Abstract
The nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor is the fourth and most recently discovered member of the opioid receptor superfamily that also includes μ, δ, and κ opioid receptor subtypes (MOR, DOR, and KOR, respectively). The widespread anatomic distribution of the NOP receptor enables the modulation of several physiologic processes by its endogenous agonist, N/OFQ. Accordingly, the NOP receptor has gained a lot of attention as a potential target for the development of ligands with therapeutic use in several pathophysiological states. NOP receptor activation frequently results in effects opposing classic opioid receptor action; therefore, regulation of the NOP receptor and conditions affecting its modulatory tone are important to understand. Mounting evidence reveals a heterologous interaction of the NOP receptor with other G protein–coupled receptors, including MOR, DOR, and KOR, which may subsequently influence their function. Our focus in this review is to summarize and discuss the findings that delineate the cellular mechanisms of NOP receptor signaling and regulation and the regulation of other receptors by N/OFQ and the NOP receptor.
Introduction
The multiplicity of opioid receptor subtypes enables opiates and endogenous opioid peptides to elicit diverse physiologic and pharmacological actions. Before the cloning of the NOP receptor [also known as opioid receptor–like 1, KOR3, OP4 (Bunzow et al., 1994; Chen et al., 1994; Fukuda et al., 1994; Mollereau et al., 1994; Wang et al., 1994; Wick et al., 1994; Pan et al., 1995)], the opioid receptor superfamily consisted of the classical MOR, DOR, and KOR. The NOP receptor sequence is approximately 50–60% identical to the classic opioid receptors; however, neither endogenous opioid peptides nor selective MOR, KOR, and DOR ligands [with the exception of KOR agonist, dynorphin A (Zhang et al., 1998)] bind to or activate the NOP receptor (Bunzow et al., 1994; Chen et al., 1994; Mollereau et al., 1994; Wang et al., 1994; Wick et al., 1994; Fukuda et al., 1997). Subsequently, two groups independently identified an endogenous neuropeptide similar to KOR agonist dynorphin A with little or no affinity for classic opioid receptor subtypes but high affinity for NOP receptor, emphasizing that this peptide and the NOP receptor are pharmacologically unique from the other opioid receptors (Meunier et al., 1995; Reinscheid et al., 1995). Reinscheid et al. named this heptadecapeptide orphanin FQ (OFQ), where “orphanin” refers to the peptide affinity for the recently cloned orphan opioid receptor; “F” (phenylalanine) and “Q” (glutamine) denote the first and last amino acids of the peptide, respectively (Reinscheid et al., 1995). Meunier et al. chose to name the peptide nociceptin (N) because of its pronociceptive activity (Meunier et al., 1995). For the purpose of this review, we have used both terms as denoted by N/OFQ for addressing this peptide.
Corresponding to the widespread distribution of the NOP receptor in the central and peripheral nervous systems [reviewed in Mollereau and Mouledous (2000) and Civelli (2008)], N/OFQ activation of the NOP receptor modulates many physiologic responses/systems, including anxiety (Jenck et al., 1997), food intake (Pomonis et al., 1996), learning (Sandin et al., 1997), locomotor (Reinscheid et al., 1995; Florin et al., 1996), respiratory (Fischer et al., 1998; Shah et al., 1998), immune (Peluso et al., 2001; Serhan et al., 2001), and cardiovascular and renal functions (Kapusta et al., 1997). To maintain this important modulatory role, the N/OFQ/-NOP receptor system therefore requires extensive and intricate regulation. Our focus in this review is to outline the findings that have advanced our understanding of NOP receptor signaling at the cellular level, how that signaling impacts NOP receptor function, and how N/OFQ and NOP receptors modulate other receptors.
NOP Receptor Signaling
The NOP receptor, like other opioid receptors, is a prototypical G protein–coupled receptor (GPCR) that couples to pertussis toxin–sensitive (Ma et al., 1997) and –insensitive (Chan et al., 1998) G proteins. After agonist activation, the NOP receptor triggers intracellular signaling events, including inhibition of adenylyl cyclase (Ma et al., 1997) and activation of protein kinase C (PKC) (Lou et al., 1997), phospholipase A (Fukuda et al., 1998) and C (Lou et al., 1997), extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Fukuda et al., 1997; Lou et al., 1997), p38 mitogen-activated protein kinase (Zhang et al., 1999a), c-Jun N-terminal kinase (JNK) (Chan and Wong, 2000), nuclear factor κB (NFκB) (Donica et al., 2011), and modulation of calcium (Connor et al., 1996b) and potassium channel conductance (Connor et al., 1996a). N/OFQ-mediated inhibition of presynaptic, voltage-gated calcium channels has been demonstrated in vitro (Connor et al., 1996b) and in vivo (Knoflach et al., 1996). Furthermore, activation of the NOP receptor opens inwardly, rectifying potassium channels, and produces a postsynaptic hyperpolarization, preventing excitation or propagation of action potentials (Vaughan et al., 1997). The significance of kinase activation and channel modulation by N/OFQ directly relates to the ability of N/OFQ to modulate neurotransmitter release, immune function, and transcriptional activation.
