Abstract
Unsafe use of alcohol results in approximately 2.5 million deaths worldwide, with cirrhosis contributing to 16.6% of reported deaths. Serum insulin levels are often elevated in alcoholism and may result in diabetes, which is why alcoholic liver disease and diabetes often are present together. Because there is a sizable population with these diseases alone or in combination, the purpose of this study was to determine whether transporter expression in human liver is affected by alcoholic cirrhosis, diabetes, and alcoholic cirrhosis coexisting with diabetes. Transporters aid in hepatobiliary excretion of many drugs and toxic chemicals and can be determinants of drug-induced liver injury. Drug transporter expression and transcription factor–relative mRNA and protein expression in normal, diabetic, cirrhotic, and cirrhosis with diabetes human livers were quantified. Cirrhosis significantly increased ABCC4, 5, ABCG2, and solute carrier organic anion (SLCO) 2B1 mRNA expression and decreased SLCO1B3 mRNA expression in the liver. ABCC1, 3–5, and ABCG2 protein expression was also upregulated by alcoholic cirrhosis. ABCC3-5 and ABCG2 protein expression was also upregulated in diabetic cirrhosis. Cirrhosis increased nuclear factor E2–related factor 2 mRNA expression, whereas it decreased pregnane-X-receptor and farnesoid-X-receptor mRNA expression in comparison with normal livers. Hierarchical cluster analysis indicated that expressions of ABCC2, 3, and 6; SLCO1B1 and 1B3; and ABCC4 and 5 were more closely related in the livers from this cohort. Overall, alcoholic cirrhosis altered transporter expression in human liver.
Introduction
Hepatobiliary excretion is an integral function necessary to excrete bile acids, bilirubin, conjugated hormones, as well as drugs and chemicals from the liver (Klaassen and Aleksunes, 2010). The process of biliary excretion relies on membrane-bound transporters localized to hepatocytes, which extract chemicals from blood and efflux chemicals into bile. The solute carrier organic anion (SLCO) and ATP-binding cassette (ABC) transporter families are two major families that mediate hepatic uptake and efflux processes.
The SLCO transporters are often described as uptake transporters because they are predominantly localized to the sinusoidal membrane and typically extract chemicals from blood into hepatocytes (reviewed by Klaassen and Aleksunes, 2010). In humans, SLCO1B1, 1B3, 2B1, and 1A2 have relatively high expression in the liver. SLCO1B1, 1B3, and 2B1 transport a diverse range of drugs, including benzylpenicillin, statins, and estradiol glucuronide (Klaassen and Aleksunes, 2010). The identification of single nucleotide polymorphisms in the SLCO1B1 gene and resulting SLCO1B1 polymorphisms results in altered disposition of statins (Generaux et al., 2011). Human SLCO mRNA expression is regulated through transcription factor–mediated pathways, such as liver-X-receptor, farnesoid-X-receptor (FXR), constitutive androstane receptor (CAR), and pregnane-X-receptor (PXR) (Svoboda et al., 2011).
The ABC transporter superfamily facilitates chemical efflux and includes multidrug resistance proteins (ABCB), multidrug resistance–associated proteins (ABCC), bile salt–export pump (ABCB11), and breast cancer–resistance protein (ABCG2). In liver, ABCC2, ABCG2, and ABCBs are localized to the canalicular membrane and facilitate biliary excretion of chemicals. ABCC1 and 3–6 are localized sinusoidally or basolaterally and efflux chemicals from hepatocytes into blood. Similar to SLCOs, human ABCC expression is modulated by transcription factors, such as nuclear factor-E2–related factor 2 (NRF2), CAR, PXR, and FXR (Klaassen and Slitt, 2005).
Alterations in transporter expression and function resulting from hepatic stress have been noted and can have significant implications on the fate of numerous drugs. Hepatic steatosis resulting from obesity or diabetes resulted in significant alterations in transporter expression in hepatocytes, as demonstrated in mouse models (Cheng et al., 2008; More and Slitt, 2011; More et al., 2012). Compared with steatosis, cirrhosis is a significant hepatic stress with replacement of normal functional tissue by scar tissue, which is unable to maintain the functions of the liver. According to Centers for Disease Control and Prevention, approximately 15,000 Americans die every year of alcoholic liver cirrhosis (Hoyert and Xu, 2012). Other major causes of cirrhosis include chronic viral hepatitis, nonalcoholic steatohepatitis (NASH), and damaged or blocked bile flow (Anand, 1999). About 30% of cirrhotic patients also suffer from diabetes (Hickman and Macdonald, 2007). Acute, as well as chronic, alcohol consumption leads to development of insulin resistance, which can progress to diabetes mellitus (Kim and Kim, 2012). Disruption of normal functions of the liver in cirrhosis may lead to hepatogenous diabetes (Garcia-Compean et al., 2009). Additionally, obesity and diabetes mellitus increase the severity of alcoholic liver disease (Raynard et al., 2002). Owing to interplay between diabetes and cirrhosis, the two conditions are often present together clinically (Baig et al., 2001).
Since many human phase I and II biotransformation enzymes are coordinately regulated by transcription factors that regulate transporter expression, representative cytochrome P450, expression of UDP glucuronosyl transferase (UGT), and Nad(p)h:quinone oxidoreductase (NQO1) mRNA was also determined. The purpose of this study was to determine whether alcoholic cirrhosis, alone or in combination with diabetes, alters transporter expression in intact human liver, as transporters are integral for the hepatobiliary clearance of drugs, bile acids, and bilirubin. Our study included analysis of livers from subjects who had steatosis or diabetes without cirrhosis, as these diseases are sometimes present in alcoholic patients. Our findings herein illustrate coordinated alterations in the expression of certain SLCO and ABC transporter members in human alcoholic cirrhotic liver tissues.
Materials and Methods
Human Liver Tissues.
Liver tissues from normal healthy, alcoholic cirrhotic, steatotic, and diabetic cirrhotic (coexistence of alcohol cirrhosis and diabetes) subjects were obtained from Liver Tissue Cell Distribution System, University of Minnesota (Minneapolis, MN). Additional liver lysates in Trizol reagent from normal and diabetic subjects were purchased from Xenotech LLC (Lenexa, KS) and analyzed only for mRNA expression. The details of subject age, gender, and ethnicity are mentioned in supplementary material (Supplemental Table 1). Exemption approval from the University of Rhode Island Institutional Review Board was granted before tissues were procured.
