Abstract
Seizures remain uncontrolled in 30% of patients with epilepsy, even with concurrent use of multiple drugs, and uncontrolled seizures result in increased morbidity and mortality. An extreme example is Dravet syndrome (DS), an infantile-onset severe epilepsy caused by heterozygous loss of function mutations in SCN1A, the gene encoding the brain type-I voltage-gated sodium channel NaV1.1. Studies in Scn1a heterozygous knockout mice demonstrate reduced excitability of GABAergic interneurons, suggesting that enhancement of GABA signaling may improve seizure control and comorbidities. We studied the efficacy of two GABA-enhancing drugs, clonazepam and tiagabine, alone and in combination, against thermally evoked myoclonic and generalized tonic-clonic seizures. Clonazepam, a positive allosteric modulator of GABA-A receptors, protected against myoclonic and generalized tonic-clonic seizures. Tiagabine, a presynaptic GABA reuptake inhibitor, was protective against generalized tonic-clonic seizures but only minimally protective against myoclonic seizures and enhanced myoclonic seizure susceptibility at high doses. Combined therapy with clonazepam and tiagabine was synergistic against generalized tonic-clonic seizures but was additive against myoclonic seizures. Toxicity determined by rotorod testing was additive for combination therapy. The synergistic actions of clonazepam and tiagabine gave enhanced seizure protection and reduced toxicity, suggesting that combination therapy may be well tolerated and effective for seizures in DS.
Introduction
Epilepsy affects 50 million persons worldwide (Duncan et al., 2006; WHO, 2012). Seizures in 30% of patients are refractory to medical therapy (Kwan and Brodie, 2000) and require treatment with multiple drugs (Beghi et al., 2003; Peltola et al., 2008), although there is little guidance as to which combinations may be most beneficial. Dravet syndrome (DS) is caused by loss-of-function mutations in the SCN1A gene encoding brain voltage-gated sodium channel type-I, NaV1.1 (De Jonghe, 2011). DS is an unusually severe genetic epilepsy in which single-drug therapy is ineffective and combined therapy with four medications is common (Chiron and Dulac, 2011; Dravet, 2011). DS is characterized by initial seizure onset at 6–9 months of age, often precipitated by elevated body temperature (Dravet et al., 2005; Oguni et al., 2005). Associated comorbidities include sleep disturbance, autistic features, and cognitive, memory, and motor impairments (Dravet, 2011). Lack of seizure control is correlated with reduced quality of life, increased risk of injury, and premature death.
Because of the small numbers of affected patients, variable phenotypic severity, and multiple concurrent medications, clinical trials of novel therapies for DS are very challenging and studies to identify beneficial drug combinations are precluded by the inability to precisely adjust drug doses. Only a single placebo-controlled trial has been performed to date (Chiron et al., 2000). Improved but incomplete seizure control has been reported with bromides (Tanabe et al., 2008), valproate (Rantala et al., 1997), topiramate (Nieto-Barrera et al., 2000; Coppola et al., 2002), stiripentol (Perez et al., 1999; Chiron et al., 2000; Inoue et al., 2009), and clobazam (Chiron et al., 2000; Thanh et al., 2002; Chiron and Dulac, 2011) in various combinations, whereas the sodium channel blockers lamotrigine and carbamazepine exacerbate seizures (Guerrini et al., 1998; Thanh et al., 2002) in a majority of patients.
Mouse genetic models of DS are remarkably faithful phenocopies of the human disease (Yu et al., 2006; Kalume et al., 2007; Ogiwara et al., 2007; Oakley et al., 2009, 2011). Seizures occur spontaneously (Yu et al., 2006; Oakley et al., 2009) and, as in patients with DS (Oguni et al., 2005), are reliably provoked by elevated body temperature (Oakley et al., 2009), providing an efficient yet clinically and physiologically relevant assay of antiepileptic drug efficacy. Mice with DS have selective loss of Na currents in GABAergic inhibitory neurons, resulting in reduced excitability and GABAergic neurotransmission (Yu et al., 2006; Kalume et al., 2007; Han et al., 2012a). Mice with heterozygous deletion of NaV1.1 only in forebrain interneurons have similar spontaneous and thermally evoked seizures and premature death (Cheah et al., 2012), supporting the hypothesis that disinhibition caused by reduced GABA signaling is the cause of this disease. These findings suggest that GABA-enhancing medications will be effective against seizures.
We studied two GABA-enhancing antiepileptic medications that act by complementary mechanisms, clonazepam (CLN) and tiagabine (TGB). CLN, a classic 1,4 benzodiazepine, is a positive allosteric modulator of the GABA-A receptor, which increases both affinity and efficacy of GABA in activating the receptor and requires concurrent binding of GABA for its actions (Macdonald, 2002). TGB is a potent and highly specific blocker of GABA reuptake into neurons (Braestrup et al., 1990; Schachter, 1999) and glia (Fraser et al., 1999) and is highly selective for GABA transporter 1 (Dalby, 2003), the predominant transporter in the forebrain (Sommerville, 2002). In DS, reduced interneuron excitability would result in decreased GABA release and less binding of GABA to its postsynaptic receptor. We hypothesized that TGB would act synergistically with CLN by increasing the concentration of GABA in the synaptic cleft during neurotransmission and, thereby, allowing the GABA-enhancing actions of CLN to be more fully engaged. We found that CLN alone provided substantial protection against thermally evoked myoclonic (MC) and generalized tonic-clonic (GTC) seizures in DS mice but was sedating at therapeutic doses, whereas TGB alone was protective against GTC seizures but was only minimally protective against MC seizures and caused increased MC seizures at high doses. Combined therapy with CLN and TGB provided synergistic protection against MC and GTC seizures, was less sedating than CLN, and did not increase MC seizure activity. These results suggest that synergistic combined therapy with CLN and TGB may enhance seizure control and reduce toxicity in DS.
Materials and Methods
All experiments were performed in accordance with animal protocols approved by the Institutional Animal Care and Use Committee of the University of Washington and in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the US National Institutes of Health.
Mouse Model of DS.
A mouse model of DS was created as previously described (Yu et al., 2006). In brief, mutant mice were generated by targeted deletion of the last exon encoding domain IV from S3 to S6 segment and the entire C-terminal tail of the NaV1.1 channel. Heterozygous mutant animals, Nav1.1 (+/−), were our DS mice disease model. Mutant mice were maintained on a C57Bl/6 background (Jackson Laboratories, Bar Harbor, ME) by crossing NaV1.1 (+/−) males with NaV1.1 (+/+) females. Genotype was confirmed using 4-oligonucleotide multiplex polymerase chain reaction of genomic DNA from tail samples as previously described (Yu et al., 2006). Male and female mice aged 27–33 days, the previously identified age of greatest seizure susceptibility (Oakley et al., 2009), were used in these studies.
Anti-Epileptic Medications.
Suspensions of CLN (Teva Pharmaceuticals, North Wales, PA) and Tiagabine (Cephalon/Teva Pharmaceuticals) were made daily in 0.5% (wt/vol) methylcellulose with concentrations of drug in suspension varied such that the injected volume for the specified dose was 0.01 ml/g mouse weight. Drugs were injected intraperitoneally 30 minutes before testing, the time to peak effect in previous studies (Dalby and Nielsen, 1997; Luszczki et al., 2006).
Thermal Induction of Seizures.
