Abstract
Background
The ventral ectodermal ridge (VER) is an important signalling centre in the mouse tail-bud following completion of gastrulation. BMP regulation is essential for VER function, but how these signals are transmitted between adjacent tissues is unclear.
Results
We investigated the idea that extracellular matrix components might be involved, using immunohistochemistry and in situ hybridisation to detect all known α, β and γ laminin chains and their mRNAs in the early tail bud. We identified an apparently novel laminin variant, comprising α5, β3 and γ2 chains, as a major component of the VER basement membrane at E9.5. Strikingly, only the mRNAs for these chains were co-expressed in VER cells, suggesting that lamin532 may be the sole basement membrane laminin at this stage. Since α6 integrin was also expressed in VER cells, this raises the possibility of cell-matrix interactions regulating BMP signalling at this site of caudal morphogenesis.
Conclusions
Laminin532 could interact with α6-containing integrin to direct differentiation of the specialised VER cells from surface ectoderm.
Keywords: mouse, embryo, tail bud, ventral ectodermal ridge, laminin, extracellular matrix, basement membrane, integrin, bone morphogenetic protein
Introduction
After completion of gastrulation, the tail begins to form in the caudal-most region of the mouse embryo, beginning with the generation of the tail-bud. From embryonic day (E) 9.5 onwards, the tail-bud contains a morphologically distinct group of ectodermal cells known as the ventral ectodermal ridge (VER). The VER is a source of signals that regulate tail development, and contains progenitor cells that contribute to the ventral midline ectoderm of the tail (Gruneberg, 1956; Goldman et al., 2000). Ablation of the VER leads to defects in somitogenesis and tail elongation (Goldman et al., 2000). However, the mechanisms through which the VER exerts its effect on these processes have not been fully elucidated.
One candidate player in mediating the effects of the VER is the bone morphogenetic protein (BMP), Bmp2. Bmp2 is expressed in the cells of the VER, while the BMP antagonist, noggin, is expressed in the immediately adjacent mesoderm of the ventral tail-bud. Noggin expression is no longer detected in the ventral mesoderm after ablation of the VER (Goldman et al., 2000). Although the signals from the VER that regulate noggin expression are unclear, it is suggested that Bmp2 could induce noggin production, as part of a negative feedback loop (Goldman et al., 2000). The signals that control the restricted expression of Bmp2 in the VER have yet to be identified.
The BMPs form a large subclass of the transforming growth factor β (TGFβ) superfamily of signalling molecules, with demonstrated involvement in embryonic events including neurulation and dorso-ventral patterning (Mehler et al., 1997). BMPs have been specifically implicated in regulating outgrowth and patterning of the Xenopus tail-bud (Beck et al., 2001) and, together with nodal, in function of the zebrafish tail organizer (Agathon et al., 2003; Fauny et al., 2009). The BMP signalling pathway is well characterised (Attisano and Wrana, 2002), and its activity can be monitored by analyzing the expression of downstream genes such as Cadherin6, Rhob, (Sela-Donenfeld and Kalcheim, 1999), Msx1 and Msx2 (Marazzi et al., 1997; Suzuki et al., 1997; Kettunen and Thesleff, 1998).
BMP signalling is regulated by extracellular antagonists including chordin, chordin-like 1 (Chrdl1; also called neuralin1), follistatin and noggin, and by the intracellular antagonists Smad6 and Smad7 (Attisano and Wrana, 2002; Rider and Mulloy, 2010). We previously described how Bmp2 signalling is modulated by its antagonists, and by sonic hedgehog (Shh), during the process of spinal neural tube closure (Ybot-Gonzalez et al., 2007). In addition to these well described BMP regulators, other factors, such as the extracellular matrix components collagen IV, heparan sulphate proteoglycans and laminins, have also been found to play a role in modulating BMP signalling (Belenkaya et al., 2004; Wang et al., 2008; Dolez et al, 2011). It is unclear whether any of these extracellular modulators are involved in the regulation of BMP signalling in the VER.
One group of potential extracellular modulators of BMP signalling are the laminins, which are major glycoprotein components of basement membranes. Laminins have been implicated in many biological processes, including cell adhesion, migration and differentiation (Colognato and Yurchenco, 2000; Miner and Yurchenco, 2004). At least 16 different laminin variants exist, and their expression in basement membranes is spatially and developmentally regulated (Tunggal et al., 2000; Yurchenco et al., 2004; Aumailley et al., 2005; Tzu and Marinkovich, 2008). Laminins are heterotrimers containing an α, β and γ chain in a cross-like three dimensional structure (Colognato and Yurchenco, 2000). To date, five distinct α chains, three β chains and three γ chains have been described, and their various combinations define the different laminin isoforms (Miner et al., 1997; Patton et al., 1997; Miner and Yurchenco, 2004). Basement membranes can contain more than one laminin isoform (Yurchenco et al., 2004; Miner, 2008) but, owing to the intracellular assembly of the laminin heterotrimer prior to its secretion, co-expression of α , β and γ chain mRNAs in a particular cell type is obligatory for production of each specific laminin isoform.
