Abstract
Organic anion-transporting polypeptides (OATPs) are multispecific transporters mediating the uptake of endogenous compounds and xenobiotics in tissues that are important for drug absorption and elimination, including the intestine and liver. Silymarin is a popular herbal supplement often used by patients with chronic liver disease; higher oral doses than those customarily used (140 mg three times/day) are being evaluated clinically. The present study examined the effect of silymarin flavonolignans on OATP1B1-, OATP1B3-, and OATP2B1-mediated transport in cell lines stably expressing these transporters and in human hepatocytes. In overexpressing cell lines, OATP1B1- and OATP1B3-mediated estradiol-17β-glucuronide uptake and OATP2B1-mediated estrone-3-sulfate uptake were inhibited by most of the silymarin flavonolignans investigated. OATP1B1-, OATP1B3-, and OATP2B1-mediated substrate transport was inhibited efficiently by silymarin (IC50 values of 1.3, 2.2 and 0.3 µM, respectively), silybin A (IC50 values of 9.7, 2.7 and 4.5 µM, respectively), silybin B (IC50 values of 8.5, 5.0 and 0.8 µM, respectively), and silychristin (IC50 values of 9.0, 36.4, and 3.6 µM, respectively). Furthermore, silymarin, silybin A, and silybin B (100 µM) significantly inhibited OATP-mediated estradiol-17β-glucuronide and rosuvastatin uptake into human hepatocytes. Calculation of the maximal unbound portal vein concentrations/IC50 values indicated a low risk for silymarin-drug interactions in hepatic uptake with a customary silymarin dose. The extent of silymarin-drug interactions depends on OATP isoform specificity and concentrations of flavonolignans at the site of drug transport. Higher than customary doses of silymarin, or formulations with improved bioavailability, may increase the risk of flavonolignan interactions with OATP substrates in patients.
Introduction
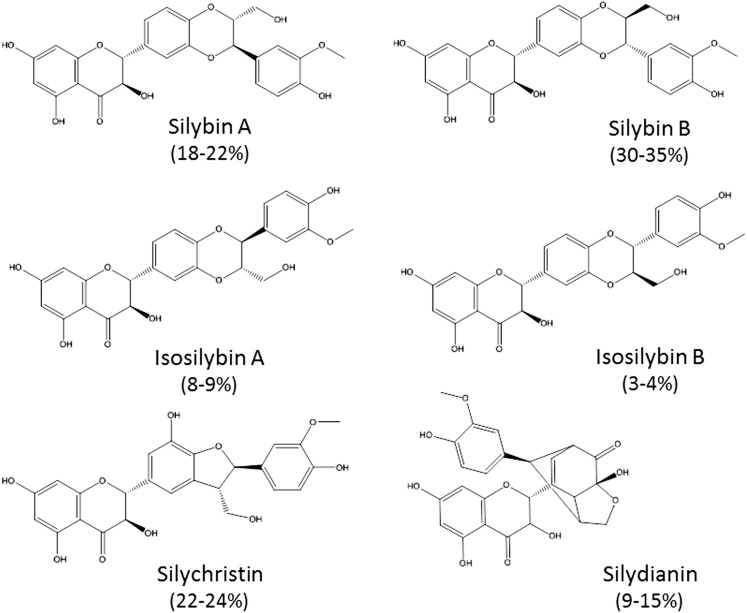
Silymarin, a purified extract from milk thistle (Silybum marianum), is a popular herbal supplement that is used by approximately one-third of patients with hepatitis C infection or chronic liver disease because of its reported hepatoprotective properties (Seeff et al., 2008; Freedman et al., 2011). A standardized milk thistle extract contains at least 70% silymarin, a complex mixture composed of mainly the flavonolignans silybin A, silybin B, silydianin, silychristin, isosilybin A, isosilybin B, and a few flavonoids, such as taxifolin (Wen et al., 2008) (Fig. 1). Legalon SIL, a commercially available formulation of silybin A and silybin B (silibinin dihemisuccinate), has been used clinically for Amanita mushroom poisoning, resulting in reduced mortality rates, compared with control-treated patients (Mengs et al., 2012). In vitro studies suggest that silibinin dihemisuccinate inhibits organic anion-transporting polypeptide (OATP)–mediated hepatic uptake of the toxin amanitin (Letschert et al., 2006).
Fig. 1.
Chemical structure of silymarin flavonolignans. The composition of individual flavonolignans in silymarin is indicated in parentheses.
OATPs are important membrane transport proteins expressed in key organs of drug disposition, including the intestine, liver, and kidneys, where they mediate the cellular uptake of a broad range of xenobiotics and endogenous compounds. Two members of this family of proteins, OATP1B1 and OATP1B3, are expressed predominantly in the basolateral membrane of the hepatocyte (Hsiang et al., 1999; Konig et al., 2000a). Other members, such as OATP2B1 and OATP1A2, show broader tissue specificity; OATP2B1, for example, is expressed in the intestine, placenta, brain, endothelial cells, and platelets (St-Pierre et al., 2002; Kobayashi et al., 2003; Grube et al., 2006b; Niessen et al., 2009). Substrates of OATP transport proteins include widely prescribed drugs, such as 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), angiotensin converting enzyme inhibitors, and antibiotics and anticancer drugs (Kobayashi et al., 2003; Hirano et al., 2004; Nozawa et al., 2005; Smith et al., 2005; Grube et al., 2006b; Ishiguro et al., 2006; Kitamura et al., 2008). Furthermore, bile acids and steroid hormone conjugates are endogenous substrates for these transporters (St-Pierre et al., 2002; Hagenbuch and Meier, 2004).
Concomitant administration of inhibitors and substrates of OATPs poses the risk of drug-drug interactions (DDIs), which can result in changes in pharmacokinetics and an increased risk of adverse events and/or reduced efficacy. Numerous studies investigating the effect of genetic polymorphisms and DDIs have highlighted the impact of altered hepatic OATP function on the pharmacokinetics of commonly used drugs. For example, coadministration of gemfibrozil, rifampicin, or cyclosporine A with statins increased the area under the plasma concentration-time profile of statins and the risk of developing rhabdomyolysis; these changes in statin disposition have been attributed primarily to decreased OATP-mediated hepatic uptake (Koenen et al., 2011).
