycles. In contrast, the CRP concentration was unaffected by changes in the pre-analytical conditions tested in this study.
Predicting pre-analytical conditions using biomarker candidates
To identify reliable high-quality biomarkers by determining the sensitivity of analytes to pre-analytical conditions, we performed linear regression analysis (Table 2). Regression analyses showed that levels of glucose and creatinine were significantly correlated with the duration of delay before blood fractionation (β=−1.387, R=0.846, p<0.05, and β=0.008, R=0.407, p<0.05, respectively), indicating that the glucose concentration decreased by 1.387 mg/dL for every hour of delay prior to blood fractionation. Landt21 reported that the initial concentration of glucose in heparinized whole blood decreased linearly over 8 h. Therefore, the duration of delay before blood fractionation could be predicted using the following formula.
 |
Table 2.
Regression Analysis for Prediction of Pre-analytic Conditions Using Biomarker Concentrations
| Condition | AST | ALT | GGT | LDH | TG | TC | CRP | BUN | creatinine | glucose | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration of delay before centrifugation | β | 0.022 | 0.021 | 0.039 | 0.865 | 0.018 | 0.212 | 0.001 | 0.050 | 0.008 | −1.387 |
| R | 0.011 | 0.014 | 0.017 | 0.136 | 0.002 | 0.068 | 0.010 | 0.144 | 0.407 | 0.846 | |
| p value | 0.770 | 0.709 | 0.646 | 0.001 | 0.965 | 0.064 | 0.792 | 0.001 | 0.001 | 0.001 | |
| Duration of delay after centrifugation | β | 0.312 | 0.115 | 0.323 | 4.725 | 0.552 | 1.502 | 0.007 | 0.128 | 0.017 | 0.352 |
| R | 0.028 | 0.014 | 0.025 | 0.131 | 0.009 | 0.087 | 0.025 | 0.066 | 0.190 | 0.080 | |
| p value | 0.556 | 0.773 | 0.597 | 0.005 | 0.856 | 0.065 | 0.600 | 0.164 | 0.000 | 0.091 | |
| Numbers of freeze/thaw cycles | β | 0.096 | −0.295 | 0.033 | 4.606 | −0.516 | −0.073 | −0.001 | 0.117 | 0.002 | 0.272 |
| R | 0.018 | 0.073 | 0.005 | 0.251 | 0.017 | 0.009 | 0.007 | 0.116 | 0.037 | 0.135 | |
| p value | 0.622 | 0.045 | 0.887 | 0.001 | 0.635 | 0.811 | 0.858 | 0.002 | 0.312 | 0.001 | |
| Plasma (Freeze/thaw) | β | −0.069 | −0.256 | −0.064 | 5.964 | −0.146 | 3.000 | −0.001 | 0.004 | −0.002 | 0.244 |
| R | 0.012 | 0.057 | 0.009 | 0.188 | 0.004 | 0.020 | 0.004 | 0.004 | 0.041 | 0.113 | |
| p value | 0.773 | 0.163 | 0.823 | 0.001 | 0.915 | 0.628 | 0.931 | 0.931 | 0.315 | 0.005 | |
In addition, the serum level of LDH decreased significantly as the number of freeze-thaw cycles increased (β=4.606, R=0.251, p<0.05); however, no significant correlation was observed with the duration of delay after centrifugation. Based on these findings, a simple constant (glucose decrease by 1.387 mg/dL per hour) was derived to estimate the length of the delay between blood collection and fractionation.