N/OFQ modulation of ion channel activity appears to be responsible for its inhibition of acetylcholine, serotonin, dopamine, β-endorphin, norepinephrine, glutamate, and GABA neurotransmission in several brain regions, including, but not limited to, the locus coeruleus, periaqueductal gray (PAG), dorsal raphe nucleus, hippocampus, hypothalamus, ventral tegmental area, spinal cord, and rostral ventral medulla (Connor et al., 1996a; Vaughan and Christie, 1996; Vaughan et al., 1997; Yu et al., 1997; Connor and Christie, 1998; Wagner et al., 1998; Vaughan et al., 2001; Zheng et al., 2002; Lu et al., 2010).
The ability of N/OFQ and the NOP receptor to inhibit neurotransmitter release is the basis for its modulation of the many biologic functions that rely on synaptic transmission, including nociception, anxiety, and reward. For instance, the influx of calcium through the N-type calcium channel plays a critical role in regulating nociceptive signaling in the spinal cord. The NOP receptor associates with and inhibits N-type calcium channels, even in the absence of N/OFQ (Beedle et al., 2004). The constitutive regulation of N-type calcium channels in the dorsal root ganglion (DRG) cells by the NOP receptor is thought to be modulated by NOP receptor expression (Beedle et al., 2004). Although there is agreement over the ability of N/OFQ-NOP receptor system to inhibit N-type calcium channels, there is still some debate about whether this involves NOP receptor–mediated internalization of those channels. For instance, N/OFQ treatment induces N-type calcium channel internalization in a PKC-dependent manner, effectively inhibiting calcium influx into the cell (Altier et al., 2006). However, a recent study examining the effect of NOP receptor activation on N-type calcium channels in a highly N/OFQ-sensitive subpopulation of rat DRG and spinal cord neurons found that, although N/OFQ treatment inhibited primary afferent excitatory postsynaptic currents on dorsal horn neurons, it did not induce internalization of N-type calcium channels in the cell body or nerve terminals of DRG neurons (Murali et al., 2012). Thus, the precise means by which the NOP receptor regulates calcium channels in vivo is still under debate.
N/OFQ and NOP receptor are expressed on lymphocytes, monocytes, and peripheral blood mononuclear cells and in T cell and B cell lines, where they modulate synthesis and release of neuromodulators (Halford et al., 1995; Wick et al., 1995; Peluso et al., 1998; Arjomand et al., 2002). N/OFQ blocks synthesis of proinflammatory cytokines in the spinal cord, astrocytes, and splenocytes (Fu et al., 2007; Miller and Fulford, 2007) and inhibits Complete Freund’s adjuvant-induced increase in proinflammatory interleukin-6, interleukin-1β, and tumor necrosis factor–α mRNA in cultured astrocytes (Fu et al., 2007). N/OFQ-NOP receptor interactions also induce neutrophil chemotaxis (Fiset et al., 2003; Serhan et al., 2001), block antibody formation in vivo and in vitro in rodent spleen cells (Anton et al., 2010), and inhibit T cell function (Waits et al., 2004). Although the mechanisms underlying these actions have yet to be determined, they undoubtedly involve signaling through one or more of the aforementioned protein kinases. We recently reported that N/OFQ activates NFκB transcription factor, a critical player in immune system regulation (Donica et al., 2011). Activation of NFκB provides one mechanism by which the NOP receptor regulates immune system function. NOP receptor activation also leads to transcriptional changes, resulting in sustained regulation of biologic processes. NOP receptor activation of ERK1/2 stimulates the Elk-1 transcription factor, which has been implicated in disorders, such as drug addiction, depression, and long-term memory (Bevan et al., 1998), whereas activation of JNK stimulates c-Jun and activating transcription factor–2 (ATF-2) (Chan and Wong, 2000). ATF-2 stimulates transcription in response to stressors, such as hypoxia and infection, and is important in development, innate immunity, and oncogenesis (Seong et al., 2012). N/OFQ activation of G16-coupled NOP receptor phosphorylates STAT3, a regulator of myeloid cell differentiation that provides another means of regulating immune function (Wu et al., 2003). Prolonged N/OFQ exposure up-regulates the transcription factors NFκB (Donica et al., 2011), activating protein-2 (Thakker and Standifer, 2002a) and Oct-2 (Thakker and Standifer, 2002b), a protein associated with development (He et al., 1989), immune cell maturation and activation (Kang et al., 1992; Humbert and Corcoran, 1997), and inflammation (Ensor et al., 1996). The ability of NOP receptor to modulate gene transcription and acute functions underscores its significance and potential as a therapeutic target.