RNA Extraction.
Total RNA from liver was isolated by phenol-chloroform extraction using RNA Bee (Tel-Test Inc, Friendswood, TX) according to the manufacturer’s protocol. Tissue lysates obtained in Trizol were directly homogenized and subject to chloroform extraction. RNA concentration was quantified by absorbance at 260 nm (Nanodrop ND1000; Thermo Fisher Scientific, Waltham, MA). Agarose gel electrophoresis followed by UV illumination was used to visualize RNA and confirm integrity.
Quantigene Plex 2.0 Assay for mRNA Quantification.
Only samples in which total RNA looked intact and were not degraded were subjected to analysis by the QuantiGene Plex 2.0 assay (Affymetrix, Santa Clara, CA). However, a benefit to this technology according to the manufacturer is that it allows for detection of partially degraded mRNA transcripts, which is desirable for RNA isolated from human tissue. The protocol for the assay is described elsewhere (Aleksunes et al., 2009). Briefly, 1.1 μg of total RNA was incubated with beads with capture probe, label extender, and blocker. On day 2, the beads were washed and incubated with amplifier and subsequently with label. Then incubation with streptavidin-containing substrate was used for detection on a Bio-plex 200 system (Bio-Rad, Hercules, CA).
Tissue Fractionation.
Approximately 100 mg of tissue was homogenized in sucrose-Tris buffer (250 mM sucrose, 10 mM Tris-HCl buffer, pH 7.4) containing a protease inhibitor cocktail (2 μg/ml; Sigma-Aldrich, St. Louis, MO). Homogenates were centrifuged at 100,000g for 60 minutes at 4°C. The resulting pellet is a typical fraction used to detect transporter expression as described in our previous publications, as well as by multiple other research groups (Trauner et al., 1997; Aleksunes et al., 2006; Campion et al., 2008; Cheng et al., 2008; Maher et al., 2008). The supernatant was saved as a cytosolic fraction to measure NQO1 and glutathione peroxidase 1 (GPX1) protein expression. Sucrose-Tris buffer (200 μl) was used to resuspend the resulting pellet. Nuclear fractions from approximately 100 mg of liver tissue were isolated using an NE-PER nuclear extraction kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Protein concentration of the membrane fractions was determined using the DC protein assay (Bio-Rad).
Western Blot Analysis.
Western blots were used to quantify the relative expression of transport proteins in human liver tissues, as described in our previous publication (More and Slitt, 2011; More et al., 2012). Briefly, the membrane/ nuclear extracts were separated on polyacrylamide gel (10% resolving, 4% stacking), transblotted on polyvinylidene fluoride membrane, and blocked with 2% nonfat dry milk in phosphate-buffered saline with Tween 20. The membranes were then incubated with specific primary and secondary antibody and then with ECL+ fluorescence reagent. The blots were then developed on X-ray films; protein bands on the resulting autoradiographs were quantified using Quantity One software v4.6.3 (Bio-Rad). Supplementary material (Supplemental Table 2) provides the antibody source and Western blot conditions. OATP1B1 and 1B3 protein expression by Western blot was not determined because of the lack of high-quality commercially available antibodies.
Statistical Analysis.
Raw data from mRNA quantification were normalized to housekeeping gene hypoxanthine phosphoribosyl transferase 1. Log-transformed normalized data were more approximately normally distributed compared with nontransformed data. Within each gene, pairwise comparison of expression between disease groups was tested by one-way analysis of variance, followed by a Tukey Honestly Significant Difference test. Data from protein quantification were plotted as percent expression and analyzed by one-way analysis of variance, followed by Dunnett’s post hoc test. A difference of P ≤ 0.05 was considered statistically significant. In the Figures, the asterisks (*) represent a statistical difference (P ≤ 0.05) from normal nonsteatotic livers, and dots (●) represent outliers. Hierarchical clustering analysis with Pearson correlation as a similarity measurement was also done to discover potential groups of genes with high correlation.
Results
Transporter mRNA Expression in Liver Is Altered by Alcohol Cirrhosis and Diabetic-Cirrhosis.
Alcoholic cirrhosis altered the mRNA expression of some transporters (Fig. 1A). SLCO1B1 mRNA expression was similar among all groups examined. SLCO1B3 mRNA expression was significantly decreased in livers from alcoholic cirrhosis patients compared with normal nonsteatotic livers. In contrast, SLCO2B1 mRNA expression was increased with alcohol cirrhosis compared with normal nonsteatotic livers.
Fig. 1.
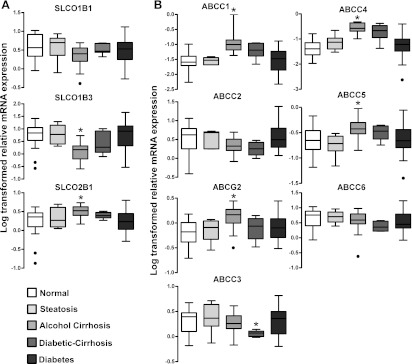
Transporter mRNA expression in livers from normal, steatotic, alcoholic cirrhotic, diabetic cirrhosis, and diabetic subjects. (A) SLCO1B1, 1B3, and 2B1 mRNA expression. (B) ABCC1-6 and ABCG2 mRNA expression. Total RNA was isolated from intact human donor liver tissue (normal, n = 22; steatosis, n = 8; alcohol cirrhosis, n = 19; diabetes, n = 20; diabetic cirrhosis, n = 9) and relative mRNA expression was quantified. Raw data were normalized to hypoxanthine phosphoribosyl transferase 1 (HPRT1) and log transformed before comparison. Asterisks (*) represent a statistical difference (P ≤ 0.05) from normal nonsteatotic livers, and dots (●) represent outliers. SLCO1B3 mRNA expression was decreased, whereas 2B1 was increased in alcohol cirrhosis compared with normal nonsteatotic livers. ABCG2, ABCC4, and ABCC5 mRNA expression was increased in livers from donors with alcoholic cirrhosis compared with normal nonsteatotic livers. ABCC3 expression decreased in diabetic cirrhosis livers.