Discussion
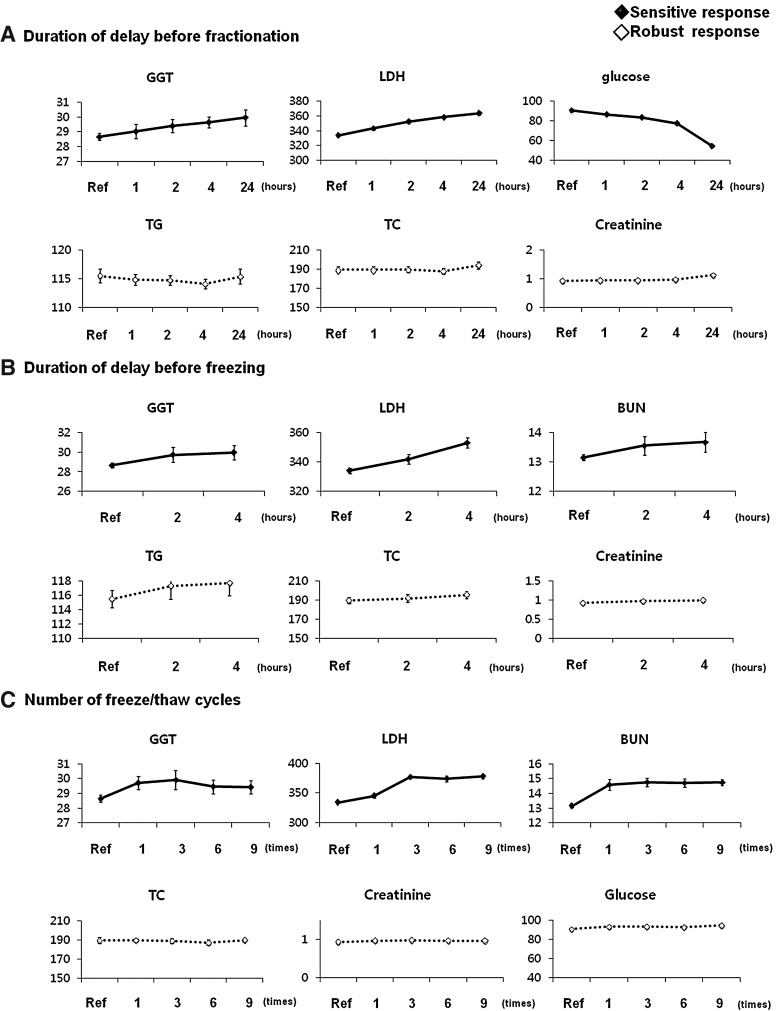
We assessed effects of pre-analytical conditions on the quality of plasma and serum samples by measuring the concentration of 10 representative analytes under conditions which are uncommon in routine laboratory tests, but occasionally may occur in the biobanking process. The aim of this study was to identify an applicable biomarker for estimating blood sample quality. Despite conflicting previous reports,2,20,22–25 the results of this study showed that delay both before and after centrifugation to separate serum from whole blood resulted in significant quantitative changes in the concentrations of some biochemical analytes, even when the delay was as short as an hour. We summarized the responses of 10 analytes according to pre-analytical status in Table 3 and Figure 1, indicating that serum GGT and LDH levels are strongly influenced by many conditions of blood sample collection and storage.
Table 3.
Summary of Response of 10 Analytes to Pre-analytical Status in Serum
| Response | Duration of delay before centrifugation | Duration of delay after centrifugation | Numbers of freeze/thaw cycles |
|---|---|---|---|
| Sensitive | GGT*, LDH*, glucose* | GGT*, LDH*, BUN* | AST§, GGT§, LDH§, BUN§ |
| Mild | AST†, CRP† | ALT¶, TG¶ | |
| Robust | AST‡, ALT‡, TG‡, TC‡, CRP‡, BUN‡, creatinine‡ | ALT‡, TG‡, TC‡, creatinine‡, glucose‡ | TC¤, CRP¤, creatinine¤, glucose¤ |
Statistically significant difference from reference within 2 h (out of SCL range and p<0.05); †Statistically significant difference from reference within 4 h (out of SCL range and p<0.05); ‡No significant difference from reference within 4 h or even after 24 h delay; §Statistically significant difference from reference after one thawing (out of SCL range and p<0.05); ¶Statistically significant difference from reference after 6 freeze-thaw periods (out of SCL range and p<0.05); ¤No significant difference from reference after 6 or even 9 freeze-thaw periods.