Cellular responses to laminin are determined in part by a group of transmembrane receptors known as integrins (Miranti and Brugge, 2002). The integrin family is composed of 24 α,β heterodimeric members that mediate the attachment of cells to the extracellular matrix (Barczyk et al., 2010). Integrins containing the α3 and α6 subunits have been described as receptors for laminin, regulating activities such as organization of the basement membrane and differentiation of several epithelial cell types (Sorokin et al., 1990; Kadoya et al., 1995; Walker and Menko, 1999). Interestingly, during osteoblast differentiation, Bmp2 has been reported to stimulate the expression of αV and β integrins which, in turn, are essential for Bmp2 activity (Lai and Cheng, 2005).
In an effort to gain insight into the factors controlling Bmp2 signalling in the VER, we have studied the mRNA expression of Bmp2 signalling components, together with the protein and mRNA expression patterns of all known laminin chains, in the tail-bud of the mouse embryo. We also examined expression of the α3 and α6 integrin subunits. Taken together, our results suggest the existence of a previously undescribed laminin variant that may be implicated in the regulation of Bmp2 responsiveness in the VER via interaction with α6-containing integrin.
Results and Discussion
Expression of BMP signalling components
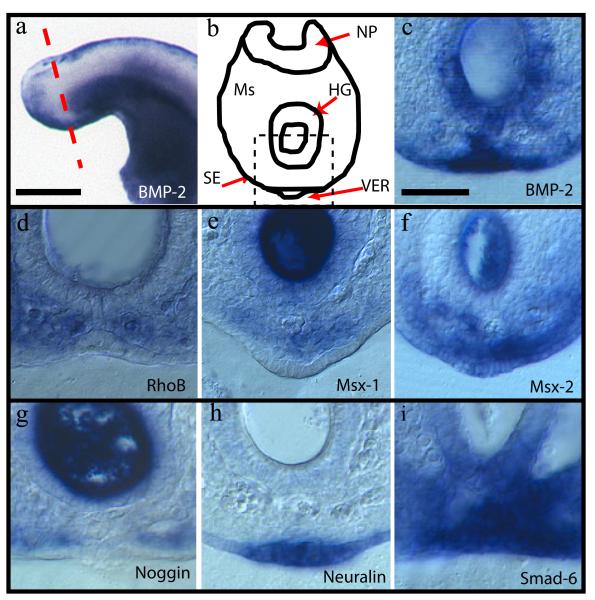
Whole mount in situ hybridisation for Bmp2 in mouse embryos at E9.5 revealed intense mRNA expression, specifically within the VER (Figure 1a-c). We asked whether this strong expression of Bmp2 might correlate with activation of the BMP signalling pathway in the vicinity of the VER. The BMP downstream genes Rhob, Msx1, and Msx2 were all expressed in the ventral mesoderm overlying the VER (Figure 1d-f). In contrast, Cdh6 (Cadherin 6) was not expressed either in the VER or in the surrounding mesoderm (data not shown and (Ybot-Gonzalez et al., 2007)). While Msx2 was also expressed in the surface ectoderm, including the VER itself, Msx1 and Rhob transcripts were strikingly excluded from this region.
Figure 1. Expression of genes in the Bmp2 signalling pathway as detected by in situ hybridisation.
(a) Expression of Bmp2 in the tail-bud of an E9.5 (25-30 somites) mouse embryo. (b) Diagram of the transverse section represented in (a) by the red dashed line. The black dashed square represents the area of this section shown at higher magnification in panels (c-i) and in Figures 2-4. The main tissues are indicated: HG, hindgut; Ms, mesenchyme; NP, neural plate; SE, surface ectoderm; VER, ventral ectodermal ridge. (c-i) mRNA distribution on 50 μm vibratome sections following whole mount in situ hybridisation of embryos with individual probes for: Bmp2 (c), Rhob (d), Msx1 (e), Msx2 (f), noggin (g), Chrdl1 (neuralin1) (h) and Smad6 (i). Scale bar in (a) represents 0.3 mm and in (c) represents 50 μm (also applies to all other panels).