In liver, ABCC transporters are localized to the canalicular (ABCC2 and ABCG2) or sinusoidal membranes (ABCC1, 3–6) of hepatocytes and mediate organic anion efflux from hepatocytes (Klaassen and Aleksunes, 2010). ABCC1, 4, and 5 mRNA expression was increased in alcoholic cirrhotic livers compared with normal nonsteatotic livers (Fig. 1B). ABCC2 mRNA expression remained unchanged between the groups compared, whereas ABCG2 expression was increased in livers from subjects with alcoholic cirrhosis (Fig. 1B). Diabetic cirrhosis decreased ABCC3 expression compared with normal nonsteatotic livers. ABCC6 mRNA expression was similar among from normal, steatotic, alcoholic cirrhotic, diabetic cirrhotic, and diabetic livers.
Correlation analysis was performed to examine transporter expression that is regulated similarly. Table 1 illustrates the correlation among mRNA expression of the above-mentioned transporters in the entire sample set. SLCO1B1 expression correlated with SLCO1B3, 2B1, ABCC2–4, and ABCC6 expression. Similarly, SLCO1B3 mRNA expression correlated with ABCC2–6 mRNA expression. SLCO2B1 expression also correlated with all ABC transporters analyzed in this study. ABCC2 mRNA expression was correlated with ABCC3, 4, 6, and ABCG2 mRNA expression. ABCC3 mRNA expression was also correlated with ABCC6 and ABCG2 mRNA expression. ABCC4 expression was correlated with ABCC5 and ABCG2 expression. Correlation was also observed between ABCC6 and ABCG2 expression.
TABLE 1.
Results for significant correlation test among transporter mRNA expression
Hierarchical clustering analysis with Pearson correlation as a similarity measurement was performed to identify potential groups of genes with high correlation.
| SLCO1B1 | SLCO1B3 | SLCO2B1 | ABCC2 | ABCC3 | ABCC4 | ABCC5 | ABCC6 | ABCG2 | |
|---|---|---|---|---|---|---|---|---|---|
| SLCO1B1 | 1.00 | ||||||||
| SLCO1B3 | 0.53* | 1.00 | |||||||
| SLCO2B1 | 0.23* | 0.00 | 1.00 | ||||||
| ABCC2 | 0.64* | 0.72* | 0.42* | 1.00 | |||||
| ABCC3 | 0.33* | 0.51* | 0.42* | 0.74* | 1.00 | ||||
| ABCC4 | −0.24* | −0.49* | 0.38* | −0.25* | −0.03 | 1.00 | |||
| ABCC5 | −0.10 | −0.30* | 0.52* | 0.05 | 0.04 | 0.52* | 1.00 | ||
| ABCC6 | 0.55* | 0.54* | 0.58* | 0.70* | 0.72* | −0.12 | 0.11 | 1.00 | |
| ABCG2 | 0.19 | 0.06 | 0.57* | 0.39* | 0.45* | 0.32* | 0.14 | 0.55* | 1.00 |
Statistically significant correlation (P ≤ 0.05) in expression.
Transporter Protein Expression Is Altered in Livers from Subjects with Steatosis, Alcoholic Cirrhosis, and Diabetic Cirrhosis.
Figure 2 illustrates the effect of steatosis, alcoholic cirrhosis, and diabetic cirrhosis on transporter protein expression in fractions from intact human liver tissue (representative blots). Alcoholic cirrhosis and diabetic cirrhosis increased ABCC1, 3, and 5 protein expression compared with normal nonsteatotic livers. ABCC2 protein remained unchanged in all the groups. ABCC4 and ABCG2 protein expression was increased in livers with steatosis, alcoholic cirrhosis, and diabetic cirrhosis. In contrast to other ABC transporters, ABCC6 protein expression decreased in livers with alcoholic cirrhosis and diabetic cirrhosis.
Fig. 2.
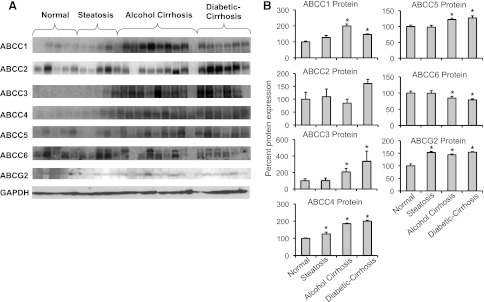
Protein expression of transporters in livers from normal, steatotic, alcoholic cirrhotic, and diabetic cirrhosis subjects by Western blot. (A) Relative ABCC1-6, and ABCG2 protein expression was determined in fractions isolated from intact human liver by Western blot. Lanes 1–5 (normal, nonsteatotic), 6–10 (steatosis), 11–20 (alcohol cirrhosis), and 21–25 (diabetic cirrhosis) represent samples analyzed. (B) Quantification of Western blots. Protein bands were quantified using Quantity One software v4.6.3 (Bio-Rad). Asterisks (*) represent a statistical difference (P ≤ 0.05) from normal nonsteatotic livers. Steatosis increased ABCC4 and ABCG2 protein expression compared with normal livers. ABCC1, 3, and 5 protein expression was increased, whereas ABCC6 expression was decreased in alcoholic cirrhotic and diabetic cirrhotic livers compared with normal nonsteatotic livers. ABCC4 and ABCG2 expression was increased in livers with steatosis, alcoholic cirrhosis, and diabetic cirrhosis.
Alcoholic Cirrhosis and Diabetic Cirrhosis Affect Transcription Factor Expression in Intact Human Liver.
Studies in recent years have revealed that several transcription factor–mediated pathways (e.g., PXR, CAR, and FXR), as well as the antioxidant response (e.g., NRF2), are important mediators of SLCO and ABC transporter regulation in the liver (Klaassen and Aleksunes, 2010). Therefore, NRF2, PXR, CAR, and FXR expression was also evaluated and correlated with transporter expression. Figure 3A depicts the PXR, CAR, FXR, and NRF2 mRNA expression in the human liver. NRF2 mRNA expression was increased in alcoholic cirrhotic and in diabetic cirrhotic livers compared with normal nonsteatotic livers. PXR mRNA expression was decreased in livers with diabetic cirrhosis compared with normal livers. CAR mRNA expression remained unchanged in all groups analyzed. FXR mRNA expression was decreased in livers with alcoholic cirrhosis and diabetic cirrhosis.