Thermal induction of seizures was performed in accordance with our previously described protocol (Oakley et al., 2009). Body temperature was measured continuously with a rectal temperature probe and controlled to ±0.3°C with a feedback temperature controller (TCAT2DF; Physitemp, Clifton, NJ) and heat lamp. Mouse body temperature was held at 37.0°C for 10 minutes and then slowly elevated by 0.5°C every 2 minutes until a generalized tonic-clonic seizure occurred or body temperature of 42.5°C was reached. Prior work combining video- electroencephalogram (EEG) recording with thermal induction in DM demonstrated that all seizures recorded by EEG had clear behavioral correlates (Oakley et al., 2009). However, implantation of EEG electrodes is invasive and may affect the type, frequency, or severity of seizures. Thus, in this study, we used behavior as an indicator of seizures. In each mouse, the time and body temperature of each MC and GTC seizure were determined from review of video.
Measurement of Motor Impairment with Rotorod.
Mice were placed on an accelerating, rotating rod (roto-rod series 8; IITC Life Science, Woodland Hills, CA), which began at 1 rpm and increased linearly to 40 rpm over 300 seconds (Mandillo et al., 2008). The rotational velocity at fall from rod was recorded. After administration of drug and before thermal induction, each mouse was tested on rotorod with 2 trials that were 5 minutes apart. Drug toxicity was associated with reduced rotational velocity at time of fall. Observed velocity was normalized to mean velocity of untreated controls and expressed as percentage reduction in motor performance.
Analysis of Single Drug Treatments.
Experimentally determined dose-effect pairs were fit with a Hill equation (IGOR; Wavemetrics, Portland, OR) using the Levenberg-Marquardt algorithm to search for coefficient values that minimize χ2. 95% confidence bands (CBs) were created using estimated standard error and Student’s t function for the degrees of freedom, assuming that error is normally distributed with zero mean; 95% CBs obtained in this way are approximations of the error incorporating only the linear term of the Taylor expansion used for fitting. A better estimate of variance over the linear portion of the sigmoidal function is obtained by linear fit of effect versus log dose. Variances provided by linear fits of log dose were nearly equivalent to (typically less than) the Hill fit approximations over the linear range of the sigmoidal function, and thus, variance from Hill fits was used in subsequent calculations.
Isobolographic Analysis of Combined Treatments.
Isobolographic analysis is a well-validated approach to study pharmacodynamic interactions (Berenbaum, 1989; Greco et al., 1995; Stafstrom, 2010; Tallarida, 2012). Dose-effect relationships of individual drugs are used to determine equally effective drug combinations, assuming dose additivity. Experimentally determined dose pairs were compared with additive predictions to identify antagonism, additivity, or synergy. Most forms of isobolographic analysis assume constant relative potency of individual drugs (Grabovsky and Tallarida, 2004), a condition that was not met by the drugs used in this study because of differing peak efficacies and slopes of linear log-dose relationships. Thus, we used a modification of the isobolographic technique for nonconstant relative potency on the basis of the method of Grabovsky and Tallarida (Grabovsky and Tallarida, 2004).
An effect level was chosen for analysis, and the dose of the more effective drug CLN (B) required to achieve this effect in monotherapy was determined from its dose-effect relationship. Then, for a given dose of the less effective drug TGB (a), an equi-effective dose of CLN (b′) was determined using the dose-effect relationship of the individual drugs from Hill fits. The additional dose of CLN (b) required to provide the effect level specified was then determined on the basis of the equation of dose additivity: B = b + b′. The solution of this equation over a range of TGB doses is the set of CLN:TGB dose pairs (the isobole) predicted to provide the chosen effect level assuming dose additivity. The variance of the predicted combined dose was determined from the individual drug variance at the specified effect level in proportion to the square of the fractional dose of the drug in the combination (Tallarida, 2000); that is, the dose of the drug in the combination divided by the dose required for the specified affect if used alone.
Predicted additive dose pairs from isobolographic analysis were compared with experimentally determined (observed) dose pairs giving the specified effect level. To study the effect of dose proportions on drug interaction, three fixed-proportion dose ratios, approximately 2:1, 1:1, and 1:4 (CLN:TGB), were studied. Fixed-proportion ratios were based on the dose of the individual drugs required to provide GTC protection to 41.0°C (0.58 mg/kg and 2 mg/kg for CLN and TB, respectively). The proportion by weight of each drug in the combination was: 2:1, 0.39 CLN and 0.61 TGB; 1:1, 0.19 CLN and 0.81 TGB; and 1:4, 0.07 CLN and 0.93 TGB.
The combined dose of a fixed-proportion combination is equal to the sum of the doses of the individual drugs in the mixture and can be considered to act like a single drug for purposes of dose-effect analysis (Tallarida, 2000). For each fixed-proportion ratio, body temperature at seizure onset was determined over a range of doses and fit with Hill function. The resulting observed dose-effect relationship and variance were compared with additive predictions from the isobole. Statistically significant variation between predicted and experimental data were determined by nonoverlapping 95% CBs at a specified effect level.
Interaction index (Tallarida, 2002), the proportion of the predicted additive dose required for the specified effect level, provides a quantitative measure of drug interaction and is given by:
| (1) |
Determination of Additive Dose-Effect Relationship.
Predicted additive dose-effect curves were generated from individual isoboles determined at multiple effect levels within the range of interest. The isobole was used to determine the additive dose and variance for each fixed-proportion ratio at each effect level. The resulting additive relationship derived from multiple dose-effect pairs was then compared with experimentally derived data. Statistically significant differences between additive predictions and experimental data were determined by nonoverlapping 95% CBs.
Results
Thermally Induced Seizures in a Mouse Model of DS.
The first seizure in a child with DS often occurs with elevated body temperature, such as during a fever or hot bath (Oguni et al., 2005). These seizures can be prolonged and are sometimes associated with a step-wise decline in function (Dravet et al., 2005). Our DS mice have a similar age- and temperature-dependent seizure susceptibility (Oakley et al., 2009). Developmental susceptibility to febrile seizures correlates with spontaneous seizure frequency in both humans (Oguni et al., 2005) and DS mice (Oakley et al., 2009), suggesting that thermal seizure sensitivity is a good predictor of overall seizure susceptibility. Therefore, in this study, antiepileptic efficacy was assayed using thermally evoked seizures. To mimic a typical fever curve, body temperature was elevated by 0.5°C every 2 minutes from a baseline temperature of 37.0°C (Oakley et al., 2009). Multiple MC seizures, consisting of brief jerk-like axial and appendicular movements, typically preceded a GTC seizure characterized by initial rearing and rhythmic clonic flexion and extension of upper extremities. As in human DS (Oguni et al., 2005), there was a clear transition to frequent, irregular MC seizures, which evolved into a GTC seizure with increasing body temperature in all untreated DS mice (Fig. 1A).
Fig. 1.
GABA-enhancing medications CLN and TGB protect against febrile (thermally induced) MC and GTC seizures. (A) Representative thermal induction experiment in an untreated DS mouse. At time 0, temperature control set point was increased to 38.0°C, and recorded body temperature (gray line) began to increase with a short latency. As body temperature increased, MC seizures began to occur with regularity (vertical hash marks, mean temperature, 38.1 ± 0.2°C; n = 17), culminating in GTC (solid black line, boxes are beginning and end of seizure, mean temperature, 38.5 ± 0.2°C; n = 17). (B and C) As body temperature was elevated, DS mice progressively experienced MC (C) and GTC (B) seizures. CLN (red) shifts seizure occurrence to higher temperatures, compared with controls (black), whereas TGB (blue) is similarly protective against GTC but has little effect on MC. Doses of CLN and TGB providing maximal protection against GTC are shown (25 mg/kg and 10 mg/kg, respectively) (D and E) The dose dependence of CLN (red) and TGB (blue) protection against thermally induced GTC (D) and MC (C). Body temperature at onset of seizure is plotted against dose (dots) and fit with Hill function (± 95% CB). The maximal protection against GTC and half maximal effective doses were 42.2 ± 0.3°C and 0.17 ± 0.07 mg/kg for CLN and 41.3 ± 0.2°C and 0.40 ± 0.08 mg/kg for TGB, respectively. The maximal protection against MC and half maximal effective doses were 41.3 ± 0.2°C and 0.09 ± 0.02 mg/kg for CLN and 38.9 ± 0.2°C and 0.14 ± 0.1 mg/kg for TGB, respectively. The mean MC temperature for TGB (40 mg/kg) was significantly lower than than that for control (box marker, 37.4 ± 0.4°C versus 38.1 ± 0.2°C, P = 0.04). TGB (40 mg/kg) was excluded from the Hill fit in (E). For (B and C), CLN: n = 42, 4–8 mice per dose; TGB: n = 44, 4–10 mice per dose.