Bmp2 signalling can be modulated by specific extracellular inhibitors: for example, noggin, which is directly implicated in the development of the tail-bud and VER (Goldman et al., 2000). We detected noggin expression in the mesoderm adjacent to the lateral aspects of the VER (Figure 1g). In previous studies, we demonstrated that beads coated in Bmp2 could induce the expression of noggin in surrounding tissues (Ybot-Gonzalez et al., 2007). Hence, the noggin expression in the mesoderm may be induced by Bmp2 activity derived from the VER. At later stages of tail-bud development, once neural tube closure is complete, noggin expression is necessary for suppression of epithelio-mesenchymal transition as gastrulation is completed (Ohta et al., 2007). Interestingly, in our study, in addition to noggin expression at the early tail-bud stages analyzed, we also detected expression of other BMP inhibitors Chrdl1 (neuralin 1) and Smad6. In contrast to noggin, Chrdl1 was expressed specifically in the VER (Figure 1h), and Smad6 was expressed in both VER and overlying mesoderm (Figure 1i). We conclude that BMP signalling is active in the vicinity of the VER, and is controlled by a complex and highly regulated system of agonists and antagonists expressed in the surface ectoderm and adjacent mesenchyme.
Expression of specific laminin isoforms
There is increasing evidence that diffusible morphogens such as Bmp2 are modulated in their activity by interacting with components of the extracellular matrix (Belenkaya et al., 2004; Lai and Cheng, 2005; Wang et al., 2008; Dolez et al., 2011). Hence, a complete understanding of the role of BMP signalling in development of the tail-bud requires an analysis of matrix components. We therefore performed in situ hybridisation and immunohistochemistry to detect mRNA and protein for all of the known laminin isoforms in the VER region.
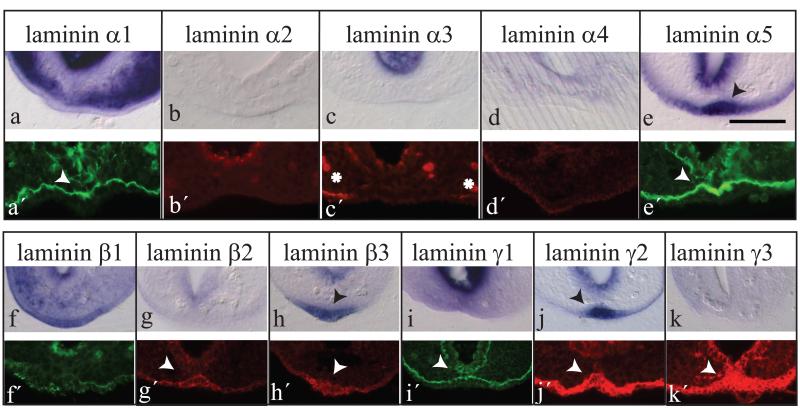
Among the α chains, laminins α1 and α5 were detectable at the protein level in the basement membrane underlying the VER (Figure 2a’, e’), while laminin α3 protein was present at the edges of the VER (Figure 2c’). Laminin proteins α2 and α4 were not detected in the vicinity of the VER (Figure 2b’, d’). Interestingly, the mRNAs for laminins α1 and α5 exhibit very different spatial distributions: while α1 mRNA is expressed solely in the mesoderm of the ventral tail bud (Figure 2a), α5 mRNA has an entirely complementary distribution, with expression in the VER and adjacent surface ectoderm, but not in the mesoderm (Figure 2e). Laminin α2, α3 and α4 mRNAs were not detectable in either mesenchyme or surface ectoderm (Figure 2b-d). Hence, laminins α1 and α5 are deposited in the VER basement membrane by different tissues: mesoderm and surface ectoderm/VER, respectively.
Figure 2. Expression of laminin chain mRNAs and proteins at the VER and surrounding tissues.
(a-k) Laminins α1-5, β1-3 and γ1-3 mRNA expression on vibratome sections following whole mount in situ hybridisation in individual mouse embryos at E9.5 (25-30 somites). (a′-k′) Protein immunolocalization for laminins α1-5, β1-3 and γ1-3 on cryosections of individual E9.5 mouse embryo tail-buds. Arrowheads point to specific labelling in the VER. Asterisks in (c′) indicate laminin α3 chain in the basement membrane of the surface ectoderm on either side of (but not overlying) the VER. Scale bar in (e) represents 50 μm (also applies to all other panels).
Considering the laminin β and γ chains: β2, β3, γ1, γ2 and γ3 proteins were all expressed throughout the basement membrane of the ventral tail bud, including the VER (Figure 2g′-k′). By contrast, the laminin β1 protein signal was discontinuous around the VER (Figure 2f′). At the mRNA level, laminins β3 and γ2 showed intense signal within the cells of the surface ectoderm, particularly the VER (Figure 2h,j), γ1 was expressed mainly in the mesoderm (Figure 2i), and β1 transcripts were present in both ectoderm and mesoderm (Figure 2f). mRNAs for laminins β2 and γ3 were not detectable (Figure 2g).