Homologous NOP Receptor Desensitization
Analogous to the cellular regulation of other GPCRs, the NOP receptor is regulated by the process of homologous desensitization. Homologous desensitization is a state of decreased responsiveness of the receptor resulting from persistent exposure to its agonist. Receptor desensitization strongly contributes to mechanisms of drug tolerance experienced after drug administration, such that more of the drug is required to produce the same response or effect. Desensitization of the NOP receptor occurs after both acute (Connor et al., 1996a; Mandyam et al., 2000; Mandyam et al., 2002; Thakker et al., 2007) and chronic (Hashimoto et al., 2002; Thakker and Standifer, 2002a) agonist exposures. Desensitization to all known NOP receptor cellular functions has been observed after agonist treatment, including inhibition of adenylyl cyclase (Cheng et al., 1997; Ma et al., 1997) and voltage-gated calcium channels (Morikawa et al., 1998) and activation of inwardly rectifying potassium channels (Connor et al., 1996a), ERK1/2 (Hawes et al., 1998), and p38 (Zhang et al., 1999b).
According to the accepted model of prototypical GPCR desensitization, GPCRs undergo three states/phases of desensitization: (1) phosphorylation, (2) internalization, and (3) down-regulation and degradation (Gainetdinov et al., 2004). NOP receptor desensitization is discussed in this context in the following paragraphs.
NOP Receptor Phosphorylation.
After activation by N/OFQ, the NOP receptor, like other GPCRs, couples to its corresponding heterotrimeric G-protein, where the Gα and βγ subunits initiate the aforementioned signaling cascades. After uncoupling from the Gα subunit, Gβγ subunits recruit intracellular G protein-coupled receptor kinases (GRKs) to the cell surface that phosphorylate the agonist-bound NOP receptor. The phosphorylated receptor is thought to undergo a rapid conformational change that prevents it from coupling to G-proteins or to bind agonists until the receptor is dephosphorylated. This is the earliest phase of desensitization.
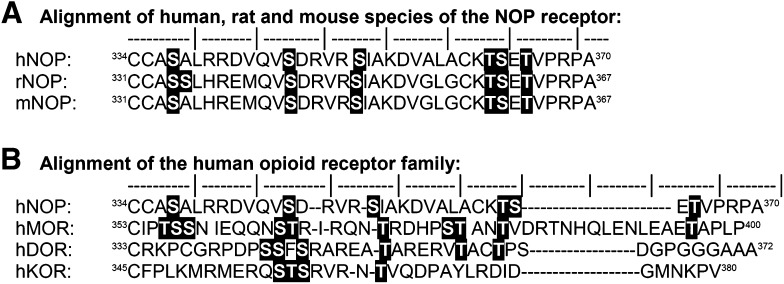
Kinases regulate GPCRs by phosphorylating threonine and serine residues. The human NOP receptor contains several serine and threonine residues in its intracellular loops and carboxyl terminus that serve as potential phosphorylation sites for intracellular serine/threonine protein kinases (highlighted residues in Fig. 1). PKC also plays an important role in mediating NOP receptor desensitization. N/OFQ binding to the NOP receptor activates PKC, as demonstrated by translocation of cytosolic PKC to the plasma membrane and increased phosphorylation of proteins by PKC in cell lines that endogenously express or have been transfected to stably express the NOP receptor (see Table 1 for specific names of cell lines, phenotype, level of expression, and use) (Lou et al., 1997; Pei et al., 1997; Pu et al., 1999; Mandyam et al., 2002; Ozsoy et al., 2005). Pretreatment with the phorbol ester, phorbol 12-myristate 13-acetate, a PKC activator, facilitates the acute desensitization of the NOP receptor (Pu et al., 1999). Depletion of PKC with persistent phorbol 12-myristate 13-acetate treatment or inhibition by nonselective and conventional PKC isoform-selective inhibitors blocks the homologous desensitization of NOP receptor after acute (≤1 hour) treatment by N/OFQ (Pei et al., 1997; Mandyam et al., 2002). Indeed, N/OFQ-induced translocation of PKC involved only conventional PKC isoforms (Lou et al., 1997; Mandyam et al., 2002).
Fig. 1.
Carboxy-terminal sequence alignment of opioid superfamily receptors. (A) Comparative view of the NOP receptor carboxy-terminal sequence among three species: human (hNOP receptor; Genbank AN: AF348323.1), rat (rNOP receptor; NCBI ref: NP_113757.1), and mouse (mNOP receptor; NCBI ref: NP_001239494.1). (B) Comparative view of sequence of the carboxy-terminal of the human NOP receptor (hNOP receptor) relative to the classic opioid receptors in the same species: μ opioid receptor (hMOR; AN: P35372.2), δ opioid receptor (hDOR; Genbank AN: AAA18789.2), and κ opioid receptor (hKOR; Genbank AN: AAC50158.1). Potential phosphorylation sites [serine (S) and threonine (T)] are highlighted in the text. ‘│’ denotes every fifth amino acid residue.
TABLE 1.