In addition to drugs, several herbal ingredients, such as flavonoids, interact with drug uptake transport proteins of the OATP family. For example, the flavonoids apigenin, quercetin, and kaempferol inhibit the function of OATP1A2 and OATP2B1, which are localized in the apical membrane of the intestine (Mandery et al., 2010). Furthermore, green tea catechins, herbal extracts, and citrus and grapefruit juice affect OATP-mediated transport (Satoh et al., 2005; Fuchikami et al., 2006; Roth et al., 2011a,b).
In light of recent trends in the clinic toward the use of higher doses and improved formulations of silymarin, the present study investigated the influence of silymarin flavonolignans on the transport function of the major hepatic OATP proteins, OATP1B1, OATP1B3, and OATP2B1, to gain insights into possible herb-drug interactions. The potential clinical implications of these findings are discussed.
Materials and Methods
Materials.
[3H]Estrone-3-sulfate (E1S; 53.4 Ci/mmol) and [3H]estradiol-17β-glucuronide (E217G; 50.3 Ci/mmol) were purchased from Perkin Elmer (Waltham, MA); [3H]rosuvastatin (10 Ci/mmol) was obtained from American Radiolabeled Chemicals (St. Louis, MO). Unlabeled E1S, E217G, silymarin, and bromosulfophtalein (BSP) were purchased from Sigma-Aldrich (St. Louis, MO). Silychristin and silydianin were purchased from ChromaDex (Santo Anna, CA) and U.S. Pharmacopoeia (Rockville, MD), respectively. Isosilybin A and isosilybin B were a generous gift from Ulrich Mengs (Madaus GmbH, Germany). Silybin A and silybin B were isolated and purified as previously described (Graf et al., 2007). Cell culture supplies were purchased from Gibco (Life Technologies, Grand Island, NY). Dimethyl sulfoxide was purchased from Fisher Scientific (Fairlawn, NJ). Bio-Safe II liquid scintillation mixture was obtained from Research Products International (Mt. Prospect, IL). All other materials were purchased from Sigma-Aldrich (St. Louis, MO) or Invitrogen (Carlsbad, CA). Silymarin flavonolignans were dissolved in dimethyl sulfoxide, and stock solutions were stored at −20°C.
Cell Culture.
The human embryonic kidney (HEK) 293-Mock, HEK293-OATP1B3, and HEK293-OATP1B1 cell lines were kindly provided by Dr. Dietrich Keppler (German Cancer Research Center, Germany). The MDCKII-OATP2B1 cell line was kindly provided by Dr. Markus Grube (University of Greifswald, Germany). HEK293-OATP1B1, HEK293-OATP1B3 and HEK293-Mock, MDCKII-OATP2B1, and the parental MDCKII cell lines were grown in 75 cm2 cell culture flasks in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 mM L-glutamine and 10% fetal calf serum. The transfected cell lines were maintained in medium containing 350 µg/ml Hygromycin B (MDCKII cell lines) or 600 µg/ml G418 (HEK293 cell lines). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Freshly isolated human hepatocytes provided by Life Technologies (Durham, NC) and Triangle Research Laboratories, LLC (Research Triangle Park, NC), were seeded in 24-well plates at a seeding density of 350,000 cells per well in DMEM containing 5% (v/v) fetal bovine serum, 10 µM insulin, 1 µM dexamethasone, 1% (v/v) minimum essential medium nonessential amino acids, 2 mM L-glutamine, 100 units penicillin G, and 100 µg of streptomycin sulfate (seeding medium). After 1 hour incubation at 37°C, 5% CO2 in a humidified incubator, medium was aspirated to remove dead and unattached cells and replaced with fresh seeding medium. At day 1, either an uptake experiment was performed or hepatocytes were overlaid with Matrigel (0.25 mg/ml) and cultured in DMEM containing 1% insulin/transferrin/selenium (ITS + premix), 0.1 µM dexamethasone, 2 mM L-glutamine, 1% MEM nonessential amino acids, 100 units of penicillin G, and 100 µg of streptomycin; medium was changed every day. Human hepatocytes were cultured up to 8 days to allow polarization and canalicular network formation. The demographic characteristics of the human liver donors are shown in Table 1.
TABLE 1.
Demographic characteristics of human liver donors
| Donor | Sex | Race | Age | BMI | Alcohol/Smoking |
|---|---|---|---|---|---|
| Hu1369 | Female | White | 47 | 19.4 | 1 Beer/day |
| Hu1416 | Male | White | 58 | 28.1 | — |
| Hu1434 | Male | White | 55 | 25.6 | Yes |
| RTL Hu 4014 | Male | White | 20 | 19 | Not reported |
Transport Assays.
For transport studies using cell lines, cells were seeded in 24-well plates and cultured to confluence; after removing the medium, cells were washed twice with phosphate-buffered saline and incubated at 37°C with Hank’s balanced salt solution (HBSS) containing the substrate (pH 7.4). At specified time points, the transport buffer was aspirated rapidly, and cells were washed four times with ice-cold HBSS. Cells were solubilized in 0.5% Triton X-100 in phosphate-buffered saline, an aliquot was dissolved in 2 ml scintillation cocktail, and samples were measured in a scintillation β-counter. In all cases, the amount of substrate transported was normalized for protein content determined by the bicinchoninic acid protein assay. To determine the concentration-dependent effects of compounds on OATP-mediated uptake, experiments were performed as described above, except that specified concentrations of silymarin and silymarin flavonolignans (0–100 µM) were used.