In our previous study of laminin secretion into mouse embryonic basement membranes (Copp et al., 2011), we could infer which of the many possible laminin heterotrimers were present from a comparison of the mRNA and protein distributions of the different chains. Lamin heterotrimers are assembled intracellularly prior to secretion (Yurchenco et al., 1997; Schneider et al., 2007; Tzu and Marinkovich, 2008) making it obligatory for the mRNAs of the constituent α, β and γ chains to be co-expressed. In the case of the tail-bud, the predominant laminin chain mRNAs in VER cells were α5, β3 and γ2, with possible minor contributions also from β1 and γ1 (Figure 2e,h,j). Previously, laminin β3 and γ2 have been described only in combination with α3, as components of laminin 5. Consequently, our results suggest the possibility of a novel laminin heterotrimer, α5β3γ2, expressed by the VER.
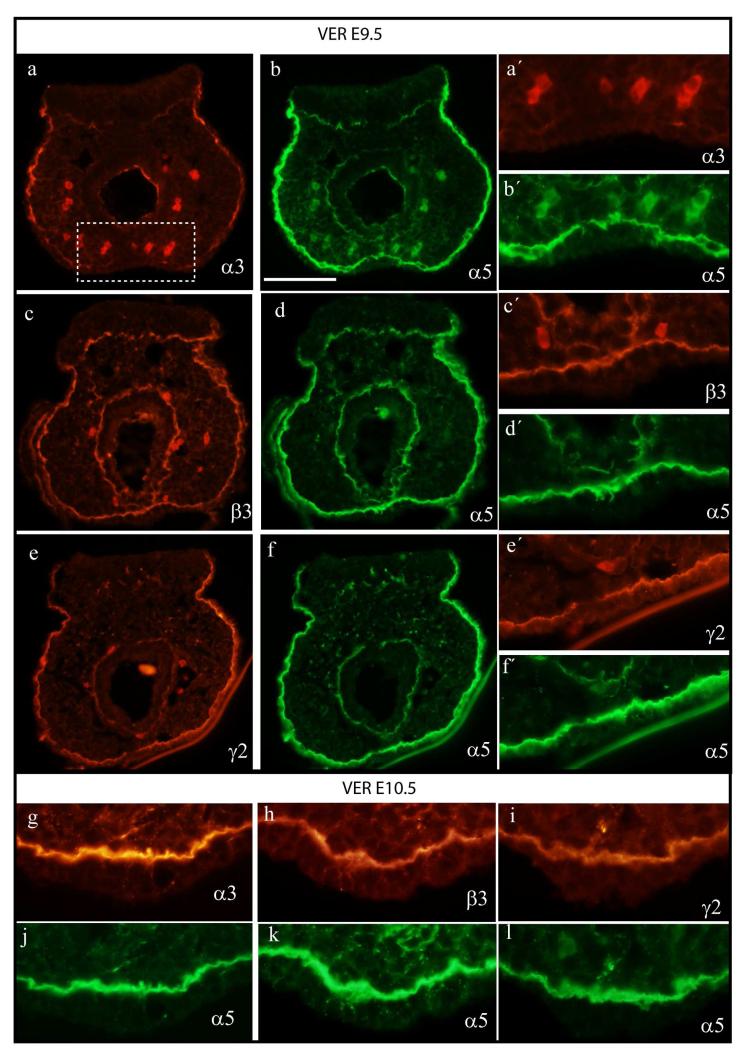
To further address the possible existence of a novel laminin isoform in the E9.5 mouse VER, we performed double immunohistochemistry for laminin α5 in combination with laminins α3, β3 or γ2. In the E9.5 tail-bud, the laminin α3 chain was clearly excluded from the basement membrane overlying the VER (Figure 3a,a’,b,b′). In contrast, robust co-labelling of α5 was observed with both laminin β3 (Figure 3c,c’,d,d’) and laminin γ2 (Figure 3e,e′,f,f′). Hence, taken together with the very specific expression of mRNAs for these lamin chains in VER cells, we conclude that α5, β3 and γ2 likely form a laminin532 heterotrimer in the basement membrane overlying the VER at E9.5.
Figure 3. Co-localization of laminin chains in the basement membrane of the VER.
Immunohistochemistry of laminin α5 together with α3 (a, a′, b, b′, g and j), β3 (c, c′, d, d′, h and k) and γ2 (e, e′, f, f′, i and l) on transverse sections of the tail-bud. Embryos were harvested at E9.5 (25-30 somites; a-f and a′-f′) and E10.5 (40-45 somites; g-l). VER region, as indicated by dotted rectangle in (a), is magnified in (a′-f′). FITC secondary antibody (green) was used for laminin α5, whereas biotin/streptavidin secondary antibody (red) was used for laminins α3, β3 and γ2. Scale bar in (b) represents 50 μm in (a-f), 20 μm in (a’-f’ and in g-l).