NOP receptor–expressing cell lines, cell derivation, NOP receptor expression levels, and cited references
NOP receptor expression was divided into the following ranges: 1–100 fmol/mg (+), 100–300 fmol/mg (++), 300–1000 fmol/mg (+++), >1 pmol/mg (++++). Cell derivation was based on documentation from American Type Culture Collection (www.atcc.org) or Sigma-Aldrich (www.sigmaaldrich.com)*.
| Cell Line and Type of NOP Receptor Expression | Cell Derivation | NOP Receptor Expression Level | References |
|---|---|---|---|
| Cell Lines Endogenously Expressing NOP Receptors | |||
| BE(2)-C Human neuroblastoma | Neuroblast cells subcloned from SK-N-BE(2) | + | Homologous/heterologous NOP receptor desensitization; NOP receptor regulation of MOR desensitization and phosphorylation (Mandyam et al., 2000, 2002, 2003; Ozsoy et al., 2005; Thakker and Standifer, 2002a,b) |
| NG108-15 Mouse neuroblastoma/ Rat glioma | Somatic cell hybrid of N18TG2 and C6.BU.1 | + | NOP receptor regulation of calcium channels; NOP receptor signaling cascades (Chan and Wong, 2000; Ma et al., 1997; Morikawa et al., 1998) |
| SH-SY5Y Human neuroblastoma | Subcloned from SK-N-SH | + | Homologous NOP receptor desensitization; Heterologous regulation by MOR and cannabinoid agonists; NOP receptor regulation of MOR; Intracellular signaling cascades (Cannarsa et al., 2012; Connor et al., 1996b; Donica et al., 2011; Mandyam et al., 2003; Thakker et al., 2007; Thakker and Standifer, 2002a,b) |
| SK-N-BE(2) Human neuroblastoma | Isolated from bone marrow biopsy of a 2-year-old male with neuroblastoma | + | Homologous NOP receptor internalization (Spampinato et al., 2001) |
| SK-N-SH Human neuroblastoma | Isolated from bone marrow biopsy of a 4-year-old female with neuroblastoma | + | Heterologous regulation of NOP receptor; NOP receptor signaling and homologous receptor regulation (Cheng et al., 1997; Zhao et al., 1998) |
| U937 Human monocyte | Isolated from adult male with histiocytic lymphoma | + | Peripheral blood mononuclear proliferation, Heterologous receptor desensitization of CXCR4 (Kaminsky and Rogers, 2011; Peluso et al., 2001) |
| Molt-4 Human T-lymphoblast | Isolated from adult male with acute lymphoblastic leukemia | + | Peripheral blood mononuclear proliferation (Peluso et al., 2001) |
| Recombinant Expression of NOP Receptors | |||
| CHO-K1 cells with hNOP receptor and Ecdysone-inducible hNOP receptor | Epithelial-like cells isolated from a biopsy of an adult Chinese hamster ovary | +, ++, ++++ | Homologous NOP receptor desensitization and Down-regulation; Intracellular signaling cascades; Heterologous desensitization of NOP; Heterodimerization (Barnes et al., 2007; Bevan et al., 1998; Fukuda et al., 1998; Fukuda et al., 1997; Hashimoto et al., 2002; Hawes et al., 1998; Lou et al., 1997; McDonald et al., 2003; Okawa et al., 1999; Pan et al., 2002; Peluso et al., 2001; Spampinato and Baiula, 2006; Spampinato et al., 2002; Waits et al., 2004) |
| COS-7 | African green monkey kidney fibroblast-like cells transformed with SV40 | ++++ | G-protein coupling and NOP receptor expression (Chan and Wong, 2000; Chan et al., 1998; Ho et al., 2002) |
| HEK293 with hNOP receptor | Human embryonic kidney epithelial cells transformed with adenovirus 5 DNA | ++++ | Homologous NOP receptor desensitization and Down-regulation; Heterodimerization, heterologous desensitization (Corbani et al., 2004; Dautzenberg et al., 2001; Wang et al., 2005, 2006; Wu et al., 2003; Zhang et al., 2012a; Zhang et al., 1998) |
| tsA-201* | Human embryonic kidney 293 epithelial cells transformed with SV40 | ++++ | Heterologous desensitization, and internalization, heterodimerization (Altier et al., 2006; Beedle et al., 2004; Evans et al., 2010) |
Despite having an important role in NOP receptor desensitization, direct phosphorylation of the NOP receptor by PKC has yet to be demonstrated. However, N/OFQ-induced activation of PKCα does promote the membrane translocation (Mandyam et al., 2000) and phosphorylation (Ozsoy et al., 2005) of GRK2/3, resulting in GRK2/3-mediated desensitization of the NOP receptor (Mandyam et al., 2002). GRKs phosphorylate serine residues 334 and 335 on the C-terminal tail of the rat NOP receptor (corresponding to Ser337 in the human NOP receptor) (Fig. 1), such that a single mutation at each site reduced receptor desensitization. A double S334A/S335A mutation almost abolished homologous desensitization and significantly impaired phosphorylation of the NOP receptor (Wang et al., 2006).
GRK2 and GRK3 also contribute to NOP receptor desensitization, which ensues after long-term (24 hour) stimulation by N/OFQ. Using two different human neuroblastoma cell lines that endogenously express the NOP receptor, we demonstrated that prolonged activation of the NOP receptor increases GRK2 and GRK3 levels by 2.2–2.5-fold (Thakker and Standifer, 2002a). PKC activation facilitates NOP receptor–mediated GRK3 up-regulation, and increases in GRK2 are ERK1/2 dependent (Thakker and Standifer, 2002a). Blockade of GRK2/3 up-regulation by PKC inhibition, ERK inhibition, or GRK antisense DNA treatment prevented NOP receptor desensitization. Of interest, homologous NOP receptor desensitization in BE(2)-C cells was mediated by GRK3; knockdown of GRK2 had no effect (Thakker and Standifer, 2002a).