FIG. 1.
Sensitive and robust responses to pre-analytical conditions. (A) The concentrations of three analytes (GGT, LDH, and glucose) in serum showed significant differences after 2 h that were dependent on the duration of delay before centrifugation. In contrast, the concentrations of TG, TC, and creatinine did not change. (B) Responses of GGT, LDH, and BUN were sensitive to the duration of delay after fractionation. (C) Levels of GGT, LDH, and BUN in plasma changed significantly with the number of freeze-thaw cycles. However, the levels of TC, creatinine, and glucose did not change after nine freeze-thaw cycles.
These changes in analyte measurements may be attributable to sustained metabolic activities of blood cells, alterations in cell membrane integrity resulting in continuous release of metabolites, or release of degradation products from clots. These findings are particularly important since variations in the time lag between blood drawing and centrifugation in the clinical laboratory are almost always encountered during blood sample processing for biobanking. Apparently, the length of time between collection and separation of serum from blood cells or clots may be critical for consistent measurements of many biological molecules. Therefore, our data, generated from artificial laboratory conditions can be used as a reference for standardizing blood processing protocols for biobanking. Moreover, these results emphasize the importance of documentation of pre-analytical conditions, and emphasize the need for investigators to control pre-analytical conditions in replicating studies. For this purpose, the SPREC (Standard PRE-analytical Code) has been proposed by the ISBER Biospecimen Working group.
The information on repeated freeze-thaw cycling is important for both the design of studies using biobanked samples and residual aliquots of samples as well as their data interpretation. Repeated freeze-thawing cycling is known to affect the stability of biological samples and, notably, to induce conformational changes in proteins that may ultimately lead to aggregation or degradation.26–28 However, previous studies have shown that some molecules are stable in serum and plasma under conditions of repeated freezing and thawing, even up to 10 cycles.29,30 The present study showed that even one freeze-thaw cycle led to changes in the concentrations of four analytes (AST, GGT, LDH, and BUN); the change in plasma LDH concentration was similar to that seen in serum (Table 3). In the case of glucose, other investigators have noted that the level of glucose in serum is relatively stable after up to six cycles of repeated freezing and thawing.26,31,32 Many pre-analytical factors, such as conditions of blood processing and storage, may cause alterations or spurious cellular release of biomolecules in vitro after sampling. This may occur as a result of ex vivo cellular injury, disintegration, cellular granule release, or the actions of proteases.
Although many studies show increased awareness of pre-analytical conditions, biobanks and individual researchers receive samples from hospitals or institutes without information on well-controlled and documented pre-analytical conditions, Instead many biobanks collect biological samples and their relevant biomedical information, including the level of clinical/diagnostic biomarkers which were used in this study. Therefore, quality control biomarkers identified from common clinical/diagnostic markers can be easily accessible for monitoring sample quality, and to estimate sample processing conditions retroactively.
The present study showed that glucose concentration could be used as an indicator of sample quality based on calculation of duration of delay time between collection and centrifugation. A previous study by Boyanton20 showed that glucose concentration changes according to the duration of the delay before sample processing. In our study, we propose the first formula to predict the duration of delay before centrifugation based on the serum levels of common biomarkers. Although GGT, LDH, and BUN showed sensitivity to time delay after blood fractionation, regression analyses of GGT and BUN concentrations showed no significant differences (p>0.05) and the LDH level exhibited higher variability (R<0.2) among individual samples. Of the four analytes (AST, GGT, LDH, and BUN) that were sensitive to freeze-thaw cycles, LDH (β=4.606, R=0.251, p<0.05) would be a better candidate biomarker for predicting the damage from freeze-thaw cycles than BUN (β=0.117, R=0.116, p<0.05) or AST or GGT (p>0.05).