At E10.5, double immunostaining also demonstrated co-localisation of laminin α5 with β3 and γ2 throughout the entire surface ectoderm, including the VER (Figure 3h,i,k,l). By this stage, laminin α3 also co-localised with α5 in the surface ectoderm of the tail-bud (Figure 3g,j), raising the possibility that two different heterotrimers, α3β3γ2 and α5β3γ2, coexist at this later stage.
Expression of integrins
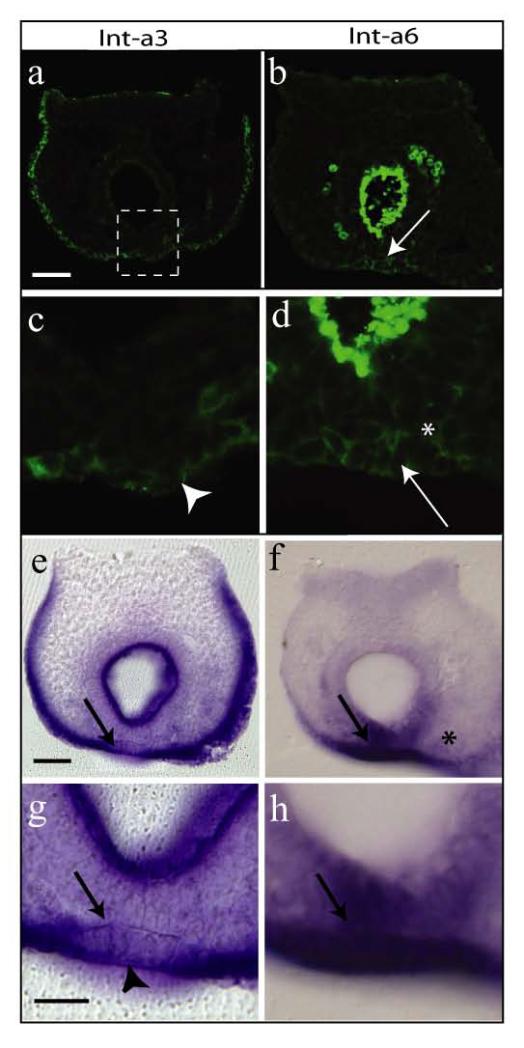
Many laminin variants interact with α6 integrins and, with the exception of α1-containing laminins, most also interact to varying degrees with α3 integrins (Barczyk et al., 2010). We therefore assessed the expression of α3 and α6 integrins by immunohistochemistry and in situ hybridization in the mouse embryonic tail bud at E9.5. While robust integrin α3 protein and mRNA expression were detected throughout most of the surface ectoderm, inmunostaining was detected solely on the outer facing surfaces of the VER cells (Figure 4a, c). Consistent with this, mRNA intensity for α3 integrin was markedly reduced in the VER compared with the rest of the surface ectoderm (Figure 4e, g). In contrast, integrin α6 mRNA expression appeared enhanced specifically in the VER and overlying mesenchyme, whereas it was largely absent from other regions of the surface ectoderm (Figure 4f, h). Immunostaining indicated the presence of α6 integrin protein on the entire surface (both inner and outer facing) surfaces of the VER cells (Figure 4b, d). Hence, the expression of integrins α3 and α6 appears complementary in the tail bud at this stage of development, with only α6 integrin being expressed on VER cell surfaces facing the basement membrane.
Figure 4. Expression of α3 and α6 integrins in the VER and surrounding tissues at E9.5.
Immunohistochemistry on cryosections (a-d) and mRNA expression on vibratome sections following whole mount in situ hybridisation (e-h) for α3 integrin (a,c,e,g) and α6 integrin (b,d,f,h). High magnification view of the boxed area in (a) is shown in (c) and similar areas are magnified from (b, e and f) to obtain panels (d, g and h) respectively. Arrows point to the VER region. Asterisks mark positive α6 signal in mesenchyme. Arrowheads point to positive α3 signal solely on the outer facing surfaces of the VER cells. Scale bar in (e) represents 50 μm (also in a, b, f) and scale bar in (g) represents 25 μm (also in c, d, h).
In the present study, we have found evidence of a putative new laminin variant (laminin532) expressed in the VER, a mid-ventral specialization of the surface ectoderm in the developing mouse tail-bud. Although final confirmation of this novel laminin would require biochemical characterisation, nevertheless, our findings suggest that the α5, β3 and γ2 laminin subunits are co-transcribed in VER cells and then co-deposited in the adjacent basement membrane. Furthermore, our observation that α6 integrin is expressed in the VER provides evidence for a spatially restricted interaction between cells of the VER and laminins within the basement membrane. Although the role of this putative novel laminin variant in the VER remains to be demonstrated, laminins and integrins are well known to be involved in regulation of cell differentiation, for example as demonstrated for laminin α5 in smooth muscle differentiation, and laminin β2 in neuromuscular differentiation (Noakes et al., 1995; Bolcato-Bellemin et al., 2003). We propose, therefore, that laminin532 could interact with α6-containing integrins to direct specialisation of the surface ectoderm during formation of the midline VER.