NOP Receptor Internalization.
Receptor internalization can occur through clathrin-dependent and/or -independent (e.g., caveolae/lipid rafts) processes. To date, there are no reports that NOP receptor internalization occurs by clathrin-independent endocytosis; thus, discussion here is limited only to the role of clathrin-dependent processes in NOP receptor internalization. Phosphorylated GPCRs serve as targets for adaptor proteins, such as β-arrestins, which further disrupt the G protein–mediated actions of the receptor (Krupnick and Benovic, 1998) and trigger the formation of clathrin-coated pits to transport surface receptors to intracellular compartments through clathrin-mediated endocytosis. Clathrin-coated vesicles are trafficked to early endosomal compartments, where the receptor is completely internalized. Agonist-induced receptor internalization is the intermediate phase of GPCR desensitization, where the receptor is now inaccessible for activation by the extracellular agonist, thereby inhibiting further agonist-mediated cellular responses. However, it is also an essential process for resensitization of receptors (Gainetdinov et al., 2004), as explained below.
It was initially reported that, although the stably transfected human NOP receptor did not internalize after N/OFQ treatment, it internalized in response to treatment with the nonpeptide agonist, Ro 64-6198 ([(1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one]), as determined by radioligand binding (Dautzenberg et al., 2001; Corbani et al., 2004). In contrast, immunofluorescent and confocal microscopic approaches and radioligand binding studies in intact cells demonstrated internalization of the NOP receptor after N/OFQ treatment in endogenously expressing or stably transfected cells (Spampinato et al., 2001, 2002; Corbani et al., 2004). These studies also confirmed the temperature and ATP dependence of NOP receptor internalization via clathrin-mediated endocytosis (Dautzenberg et al., 2001; Spampinato et al., 2001, 2002; Corbani et al., 2004). Variability between studies may be attributable to possible differences in the cellular components of the cell lines [human embryonic kidney (HEK)293, Chinese hamster ovary (CHO), SK-N-BE] that are crucial for NOP receptor internalization, levels of NOP receptor expression, and/or to the efficacy of peptide versus nonpeptide agonists. NOP receptor expression in HEK293 cells (1.3 pmol/mg protein; Table 1) (Dautzenberg et al., 2001) was 10–20-fold higher than that in CHO (110 fmol/mg protein) or SK-N-BE (38 fmol/mg protein) cells used by Spampinato et al. (2001, 2002). It is possible that little internalization was noted because of the presence of numerous spare receptors in the Dautzenberg study (Dautzenberg et al., 2001). Of interest, partial agonists did not induce NOP receptor endocytosis and recruited less GRK2 to the membrane, but did produce robust NOP receptor desensitization after 1 hour of pretreatment (Spampinato et al., 2001; Corbani et al., 2004; Spampinato and Baiula, 2006). This indicates that endocytosis is important for NOP receptor resensitization.
GPCR internalization is a net result of receptor endocytosis and recycling back to the surface. This process involves acidification of endocytic vesicles and activation of GPCR phosphatases for dephosphorylating the internalized NOP receptor (Spampinato et al., 2001, 2002). Dephosphorylated receptors then transit in a recycling compartment or recycle directly back to the cell surface, ready for reactivation by their agonist. The percentage of internalized receptors at any given time, therefore, depends on the net result of endocytosis and recycling to the surface. Interfering with vesicular acidification not only reduces NOP receptor recycling to the cell surface, but also prolongs NOP receptor desensitization (Spampinato et al., 2001, 2002). NOP receptor recycling to the cell surface is a default trafficking pathway that occurs regardless of whether the agonist remains bound (Spampinato et al., 2001).
Although receptor recycling contributes to the rapid recovery of many cell surface NOP receptors, some receptors remain internalized for as long as 2 hours after washout of N/OFQ (Spampinato et al., 2001, 2002). Agonist-induced phosphorylation is required for efficient NOP receptor internalization. Agonists that induce robust NOP receptor internalization enable the NOP receptor to resume signaling more rapidly than do agonists that induce slower NOP receptor internalization (Spampinato et al., 2001; Corbani et al., 2004; Spampinato and Baiula, 2006).
Increasing expression of β-arrestin 2 increases the rate of NOP receptor internalization (Spampinato et al., 2001). Conversely, reducing the expression of β-arrestin 2 and GRK3 via siRNA significantly inhibits N/OFQ-induced NOP receptor internalization (Zhang et al., 2012a). When Ser363, a putative GRK phosphorylation site on the NOP receptor, was mutated to an alanine, cytosolic β-arrestin 2 was not recruited to the cell surface after N/OFQ treatment and the mutant S363A demonstrated significantly reduced NOP receptor internalization (Zhang et al., 2012a). Of interest, Ser363 of the NOP receptor aligns with the putative GRK2 phosphorylation sites of MOR and DOR (Fig. 1). Development of phospho-specific antibodies will be very useful to confirm the role of NOP receptor phosphorylation in internalization, recycling, and postendocytic receptor trafficking.