Fig. 3.
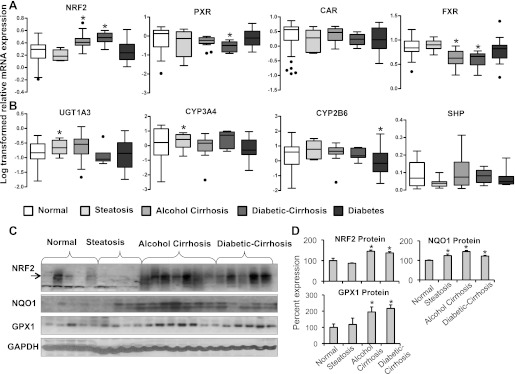
Transcription factor and biotransformation enzyme expression in livers from normal, steatotic, alcoholic cirrhotic, diabetic cirrhotic, and diabetic subjects. (A) Relative PXR, CAR, FXR, and NRF2 mRNA expression in human liver. Total RNA was isolated from intact human donor liver tissue (normal, n = 22; steatosis, n = 8; alcohol cirrhosis, n = 19; diabetes, n = 20; diabetic cirrhosis, n = 9), and relative mRNA expression was quantified. Alcohol cirrhosis and diabetic cirrhosis increased NRF2 but decreased FXR mRNA levels. Diabetic cirrhosis also decreased PXR mRNA levels. (B) CYP3A4, 2B6, UGT1A3, and 2B7 mRNA expression in liver. Total RNA was isolated from intact human donor liver tissue (normal, n = 22; steatosis, n = 8; alcohol cirrhosis, n = 19; diabetes, n = 20; diabetic cirrhosis, n = 9). Steatosis increased CYP3A4 and UGT1A3 mRNA expression compared with normal nonsteatotic livers. CYP2B6 mRNA expression was decreased in diabetic livers compared with normal nonsteatotic livers. (C) Relative NRF2, NQO1, and GPX1 protein expression in nuclear (NRF2) and cytosolic (NQO1, GPX1) fractions in livers of normal, steatotic, cirrhotic, and diabetic cirrhotic subjects. Lanes 1–5 (normal, nonsteatotic), 6–9 (steatosis), 10–16 (alcohol cirrhosis), and 17–21 (diabetic cirrhosis) represent samples analyzed. (D) Quantification of NRF2, NQO1, and GPX1 Western blots. Asterisks (*) represent a statistical difference (P ≤ 0.05) from normal nonsteatotic livers, and dots (●) represent outliers. Nuclear NRF2 (approximately 110 kDa) and cytosolic NQO1, GPX1 protein (31 kDa and 23 kDa) levels were increased in livers of alcohol cirrhosis and diabetic cirrhosis subjects compared with normal livers. NQO1 was also increased in steatotic livers.
Table 2 illustrates the correlation among abovementioned transcription factor mRNA expressions. CAR mRNA expression correlated significantly with FXR and PXR mRNA expression, and PXR expression correlated with FXR expression.
TABLE 2.
Results for significant correlation test among mRNA expression of transcription factors CAR, FXR, NRF2, and PXR
Hierarchical clustering analysis with Pearson correlation as a similarity measurement was performed to identify potential groups of genes with high correlation.
Statistically significant correlation in the expression of corresponding transcription factor, with P < 0.05.
Alcohol Cirrhosis Affects Phase I and Phase II Drug-Metabolizing Enzymes mRNA Expression.
Correspondingly, Fig. 3B depicts mRNA expression for representative cytochrome P450 and UGTs, along with FXR target gene, small heterodimer protein. UGT1A3 mRNA expression was increased in steatotic livers compared with normal livers. CYP3A4 mRNA expression was increased in livers with steatosis, but similar to normal livers in the other disease conditions. CYP2B6 mRNA expression was decreased in livers with diabetic cirrhosis compared with normal nonsteatotic livers. Small heterodimer protein mRNA expression was similar among all the disease conditions tested in the study. Other cytochrome and UGT isoforms, including CYP2D6, UGT1A1, 1A4 mRNA expressions, were also studied and remained unchanged between the groups (unpublished data).
Alcohol Cirrhosis Increases NRF2, NQO1, and Glutathione Peroxidase Protein Expression.
NRF2 protein expression in liver fractions was correspondingly increased in alcoholic cirrhotic and diabetic cirrhotic livers compared with normal nonsteatotic livers (Fig. 3, C and D). NQO1 and GPX1, enzymes, which are regulated via NRF2, were also quantified at the protein level. NQO1 protein expression was increased in steatotic, alcoholic cirrhotic, and diabetic cirrhotic livers compared with normal livers; the most prominent increase was in alcoholic cirrhosis. GPX1 protein expression was increased in liver fractions from subjects with alcoholic cirrhosis and diabetic cirrhosis.
Alcohol Cirrhosis Increases Inflammatory Cytokine mRNA Expression.