One of the characteristics of the VER is its involvement in the process of EMT, which is subject to regulation by BMP signalling at this stage of tail-bud development (Ohta et al., 2007). Given the well described role of laminins and integrins in cell migration (Tzu and Marinkovich, 2008), it is possible that laminin532 could act via integrin α6, in concert with BMP pathway activation, to regulate EMT. Indeed, the laminin γ2 chain is known to undergo proteolytic processing, thereby releasing a fragment (DIII) that binds to the epidermal growth factor receptor (EGFR) to trigger cell migration (Koshikawa et al., 2005). Although most of the work on proteolysis of laminin γ2 has been undertaken with laminin322, it is possible that similar processing could occur with the laminin532 variant. In order to address the role of laminin532 in regulating BMP signalling and EMT in the VER, it will be necessary to explore the effects of interfering directly with the laminin-integrin interactions in the developing tail-bud.
Experimental procedures
cDNA probes
First-strand cDNA was prepared using reverse transcriptase (Bioline) with total RNA extracted from E9.5 CD1 mouse embryos using TRIzol reagent (Invitrogen, Carlsbad, California.U.S.A.). A cDNA probe for integrin α3 was prepared using the primers5′ggtgatgactataccaaccg3′-5′gataaatcccagtccttccg3′, which amplify a 463-bp region located between base pairs 619 and 1082 (GenBank accession no BC053031.1). A cDNA probe for integrin α6 was prepared using the primers 5′ccaaggagattagcaatgg3′-5′atctctcgctcttctttccg3′, which amplify a 620-bp region located between base pairs 2692 and 3312 (GenBank accession no BC058095). PCR products were cloned into pGEMt (Promega, Fichburg, Wisconsin U.S.A.) and sequenced to confirm identity. cDNA probes for Bmp2, Noggin, Neuralin (Chrdl1), Cadherin6, Msx1, Msx2, RhoB, and laminins α1, α2, α3, α4, α5, β1, β2, β3, γ1, γ2 and γ3 are described elsewhere (Henderson et al., 2000; Ybot-Gonzalez et al., 2007; Copp et al., 2011).
In situ hybridisation
Whole mount in situ hybridisation was carried out using sense and antisense digoxygenin–labelled riboprobes prepared using a digoxigenin RNA labelling kit (Roche, Basel Switzerland)) according to the manufacturer’s instructions. E9.5 (25-30 somites) mouse embryos were washed twice in DEPC-treated PBS and fixed overnight in 4% paraformaldehyde. To reveal sites of mRNA expression, embryos were processed as described previously (Ybot-Gonzalez et al., 2005). Selected embryos, labelled with individual probes, were embedded in a gelatine-sucrose-albumin (1:1.5:60) solution, solidified by addition of 2.5% of glutaraldehyde. A vibratome was used to obtain 50 μm sections, which were mounted with 50% glycerol, and photographed with an Axiophot (Zeiss) photomicroscope. Sense-strand riboprobes used as a control for specificity gave no specific signal.
Immunohistochemistry
The method was as described previously (Copp et al., 2011), but with variations as follows. E9.5 (25-30 somites) and E10.5 (35-40 somites) mouse embryos were fixed overnight in either 4% paraformaldehyde in PBS at 4°C, for immunostaining with anti-integrin α6 or laminin α2 antibodies, or in zinc fixative (3 mM calcium acetate, 0.023 M zinc acetate, 0.036 M zinc chloride in 0.1 M Tris buffer pH 7.4) at room temperature and overnight, for anti-integrin α3 and all other anti-laminin antibodies. Prior to blocking, sections for anti-laminin staining were treated with 0.05% hyaluronidase in PBS for 2h at 37°C. Primary antibodies are shown in Table 1. Secondary antibodies were: goat anti-rat FITC conjugated (Abcam, Cambridge, UK; ab97056), goat anti-rat Biotin conjugated (Sigma, St louis, Missouri, USA; B7139), and goat anti-rabbit Biotin conjugated (Life Technologies, Carlsbad, California USA; 65-6140) all used at 1:250 dilution. Streptavidin was obtained from Sigma (S6402). Representative sections were selected from at least three serially sectioned embryos of each staining type, and photographed with an Olympus (Tokyo, Japan) BX-61 photomicroscope.