NOP Receptor Down-Regulation.
Receptors that remain internalized are often degraded, resulting in NOP receptor down-regulation. Long-term treatment with agonist often causes internalized receptors to be transported from endosomes to either proteosomes or lysosomes for degradation. The consequent decrease in total receptor number is referred to as receptor down-regulation, which generally ensues after chronic (hours or days) activation by an agonist. Down-regulation is the last phase of desensitization, where the total number of receptors in the cell is reduced, and therefore, responsiveness to the agonist is reduced until new receptors are synthesized and targeted to the cell surface.
NOP receptor down-regulation was observed after acute (≤1 hour) exposure to the nonpeptide agonist, Ro 64-6198 (Dautzenberg et al., 2001), but not the peptide agonist, N/OFQ, in HEK293 (stably transfected) or BE(2)-C cells (endogenously) expressing the human NOP receptor (Dautzenberg et al., 2001; Mandyam et al., 2002). Prolonged (24–48 hours) incubation with N/OFQ resulted in marked NOP receptor down-regulation in CHO cells expressing recombinant NOP receptor (Hashimoto et al., 2002). Significant NOP receptor down-regulation was also observed in membranes from rat brain homogenates 3 hours after a single intraperitoneal injection of Ro 64-6198 (Dautzenberg et al., 2001). NOP receptor levels recovered 24 hours after Ro 64-6198 injection, with a half-life of approximately 5.5 hours. Of interest, when Ro 64-6198 was administered intraperitoneally once daily for 15 days, tolerance to the drug (as indicated by loss of anti–anxiety-like behavior) was not noted, correlating well with no net loss of NOP receptor (Dautzenberg et al., 2001). This suggests that repeated long-term exposure to a NOP receptor nonpeptide agonist activates compensatory mechanisms to ensure that NOP receptor expression is maintained in vivo or that intermittent dosing regimens that allow receptor expression to recover after down-regulation is different from sustained dosing regimens that ultimately lead to degradation and down-regulation. It is possible that peptide NOP receptor agonists, such as N/OFQ, differentially induce down-regulation in a time-dependent manner, compared with nonpeptide (Ro 64-6198) agonists, because acute but not chronic Ro 64-6198 treatment produced NOP receptor down-regulation, whereas the reverse is true with N/OFQ. Furthermore, Ro 64-6198 also modulates NOP receptor function differently than N/OFQ in the vas deferens, ileum, and ventrolateral PAG (Calo et al., 1996; Rizzi et al., 2001; Chiou et al., 2004). For instance, Ro 64-6198 regulates only 60% of N/OFQ-sensitive NOP receptors in rat PAG slices, suggesting that Ro 64-6198 only regulates a specific subset of NOP receptors (Chiou et al., 2004). Whether this can be attributed to expression of NOP receptor splice variants (Pan et al., 1995, 1998; Xie et al., 1999), spare receptors, or receptor dimerization (Pan et al., 2002) is not clear. Differences in neuronal (rat brain) versus nonneuronal cell phenotype (CHO and HEK cells) expression in different backgrounds (native versus recombinant) and in levels of NOP receptor expression also may account for some of these differences.
These differences underscore the need to investigate the role of NOP receptor expression levels and neuronal cellular environment on NOP receptor regulation. To address the role of NOP receptor density, an ecdysone-inducible expression system was used to express human NOP receptors at different levels in CHO cells to determine the functional activity of several partial agonists (McDonald et al., 2003). These studies found that receptor density dictated the level of agonist or antagonist effect on receptor binding and cAMP inhibition (McDonald et al., 2003). For instance, with use of 35S-GTPγS binding as a functional readout, N/OFQ(1–13)–NH2 was a full NOP receptor agonist when the NOP receptor was expressed at approximately 25 fmol/mg, whereas [F/G]N/OFQ(1–13)–NH2 failed to stimulate any 35S-GTPγS binding at this expression level. In fact, [F/G]N/OFQ(1–13)–NH2 was a full NOP receptor agonist only after NOP receptor levels reached ∼70 fmol/mg (McDonald et al., 2003). When measuring inhibition of cAMP accumulation, N/OFQ(1–13)–NH2 remained a full agonist at all receptor densities (25–1100 fmol/mg), whereas [F/G]N/OFQ(1–13)–NH2 was a full agonist only at high receptor density (McDonald et al., 2003). Increasing receptor expression from physiologic (194 fmol/mg) to supra-physiologic (473 fmol/mg) levels increased N/OFQ-induced 35S-GTPγS binding and, thus, increased N/OFQ efficacy via G-protein coupling to the NOP receptor (McDonald et al., 2003; Barnes et al., 2007). However, N/OFQ potency was unaffected by NOP receptor density (Barnes et al., 2007). Furthermore, pretreatment with N/OFQ produced similar desensitization of the NOP receptor response, regardless of receptor density. Of note, low-density receptor expression (25 fmol/mg) in CHO cells using the ecdysone-inducible system was similar to NOP receptor levels in SK-N-BE and SH-SY5Y human neuroblastoma cells, whereas mid-range (200 fmol/mg) and high expression (1100 fmol/mg) were similar to levels seen in rat cortex and stably transfected CHO cells, respectively (Okawa et al., 1999; Peluso et al., 2001). Altogether, this suggests that, in non-neuronal cell background, NOP receptor desensitization induced by N/OFQ is not dependent on the amount of NOP receptor (Barnes et al., 2007). The role of receptor density on NOP receptor regulation in a neuronal cell background remains to be addressed.