We demonstrated that the length of the time delay in blood processing could be retroactively estimated by a simple formula with the regression coefficient β=−1.387 if the baseline levels of sensitive biomarkers were available. In practice, however, it is likely that the baseline concentrations of selected biomarkers, such as LDH and glucose, are not provided when biobanked samples are distributed to researchers. Moreover, in general, biochemical measurements of biomarkers may vary depending on instrumentation and methodology. Thus, we propose a normalization-based formula by which the concentration of a biomarker sensitive to pre-analytical conditions, such as GGT, LDH, glucose, or BUN, is subtracted from the concentration of an insensitive biomarker, such as TC. For example, Δ(LDH-TC) and Δ(glucose-TC) significantly reflected pre-analytical conditions, such as delayed time of blood processing, subsequent freezing, and numbers of freeze-thaw cycles (Supplementary Fig. S1 and Table 4). These formulas would be easily applicable and useful for assessing sample quality, even in biobanks and laboratories, in which a variety of methods are used for making biochemical measurements of samples of serum and plasma.
Table 4.
Summary of Differences of 5 Adjusted Markers in Serum
| |
|
Δ(AST-TC) |
Δ(GGT-TC) |
Δ(LDH-TC) |
Δ(BUN-TC) |
Δ(Glucose-TC) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ(ConcentrationRef-TCRef) | 163.9 | p value* | 161.0 | p value* | 144.4 | p value* | 176.5 | P-value* | 99.1 | P-value* | |
| Duration of delay before centrifugation | 1 | 163.6 | .673 | 160.7 | .716 | 153.7 | .001 | 176.6 | .960 | 103.2 | .001 |
| 2 | 163.7 | .632 | 160.5 | .334 | 162.7 | .001 | 176.6 | .832 | 106.4 | .001 | |
| 4 | 162.1 | .002 | 158.5 | .001 | 170.3 | .001 | 174.8 | .003 | 110.6 | .001 | |
| 24 | 167.9 | .001 | 164.4 | .001 | 169.5 | .001 | 180.0 | .001 | 139.8 | .001 | |
| Duration of delay after centrifugation | 2 | 165.5 | .019 | 162.3 | .055 | 149.8 | .001 | 178.4 | .004 | 100.5 | .021 |
| 4 | 168.7 | .001 | 165.7 | .001 | 157.3 | .001 | 182.0 | .001 | 103.7 | .001 | |
| Numbers of freeze/thaw cycles | 1 | 162.9 | .207 | 160.1 | .322 | 155.2 | .001 | 175.3 | .180 | 96.8 | .034 |
| 3 | 161.8 | .028 | 159.3 | .070 | 188.0 | .001 | 174.4 | .039 | 95.7 | .004 | |
| 6 | 160.3 | .001 | 157.8 | .001 | 186.8 | .001 | 172.6 | .001 | 94.6 | .001 | |
| 9 | 162.8 | .260 | 160.5 | .581 | 188.3 | .001 | 175.2 | .181 | 95.6 | .002 | |
Statistically significant difference from Δ(ConcentrationRef-TCRef) (repeated-measure ANOVA).
Inappropriate collection, handling, and storage of samples, as well as errors in data analysis and documentation, may all contribute to generation of irreproducible and, more important, unreliable data. Therefore, it is recommended that whenever data generated from specimens obtained from such a biobank are published, the report should contain a detailed description of all parameters that could have influenced the findings. Our findings demonstrate the effects of pre-analytical conditions on blood samples, and may provide a reliable reference biomarker to determine sample quality and retroactively estimate sample processing conditions for biobanks, as well as for researchers analyzing the same specimens.
Supplementary Material
Abbreviations Used
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- CRP
C-reactive protein
- GGT
gamma-glutamyltransferase
- LDH
lactate dehydrogenase
- TC
total cholesterol
- TG
triglycerides
Disclosure Statement
This work was supported by Grants 2008-E74010, 2009-N74001 of Korea National Institute of Health, Korea Centers for Disease Control and Prevention.