GABA-Enhancing Therapy Provides Protection against Thermally Induced Seizures.
Because reduction of sodium currents in GABAergic interneurons causes DS in mice (Yu et al., 2006; Kalume et al., 2007; Cheah et al., 2012; Han et al., 2012a), we hypothesized that CLN and TGB, two well characterized, clinically available medications that enhance GABA neurotransmission through complementary mechanisms, would be effective in seizure control. We measured the ability of each drug individually to increase the temperature required for induction of seizures. At the beginning of each experiment, all mice were seizure-free (100%; Fig. 1, B and C). As temperature was increased in the absence of drug, mice progressively experienced seizures, and eventually, no mice remained seizure-free (0%; Fig. 1, B and C). The temperature at which animals have seizures provides a quantitative estimate of seizure susceptibility (Fig. 1, B and C). Increasing doses of CLN (Fig. 1, B and C, red) or TGB (Fig. 1, B and C, blue) shift the thermal induction curves to higher temperatures.
We plotted body temperature at seizure versus dose to generate dose-response relationships for prevention of seizures (Fig. 1, D and E). Both CLN and TGB provided significant protection against GTC seizures, but only CLN provided significant protection against MC seizures (Fig. 1, D and E; Tables 1 and 2). Mean body core temperature at GTC seizure increased with CLN up to 25 mg/kg, providing protection up to 42.2 ± 0.3°C (Fig. 1D). TGB protected against GTC seizures nearly as well, providing protection up 41.3 ± 0.2°C at doses above 10 mg/kg (Fig. 1D). CLN also provided protection against MC seizures to a maximal temperature of 41.3 ± 0.2°C at doses above 2.5 mg/kg (Fig. 1E). In contrast, TGB was only minimally effective against MC protecting to a maximal to temperature of 38.9 ± 0.2°C at doses of 1–10 mg/kg (Fig. 1E). Moreover, TGB was pro-myoclonic at 40 mg/kg, lowering the body temperature at onset of MC below that of control (Fig. 1E; marker, P = 0.04), even though it retained efficacy against GTC seizures at that dose (Fig. 1D). Remarkably, these results reveal similar efficacy of CLN and TGB in prevention of GTC seizures but striking differential efficacy in preventing MC seizures.
TABLE 1.
Summary of doses for protection against GTC to 41.0°C from isobolographic analysis
| Clonazepam:Tiagabine (Ratio) |
Clonazepam | Tiagabine | Interaction index |
|---|---|---|---|
| Dose mg/kg (95% confidence interval) | Observed/Predicted (95% confidence interval) | ||
| Observed: Alone | 0.55 (0.30–1.1) | 2.0 (1.0–6.8) | |
| 2:1 | 0.22 (0.14–0.32) | 0.33 (0.22–0.50) | 0.54 (0.34–0.82) |
| 1:1 | 0.11 (0.091–0.14) | 0.46 (0.38–0.60) | 0.44 (0.25–0.76) |
| 1:4 | 0.063 (0.045–0.084) | 0.85 (0.60–1.1) | 0.57 (0.24–0.96) |
| Predicted | |||
| 2:1 | 0.40 (0.35–0.48) | 0.62 (0.55–0.74) | |
| 1:1 | 0.25 (0.23–0.29) | 1.0 (0.97–1.2) | |
| 1:4 | 0.11 (0.098–0.14) | 1.5 (1.3–1.9) | |
TABLE 2.
Summary of doses for protection against MC to 40.0°C from isobolographic analysis
| Clonazepam:Tiagabine (Ratio) |
Clonazepam | Tiagabine | Interaction index |
|---|---|---|---|
| Dose mg/kg (95% confidence interval) | Observed/Predicted (95% confidence interval) | ||
| Observed: Alone | 0.55 (0.30–1.1) | 2.0 (1.0–6.8) | |
| 2:1 | 0.14 (0.10–0.21) | 0.22 (0.15–0.33) | 1.7 (1.0–2.6) |
| 1:1 | 0.062 (0.044–0.079) | 0.26 (0.18–0.33) | 0.86 (0.58–1.2) |
| 1:4 | 0.046 (0.027–0.078) | 0.62 (0.36–1.04) | 0.69 (0.34–1.2) |
| Predicted | |||
| 2:1 | 0.086 (0.065–0.11) | 0.13 (0.10–0.17) | |
| 1:1 | 0.073 (0.054–0.095) | 0.30 (0.27–0.40) | |
| 1:4 | 0.067 (0.048–0.09) | 0.9 (0.64–1.2) | |
Combination Drug Therapy Is Synergistic against Thermally Induced GTC Seizures.
Isobolographic analysis (Tallarida, 2012) was used to study pharmacodynamic interactions of CLN and TGB and to determine how these interactions varied with drug proportion and effect level. To determine the influence of drug proportion, three fixed proportions of CLN:TGB (2:1, 1:1, and 1:4) were studied at an effect level of 41.0°C, because this is the highest temperature expected in a febrile child. Drug proportions were based on the individual drug dose required to protect against GTC to 41.0°C.
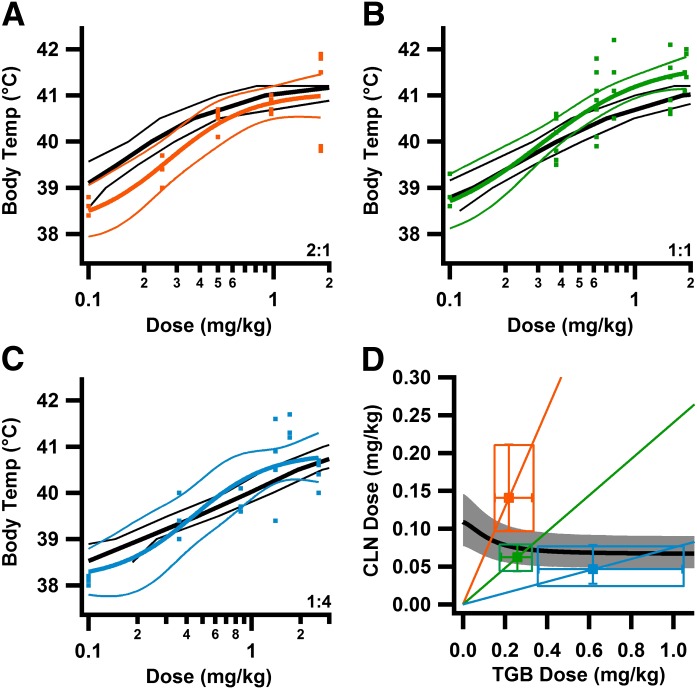
Body core temperatures at GTC were plotted against effective dose for fixed proportion drug ratios of 2:1 (Fig. 2A), 1:1 (Fig. 2B), and 1:4 (Fig. 2C) to determine the dose required to protect against GTC to 41.0°C. With use of the individual dose-effect relations of CLN and TGB, a set of equi-effective dose pairs (the isobole) was determined, assuming dose additivity (Fig. 2D). These predicted doses for additivity were compared with experimentally determined (observed) doses. Observed doses less than predicted demonstrate synergy, observed doses equal to predicted demonstrate additivity, and observed doses greater than predicted demonstrate antagonism. Observed doses were lower than predicted at all three dose ratios (Fig. 2D).