Additional evidence for N/OFQ regulation of NOP receptor expression comes from pre-pro-N/OFQ knockout (KO) mice. Clarke et al. (2003) observed significant NOP receptor up-regulation in several brain regions of the pre-pro-N/OFQ KO mice. This up-regulation may represent a type of denervation supersensitivity of postsynaptic cells, resulting in increased NOP receptor expression in response to the loss of tonic neurotransmitter (N/OFQ) release from the presynaptic nerve terminals (Clarke et al., 2003) in the N/OFQ KO mice. Alternatively, other compensatory changes during the development of pre-pro-N/OFQ KO mice may also account for such regulation of the NOP receptor (Clarke et al., 2003). Further characterization of these events is required to confer a definitive role for N/OFQ in regulating NOP receptor expression in vivo.
Heterologous Regulation of the NOP Receptor
In addition to the homologous regulation by its own agonists, heterologous regulation of the NOP receptor occurs in a cell-specific manner and may increase or decrease NOP receptor activity or expression. Heterologous receptor desensitization is a state of reduced responsiveness of a receptor to its agonist because of activation of a second receptor system. This can occur by a simple heterologous effect or by heterologous modulation of homologous desensitization.
Regulation by MOR Agonists.
MOR agonists induced heterologous desensitization of the NOP receptor in a cell-specific manner. Short-term pretreatment (1 hour) of MOR and NOP receptor with the MOR agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) robustly desensitized N/OFQ/NOP receptor–induced inhibition of cAMP accumulation in BE(2)-C human neuroblastoma cells (Mandyam et al., 2000, 2003). However, the same treatment had no effect on the N/OFQ response in SH-SY5Y cells, despite the fact that both cell lines expressed both MOR and NOP receptor (Mandyam et al., 2003). This may result from differences in signal transduction components in the two cell lines, as described above. In SH-SY5Y cells, MOR agonists increased PKCε translocation, not translocation of a conventional isoform (such as PKCα) that produced homologous NOP receptor desensitization in BE(2)-C cells (Mandyam et al., 2002). Similar results were noted in HEK 293 or CHO cells expressing recombinant NOP receptor and MOR. In those studies, pretreatment with a MOR agonist had no effect on NOP receptor–mediated inhibition of cAMP (Wang et al., 2005) or stimulation of ERK1/2 (Hawes et al., 1998). In contrast, MOR agonist treatment internalized recombinant NOP receptor expressed with MOR in tsA-201 cells (Evans et al., 2010). Therefore, because the specific components of signaling cascades, especially kinase isoforms, activated by MOR agonists vary between cell types, the ability of MOR agonists to regulate NOP receptor activity also varies among cell types.
MOR agonist treatment also heterologously regulates homologous NOP receptor desensitization (e.g., 10 minutes incubation with N/OFQ to measure NOP receptor inhibition of cAMP accumulation; NOP receptor desensitization of this response normally does not occur within 10 minutes). However, if GRK2 or 3 levels at the plasma membrane (Mandyam et al., 2003) or in the cell (Thakker and Standifer, 2002a) were up-regulated by 1 hour or 24 hours of DAMGO pretreatment, respectively, the rate of NOP receptor desensitization increased; the subsequent 10 minutes exposure to N/OFQ resulted in a rightward shift or flattening of the concentration-response curve. Down-regulation of GRK2 and 3 or blockade of their up-regulation prevented NOP receptor desensitization (Thakker and Standifer, 2002a; Mandyam et al., 2003). MOR-mediated NOP receptor desensitization in SH-SY5Y cells involved GRK2, whereas NOP receptor desensitization in BE(2)-C cells was PKC and GRK3 dependent. Therefore, the ability of a MOR agonist to up-regulate or translocate GRK2 or 3 serves as a critical factor for producing heterologous modulation of homologous NOP receptor desensitization.
The role of the MOR in regulating NOP receptor expression also was examined in vivo using MOR knockout mouse strains and chronic MOR agonist treatment. NOP receptor expression in the brains of MOR knockout mice did not differ from levels in wild-type mice (Slowe et al., 2001), suggesting that activation of MOR by endogenous agonists does not regulate the expression of NOP receptor supraspinally. In contrast, chronic activation of MOR with morphine increased NOP receptor expression in the dorsal spinal cord in mice and rats (Gouarderes et al., 1999; Ueda et al., 2000; Zhang et al., 2012b). Whether chronic morphine treatment also increases NOP receptor expression supraspinally is not yet known.
Regulation by DOR or KOR Agonists.