For hepatocyte experiments, hepatocytes were rinsed twice with HBSS at 37°C. Subsequently, hepatocytes were incubated with [3H]rosuvastatin (0.5 µM, 1.5 minutes) or [3H]E217G (1 µM, 1 minute) in the presence of test compound or vehicle in HBSS at 37°C. After incubation, the dosing solution was aspirated and cells were washed 3 times with ice-cold HBSS. Cells were lysed in 0.2 ml of 0.5% (v/v) Triton-X100 in phosphate-buffered saline, and an aliquot was measured in a scintillation β-counter.
Because substrate uptake experiments were performed under sink conditions, apparent hepatic uptake clearance (CLuptake,app) of substrates, which includes total cellular accumulation resulting from passive diffusion, active transport, binding, and/or membrane partitioning, was calculated according to Eq. 1:
| (1) |
Inhibition of OATP-mediated uptake of E217G and rosuvastatin by silymarin flavonolignans in human hepatocytes was determined after subtraction of the non–OATP-mediated component of accumulation, which was determined as the accumulation of the respective substrate in the presence of 100 µM BSP (an inhibitor of OATPs). For each experiment, this OATP-mediated accumulation value was set at 100%, data were expressed as a percentage of vehicle control, and the mean and S.E.M. of three experiments was reported. Uptake was corrected for nonspecific binding by incubating collagen-coated wells without hepatocytes. Furthermore, accumulation and clearance values were normalized for protein content measured by bicinchoninic acid protein assay.
Statistics.
The OATP-mediated net uptake was calculated by subtracting the substrate uptake in the parental cell lines (OATP-negative) from the uptake in OATP-expressing cells. Uptake inhibition, presented as percentage of control, was calculated from control experiments in the presence of vehicle, which was set as 100% uptake. Results are presented as mean ± S.D., mean ± range, or mean ± S.E.M., as indicated. The half-maximal inhibitory concentration (IC50) values were calculated by fitting dose-response curves to the data by nonlinear regression using Prism software 5.0 (GraphPad Software Inc., La Jolla, CA), with use of three models: (1) four parameter fit, (2) three parameter fit with a fixed bottom, and (3) three parameter fit with a fixed top. IC50 values are reported from the model that best described each data set based on goodness of fit parameters.
Statistical analysis of the data was conducted using a repeated-measures analysis of variance, followed by Dunnet’s post hoc test. Differences were considered to be statistically significant when P ≤ 0.05.
Estimation of Portal Vein Concentrations.
Maximal unbound portal vein concentrations (Cu,max,in) were estimated using the method described by Ito et al. to assess the relevance of the inhibitory effect of silymarin flavonolignans on the transport function of the investigated OATPs according to Eq. 2 (Ito et al., 1998):
| (2) |
The maximal plasma concentration (Cmax), the dose (D), the fraction absorbed from the gastrointestinal tract (Fa), and hepatic blood flow (Q) were obtained from the literature (Table 3). We previously determined an absorption rate constant (ka) of 0.669 hour−1 for silybin A in patients with liver disease with use of a population pharmacokinetic approach (unpublished data). However, because this study was conducted in a diseased population and the absorption rate constant (ka) for other silymarin flavonolignans has not been described in the literature, ka was set to the theoretical maximal value (0.1 minute−1, which is the maximal gastric emptying rate constant in humans) (Table 3). The fraction unbound (fu) of silibinin (silybin A and silybin B 1:1) was 0.05-0.1 (personal communication. Dr. Ulrich Mengs, Madaus GmbH).
TABLE 3.
Estimation of portal vein concentrations for individual parent flavonolignans
A bold [I]/IC50 number indicates that the value is above the cutoff of 0.1.
| Variable | Silybin A | Silybin B | Reference | ||
|---|---|---|---|---|---|
| Dose of silymarin (mg) | 700 | 140 | 700 | 140 | (Hawke et al., 2010) |
| [Dose of individual flavonolignans (mg)] | [116] | [23.2] | [160] | [32] | |
| Cmax (µg/ml) | 0.58 | 0.04 | 0.22 | 0.008 | (Hawke et al., 2010) |
| ka (Absorption rate constant; min−1) | 0.1 | (Ito et al., 1998) | |||
| Q (Hepatic blood flow; ml/min) | 1500 | (Wynne et al., 1989) | |||
| fu (Fraction unbound) | 0.05 | Dr. Mengs, Madaus GmbH, Germany | |||
| fa (Fraction absorbed) | 0.2 | 0.62 | From rat (Pade, 2007) | ||
| Cu,max,in (unbound portal vein conc.; µM) | 0.22 | 0.04 | 0.70 | 0.13 | Calculated |
| [I]/IC50 ratio OATP1B1 | 0.02 | 0.004 | 0.08 | 0.02 | Calculated |
| [I]/IC50 ratio OATP1B3 | 0.08 | 0.01 | 0.14 | 0.03 | Calculated |
| [I]/IC50 ratio OATP2B1 | 0.05 | 0.01 | 0.88 | 0.16 | Calculated |
Results
Characterization of OATP-Overexpressing Cell Lines.
HEK293-OATP1B1, HEK293-OATP1B3, and MDCKII-OATP2B1 cells have been described previously (Konig et al., 2000a,b; Grube et al., 2006a). Uptake of E1S (1 µM; OATP2B1) and E217G (1 µM; OATP1B1 and OATP1B3) was linear up to 5 minutes (unpublished data). Therefore, subsequent experiments using these cell lines were performed at 3 minutes.
Influence of Silymarin Flavonolignans on OATP-Mediated Uptake in Overexpressing Cell Lines.