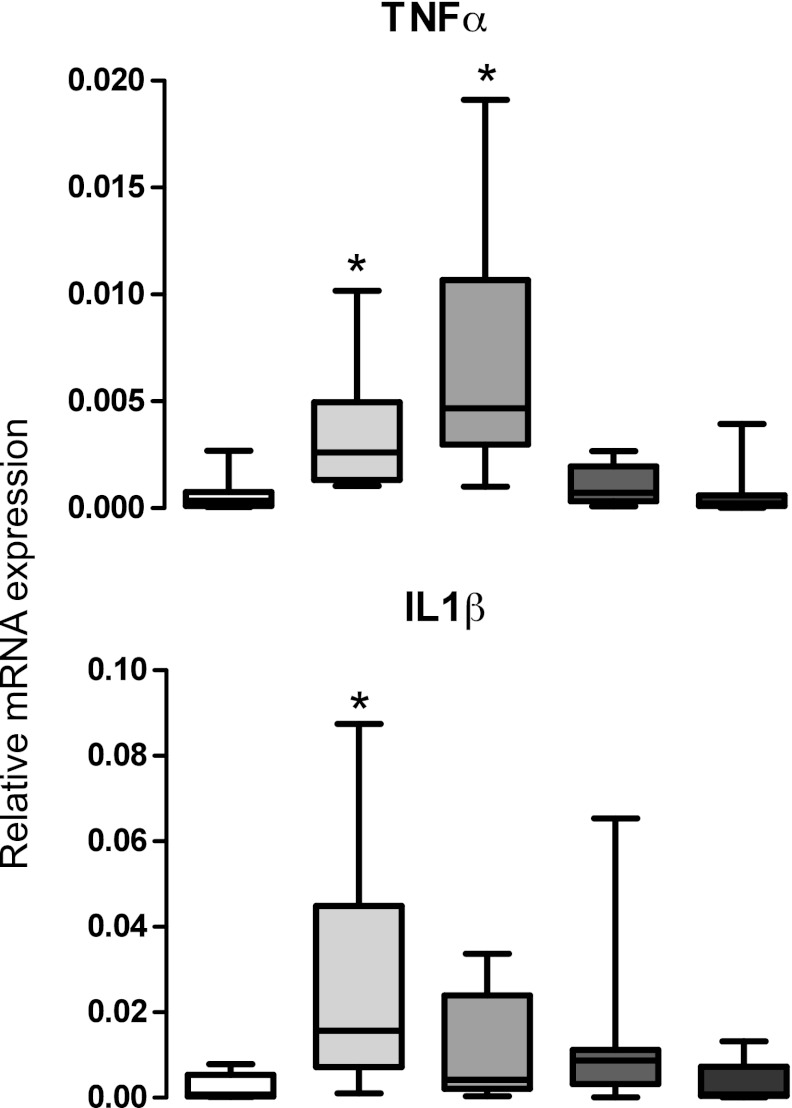
Figure 4 demonstrates mRNA expression of inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-β (IL-β) in livers. TNF-α mRNA expression was increased in both steatosis and alcoholic cirrhosis groups compared with normal nonsteatotic livers. IL-β expression was increased only with steatosis compared with normal livers.
Fig. 4.
Inflammatory cytokine mRNA expression in livers from normal, steatotic, alcohol cirrhotic, diabetic cirrhotic, and diabetic subjects. Inflammatory cytokine TNF-α and interleukin 1β (IL-1β) mRNA expression. Steatosis increased mRNA expression of both TNF-α and IL-1β, and alcohol cirrhosis increased expression of only TNF-α compared with normal nonsteatotic livers.
Hierarchical Cluster Analysis of Transporter and Transcription Factor mRNA Expression.
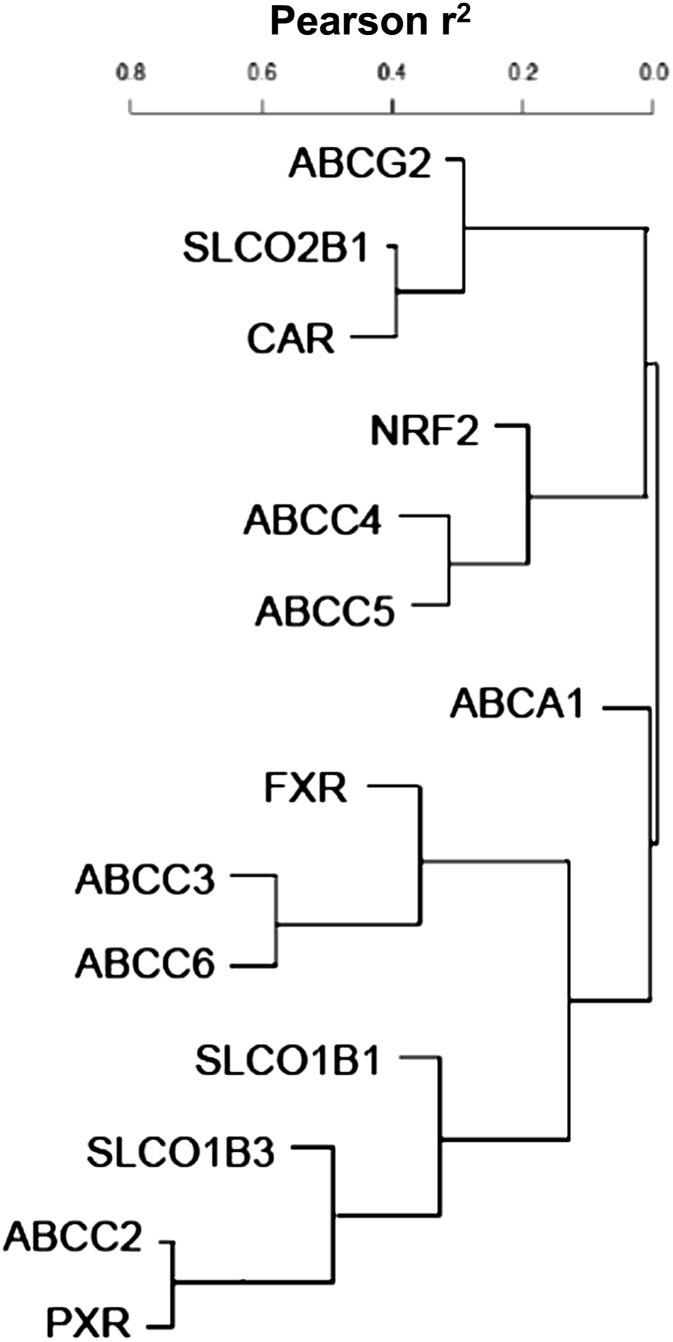
Figure 5 depicts the correlations between transcription factor and transporter mRNA expression. ABCG2 and SLCO2B1 expression were closely related to CAR expression. Similarly, expression of ABCC4, ABCC5, and NRF2 were closely related. Expression of ABCC2 and PXR were also closely related and more distantly related to SLCO1B3 and 1B1 expression.
Fig. 5.
Hierarchical cluster analysis of different transporters and transcription factors. Target gene expression was normalized to hypoxanthine phosphoribosyl transferase 1 (HPRT1) and log transformed to use for cluster analysis. Cluster analysis was performed by using squared Pearson’s correlation (r2) as a similarity measure. Genes were clustered as a group with greater r2. ABCG2 and SLCO2B1 expression were closely related to CAR expression. Similarly, expression of ABCC4, ABCC5, and NRF2 were closely related to each other. Expression of ABCC2 and PXR were also closely related and further related to SLCO1B3 and 1B1 expression.