Table 1. Primary antibodies used in the immunohistochemistry studies.
| Target | Antibody details | Source | References |
|---|---|---|---|
| Integrin α3 | Anti-C terminus of chicken integrin α3, 1:100 dilution |
rabbit | DiPersio et al., 1995 |
| Integrin α6 | Anti-human integrin α6, 1:100 dilution |
rat | Abc-serotec, Cambridge, U.K. (MCA699) |
| Laminin α1 | Anti-mouse laminin α1, conditioned medium used undiluted |
rat | Sorokin et al, 1992 |
| Laminin α2 | Anti-mouse laminin α2, conditioned medium used undiluted |
rat | Schuler and Sorokin, 1995 |
| Laminin α3 | Anti-α3 IIIa antiserum, 1:500 dilution |
rabbit | Sasaki et al., 2001 |
| Laminin α4 | Anti-α4 LG1 antiserum, 1:500 dilution |
rabbit | Talts et al., 2000 |
| Laminin α5 | Anti-mouse laminin α5, conditioned medium used undiluted |
rat | Sorokin et al., 1997 |
| Laminin β1 | Anti-mouse laminin β1, conditioned medium used undiluted |
rat | Sixt et al., 2001 |
| Laminin β2 | Anti-mouse laminin β2, 1:500 dilution |
rabbit | Agrawal et al., 2006 |
| Laminin β3 | Anti-β3 VI/V antiserum, 1:500 dilution |
rabbit | Sasaki et al., 2001 |
| Laminin γ1 | Anti-γ1, 1:100 dilution |
rat | Chemicon, Billerica, Massachusetts, U.S.A. (MAB1914) |
| Laminin γ2 | Anti-γ2 antiserum, 1:500 dilution |
rabbit | Sasaki et al., 2001 |
| Laminin γ3 | Anti-γ3 III3-5 antiserum, 1:500 dilution |
rabbit | Gersdorff et al., 2005 |
Laminin α5, β3 and γ2 chains are co-expressed by cells of the ventral ectodermal ridge (VER) in the E9.5 mouse embryo.
A possible new laminin532 variant is expressed by VER cells.
Laminin 532 may interact with α6-containing integrins to mediate BMP signalling during development of the mouse tail bud.
Acknowledgements
The authors gratefully acknowledge Lydia Sorokin and Iain Patten for valuable comments during writing of the manuscript. We also thank Loren Valbuena and Pilar Jaraquemada for technical assistance. Brigid Hogan, Paul Sharpe and Eddy de Robertis provided cDNA probes, and Lydia Sorokin and Richard Hynes provided antibodies. This work was supported by de Instituto de Salud Carlos III project CP08/00111 and PS09/00050 (to PY-G), la Consejería de Salud de la Junta de Andalucia project PI-0438-2010 (to P Y-G) and the Wellcome Trust (to AJC).
References
- Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–452. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CW, Whitman M, Slack JM. The role of BMP signaling in outgrowth and patterning of the Xenopus tail bud. Dev Biol. 2001;238:303–314. doi: 10.1006/dbio.2001.0407. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–244. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Bolcato-Bellemin AL, Lefebvre O, Arnold C, Sorokin L, Miner JH, Kedinger M, Simon-Assmann P. Laminin alpha5 chain is required for intestinal smooth muscle development. Dev Biol. 2003;260:376–390. doi: 10.1016/s0012-1606(03)00254-9. [DOI] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Carvalho R, Wallace A, Sorokin L, Sasaki T, Greene ND, Ybot-Gonzalez P. Regional differences in the expression of laminin isoforms during mouse neural tube development. Matrix Biol. 2011;30:301–309. doi: 10.1016/j.matbio.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Shah S, Hynes RO. alpha 3A beta 1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. J Cell Sci. 1995;108(Pt 6):2321–2336. doi: 10.1242/jcs.108.6.2321. [DOI] [PubMed] [Google Scholar]
- Dolez M, Nicolas JF, Hirsinger E. Laminins, via heparan sulfate proteoglycans, participate in zebrafish myotome morphogenesis by modulating the pattern of Bmp responsiveness. Development. 2011;138:97–106. doi: 10.1242/dev.053975. [DOI] [PubMed] [Google Scholar]
- Fauny JD, Thisse B, Thisse C. The entire zebrafish blastula-gastrula margin acts as an organizer dependent on the ratio of Nodal to BMP activity. Development. 2009;136:3811–3819. doi: 10.1242/dev.039693. [DOI] [PubMed] [Google Scholar]
- Gersdorff N, Kohfeldt E, Sasaki T, Timpl R, Miosge N. Laminin gamma3 chain binds to nidogen and is located in murine basement membranes. J Biol Chem. 2005;280:22146–22153. doi: 10.1074/jbc.M501875200. [DOI] [PubMed] [Google Scholar]
- Goldman DC, Martin GR, Tam PP. Fate and function of the ventral ectodermal ridge during mouse tail development. Development. 2000;127:2113–2123. doi: 10.1242/dev.127.10.2113. [DOI] [PubMed] [Google Scholar]
- Gruneberg H. A ventral ectodermal ridge of the tail in mouse embryos. Nature. 1956;177:787–788. doi: 10.1038/177787b0. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Ybot-Gonzalez P, Copp AJ. RhoB is expressed in migrating neural crest and endocardial cushions of the developing mouse embryo. Mech Dev. 2000;95:211–214. doi: 10.1016/s0925-4773(00)00333-6. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995;129:521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen P, Thesleff I. Expression and function of FGFs-4, -8, and -9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev Dyn. 1998;211:256–268. doi: 10.1002/(SICI)1097-0177(199803)211:3<256::AID-AJA7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. J Biol Chem. 2005;280:88–93. doi: 10.1074/jbc.M411824200. [DOI] [PubMed] [Google Scholar]