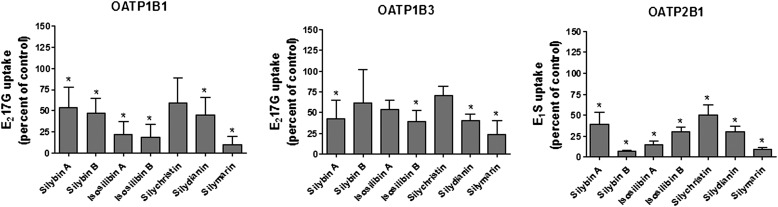
To determine the effect of silymarin on OATP function, OATP-mediated uptake of E217G (OATP1B1, OATP1B3) and E1S (OATP2B1) was measured in the presence of silymarin or individual silymarin flavonolignans (10 μM each). As illustrated in Fig. 2, all tested silymarin flavonolignans significantly inhibited OATP2B1-mediated E1S uptake. For OATP1B1, all flavonolignans except silychristin significantly inhibited E217G uptake, whereas OATP1B3-mediated E217G transport was significantly inhibited only by silybin A, isosilybin B, silydianin, and silymarin.
Fig. 2.
Effect of silymarin flavonolignans on OATP-mediated substrate uptake. Cells were incubated with [3H]E217G (1 μM; OATP1B1, OATP1B3) or [3H]E1S (1 μM, OATP2B1) and 10 μM of the flavonolignans indicated or with the vehicle control for 3 minutes at 37°C. OATP-mediated uptake was calculated after correcting for protein by subtracting uptake into empty vector (OATP1B1, OATP1B3) or nontransfected control cells (OATP2B1). Values are expressed as a percentage of control; each value is presented as the mean ± S.D. of at least three independent experiments performed in triplicate. *P ≤ 0.05, compared with control.
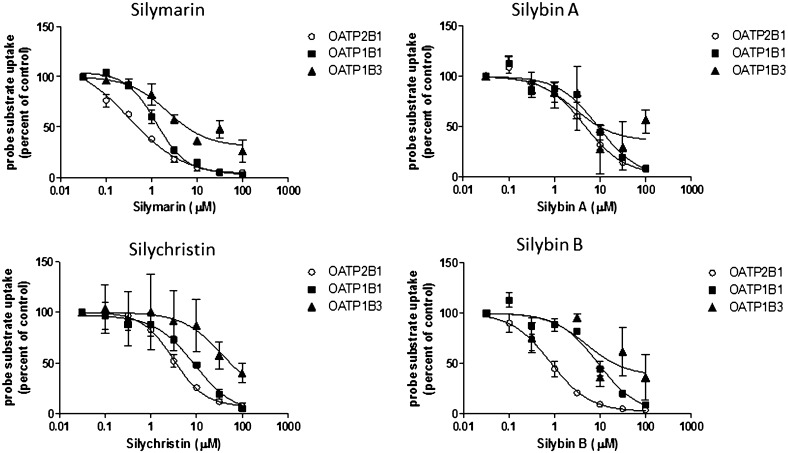
The concentration-dependent inhibition of OATP-mediated substrate uptake was investigated further for silymarin and the individual flavonolignans silybin A, silybin B, and silychristin, which are the main constituents of silymarin, each comprising up to 35% of the mixture. As shown in Fig. 3 and Table 2, the interaction of individual silymarin flavonolignans with OATP2B1, compared with inhibition of OATP1B1- and 1B3-mediated substrate uptake, appeared to be very potent, in general. Silymarin was the strongest inhibitor of all OATPs (IC50 values of 1.3, 2.2, and 0.3 µM for OATP1B1, OATP1B3, and OATP2B1, respectively). Silybin B inhibited OATP2B1 uptake more than silybin A or silychristin (IC50 values of 0.8 µM versus 4.5 µM and 3.6 µM, respectively). Silybin A, silybin B, and silychristin were almost equipotent in inhibiting OATP1B1-mediated substrate uptake, whereas OATP1B3-mediated substrate uptake was inhibited more strongly by silybin A and silybin B (IC50 values of 2.7 and 5.0 µM, respectively), compared with silychristin (IC50 value of 36.4 µM).
Fig. 3.
Concentration-dependent modulation of E1S and E217G uptake into OATP-overexpressing cell lines by silymarin and silymarin flavonolignans. Cells were coincubated with silymarin flavonolignans (0–100 μM) and [3H]E1S (0.25 µCi/ml; 1 µM; OATP2B1) or [3H]E217G (0.25 µCi/ml; 1 µM; OATP1B1, OATP1B3) for 3 minutes at 37°C. Values are expressed as percentage of vehicle control; each value represents the mean ± range of two independent experiments performed in duplicate.
TABLE 2.
IC50 values and 95% confidence intervals (CIs) for inhibition of OATP-mediated transport (E217G for OATP1B1 and OATP1B3 and E1S for OATP2B1)
| Cell Line | Compound | IC50 | 95% CI |
|---|---|---|---|
| μM | |||
| OATP1B1 | Silymarin | 1.3a | 1.0–1.6 |
| Silybin A | 9.7b | 5.3–17.7 | |
| Silybin B | 8.5b | 5.6–12.9 | |
| Silychristin | 9.0b | 6.0–13.4 | |
| OATP1B3 | Silymarin | 2.2c | 1.1–4.7 |
| Silybin A | 2.7c | 0.7–11.0 | |
| Silybin B | 5.0c | 0.5–52.4 | |
| Silychristin | 36.4c | 1.6–855 | |
| OATP2B1 | Silymarin | 0.3a | 0.2–0.7 |
| Silybin A | 4.5b | 2.7–7.8 | |
| Silybin B | 0.8b | 0.6–1.1 | |
| Silychristin | 3.6a | 2.5–4.0 |
Four parameter fit.
Three parameter fit with fixed bottom.
Three parameter fit with fixed top; IC50 values were estimated from a maximal inhibition that was less than 100%.
Estimation of Portal Vein Concentrations.
To evaluate the potential impact of silymarin flavonolignans on inhibition of hepatic OATPs, maximal unbound portal vein concentrations (Cu,max,in) were estimated on the basis of a method described by Ito et al. (Ito et al., 1998). Because the absorbed fraction of silymarin flavonolignans in humans has not been reported, absolute bioavailability data from rats were used under the assumption that the fraction absorbed is at least equal to absolute bioavailability and similar in rat and human. To determine the inhibition potential for hepatic OATPs, the unbound fraction must also be taken into account. Although no protein binding data have been reported in humans for these compounds, the protein binding of silibinin (silybin A and silybin B) is 70% in rat (Wu et al., 2007). With use of the ADMET predictor, the unbound fraction of silybin A and silybin B was estimated to be approximately 1.5% in humans. An unbound fraction of 5%–10% in human plasma was determined experimentally for silibinin (silybin A and B 1:1) (personal communication, Dr. Ulrich Mengs, Madaus GmbH). To calculate the unbound portal vein concentration an unbound fraction of 5% was assumed (Table 3).