Biospecimens used in this study were provided by the National Biobank of Korea.
No competing financial interests exist.
References
- 1.Anderson NL. Anderson NG. The human plasma proteome: History, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 2.Elliott P. Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–244. doi: 10.1093/ije/dym276. [DOI] [PubMed] [Google Scholar]
- 3.Adkins JN. Monroe ME. Auberry KJ, et al. A proteomic study of the HUPO Plasma Proteome Project's pilot samples using an accurate mass and time tag strategy. Proteomics. 2005;5:3454–3466. doi: 10.1002/pmic.200401333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson NL. Anderson NG. Pearson TW, et al. A human proteome detection and quantitation project. Mol Cell Proteomics. 2009;8:883–886. doi: 10.1074/mcp.R800015-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaigneau C. Cabioch T. Beaumont K, et al. Serum biobank certification and the establishment of quality controls for biological fluids: Examples of serum biomarker stability after temperature variation. Clin Chem Lab Med. 2007;45:1390–1395. doi: 10.1515/CCLM.2007.160. [DOI] [PubMed] [Google Scholar]
- 6.Thorpe JD. Duan X. Forrest R, et al. Effects of blood collection conditions on ovarian cancer serum markers. PLoS One. 2007;2:e1281. doi: 10.1371/journal.pone.0001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyna R. Traynor KD. Hines G, et al. Repeated freezing and thawing does not generally alter assay results for several commonly studied reproductive hormones. Fertil Steril. 2001;76:823–825. doi: 10.1016/s0015-0282(01)01986-0. [DOI] [PubMed] [Google Scholar]
- 8.Peakman TC. Elliott P. The UK Biobank sample handling and storage validation studies. Int J Epidemiol. 2008;37:i2–6. doi: 10.1093/ije/dyn019. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G. Guidi GC. Mattiuzzi C, et al. Preanalytical variability: The dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–365. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 10.Carraro P. Plebani M. Errors in a stat laboratory: Types and frequencies 10 years later. Clin Chem. 2007;53:1338–1342. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 11.Romero A. Munoz M. Ramos JR, et al. Identification of preanalytical mistakes in the stat section of the clinical laboratory. Clin Chem Lab Med. 2005;43:974–975. doi: 10.1515/CCLM.2005.168. [DOI] [PubMed] [Google Scholar]
- 12.Plebani M. Carraro P. Mistakes in a stat laboratory: Types and frequency. Clin Chem. 1997;43:1348–1351. [PubMed] [Google Scholar]
- 13.Sweep FC. Fritsche HA. Gion M, et al. Considerations on development, validation, application, and quality control of immuno(metric) biomarker assays in clinical cancer research: An EORTC-NCI working group report. Int J Oncol. 2003;23:1715–1726. [PubMed] [Google Scholar]
- 14.Schrohl AS. Wurtz S. Kohn E, et al. Banking of biological fluids for studies of disease-associated protein biomarkers. Mol Cell Proteomics. 2008;7:2061–2066. doi: 10.1074/mcp.R800010-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark S. Youngman LD. Chukwurah B, et al. Effect of temperature and light on the stability of fat-soluble vitamins in whole blood over several days: Implications for epidemiological studies. Int J Epidemiol. 2004;33:518–525. doi: 10.1093/ije/dyh064. [DOI] [PubMed] [Google Scholar]
- 16.Mejia LA. Arroyave G. Determination of vitamin A in blood. Some practical considerations on the time of collection of the specimens and the stability of the vitamin. Am J Clin Nutr. 1983;37:147–151. doi: 10.1093/ajcn/37.1.147. [DOI] [PubMed] [Google Scholar]