- Lai CF, Cheng SL. Alphavbeta integrins play an essential role in BMP-2 induction of osteoblast differentiation. J Bone Miner Res. 2005;20:330–340. doi: 10.1359/JBMR.041013. [DOI] [PubMed] [Google Scholar]
- Marazzi G, Wang Y, Sassoon D. Msx2 is a transcriptional regulator in the BMP4-mediated programmed cell death pathway. Dev Biol. 1997;186:127–138. doi: 10.1006/dbio.1997.8576. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–317. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008;71:349–356. doi: 10.1002/jemt.20563. [DOI] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Ohta S, Suzuki K, Tachibana K, Tanaka H, Yamada G. Cessation of gastrulation is mediated by suppression of epithelial-mesenchymal transition at the ventral ectodermal ridge. Development. 2007;134:4315–4324. doi: 10.1242/dev.008151. [DOI] [PubMed] [Google Scholar]
- Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CC, Mulloy B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J. 2010;429:1–12. doi: 10.1042/BJ20100305. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Gohring W, Mann K, Brakebusch C, Yamada Y, Fassler R, Timpl R. Short arm region of laminin-5 gamma2 chain: structure, mechanism of processing and binding to heparin and proteins. J Mol Biol. 2001;314:751–763. doi: 10.1006/jmbi.2001.5176. [DOI] [PubMed] [Google Scholar]
- Schneider H, Muhle C, Pacho F. Biological function of laminin-5 and pathogenic impact of its deficiency. Eur J Cell Biol. 2007;86:701–717. doi: 10.1016/j.ejcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci. 1995;108(Pt 12):3795–3805. doi: 10.1242/jcs.108.12.3795. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D, Kalcheim C. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development. 1999;126:4749–4762. doi: 10.1242/dev.126.21.4749. [DOI] [PubMed] [Google Scholar]
- Sixt M, Hallmann R, Wendler O, Scharffetter-Kochanek K, Sorokin LM. Cell adhesion and migration properties of beta 2-integrin negative polymorphonuclear granulocytes on defined extracellular matrix molecules. Relevance for leukocyte extravasation. J Biol Chem. 2001;276:18878–18887. doi: 10.1074/jbc.M010898200. [DOI] [PubMed] [Google Scholar]
- Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the alpha 6 subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol. 1990;111:1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin LM, Conzelmann S, Ekblom P, Battaglia C, Aumailley M, Timpl R. Monoclonal antibodies against laminin A chain fragment E3 and their effects on binding to cells and proteoglycan and on kidney development. Exp Cell Res. 1992;201:137–144. doi: 10.1016/0014-4827(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Sorokin LM, Pausch F, Frieser M, Kroger S, Ohage E, Deutzmann R. Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol. 1997;189:285–300. doi: 10.1006/dbio.1997.8668. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ueno N, Hemmati-Brivanlou A. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development. 1997;124:3037–3044. doi: 10.1242/dev.124.16.3037. [DOI] [PubMed] [Google Scholar]
- Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, Timpl R. Structural and functional analysis of the recombinant G domain of the laminin alpha4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275:35192–35199. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- Tunggal P, Smyth N, Paulsson M, Ott MC. Laminins: structure and genetic regulation. Microsc Res Tech. 2000;51:214–227. doi: 10.1002/1097-0029(20001101)51:3<214::AID-JEMT2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol. 2008;40:199–214. doi: 10.1016/j.biocel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Menko AS. alpha6 Integrin is regulated with lens cell differentiation by linkage to the cytoskeleton and isoform switching. Dev Biol. 1999;210:497–511. doi: 10.1006/dbio.1999.9277. [DOI] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Copp AJ, Greene ND. Expression pattern of glypican-4 suggests multiple roles during mouse development. Dev Dyn. 2005;233:1013–1017. doi: 10.1002/dvdy.20383. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–3211. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Quan Y, Colognato H, Mathus T, Harrison D, Yamada Y, O’Rear JJ. The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci U S A. 1997;94:10189–10194. doi: 10.1073/pnas.94.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]