Discussion
This study demonstrated predominant increased mRNA and protein of efflux transporters, such as ABCG2, ABCC1, 3–5 in intact livers of human subjects with alcohol cirrhosis. Uptake transporter expression was less consistent, with decreased SLCO1B3 and increased SLCO2B1 mRNA expression occurring in livers with alcoholic cirrhosis. Transcription factors that regulate transporter expression were also correspondingly altered. NRF2 mRNA and protein expression was increased in alcoholic cirrhotic livers, whereas FXR mRNA expression was decreased.
Hierarchical cluster analysis of transcription factors and transporters obtained in this study is in agreement with the findings in literature. In rodents, as well as in humans, NRF2 is known to regulate the expression of efflux transporters ABCC2–5 (Klaassen and Slitt, 2005). In the cluster analysis in the present study, ABCC4 and 5 were expressed together with NRF2. Similarly, SLCO2B1 and CAR were expressed together, as observed in rodents (Cheng et al., 2005). ABCC2 and PXR were also clustered together, as also described (Klaassen and Slitt, 2005). SLCO1B1 and 1B3 are reported to be regulated by the same transcription factors—hepatocyte nuclear factor-1α, aryl hydrocarbon receptor, and CAR (Klaassen and Aleksunes, 2010)—and were clustered together in the present data. CAR and PXR activation have been shown to increase ABCC2 and 3 expression in hepatocytes (Teng and Piquette-Miller, 2005), indicating that these two transporters also have significant correlation in expression. CAR is known to regulate ABCC2 and 3, as well as SLCO1B1, indicating significant correlation in the expression of these three transporters.
Transporter expression in human livers with alcoholic cirrhosis has not been characterized comprehensively before this study. Rodent models for alcohol-induced liver disease display steatosis and some degree of fibrosis, but no model fully progresses to the human level of cirrhotic liver (Lieber et al., 1965; Tsukamoto et al., 1986). Previous studies with hepatic transporter expression are with small sample size or different liver pathologies like hepatitis C, hepatocellular carcinoma, NASH, or from nondiseased human livers (Nishimura and Naito, 2005; Hilgendorf et al., 2007; Ogasawara et al., 2010; Doi et al., 2011). Hepatitis C virus (HCV) –related cirrhosis increased ABCC4 mRNA and protein expression and ABCC1 mRNA expression in human livers (Ogasawara et al., 2010). It was also noted that ABCG2, SLCO1B1, and 1B3 mRNA expression was decreased in HCV-related cirrhosis. Findings of the present study for ABCC1, 4, and SLCO1B3 are similar to those observed with HCV-related cirrhosis, but ABCG2 and SLCO1B1 differed. Efflux transporter expression in human livers with primary biliary cirrhosis (PBC) was also similar to that observed in alcoholic cirrhotic livers (Zollner et al., 2003). ABCC3 protein expression was increased in PBC and alcohol cirrhosis. The uptake transporter, SLCO1B1, however, remained unchanged with alcohol cirrhosis but went down with PBC (Zollner et al., 2003). Fatty and nonfatty NASH also enhanced the mRNA and protein expression of ABCC1, 4, and 5 in human livers (Hardwick et al., 2011). These comparisons of the present study with existing findings suggest that these alterations in transporter expression are likely a general response to cirrhosis of any cause.
Another study reported a patient having lowered SLCO1B3 expression in a hepatocellular carcinoma nodule (Doi et al., 2011), which is consistent with the present data that illustrate decreased SLCO1B3 mRNA expression in alcoholic cirrhosis, too. As other models of liver injury (e.g., acetaminophen, carbon tetrachloride, cholestasis) also increase efflux transporter expression, we acknowledge that the observation was anticipated. However, because alcohol cirrhosis plagues about 20% of the alcoholic people worldwide, knowing whether aberrant transporter and nuclear receptor expression is present in the liver is of toxicological significance because it can provide mechanistic understanding of drug-induced liver injury or altered drug efficacy in patients with alcoholic liver disease.
Transporters facilitate absorption, distribution, and elimination of xenobiotics, as well as endobiotics such as bile acids, cholesterol, and conjugated hormones (e.g., estrogens and thyroid hormones) (Klaassen and Aleksunes, 2010). Alterations in the transporter expression or polymorphisms have been associated with alterations in disposition and adverse effects/ protection against adverse effects of certain xenobiotics. Simvastatin-induced myopathy, which is a concentration-dependent side effect, was found to be associated with SLCO1B1 polymorphism in human subjects (reviewed by Niemi et al., 2011). In another study with methotrexate, variants of SLCO1B1 were associated with increased clearance and gastrointestinal toxicity as a side effect in children with acute lymphoblastic leukemia (Trevino et al., 2009). In a different study, mice with increased Abcc3 and 4 expression in the liver had enhanced metabolite excretion and were protected against acetaminophen-induced hepatocyte injury (Slitt et al., 2003; Aleksunes et al., 2008), and mice lacking Abcc2, 3, and Abcg2 demonstrate mild hepatotoxicity when administered diclofenac (Lagas et al., 2010). The present study illustrates that intact livers from subjects with alcoholic cirrhosis have alterations in major drug transporter mRNA and protein expression in liver. As transporters play a vital role in drug disposition, the findings in this study imply that subjects with the above-mentioned disease conditions need a consideration while administering drugs that form glucuronide, which are pharmacologically active.