Influence of Silymarin Flavonolignans on E217G and Rosuvastatin Uptake in Human Hepatocytes.
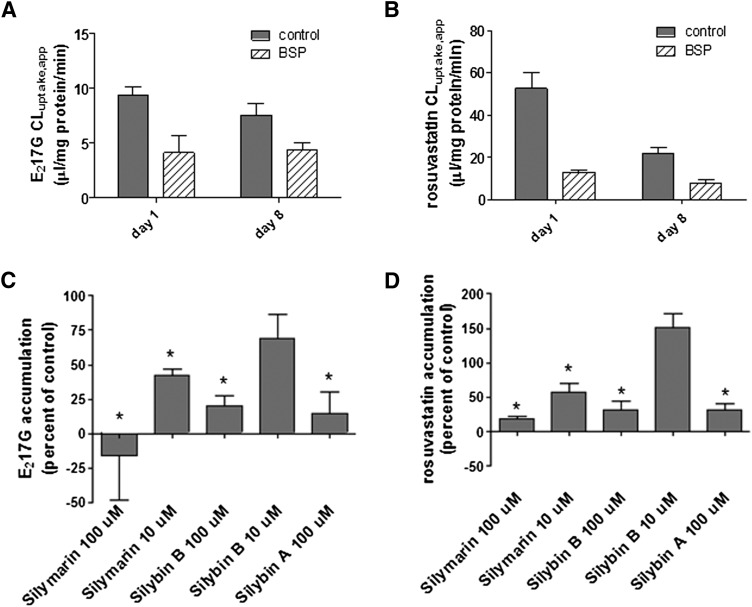
E217G and rosuvastatin were used to investigate the effect of silymarin, silybin A, and silybin B on OATP-mediated uptake in human hepatocytes. BSP, a potent inhibitor of OATPs, was used to assess passive permeation and other active uptake processes in the absence of OATP-mediated substrate transport. BSP (100 µM) decreased substrate uptake into OATP1B1-, OATP1B3-, and OATP2B1-overexpressing cell lines to values observed in the parental cell lines (unpublished data).
In sandwich-cultured human hepatocytes, the apparent uptake clearance of E217G was 9.7 and 7.5 µl/mg protein/min for day 1 and 8 of culture, respectively. For rosuvastatin, apparent uptake clearance values of 52.6 µl/mg protein/min and 21.7 µl/mg protein/min on days 1 and 8, respectively, were observed. BSP inhibited the apparent uptake clearance of E217G in sandwich-cultured hepatocytes to ∼45% and ∼60% of control values on day 1 and day 8 of culture, respectively; BSP inhibited the apparent uptake clearance of rosuvastatin to ∼25% and ∼36% of uptake in control-treated cells on day 1 and day 8 of culture, respectively. These data suggested that some passive diffusion, non–OATP-mediated transport and/or binding/partitioning processes were involved in accumulation of these probe substrates (Fig. 4, A and B). On day 1, OATP-mediated uptake of E217G and rosuvastatin was inhibited significantly by silymarin, silybin B, and silybin A (100 µM) and 10 µM silymarin (Fig. 4, C and D). Similar results were obtained for sandwich-cultured hepatocytes on day 8; however, in contrast to day 1, the E217G uptake was not significantly inhibited by silybin A at a concentration of 100 µM (inhibition to 58% ± 35% of control) (unpublished data).
Fig. 4.
Inhibition of OATP-mediated E217G and rosuvastatin accumulation in sandwich-cultured human hepatocytes by silymarin and silymarin flavonolignans. (A and B) Apparent uptake clearance (CLuptake,app) of E217G (0.25 µCi/ml; 1 µM, 1 minute) and rosuvastatin (0.05 µCi/ml; 0.5 µM, 1.5 minute) in sandwich-cultured human hepatocytes on day 1 and day 8 of culture determined in the absence (control, solid bars) or presence (hatched bars) of 100 µM BSP. Values represent the mean ± S.E.M. of three (rosuvastatin) or four (E217G) independent experiments performed in triplicate. (C and D) Human hepatocytes (day 1) were coincubated with silymarin flavonolignans and [3H]E217G (0.25 µCi/ml; 1 µM) for 1 minute or [3H]rosuvastatin (0.05 µCi/ml; 0.5 µM) for 1.5 minute at 37°C. To calculate OATP-mediated uptake, substrate accumulation was determined in the presence of 100 µM BSP; after subtraction of this value, accumulation in the presence of silymarin or silymarin flavonolignans was expressed as a percentage of vehicle control. Values represent the mean ± S.E.M. of three independent experiments performed in triplicate. *P ≤ 0.05, compared with control.
Discussion
Silymarin, the extract of milk thistle, is widely used as a dietary supplement by patients with liver and biliary tract disease because of its reported hepatoprotective properties. Because of the common perception that herbal supplements are generally safe, silymarin is often comedicated with conventional drugs, raising the potential for herb-drug interactions. Members of the OATP family of transport proteins are responsible for the hepatic uptake of many clinically important drugs, including statins, angiotensin-converting enzyme inhibitors, and anticancer therapeutics, and some endogenous compounds, such as bilirubin. Several clinical studies have shown that the activity of these transporters can determine the efficacy and adverse events of drugs (Koenen et al., 2011). Therefore, the goal of the present study was to investigate the interaction potential of individual constituents of silymarin extract with hepatic OATPs to gain insights into possible silymarin-drug interactions.