Fig. 2.
Combined treatment with CLN and TGB provides supra-additive (synergistic) protection against GTC seizures. (A–C) Synergism is seen at higher effect levels. Additive predictions based on isobolographic analysis (as in D) for temperatures between 38.5 and 41.0°C in 0.5°C increments (black lines) are compared with observed results (colored lines) over the range of doses tested at 2:1 (A), 1:1 (B), and 1:4 (D) dose ratios. As dose increased, observed protection against GTC was greater than additive prediction, becoming significant at 1.0 mg/kg, > 41.0°C for 2:1; 0.53 mg/kg, > 40.6°C for 1:1; and 1.2 mg/kg, > 40.9°C for 1:4 ratios (nonoverlapping 95% CB). (D) Synergy depends on dose ratio. Isobole plot of GTC protection at 41.0°C. Black line is the isobole, gray area 95% CB; Radial lines are fixed-proportion CLN:TGB ratios, 2:1 orange, 1:1 green, and 1:4 light blue. For each dose ratio, the predicted additive dose is given by the intersection of the fixed proportion radial line and the isobole. Markers are the observed dose pairs required for 41.0°C protection ± 95% confidence interval for each drug. Observed doses are significantly less than additive predictions, demonstrating dose supra-additivity (Table 1). For observed dose-effect relationship (A–C): 2:1 ratio: n = 20, 4–8 mice/dose; 1:1 ratio: n = 35, 4–10 mice/dose; 1:4 ratio: n = 23, 3–4 mice/dose. Colored lines are Hill fits ± 95% CB.
To determine how drug interactions varied by effect level, predicted additive dose pairs determined from isobolographic analysis at effect levels of 39–41.5°C were used to create an additive dose-effect relationship for each fixed proportion (Fig. 2, A–C). Experimentally observed results (Fig. 2, A–C) were compared with the predictions for additivity. For each fixed-proportion treatment, the slope of the observed dose-response relationship was greater than the line of additivity (Fig. 2, A–C). At low effective doses, the curve for each fixed proportion was near or below the line of additivity (Fig. 2, A–C). On the other hand, at higher doses, the curves for all three fixed-proportion treatments were significantly greater than the line of additivity, as judged by comparison with 95% confidence limits (Fig. 2, A–C). These curves suggest significant synergy for each fixed-proportion drug ratio given at a high effective drug dose.
To quantify the degree of synergy, we determined the interaction index for protection against GTC to 41.0°C (Eq. 1; Table 1; Fig. 2D). The interaction index was most favorable for the 1:1 ratio and was significantly lower than the line of additivity, as judged by 95% confidence limits (Fig. 2D). Equi-effective fixed-proportion doses were approximately 2.5-fold lower than predicted for additivity. The interaction indices for 2:1 or 1:4 fixed proportions also indicate synergy. At each of these fixed proportions, synergy reduced the total amount of drug required by approximately 2-fold. These results demonstrate substantial synergy in prevention of GTC seizures by CLN and TGB.
Combination Drug Therapy Is Additive against MC Seizures.
Isobolographic analysis was also used to determine drug interactions against MC seizures. The influence of fixed-proportion treatment on synergy of drug interaction was studied at 40.0°C (Fig. 3D) rather than 41.0°C, because MC seizures begin at lower body temperatures than do GTC seizures, and this level of protection was provided by all combinations tested. Dose-response curves for these fixed-proportion treatments all followed the line of additivity closely and did not provide evidence for synergistic interactions (Fig. 3, A–C). Similarly, the interaction indices also indicated additivity for treatment at 2:1, 1:1, and 1:4 fixed proportions (Fig. 3D; Table 2).
Fig. 3.
Combined treatment with CLN and TGB provides additive protection against MC seizures. (A–C) Additive dose-effect relationships from isobolographic analysis, compared with observed data for MC seizures as in Fig. 2. Additive predictions and experimental data are not different across the range of doses tested. Orange 2:1 ratio, green 1:1 ratio, light blue 1:4 ratio. (D) Isobole plot of MC seizure protection at 40.0°C as in Fig. 2D. Experimentally determined doses are not different than additive predictions (Table 2). For observed dose-effect relationship (A–C): n and mice/dose as in Fig. 2.
Tiagabine Increases MC Seizures before GTC Seizures.
Tiagabine has been associated with MC seizures or absence status epilepticus in rodent models of generalized epilepsy (Coenen et al., 1995; Hosford and Wang, 1997; Lancel et al., 1998), in individuals with epilepsy (Eckardt and Steinhoff, 1998; Ettinger et al., 1999; Mangano et al., 2003; Skardoutsou et al., 2003; Koepp et al., 2005), and in nonepileptic individuals in overdose (Zhu and Vaughn, 2002; Leikin et al., 2008). In DS mice, we observed a substantial, dose-dependent increase in the number of MC seizures before the first GTC seizure with TGB (Fig. 4A). The mean number of MC seizures occurring before the first GTC seizure increased with TGB treatment from 21 ± 5 in control mice to 125 ± 29 at 0.6 mg/kg, 300 ± 29 at 10 mg/kg, and 450 ± 83 at 40 mg/kg. In contrast, equally protective doses of CLN resulted in 17 ± 7 MC seizures before the first GTC seizure, which was not different from control (Fig. 4A; P = 0.37). Remarkably, equally effective treatment with the 1:1 fixed proportion resulted in only 11 ± 4 MC seizures before the first GTC seizure, not different than control (Fig. 4A; P = 0.14).
Fig. 4.
Increased number of MC before GTC occurs with TGB but not CLN or combined treatment because of increased duration and peak rate of MC before GTC. (A) TGB dose-dependently increases number of MC before GTC. The mean number of MC before GTC (± S.E.M.) is shown. (B) TGB increases duration and peak rate of MC. Mean MC rate leading up to GTC (time 0) for equally effective doses of CLN, TGB, and 1:1 ratio (red CLN 2.5 mg/kg; blue TGB 10 mg/kg; green 1:1 ratio, 0.6 mg/kg; control black). MC seizures began earliest (−700 seconds) and reached the highest rate (0.9 MC/s) with TGB treatment. Onset relative to GTC and maximal rate of MC was not different among control (−300 seconds, 0.1 MC/s), CLN (−300 seconds, 0.19 MC/s), and 1:1 ratio (−300 seconds, 0.15 MC/s). (C) Increased duration of MC activity with TGB treatment is associated with a greater difference in temperature between MC and GTC. Number of MC seizures in each 0.1°C temperature bin before GTC at the maximally effective dose of TGB (10 mg/kg) and doses of CLN (2.5 mg/kg) and 1:1 ratio (0.6 mg/kg) providing equal protection against GTC. MC begins at slightly higher temperatures than does control with TGB (inset), and the number of MC increases substantially with temperature. MC does not increase until high temperatures with CLN or 1:1 ratio. Mean temperatures (± S.E.M.) at MC and GTC are as follows: control, 38.1 ± 0.2°C and 38.5 ± 0.2°C; CLN, 41.0 ± 0.2°C and 41.4 ± 0.1°C; 1:1 ratio, 41.0 ± 0.2°C and 41.1 ± 0.3°C; and TGB, 38.9 ± 0.3°C and 41.2 ± 0.2°C. In B and C, CLN, red, n = 8; 1:1 ratio, green, n = 10; TGB, blue, n = 10.