With progression of nonalcoholic fatty liver disease, the expression of Nrf2 and its target genes increases, as determined by immunohistochemistry in human livers (Hardwick et al., 2010). Alcohol-induced oxidative stress also activates Nrf2 in human hepatocytes (Nussler et al., 2010). Alcohol induces lipid deposition in liver, and metabolism of fatty acids as well as ethanol causes generation of oxidative stress in liver (Syn et al., 2009). Alcoholic cirrhotic livers in the current study also displayed increased Nrf2 protein levels in nuclear fractions, which is likely a response to increased oxidative stress in the alcoholic liver. PXR is indicated in therapeutic applications against inflammatory liver diseases. PXR activation by pregnenolone-16-alpha carbonitrile leads to decreased carbon tetrachloride-induced fibrogenesis in rats (Marek et al., 2005). The decreased PXR expression may be an indicator that PXR deficiency correlates with increased risk for liver disease. FXR regulates bile acid homeostasis, triglyceride, and cholesterol metabolism, glucose homeostasis and fibrogenesis in liver (reviewed by Fuchs, 2012). FXR activation by bile acids induces PPARα expression, and this increases β-oxidation of fatty acids (Pineda Torra et al., 2003). Thus FXR activation may protect the liver from fat deposition in both alcoholic, as well as, nonalcoholic liver diseases. In the present study, FXR mRNA expression was decreased in alcohol cirrhosis, suggesting that FXR suppression might occur during alcoholic liver disease, which could be a mechanism for alcoholic liver injury. Inflammation could be a possible factor contributing to the alterations in nuclear receptors analyzed in this study. Lipopolysaccharide treatment of mice resulted in decreased PXR signaling and target gene expression in mice (Moriya et al., 2012). Similarly, treatment of Huh7 cells with inflammatory cytokines TNF-α and IL-6 resulted in a marked decrease in FXR target transporter bile salt exporter pump (Chen et al., 2012). As disease progression of cirrhosis involves an increase in inflammation, decreased mRNA expression of PXR/ FXR in alcoholic cirrhosis or diabetic cirrhosis could possibly be explained. Further studies are necessary to elucidate why PXR and FXR expression is decreased in alcoholic cirrhosis and whether the decreased expression contributes to the development of alcoholic cirrhosis.
Elbekai et al. (2004) reported that certain phase I biotransformation enzyme expression was altered expression in livers of cirrhotic subjects. CYP1A and CYP3A showed reduced expression with cirrhosis, whereas CYP2C, 2A, and 2B remained unaltered (Elbekai et al., 2004). The present data display little or no change in CYP isoform mRNA expression. Similarly, glucuronidation activity in liver is reported to be unaltered with cirrhosis (Elbekai et al., 2004). The present study had results consistent with this observation; UGT1A1, 1A3, 1A4, and 2B7 expression remained unchanged between normal and alcoholic cirrhotic livers, although it should be noted that UGT1A3 was decreased in diabetic cirrhosis livers and UGT2B7 was decreased in diabetic livers.
In summary, we demonstrate that alcoholic cirrhosis significantly alters transporter expression in human liver, most notably altering ABCC3, ABCC4, and, ABCC5, which was associated with altered NRF2, CAR, and FXR mRNA expression. In this study, diabetes did not significantly alter mRNA expression of the transporters analyzed. However, as this is a small sample set, and expression was quantified only on the mRNA level, further studies are needed to address comprehensively the effect of diabetes on transporters. Additionally, the presence of diabetes in combination with cirrhosis did not augment the effect of cirrhosis on transporter expression. Significant correlations between transporter and nuclear receptor expression were observed in the cohort of livers analyzed. Overall, the data herein illustrate alterations in hepatic transporter expression in the alcoholic cirrhotic liver that correlates to changes in nuclear receptor expression. Alterations in nuclear receptor and drug transporter expression in alcoholic liver should be given consideration when evaluating altered drug toxicities.
Supplementary Material
Acknowledgments
The authors thank Rhiannon Hardwick, University of Arizona, and Prajakta Shimpi, University of Rhode Island, for assistance in manuscript preparation. The authors also thank Supriya Kulkarni, University of Rhode Island, for technical assistance.
Abbreviations
- ABC
ATP binding cassette family
- ABCB
multidrug resistance protein
- ABCB11
bile salt–export pump
- ABCC
multidrug resistance–associated protein
- ABCG2
breast cancer–resistance protein
- CAR
constitutive androstane receptor
- FXR
farnesoid-X-receptor
- GPX
glutathione peroxidase
- HCV
hepatitis C virus
- IL-β
interleukin-β
- NASH
nonalcoholic steatohepatitis
- NQO1
Nad(p)h:quinone oxidoreductase
- NRF2
nuclear factor E2–related factor 2
- PBC
primary biliary cirrhosis
- PXR
pregnane-X-receptor
- SLCO
solute carrier organic anion
- TNF-α
tumor necrosis factor-α
- UGT
UDP glucuronosyl transferase
Authorship Contributions
Participated in research design: More, Slitt, Cherrington, Cheng, Buckley.
Conducted experiments: More, Cheng, Donepudi.
Contributed new reagents or analytic tools: Buckley.
Performed data analysis: More, Slitt, Lu.
Wrote or contributed to the writing of the manuscript: More, Slitt, Cherrington, Lu, Buckley.