The present study indicated that silymarin flavonolignans significantly inhibited OATP transport in overexpressing cell lines and human hepatocytes (Figs. 2–4). Of interest, despite the structural similarity and identical molecular weight (Fig. 1), the individual silymarin flavonolignans differentially inhibited OATP-mediated transport. These findings suggest that stereo- and regiochemistry modify the interaction potential with OATP transport proteins; IC50 values varied by ∼10-fold among the individual flavonolignans (Table 2). This is consistent with results reported from recent clinical and in vitro metabolism studies in which the diastereomers of silybin (A and B), and the isomers isosilybin A and isosilybin B exhibited different pharmacokinetic properties and inhibition potential for CYP-mediated metabolism (Brantley et al., 2010; Hawke et al., 2010).
To assess the clinical interaction potential of drugs/compounds with uptake transport proteins, the International Transporter Consortium recently recommended a cutoff value of [I]/IC50 > 0.1, where [I] represents the inhibitor concentration, for performing in vivo drug interaction studies (Giacomini et al., 2010). Of note, the total (bound and unbound) systemic concentrations of silymarin flavonolignans are generally low. The maximal steady-state concentrations (Cmax) of total unconjugated silybin A and silybin B, after a dose of 140 mg of silymarin three times per day in patients with chronic hepatitis C was 40 ng/ml (0.08 µM) and 8 ng/ml (0.016 µM), respectively, which is at least 10-fold lower than the IC50 values reported for OATP inhibition in the current study (Hawke et al., 2010). However, in a recently completed large, placebo-controlled clinical trial in patients with hepatitis C virus infection receiving silymarin doses of 420 and 700 mg three times per day, plasma concentrations up to 2048 ng/ml (4.2 µM) were observed for silybin A (Fried et al., 2012). Assuming an unbound fraction of 5%, the unbound concentration of silybin A achieved in this clinical study was approximately 10–50-fold lower than the concentrations associated with the IC50 values observed for inhibition of OATP-mediated uptake in the present study. Because the low customary doses of silymarin, which are used by patients with liver disease, do not achieve high systemic concentrations of silymarin flavonolignans, the potential for DDIs appears to be low. However, systemic concentrations may not be the best measure to assess the interaction potential with hepatic uptake transport proteins; for compounds that undergo extensive presystemic elimination, unbound portal vein concentrations are more applicable. On the basis of estimated unbound portal vein concentrations (Ito et al., 1998), the silybin B concentrations after high-dose silymarin supplementation are within the same micromolar range as the IC50 value determined for inhibition of OATP2B1 (Tables 2 and 3). Furthermore, the overall lower IC50 values for silymarin, compared with the individual flavonolignans, suggest that there might be synergy between the silymarin constituents with respect to OATP inhibition. However, because of differential pharmacokinetics of these compounds, the actual unbound portal vein concentrations of each component are difficult to assess in humans. On the basis of estimations, the maximal total unbound portal vein concentrations of the major flavonolignans in the systemic circulation (silybin A and silybin B) add up to ∼0.17 and 0.92 µM for a dose of 140 mg and 700 mg silymarin, respectively; the [I]/IC50 ratio observed for inhibition of OATP-mediated transport by silymarin (IC50 values of 1.3, 2.2, and 0.3 µM for OATP1B1, OATP1B3, and OATP2B1, respectively) yielded values that were above the cutoff of 0.1 recommended by the International Transporter Consortium to initiate further studies to assess the interaction potential with uptake transport proteins. Other flavonolignans present in the silymarin extract were excluded from this calculation, because their systemic exposure is negligible after oral silymarin administration. However, this does not exclude the possibility that portal concentrations of these flavonolignans could be significant and contribute to inhibition of OATPs.
Several in vivo studies have investigated the interaction between silymarin and drugs, such as digoxin, nifedipine, indinavir, ranitidine, and rosuvastatin (Wu et al., 2009). Of those drugs, only digoxin and rosuvastatin have been described as OATP substrates, although the role of OATPs in digoxin transport has been questioned (Taub et al., 2011). Silymarin administration (140 mg three times per day) did not affect the pharmacokinetics of the OATP substrate rosuvastatin in healthy subjects, despite inhibition of OATP1B1 function by silymarin in OATP1B1-overexpressing oocytes (Deng et al., 2008). The authors concluded that pretreatment with silymarin does not result in a risk for drug interactions between silymarin and rosuvastatin in vivo. However, in the study by Deng et al., a customary dose of 140 mg silymarin was administered three times daily. We previously demonstrated that silymarin flavonolignans do not reach peak plasma concentrations above 0.2 µM with use of this dose regimen (Hawke et al., 2010); these concentrations are significantly lower than those associated with drug interaction risk identified in the present study. However, higher oral doses and improved formulations of silymarin (e.g., nanoemulsions) are being evaluated to increase systemic/tissue concentrations to achieve desired clinical outcomes (Flaig et al., 2010; Li et al., 2010; Wang et al., 2012). On the basis of the present study, these higher doses and/or improved formulations may increase the risk of OATP-mediated interactions.
In hepatocytes at day 1 of culture, 100 µM silymarin, silybin A, or silybin B significantly inhibited E217G and rosuvastatin transport. These concentrations were above the observed IC50 value for all investigated OATPs; thus, almost complete ablation of OATP-mediated transport would be expected. Silymarin and individual flavonolignan concentrations of 10 µM are close to the observed IC50 values for OATP inhibition, resulting in less pronounced inhibition of transport (Fig. 4; Table 2). Indeed, for silybin B, no significant reduction in substrate uptake was observed. To assess the overall effect on hepatic substrate uptake, it is important to appreciate the contribution of individual transport proteins to substrate uptake, the relative protein expression levels, and the potency of the inhibitor toward the respective transport protein. For example, OATP1B1 is primarily involved in rosuvastatin uptake, with only 16–34% attributable to OATP1B3. Because of the lower expression levels of OATP2B1 in hepatocytes, the contribution of this transport protein to hepatic uptake of rosuvastatin was deemed to be negligible (Kitamura et al., 2008). However, for other substrates, the contribution of the individual transport proteins might differ. Compounds will affect the overall substrate uptake differently, based on their inhibition potential for the individual proteins, which was highlighted by predictions in the study by Karlgren et al. (2012).