Increased numbers of MC seizures could result from a greater period of high-frequency MC seizures before the first GTC seizure or from an increased rate of MC seizures over a similar time frame. To assess these two possibilities, we determined the rate of MC seizures at times before the first GTC seizure by comparing the maximally effective dose of TGB, 10 mg/kg, with untreated control mice. There was a longer period during which frequent MC seizures occurred in TGB-treated DS mice, with MC seizures beginning 700 seconds before the GTC seizure, compared with 300 seconds in controls (Fig. 4B). A higher peak rate of occurrence of MC seizures was also observed: 0.9 MC/s with TGB versus 0.1 MC/s in controls (Fig. 4B). A similar increase in MC seizure activity was not seen with equally effective doses of CLN (2.5 mg/kg) or the 1:1 fixed proportion (0.6 mg/kg), which resulted in a duration and peak rate of MC seizure activity not different than in controls (Fig. 4B). Despite the frequent MC activity, DS mice continued to explore the environment between seizures, suggesting that they retained consciousness. Although frequent, MC seizures were irregular and were behaviorally different from GTC seizures, which consisted of rearing and higher frequency forelimb clonus, followed by running. This degree of MC seizure activity would not be tolerable in humans and likely would be described as status epilepticus.
To determine how the frequency and time course of TGB-induced MC seizures varied with temperature, the seizure-provoking stimulus, we determined the number of MC seizures occurring at each temperature before the first GTC seizure in 0.1°C increments for 10 mg/kg TGB and controls (Fig. 4C). MC seizures began at higher temperatures in TGB-treated mice than in controls, again demonstrating a small protective effect (Fig. 4C). Because TGB was more effective against GTC seizures than MC seizures, there was a significantly greater difference in temperature of onset between MC and GTC seizures in TGB-treated DS mice, compared with controls (2.5 ± 0.4°C versus 0.4 ± 0.1°C). Treatment with a higher dose of TGB, 40 mg/kg, was pro-epileptic, lowering the temperature at onset of MC below that of controls (37.4 ± 0.4°C versus 38.1 ± 0.2°C; P = 0.04; Fig. 1C) although protection against GTC seizures remained maximal (Fig. 1B), resulting in an even larger temperature window for MC seizures before the first GTC seizure (4.0 ± 0.5°C). In contrast to these results with TGB alone, the temperature difference between MC and GTC seizures in CLN and 1:1 fixed-proportion treatment in DS mice was not different than that in controls.
Motor Impairment Is Additive with Combined Drug Therapy.
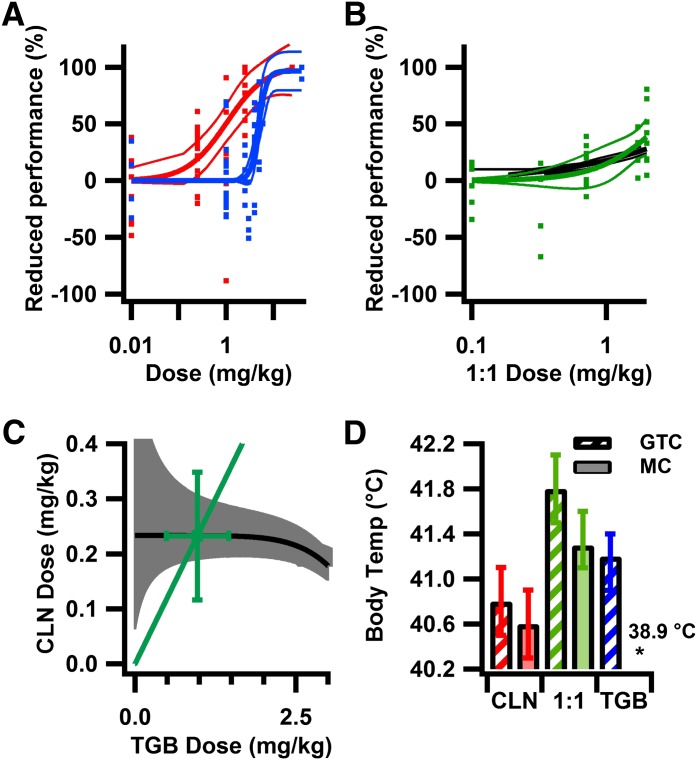
Drug-related motor impairment in DS mice was determined by rotorod performance, a test of the ability to walk on an accelerating, rotating rod. Only the 1:1 fixed-proportion drug ratio was tested, because this treatment resulted in the best combination of protection against MC and GTC seizures. Increasing doses of CLN and TGB resulted in progressive impairment of rotorod performance (Fig. 5A). The 1:1 fixed-proportion treatment reduced motor performance beginning at 1 mg/kg, with up to 40 ± 18% reduction in performance seen at the highest dose tested (Fig. 5B). An example isobole is shown for a 20% reduction in motor performance, because this level was achieved by all drug treatments and likely reflects a noticeable level of toxicity in humans. The 1:1 fixed-proportion drug treatment (Fig. 5C) was not different than the additive prediction from isobolographic analysis (Fig. 5C; Table 3). To study drug interaction across the range of combined doses tested, additive predictions were determined from isobolographic analysis (Fig. 5B) and compared with observed results (Fig. 5B). Toxicity was additive throughout the range of doses tested (Fig. 5B).
Fig. 5.
Combined treatment at the 1:1 fixed-proportion ratio is additive for motor toxicity and provides greater seizure protection at equally toxic doses. (A) CLN reduces motor performance at lower doses than does TGB. Performance on rotorod expressed as percentage reduction in motor performance, compared with controls, as a function of dose. Half maximal toxic dose (95% confidence interval): CLN, 0.72 mg/kg (0.43–1.25 mg/kg); and TGB, 5.41 mg/kg (4.71–6.95 mg/kg). (B) Predicted additive dose-effect relationship from isobole analysis (as in C) for reduction in performance of 5–30% in 5% increments (black line, ± 95% CB), compared with observed data at the 1:1 ratio (dots). The additive prediction is not different than observed across the range of toxicities tested. (C) Isobole for 20% reduction in motor performance (black line, ± 95% CB gray area). The observed dose (marker) is not different than additive prediction (nonoverlapping 95% CB). (D) Combined drug therapy results in greater efficacy at equally toxic doses. The predicted mean body temperature (± 95% CI) at MC (solid) and GTC (striped) seizure at doses resulting in 20% reduction in motor performance from A and B (CLN, 0.16 mg/kg; TGB, 4.3 mg/kg; and 1:1 ratio, 1.5 mg/kg). Predicted body temperature at MC: CLN, 40.4°C (40.1–40.8°C); TGB, 38.9°C (38.6–39.3°C); and 1:1 ratio, 41.4°C (41.1–41.7°C). Predicted body temperature at GTC: CLN, 40.3°C (40.1–40.6°C); TGB, 41.2°C (40.9–41.4°C); and 1:1 ratio, 41.9°C (41.6–42.3°C). For observed dose-toxicity relationship, (A and B): CLN, 52 trials, 26 mice, 3–8 mice/dose; TGB, 62 trials, 31 mice, 3–8 mice/dose; 1:1 ratio, 32 trials, 16 mice, 3–5 mice/dose. Colored lines are Hill fits ± 95% CB.
TABLE 3.