Footnotes
This work was supported by the National Institutes of Health [Grants 1R01ES016042 and 5K22ES013782]; the National Institutes of Health National Institute of General Medical Sciences [Grant 8P20 GM103430-11]; and the National Institutes of Health National Center for Research Resources [Grant 5P20RR016457-11].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Aleksunes LM, Campion SN, Goedken MJ, Manautou JE. (2008) Acquired resistance to acetaminophen hepatotoxicity is associated with induction of multidrug resistance-associated protein 4 (Mrp4) in proliferating hepatocytes. Toxicol Sci 104:261–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AL, Maher JM, Dieter MZ, Knight TR, Goedken M, Cherrington NJ, Chan JY, Klaassen CD, Manautou JE. (2006) Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H:quinone oxidoreductase 1 during cholestasis. Cell Stress Chaperones 11:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Yeager RL, Klaassen CD. (2009) Application of multivariate statistical procedures to identify transcription factors that correlate with MRP2, 3, and 4 mRNA in adult human livers. Xenobiotica 39:514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand BS. (1999) Cirrhosis of liver. West J Med 171:110–115 [PMC free article] [PubMed] [Google Scholar]
- Baig NA, Herrine SK, Rubin R. (2001) Liver disease and diabetes mellitus. Clin Lab Med 21:193–207 [PubMed] [Google Scholar]
- Campion SN, Johnson R, Aleksunes LM, Goedken MJ, van Rooijen N, Scheffer GL, Cherrington NJ, Manautou JE. (2008) Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am J Physiol Gastrointest Liver Physiol 295:G294–G304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Song X, Valanejad L, Vasilenko A, More V, Qiu X, Chen W, Lai Y, Slitt A, Stoner M,, et al. (2012) Bile salt export pump is dysregulated with altered farnesoid x receptor isoform expression in patients with hepatocellular carcinoma tissues. Hepatology DOI: 10.1002/hep.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, Slitt AL. (2008) Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm 5:77–91 [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD. (2005) Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos 33:1276–1282 [DOI] [PubMed] [Google Scholar]
- Doi N, Tomiyama Y, Kawase T, Nishina S, Yoshioka N, Hara Y, Yoshida K, Korenaga K, Korenaga M, Moriya T, et al. (2011) Focal nodular hyperplasia-like nodule with reduced expression of organic anion transporter 1B3 in alcoholic liver cirrhosis. Intern Med 50:1193–1199 [DOI] [PubMed] [Google Scholar]
- Elbekai RH, Korashy HM, El-Kadi AO. (2004) The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab 5:157–167 [DOI] [PubMed] [Google Scholar]
- Fuchs M. (2012) Non-alcoholic fatty liver disease: the bile acid-activated farnesoid x receptor as an emerging treatment target. J Lipids 2012:934–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. (2009) Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol 15:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Generaux GT, Bonomo FM, Johnson M, Doan KM. (2011) Impact of SLCO1B1 (OATP1B1) and ABCG2 (BCRP) genetic polymorphisms and inhibition on LDL-C lowering and myopathy of statins. Xenobiotica 41:639–651 [DOI] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. (2010) Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 38:2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. (2011) Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:2395–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman IJ, Macdonald GA. (2007) Impact of diabetes on the severity of liver disease. Am J Med 120:829–834 [DOI] [PubMed] [Google Scholar]
- Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. (2007) Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos 35:1333–1340 [DOI] [PubMed] [Google Scholar]
- Hoyert DL, Xu JQ. (2012) Deaths: Preliminary data for 2011. National Vital Statistics Report 61:1–51 [PubMed] [Google Scholar]
- Kim SJ, Kim DJ. (2012) Alcoholism and diabetes mellitus. Diabetes Metab J 36:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL. (2005) Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab 6:309–328 [DOI] [PubMed] [Google Scholar]
- Lagas JS, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. (2010) Hepatic clearance of reactive glucuronide metabolites of diclofenac in the mouse is dependent on multiple ATP-binding cassette efflux transporters. Mol Pharmacol 77:687–694 [DOI] [PubMed] [Google Scholar]
- Lieber CS, Jones DP, Decarli LM. (1965) Effects of prolonged ethanol intake: production of fatty liver despite adequate diets. J Clin Invest 44:1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Aleksunes LM, Dieter MZ, Tanaka Y, Peters JM, Manautou JE, Klaassen CD. (2008) Nrf2- and PPAR alpha-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol Sci 106:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek CJ, Tucker SJ, Konstantinou DK, Elrick LJ, Haefner D, Sigalas C, Murray GI, Goodwin B, Wright MC. (2005) Pregnenolone-16alpha-carbonitrile inhibits rodent liver fibrogenesis via PXR (pregnane X receptor)-dependent and PXR-independent mechanisms. Biochem J 387:601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More VR, Slitt AL. (2011) Alteration of hepatic but not renal transporter expression in diet-induced obese mice. Drug Metab Dispos 39:992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More VR, Wen X, Thomas PE, Aleksunes LM, Slitt AL. (2012) Severe diabetes and leptin resistance cause differential hepatic and renal transporter expression in mice. Comp Hepatol 11:1 DOI: 10.1186/1476-5926-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya N, Kataoka H, Fujino H, Nishikawa J, Kugawa F. (2012) Effect of lipopolysaccharide on the xenobiotic-induced expression and activity of hepatic cytochrome P450 in mice. Biol Pharm Bull 35:473–480 [DOI] [PubMed] [Google Scholar]
- Niemi M, Pasanen MK, Neuvonen PJ. (2011) Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev 63:157–181 [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. (2005) Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet 20:452–477 [DOI] [PubMed] [Google Scholar]
- Nussler AK, Hao L, Knobeloch D, Yao P, Nussler NC, Wang Z, Liu L, Ehnert S. (2010) Protective role of HO-1 for alcohol-dependent liver damage. Dig Dis 28:792–798 [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Terada T, Katsura T, Hatano E, Ikai I, Yamaoka Y, Inui K. (2010) Hepatitis C virus-related cirrhosis is a major determinant of the expression levels of hepatic drug transporters. Drug Metab Pharmacokinet 25:190–199 [DOI] [PubMed] [Google Scholar]
- Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. (2003) Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol 17:259–272 [DOI] [PubMed] [Google Scholar]
- Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. (2002) Risk factors of fibrosis in alcohol-induced liver disease. Hepatology 35:635–638 [DOI] [PubMed] [Google Scholar]
- Slitt AL, Cherrington NJ, Maher JM, Klaassen CD. (2003) Induction of multidrug resistance protein 3 in rat liver is associated with altered vectorial excretion of acetaminophen metabolites. Drug Metab Dispos 31:1176–1186 [DOI] [PubMed] [Google Scholar]
- Svoboda M, Riha J, Wlcek K, Jaeger W, Thalhammer T. (2011) Organic anion transporting polypeptides (OATPs): regulation of expression and function. Curr Drug Metab 12:139–153 [DOI] [PubMed] [Google Scholar]
- Syn WK, Teaberry V, Choi SS, Diehl AM. (2009) Similarities and differences in the pathogenesis of alcoholic and nonalcoholic steatohepatitis. Semin Liver Dis 29:200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. (2005) The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther 312:841–848 [DOI] [PubMed] [Google Scholar]
- Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, Keppler D, Boyer JL. (1997) The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology 113:255–264 [DOI] [PubMed] [Google Scholar]
- Treviño LR, Shimasaki N, Yang W, Panetta JC, Cheng C, Pei D, Chan D, Sparreboom A, Giacomini KM, Pui CH, et al. (2009) Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol 27:5972–5978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Towner SJ, Ciofalo LM, French SW. (1986) Ethanol-induced liver fibrosis in rats fed high fat diet. Hepatology 6:814–822 [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. (2003) Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol 38:717–727 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.