Because of the low estimated portal vein concentrations (Table 3), interaction with hepatic OATP-mediated uptake processes is relatively unlikely, especially at the low dose of silymarin recommended for supplementary use. However, our results demonstrate that silymarin flavonolignans not only inhibit OATP1B1 but also OATP2B1. While OATP1B1 and OATP1B3 are expressed almost exclusively in hepatocytes, OATP2B1 exhibits ubiquitous expression and localization in the apical plasma membrane of enterocytes, where this transport protein is believed to play an important role in uptake of substrates from the intestinal lumen into enterocytes (Nozawa et al., 2004). A dose containing 140 mg of silymarin extract as a dietary supplement would result in a theoretical maximal gastrointestinal concentration of ∼1 mM if taken with 250 ml water. Because of silymarin’s low water solubility of 0.4 mg/ml (Woo et al., 2007), concentrations in the range of 0.8 mM may be more realistic. Regardless, the estimated maximal gastrointestinal concentration of silymarin is significantly greater than the IC50 for OATP2B1 inhibition, which could result in lower bioavailability of OATP2B1 substrates when administered orally with silymarin. Although drugs that are predominately absorbed by an OATP2B1-mediated process have yet to be identified, current drugs that may be partly dependent on OATP2B1-mediated transport include aliskiren, montelukast, and glibenclamide (Vaidyanathan et al., 2008; Mougey et al., 2009, 2011; Tapaninen et al., 2011). Recently, scutallarin, an active flavonoid in Erigheron brevisacapus extract was demonstrated to be a specific substrate for OATP2B1 (Gao et al., 2012).
In conclusion, the present data suggest that silymarin flavonolignans inhibit the transport of OATP substrates in overexpressing cell lines and in human hepatocytes. Estimations of the maximal portal vein concentrations indicate a low risk for silymarin-drug interactions at the hepatic transport protein level, especially at the recommended silymarin dose of 140 mg. However, the use of higher silymarin doses or silymarin formulations with improved bioavailability might increase portal vein concentrations and, thus, may increase the risk of OATP-mediated drug interactions.
Acknowledgments
The HEK293-Mock, HEK293-OATP1B1, and HEK293-OATP1B3 cell lines were kindly provided by Dr. Dietrich Keppler (German Cancer Research Center, Germany). The MDCKII-OATP2B1 cells were kindly provided by Dr. Markus Grube (University of Greifswald, Germany). Freshly isolated human hepatocytes were generously provided by Life Technologies (Durham, NC) and Triangle Research Laboratories, LLC (Research Triangle Park, NC).
Abbreviations
- BSP
bromosulfophthalein
- DDI
drug-drug interaction
- DMEM
Dulbecco’s modified Eagle’s medium
- E1S
estrone-3-sulfate
- E217G
estradiol-17β-glucuronide
- HBSS
Hanks’ balanced salt solution
- HEK
human embryonic kidney
- OATP
organic anion-transporting polypeptide
Authorship Contributions
Participated in research design: Köck, Brouwer, Ying, Hawke.
Conducted experiments: Köck.
Contributed new reagents or analytic tools: Oberlies.
Performed data analysis: Köck.
Wrote or contributed to the writing of the manuscript: Köck, Brouwer, Ying, Oberlies, Hawke.
Footnotes
This work was supported by the National Institutes of Health National Center for Research Resources and the National Center for Advancing Translational Sciences [Grant UL1TR000083]; the National Institutes of Health National Institute of General Medical Sciences [Grant R01GM41935]; and Deutsche Forschungsgemeinschaft [Grant Ko4186/1-1]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Brantley SJ, Oberlies NH, Kroll DJ, Paine MF. (2010) Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations. J Pharmacol Exp Ther 332:1081–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JW, Shon JH, Shin HJ, Park SJ, Yeo CW, Zhou HH, Song IS, Shin JG. (2008) Effect of silymarin supplement on the pharmacokinetics of rosuvastatin. Pharm Res 25:1807–1814 [DOI] [PubMed] [Google Scholar]
- Flaig TW, Glodé M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, Su LJ, Li Y, Harrison G, Agarwal R, et al. (2010) A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate 70:848–855 [DOI] [PubMed] [Google Scholar]
- Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, Sinha R, Everhart JE, HALT-C Trial Group (2011) Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. Aliment Pharmacol Ther 33:127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, Doo E, Meyers CM, Reddy KR, Silymarin in NASH and C Hepatitis (SyNCH) Study Group (2012) Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA 308:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y. (2006) Effects of herbal extracts on the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 34:577–582 [DOI] [PubMed] [Google Scholar]
- Gao C, Zhang H, Guo Z, You T, Chen X, Zhong D. (2012) Mechanistic studies on the absorption and disposition of scutellarin in humans: selective OATP2B1-mediated hepatic uptake is a likely key determinant for its unique pharmacokinetic characteristics. Drug Metab Dispos 40:2009–2020 [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf TN, Wani MC, Agarwal R, Kroll DJ, Oberlies NH. (2007) Gram-scale purification of flavonolignan diastereoisomers from Silybum marianum (Milk Thistle) extract in support of preclinical in vivo studies for prostate cancer chemoprevention. Planta Med 73:1495–1501 [DOI] [PubMed] [Google Scholar]
- Grube M, Köck K, Karner S, Reuther S, Ritter CA, Jedlitschky G, Kroemer HK. (2006a) Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol 70:1735–1741 [DOI] [PubMed] [Google Scholar]
- Grube M, Köck K, Oswald S, Draber K, Meissner K, Eckel L, Böhm M, Felix SB, Vogelgesang S, Jedlitschky G, et al. (2006b) Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther 80:607–620 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B and Meier PJ (2004) Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665. [DOI] [PubMed]
- Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, et al. SyNCH Trial Group (2010) Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol 50:434–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. (2004) Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther 311:139–146 [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. (1999) A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem 274:37161–37168 [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Maeda K, Kishimoto W, Saito A, Harada A, Ebner T, Roth W, Igarashi T, Sugiyama Y. (2006) Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos 34:1109–1115 [DOI] [PubMed] [Google Scholar]