Summary of rotorod drug toxicity from isobolographic analysis at 20% reduction in motor performance
| Clonazepam:Tiagabine (Ratio) |
Clonazepam | Tiagabine |
|---|---|---|
| Dose mg/kg (95% confidence interval) | ||
| Observed: Alone | 0.27 (0.10–0.55) | 3.8 (3.1–4.4) |
| 1:1 | 0.23 (0.12–0.35) | 0.97 (0.48–1.5) |
| Predicted | ||
| 1:1 | 0.23 (0.17–0.35) | 0.97 (0.72–1.5) |
Because the therapeutic benefit of a drug depends on the ratio of its efficacy and toxicity, we compared protection against seizures at equally toxic doses resulting in a 20% reduction in motor performance on rotorod (Fig. 5D). An estimate of efficacy was determined from dose-effect relationships for prevention of MC and GTC seizures at the dose resulting in 20% reduction in motor performance. The 1:1 fixed-proportion treatment provided significantly greater protection against GTC and MC seizures than did either TGB or CLN (Fig. 5D), demonstrating improved therapeutic benefit of combined therapy in DS mice.
Discussion
Current Treatments for DS Are Ineffective.
DS is one of the most pharmacoresistant epilepsies with recurrent, prolonged seizures often associated with elevated body temperature (Dravet et al., 2005). Seizure control is complicated by multiple seizure types and prolonged seizures (Oguni et al., 2005). A wide range of seizure medications has been used (Chiron and Dulac, 2011) but evidence for best single drug or combination therapy from clinical trials is lacking. Recent case series suggest that bromides (Ernst et al., 1988; Tanabe et al., 2008), topiramate (Nieto-Barrera et al., 2000; Coppola et al., 2002), and levetiracetam (Striano et al., 2007) may be helpful but provide incomplete seizure protection and are associated with significant adverse effects. Monotherapy with any medication tested to date is insufficient to provide adequate seizure control, indicating that combination drug therapy is required. (Chiron and Dulac, 2011).
A placebo-controlled, double-blind clinical trial (Chiron et al., 2000; Kassai et al., 2008) demonstrated that stiripentol, in combination with valproate and clobazam, provided significant although incomplete seizure control. Half of patients in this trial reported adverse events, including sedation, mental slowing, ataxia, diplopia, nausea, abdominal pain, and weight loss. A smaller open-label trial (Inoue et al., 2009) suggested that stiripentol provides additional seizure protection when combined with clonazepam, phenobarbital, or bromides. However, it is clear that current drug combinations fall short of the goal of no seizures and no adverse effects, and additional therapeutic strategies are urgently needed.
Testing Drug Efficacy and Toxicity in DS Mice.
Because of the difficulties of clinical trials, analysis of antiepileptic drug combinations has relied on rodent models of induced seizures (Stafstrom, 2010). However, because seizures in these models result from a proconvulsant drug or electrical shock, it is unclear whether findings of beneficial drug interactions will translate into clinical practice. Our DS mice recapitulate DS remarkably well, including age-dependent spontaneous and thermally induced MC and GTC seizures (Yu et al., 2006; Oakley et al., 2009), and comorbidities of cognitive dysfunction (Han et al., 2012a), autistic features (Han et al., 2012a), ataxia (Kalume et al., 2007), and circadian rhythm disruption (Han et al., 2012b). In close correlation with the human disease, DS mice develop normally until postnatal day 20, when MC seizures progressing to GTC seizures can be induced by controlled elevation of body temperature (Oakley et al., 2009). Spontaneous seizures occur with increasing frequency over subsequent days, and substantial premature death coincides with the period of greatest seizure activity. The ability to reliably induce MC and GTC seizures with small elevations in body temperature provides an efficient and clinically relevant assay of drug efficacy.
Narrow Therapeutic Window for Monotherapy with CLN in DS Mice.
CLN is a potent anticonvulsant used in acute management of status epilepticus and recurrent seizure clusters (Schmidt, 2002). Small trials in humans have demonstrated efficacy against generalized absence and myoclonic seizures (Schmidt, 2002). In DS, reports suggest partial benefit of CLN and a related benzodiazepine clobazam (Chiron and Dulac, 2011) when used as adjunctive therapy, but no clinical trials have been performed to validate this impression. Chronic use of CLN has been limited by development of dose-dependent adverse effects, including sedation, motor impairment, and tolerance, leading to reduced drug efficacy over time and withdrawal symptoms (Michelucci and Tassinari, 2002). In this study, CLN was effective in preventing seizures but produced increasing motor impairment at doses that nearly overlapped its range of therapeutic doses, which is consistent with the clinical impression of narrow therapeutic window.
Increased MC Seizures in Monotherapy with TGB in DS Mice.
In adult and pediatric trials as adjunctive therapy, TGB demonstrated efficacy against partial seizures but was ineffective against generalized seizures in children (Kalviainen, 2002). TGB treatment is associated with nervousness, lack of energy, and difficulty with concentration and word-finding, but not substantial motor impairment or sedation (Schachter, 1999; Kalviainen, 2002). TGB has not been widely used in generalized epilepsy, because case reports of nonconvulsive absence and myoclonic status epilepticus associated with TGB treatment suggest dose-dependent proconvulsant effects (Eckardt and Steinhoff, 1998; Ettinger et al., 1999; Mangano et al., 2003; Skardoutsou et al., 2003; Koepp et al., 2005).
In traditional rodent models, TGB was ineffective against maximal electroshock-induced seizures at doses up to 30 mg/kg (Dalby and Nielsen, 1997), whereas it was effective against pentylenetetrazole-induced clonic seizures at lower doses (ED50 = 2 mg/kg) but became ineffective at 30 mg/kg (Dalby and Nielsen, 1997). TGB induced 1–10-second hypersynchronous 4–7-Hz EEG waves in rats at 10 mg/kg (Lancel et al., 1998). In WAG/Rij rats, a model of generalized spike-wave epilepsy, TGB produced dose-related increases in number and duration of spike-wave discharges at 1–10 mg/kg (Coenen et al., 1995). In lethargic (lh,lh) mice, TGB increased the number and duration of absence seizures above 1 mg/kg and produced absence status epilepticus at 11 mg/kg (Hosford and Wang, 1997). Thus, studies in rodent models mirror clinical experience in showing weak efficacy and substantial proconvulsant activity.
In DS mice, TGB provided protection against GTC seizures, although less than CLN, with saturation of efficacy at 1–10 mg/kg. TGB began to impair motor performance at doses above those required for maximal efficacy, providing a narrow window for seizure protection without motor impairment at carefully selected doses. However, TGB caused a substantial dose-dependent increase in the number of MC seizures before the first GTC seizure, which resulted from both a prolonged period of high-frequency MC seizures and a greater peak MC seizure rate. The prolonged period of high-frequency MC seizures was caused by an increased temperature difference between onset of MC and GTC seizures, which increased with dose at 0.6–10 mg/kg as GTC protection increased and MC protection saturated. A further increase in this temperature difference occurred at 40 mg/kg, a pro-myoclonic dose that reduced the temperature at onset of MC seizures below controls, even while GTC protection remained maximal. These results do not support use of TGB in monotherapy of DS.
Synergy Increases Efficacy and Reduces Toxicity in Combination Therapy in DS Mice.
Because of their complementary molecular mechanisms of enhancing GABAergic neurotransmission, we hypothesized that combined therapy with CLN and TGB would be synergistic, potentially increasing efficacy and reducing toxicity. In support of this hypothesis, the combined dose of CLN and TGB required to protect against GTC seizures in DS mice was 2.5-fold less than predicted from additivity, providing strong evidence for drug synergy. CLN alone provided equivalent maximal protection against GTC seizures to combined drug therapy, but the combination therapy had much reduced motor impairment.