- 17.van Eijsden M. van der Wal MF. Hornstra G, et al. Can whole-blood samples be stored over 24 hours without compromising stability of C-reactive protein, retinol, ferritin, folic acid, and fatty acids in epidemiologic research? Clin Chem. 2005;51:230–232. doi: 10.1373/clinchem.2004.042234. [DOI] [PubMed] [Google Scholar]
- 18.Holland NT. Smith MT. Eskenazi B, et al. Biological sample collection and processing for molecular epidemiological studies. Mutat Res. 2003;543:217–234. doi: 10.1016/s1383-5742(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell BL. Yasui Y. Li CI, et al. Impact of freeze-thaw cycles and storage time on plasma samples used in mass spectrometry based biomarker discovery projects. Cancer Inform. 2005;1:98–104. [PMC free article] [PubMed] [Google Scholar]
- 20.Boyanton BL., Jr. Blick KE. Stability studies of twenty-four analytes in human plasma and serum. Clin Chem. 2002;48:2242–2247. [PubMed] [Google Scholar]
- 21.Landt M. Glyceraldehyde preserves glucose concentrations in whole blood specimens. Clin Chem. 2000;46:1144–1149. [PubMed] [Google Scholar]
- 22.Marshall J. Kupchak P. Zhu W, et al. Processing of serum proteins underlies the mass spectral fingerprinting of myocardial infarction. J Proteome Res. 2003;2:361–372. doi: 10.1021/pr030003l. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg PR. Sherman LA. Tiefenbrunn AJ, et al. Sustained fibrinolysis after administration of t-PA despite its short half-life in the circulation. Thromb Haemost. 1987;57:35–40. [PubMed] [Google Scholar]
- 24.Drammeh BS. Schleicher RL. Pfeiffer CM. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin Chem. 2008;54:1883–1891. doi: 10.1373/clinchem.2008.108761. [DOI] [PubMed] [Google Scholar]
- 25.Dunn WB. Broadhurst D. Ellis DI, et al. A GC-TOF-MS study of the stability of serum and urine metabolomes during the UK Biobank sample collection and preparation protocols. Int J Epidemiol. 2008;37:i23–30. doi: 10.1093/ije/dym281. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar BS. Bogner RH. Pikal MJ. Protein stability during freezing: Separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12:505–523. doi: 10.1080/10837450701481157. [DOI] [PubMed] [Google Scholar]
- 27.Dong A. Prestrelski SJ. Allison SD, et al. Infrared spectroscopic studies of lyophilization- and temperature-induced protein aggregation. J Pharm Sci. 1995;84:415–424. doi: 10.1002/jps.2600840407. [DOI] [PubMed] [Google Scholar]
- 28.Klibanov AM. Schefiliti JA. On the relationship between conformation and stability in solid pharmaceutical protein formulations. Biotechnol Lett. 2004;26:1103–1106. doi: 10.1023/b:bile.0000035520.47933.a6. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh SY. Zhuang FH. Wu YT, et al. Profiling the proteome dynamics during the cell cycle of human hepatoma cells. Proteomics. 2008;8:2872–2884. doi: 10.1002/pmic.200800196. [DOI] [PubMed] [Google Scholar]
- 30.Reimers TJ. McCann JP. Cowan RG, et al. Effects of storage, hemolysis, and freezing and thawing on concentrations of thyroxine, cortisol, and insulin in blood samples. Proc Soc Exp Biol Med. 1982;170:509–516. doi: 10.3181/00379727-170-41466. [DOI] [PubMed] [Google Scholar]
- 31.DiMagno EP. Corle D. O'Brien JF, et al. Effect of long-term freezer storage, thawing, and refreezing on selected constituents of serum. Mayo Clin Proc. 1989;64:1226–1234. doi: 10.1016/s0025-6196(12)61285-3. [DOI] [PubMed] [Google Scholar]
- 32.Bao Y. Zuo L. Effect of repeated freeze-thaw cycles on urinary albumin-to-creatinine ratio. Scand J Clin Lab Invest. 2009;69:886–888. doi: 10.3109/00365510903323209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.