- Ito K, Iwatsubo T, Kanamitsu S, Ueda K, Suzuki H, Sugiyama Y. (1998) Prediction of pharmacokinetic alterations caused by drug-drug interactions: metabolic interaction in the liver. Pharmacol Rev 50:387–412 [PubMed] [Google Scholar]
- Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, Haglund U, Artursson P. (2012) Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem 55:4740–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Maeda K, Wang Y, Sugiyama Y. (2008) Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos 36:2014–2023 [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2003) Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther 306:703–708 [DOI] [PubMed] [Google Scholar]
- Koenen A, Kroemer HK, Grube M, and Meyer zu Schwabedissen HE (2011) Current understanding of hepatic and intestinal OATP-mediated drug-drug interactions. Expert review of clinical pharmacology 4:729-742. [DOI] [PubMed]
- König J, Cui Y, Nies AT, Keppler D. (2000a) Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275:23161–23168 [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000b) A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol 278:G156–G164 [DOI] [PubMed] [Google Scholar]
- Letschert K, Faulstich H, Keller D, and Keppler D (2006) Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci 91:140–149. [DOI] [PubMed]
- Li X, Yuan Q, Huang Y, Zhou Y, Liu Y. (2010) Development of silymarin self-microemulsifying drug delivery system with enhanced oral bioavailability. AAPS PharmSciTech 11:672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandery K, Bujok K, Schmidt I, Keiser M, Siegmund W, Balk B, König J, Fromm MF, Glaeser H. (2010) Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol 80:1746–1753 [DOI] [PubMed] [Google Scholar]
- Mengs U, Pohl RT, Mitchell T. (2012) Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharm Biotechnol 13:1964–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougey EB, Feng H, Castro M, Irvin CG, Lima JJ. (2009) Absorption of montelukast is transporter mediated: a common variant of OATP2B1 is associated with reduced plasma concentrations and poor response. Pharmacogenet Genomics 19:129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougey EB, Lang JE, Wen X, Lima JJ. (2011) Effect of citrus juice and SLCO2B1 genotype on the pharmacokinetics of montelukast. J Clin Pharmacol 51:751–760 [DOI] [PubMed] [Google Scholar]
- Niessen J, Jedlitschky G, Grube M, Bien S, Schwertz H, Ohtsuki S, Kawakami H, Kamiie J, Oswald S, Starke K, et al. (2009) Human platelets express organic anion-transporting peptide 2B1, an uptake transporter for atorvastatin. Drug Metab Dispos 37:1129–1137 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. (2004) Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther 308:438–445 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. (2005) Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos 33:434–439 [DOI] [PubMed] [Google Scholar]
- Pade DS(2007) Use of in silico predictors, solubility and permeability to select bioavailability and bioequivalence markers in herbal supplements, University of Texas at Austin, Thesis. [Google Scholar]
- Roth M, Araya JJ, Timmermann BN, Hagenbuch B. (2011a) Isolation of modulators of the liver-specific organic anion-transporting polypeptides (OATPs) 1B1 and 1B3 from Rollinia emarginata Schlecht (Annonaceae). J Pharmacol Exp Ther 339:624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Timmermann BN, Hagenbuch B. (2011b) Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos 39:920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. (2005) Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 33:518–523 [DOI] [PubMed] [Google Scholar]
- Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, Lindsay KL, Lok AS, Di Bisceglie AM, et al. HALT-C Trial Group (2008) Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology 47:605–612 [DOI] [PubMed] [Google Scholar]
- Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. (2005) Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther 4:815–818 [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. (2002) Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab 87:1856–1863 [DOI] [PubMed] [Google Scholar]
- Tapaninen T, Neuvonen PJ, Niemi M. (2011) Orange and apple juice greatly reduce the plasma concentrations of the OATP2B1 substrate aliskiren. Br J Clin Pharmacol 71:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub ME, Mease K, Sane RS, Watson CA, Chen L, Ellens H, Hirakawa B, Reyner EL, Jani M, Lee CA. (2011) Digoxin is not a substrate for organic anion-transporting polypeptide transporters OATP1A2, OATP1B1, OATP1B3, and OATP2B1 but is a substrate for a sodium-dependent transporter expressed in HEK293 cells. Drug Metab Dispos 39:2093–2102 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S, Camenisch G, Schuetz H, Reynolds C, Yeh CM, Bizot MN, Dieterich HA, Howard D, Dole WP. (2008) Pharmacokinetics of the oral direct renin inhibitor aliskiren in combination with digoxin, atorvastatin, and ketoconazole in healthy subjects: the role of P-glycoprotein in the disposition of aliskiren. J Clin Pharmacol 48:1323–1338 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang L, Liu Z, Zhang D, and Zhang Q (2012) In vivo evaluation of silybin nanosuspensions targeting liver. J Biomed Nanotechnol 8:760–769. [DOI] [PubMed]
- Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. (2008) Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos 36:65–72 [DOI] [PubMed] [Google Scholar]
- Woo JS, Kim TS, Park JH, Chi SC. (2007) Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch Pharm Res 30:82–89 [DOI] [PubMed] [Google Scholar]
- Wu JW, Lin LC, Hung SC, Chi CW, Tsai TH. (2007) Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J Pharm Biomed Anal 45:635–641 [DOI] [PubMed] [Google Scholar]
- Wu JW, Lin LC, Tsai TH. (2009) Drug-drug interactions of silymarin on the perspective of pharmacokinetics. J Ethnopharmacol 121:185–193 [DOI] [PubMed] [Google Scholar]
- Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. (1989) The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 9:297–301 [DOI] [PubMed] [Google Scholar]