The efficacy of combined CLN and TGB against MC seizures was additive rather than synergistic, indicating that TGB does not enhance or antagonize the MC protection provided by CLN. TGB provided some protection against MC seizures, such that CLN in combination with TGB was effective at 2-fold lower dose at 40.0°C. Although TGB was less effective against MC seizures, the peak efficacy of the combined drugs was similar to that of CLN alone and caused much lower incidence of MC seizures than did TGB alone.
Combining CLN and TGB at a 1:1 fixed proportion resulted in additive motor impairment at all doses tested. Synergistic efficacy and additive toxicity should enhance the therapeutic benefit of combined drug therapy (Stafstrom, 2010; Brodie and Sills, 2011). As expected, the 1:1 fixed proportion of CLN and TGB provided greater protection against MC and GTC seizures at equally toxic doses than did CLN or TGB alone. These results demonstrate that CLN and TGB in combination are superior to either drug alone in control of MC and GTC seizures in DS mice.
Combination therapy also reduces toxicity with respect to the frequency of MC seizures. Although TGB in monotherapy increased MC seizures at elevated body temperature and was pro-myoclonic at normal body temperature at 40 mg/kg, the combination of CLN and TGB did not increase MC seizures, suggesting that TGB may be safe if used at low doses in combination with CLN. These findings demonstrate that synergistic combined therapy with CLN and TGB improves seizure control and minimizes drug-related toxicity in DS mice.
Combined therapy with CLN and TGB may be uniquely effective in DS because of the failure of GABA release in this disease (Yu et al., 2006; Cheah et al., 2012). CLN is only effective in enhancing GABA actions; it does not activate GABA-A receptors by itself (Macdonald, 2002). Inhibition of GABA re-uptake by low doses of TGB may return GABA concentrations in the synaptic cleft toward normal values during GABAergic neurotransmission and, thereby, enhance the efficacy of CLN as a coactivator of GABA-A receptors. The increased concentration of GABA in the synaptic cleft and the enhanced activation of GABA-A receptors by CLN plus GABA may reverse the disinhibition caused by impaired excitability of GABAergic interneurons and restore the normal balance of excitation and inhibition, leading to effective prevention of seizures with minimal adverse effects in DS mice.
Acknowledgments
The authors thank Dr. Frank H. Yu for providing Dravet mice, and Drs. Franck Kalume and Ruth Westenbroek for assistance with animal care and breeding.
Abbreviations
- CB
confidence bands
- CLN
clonazepam
- DS
Dravet syndrome
- EEG
electroencephalogram
- GTC
generalized tonic-clonic seizure
- MC
myoclonic seizure
- SCN1A
brain type-I voltage-gated sodium channel NaV1.1
- TGB
tiagabine
Authorship Contributions
Participated in research design: Oakley, Scheuer, Catterall.
Conducted experiments: Oakley, Cho, Cheah.
Contributed new reagents or analytic tools: Oakley.
Performed data analysis: Oakley, Cho.
Wrote or contributed to the writing of the manuscript: Oakley, Cheah, Catterall.
Footnotes
This work was supported by the National Institutes of Health [Grants R01 NS25704 (to W.A.C.) and K08 NS071193-01A1 (to J.O.)]; and the McKnight Foundation.
References
- Beghi E, Gatti G, Tonini C, Ben-Menachem E, Chadwick DW, Nikanorova M, Gromov SA, Smith PE, Specchio LM, Perucca E, BASE Study Group (2003) Adjunctive therapy versus alternative monotherapy in patients with partial epilepsy failing on a single drug: a multicentre, randomised, pragmatic controlled trial. Epilepsy Res 57:1–13 [DOI] [PubMed] [Google Scholar]
- Berenbaum MC. (1989) What is synergy? Pharmacol Rev 41:93–141 [PubMed] [Google Scholar]
- Braestrup C, Nielsen EB, Sonnewald U, Knutsen LJ, Andersen KE, Jansen JA, Frederiksen K, Andersen PH, Mortensen A, Suzdak PD. (1990) (R)-N-[4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl]nipecotic acid binds with high affinity to the brain gamma-aminobutyric acid uptake carrier. J Neurochem 54:639–647 [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Sills GJ. (2011) Combining antiepileptic drugs—rational polytherapy? Seizure 20:369–375 [DOI] [PubMed] [Google Scholar]
- Cheah CS, Yu FH, Westenbroek RE, Kalume FK, Oakley JC, Potter GB, Rubenstein JL, Catterall WA. (2012) Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA 109:14646–14651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Dulac O. (2011) The pharmacologic treatment of Dravet syndrome. Epilepsia 52 (Suppl 2):72–75 [DOI] [PubMed] [Google Scholar]
- Chiron C, Marchand MC, Tran A, Rey E, d’Athis P, Vincent J, Dulac O, Pons G. (2000) Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet 356:1638–1642 [DOI] [PubMed] [Google Scholar]
- Coenen AM, Blezer EH, van Luijtelaar EL. (1995) Effects of the GABA-uptake inhibitor tiagabine on electroencephalogram, spike-wave discharges and behaviour of rats. Epilepsy Res 21:89–94 [DOI] [PubMed] [Google Scholar]
- Coppola G, Capovilla G, Montagnini A, Romeo A, Spanò M, Tortorella G, Veggiotti P, Viri M, Pascotto A. (2002) Topiramate as add-on drug in severe myoclonic epilepsy in infancy: an Italian multicenter open trial. Epilepsy Res 49:45–48 [DOI] [PubMed] [Google Scholar]
- Dalby NO. (2003) Inhibition of gamma-aminobutyric acid uptake: anatomy, physiology and effects against epileptic seizures. Eur J Pharmacol 479:127–137 [DOI] [PubMed] [Google Scholar]
- Dalby NO, Nielsen EB. (1997) Comparison of the preclinical anticonvulsant profiles of tiagabine, lamotrigine, gabapentin and vigabatrin. Epilepsy Res 28:63–72 [DOI] [PubMed] [Google Scholar]
- De Jonghe P. (2011) Molecular genetics of Dravet syndrome. Dev Med Child Neurol 53 (Suppl 2):7–10 [DOI] [PubMed] [Google Scholar]
- Dravet C. (2011) The core Dravet syndrome phenotype. Epilepsia 52 (Suppl 2):3–9 [DOI] [PubMed] [Google Scholar]
- Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. (2005) Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol 95:71–102 [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. (2006) Adult epilepsy. Lancet 367:1087–1100 [DOI] [PubMed] [Google Scholar]
- Eckardt KM, Steinhoff BJ. (1998) Nonconvulsive status epilepticus in two patients receiving tiagabine treatment. Epilepsia 39:671–674 [DOI] [PubMed] [Google Scholar]
- Ernst JP, Doose H, Baier WK. (1988) Bromides were effective in intractable epilepsy with generalized tonic-clonic seizures and onset in early childhood. Brain Dev 10:385–388 [DOI] [PubMed] [Google Scholar]
- Ettinger AB, Bernal OG, Andriola MR, Bagchi S, Flores P, Just C, Pitocco C, Rooney T, Tuominen J, Devinsky O. (1999) Two cases of nonconvulsive status epilepticus in association with tiagabine therapy. Epilepsia 40:1159–1162 [DOI] [PubMed] [Google Scholar]
- Fraser CM, Sills GJ, Butler E, Thompson GG, Lindsay K, Duncan R, Howatson A, Brodie MJ. (1999) Effects of valproate, vigabatrin and tiagabine on GABA uptake into human astrocytes cultured from foetal and adult brain tissue. Epileptic Disord 1:153–157 [PubMed] [Google Scholar]
- Grabovsky Y, Tallarida RJ. (2004) Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther 310:981–986 [DOI] [PubMed] [Google Scholar]
- Greco WR, Bravo G, Parsons JC. (1995) The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385 [PubMed] [Google Scholar]
- Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. (1998) Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia 39:508–512 [DOI] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. (2012a) Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Yu FH, Schwartz MD, Linton JD, Bosma MM, Hurley JB, Catterall WA, de la Iglesia HO. (2012b) Na(V)1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc Natl Acad Sci USA 109:E368–E377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford DA, Wang Y. (1997) Utility of the lethargic (lh/lh) mouse model of absence seizures in predicting the effects of lamotrigine, vigabatrin, tiagabine, gabapentin, and topiramate against human absence seizures. Epilepsia 38:408–414 [DOI] [PubMed] [Google Scholar]
- Inoue Y, Ohtsuka Y, Oguni H, Tohyama J, Baba H, Fukushima K, Ohtani H, Takahashi Y, Ikeda S. (2009) Stiripentol open study in Japanese patients with Dravet syndrome. Epilepsia 50:2362–2368 [DOI] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. (2007) Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci 27:11065–11074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalviainen R (2002) Tiagabine clinical efficacy and use in epilepsy, in Antiepileptic drugs (Levy R, Mattson R, Meldrum B, and Perucca E eds) pp 698–704, Lippincott Williams & Wilkins, Philadelphia.
- Kassaï B, Chiron C, Augier S, Cucherat M, Rey E, Gueyffier F, Guerrini R, Vincent J, Dulac O, Pons G. (2008) Severe myoclonic epilepsy in infancy: a systematic review and a meta-analysis of individual patient data. Epilepsia 49:343–348 [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Edwards M, Collins J, Farrel F, Smith S. (2005) Status epilepticus and tiagabine therapy revisited. Epilepsia 46:1625–1632 [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319 [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Deisz RA. (1998) Effect of the GABA uptake inhibitor tiagabine on sleep and EEG power spectra in the rat. Br J Pharmacol 123:1471–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikin JB, Benigno J, Dubow JS, Fisher M. (2008) Status epilepticus due to tiagabine ingestion. Am J Ther 15:290–291 [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Ratnaraj N, Patsalos PN, Czuczwar SJ. (2006) Characterization of the anticonvulsant, behavioral and pharmacokinetic interaction profiles of stiripentol in combination with clonazepam, ethosuximide, phenobarbital, and valproate using isobolographic analysis. Epilepsia 47:1841–1854 [DOI] [PubMed] [Google Scholar]
- Macdonald RL. (2002) Benzodiazepines mechanism of action, in Antiepileptic Drugs (Levy R, Mattson R, Meldrum B, Perucca E, eds, ed) pp 179–186, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- Mandillo S, Tucci V, Hölter SM, Meziane H, Banchaabouchi MA, Kallnik M, Lad HV, Nolan PM, Ouagazzal AM, Coghill EL, et al. (2008) Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics 34:243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano S, Cusumano L, Fontana A. (2003) Non-convulsive status epilepticus associated with tiagabine in a pediatric patient. Brain Dev 25:518–521 [DOI] [PubMed] [Google Scholar]
- Michelucci R and Tassinari C (2002) Benzodiazepines adverse effects, in Antiepileptic drugs (Levy R, Mattson R, Meldrum B, and Perucca E eds) pp 215–223, Lippincott Williams & Wilkins, Philadelphia.
- Nieto-Barrera M, Candau R, Nieto-Jimenez M, Correa A, del Portal LR. (2000) Topiramate in the treatment of severe myoclonic epilepsy in infancy. Seizure 9:590–594 [DOI] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Catterall WA. (2011) Insights into pathophysiology and therapy from a mouse model of Dravet syndrome. Epilepsia 52 (Suppl 2):59–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. (2009) Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci USA 106:3994–3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, et al. (2007) Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 27:5903–5914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguni H, Hayashi K, Osawa M, Awaya Y, Fukuyama Y, Fukuma G, Hirose S, Mitsudome A, Kaneko S. (2005) Severe myoclonic epilepsy in infancy: clinical analysis and relation to SCN1A mutations in a Japanese cohort. Adv Neurol 95:103–117 [PubMed] [Google Scholar]
- Peltola J, Peltola M, Raitanen J, Keränen T, Kharazmi E, Auvinen A. (2008) Seizure-freedom with combination therapy in localization-related epilepsy. Seizure 17:276–280 [DOI] [PubMed] [Google Scholar]
- Perez J, Chiron C, Musial C, Rey E, Blehaut H, d’Athis P, Vincent J, Dulac O. (1999) Stiripentol: efficacy and tolerability in children with epilepsy. Epilepsia 40:1618–1626 [DOI] [PubMed] [Google Scholar]
- Rantala H, Tarkka R, Uhari M. (1997) A meta-analytic review of the preventive treatment of recurrences of febrile seizures. J Pediatr 131:922–925 [DOI] [PubMed] [Google Scholar]
- Schachter SC. (1999) A review of the antiepileptic drug tiagabine. Clin Neuropharmacol 22:312–317 [PubMed] [Google Scholar]
- Schmidt D (2002) Benzodiazepines clinical efficacy and use in epilepsy, in Antiepileptic drugs (Levy R, Mattson R, Meldrum B, Perucca E, eds, ed) pp 206–214, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- Skardoutsou A, Voudris KA, Vagiakou EA. (2003) Non-convulsive status epilepticus associated with tiagabine therapy in children. Seizure 12:599–601 [DOI] [PubMed] [Google Scholar]
- Sommerville K. (2002) Tiagabine drug interactions, in Antiepileptic drugs (Levy R, Mattson R, Meldrum B, Perucca E, eds, ed) pp 691–697, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- Stafstrom CE. (2010) Mechanisms of action of antiepileptic drugs: the search for synergy. Curr Opin Neurol 23:157–163 [DOI] [PubMed] [Google Scholar]
- Striano P, Coppola A, Pezzella M, Ciampa C, Specchio N, Ragona F, Mancardi MM, Gennaro E, Beccaria F, Capovilla G, et al. (2007) An open-label trial of levetiracetam in severe myoclonic epilepsy of infancy. Neurology 69:250–254 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. (2000) Drug Synergism and Dose-Effect Data Analysis, Chapman & Hall/CRC, Boca Raton [Google Scholar]
- Tallarida RJ. (2002) The interaction index: a measure of drug synergism. Pain 98:163–168 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. (2012) Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther 342:2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Awaya Y, Matsuishi T, Iyoda K, Nagai T, Kurihara M, Yamamoto K, Minagawa K, Maekawa K. (2008) Management of and prophylaxis against status epilepticus in children with severe myoclonic epilepsy in infancy (SMEI; Dravet syndrome)—a nationwide questionnaire survey in Japan. Brain Dev 30:629–635 [DOI] [PubMed] [Google Scholar]
- Thanh TN, Chiron C, Dellatolas G, Rey E, Pons G, Vincent J, Dulac O. (2002) [Long-term efficacy and tolerance of stiripentaol in severe myoclonic epilepsy of infancy (Dravet’s syndrome)]. Arch Pediatr 9:1120–1127 [DOI] [PubMed] [Google Scholar]
- WHO (2012) WHO fact sheet No. 999, Epilepsy. Geneva: WHO..
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. (2006) Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 9:1142–1149 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Vaughn BV. (2002) Non-convulsive status epilepticus induced by tiagabine in a patient with pseudoseizure. Seizure 11:57–59 [DOI] [PubMed] [Google Scholar]