Heterologous regulation of the NOP receptor by DOR or KOR agonists is far less characterized than that by MOR agonists. DOR agonists were unable to desensitize NOP receptor–mediated inhibition of cAMP accumulation (Cheng et al., 1997; Ma et al., 1997) or activation of p38 (Zhang et al., 1999b) in SK-N-SH human neuroblastoma and NG108-15 rat neuroblastoma-glioma hybrid cells that natively express NOP receptor and DOR. Of interest, significant NOP receptor up-regulation was observed in the brains of DOR KO mice, suggesting that basal activation of DOR by endogenous peptides exerted inhibitory control over NOP receptor expression (Slowe et al., 2001).
In contrast to DOR KO mice, NOP receptors were down-regulated in the brains of KOR KO mice (Slowe et al., 2001), indicating that endogenous KOR signaling increased NOP receptor expression or prevented its degradation. NOP receptor coupling to G16 (a pertussis toxin–insensitive G protein commonly expressed in hematopoietic cells) also increased after KOR and NOP receptor coexpression of the NOP receptor with the KOR in COS-7 African green monkey kidney cells (Ho et al., 2002). The mechanism of this enhanced coupling of NOP receptor to the G16 by increased KOR expression is unknown.
Regulation by GABAB Receptor Agonists.
In addition to opioid receptors, the metabotropic GABAB receptor is another GPCR that modulates NOP receptor function. Only a brief (1 minute) activation of the GABAB receptor is sufficient to desensitize the NOP receptor in hippocampal neurons (Pu et al., 1999). Both GABAB receptor and NOP receptor homologous desensitization are PKC dependent (Pei et al., 1997; Pu et al., 1999; Mandyam et al., 2002); however, whether heterologous desensitization of NOP receptor by GABAB occurs via PKC activation remains to be determined. GABAB and NOP receptors are important for the modulation of pain (Malcangio and Bowery, 1995; Mogil and Pasternak, 2001) and are colocalized in several neurons implicated in the actions of opioids (Vaughan and Christie, 1996; Connor and Christie, 1998; Pu et al., 1999). Therefore, a detailed investigation of their interaction will possibly shed more light on the diverse actions of N/OFQ on pain perception and its regulation of opioid actions via GABAB receptors.
Regulation by CB1 Receptor Agonists.
Cannabinoids have pharmacological characteristics and physiologic effects similar to those of the NOP receptor system (Pertwee, 2008). Long-term (24 hours) treatment with the CB1 agonist, delta9-tetrahydrocannabinol, dose-dependently decreased NOP receptor mRNA and protein expression in SH-SY5Y human neuroblastoma cells (Cannarsa et al., 2012). However, the molecular mechanism responsible for NOP receptor down-regulation by delta9-tetrahydrocannabinol is not known.
Regulation by N-Methyl-D-Aspartate Receptor Ligands.
N-methyl-D-aspartate (NMDA) receptors also regulate NOP receptor function. Stimulation of the NMDA receptor promotes calcium influx and augmentation of intracellular calcium, which in turn, leads to the activation of PKC (Etoh et al., 1991). Because enhanced calcium levels are required for NOP receptor desensitization (Pu et al., 1999), short-term exposure (5 minutes) to NMDA promotes the desensitization of NOP receptor in neuronal cells. NMDA receptor antagonists in the same cells also inhibit homologous NOP receptor desensitization by blocking calcium influx (Zhao et al., 1998). As mentioned above, presynaptic NOP receptor activation decreases calcium influx and inhibits neurotransmitter release. Desensitization of NOP receptor by acute NMDA receptor activation would permit calcium influx and, thus, facilitate neurotransmission.
Heterologous Regulation by N/OFQ
Activation of the NOP receptor by N/OFQ or other NOP receptor agonists can also heterologously regulate the activity of other receptors.
Classic Opioid Receptors.
Because of its ability to modulate morphine analgesia and morphine tolerance, it is important to understand how N/OFQ can regulate the MOR. Indeed, N/OFQ pretreatment produced MOR desensitization (Hawes et al., 1998; Mandyam et al., 2000, 2002; Thakker and Standifer, 2002a; Ozsoy et al., 2005), phosphorylation (Ozsoy et al., 2005), internalization (Evans et al., 2010), and down-regulation (Mandyam et al., 2002) in cells endogenously expressing and stably transfected with NOP receptor. However, mechanisms of N/OFQ-mediated MOR desensitization also are cell, time, and signal pathway specific. Short-term N/OFQ pretreatment reduced the ability of the MOR agonists DAMGO (Mandyam et al., 2002; Wang et al., 2005) and morphine (Mandyam et al., 2000) to inhibit cAMP and to stimulate mitogen-activated protein kinase (Hawes et al., 1998). Acute desensitization of MOR responses by N/OFQ in BE(2)-C cells involves translocation of PKCα, GRK2, and GRK3 to the plasma membrane. By increasing GRK2 levels at the plasma membrane during N/OFQ pretreatment, MOR phosphorylation was enhanced significantly during subsequent challenge with DAMGO, compared with MOR phosphorylation in the absence of N/OFQ pretreatment (Mandyam et al., 2002; Ozsoy et al., 2005). N/OFQ-mediated MOR desensitization and phosphorylation was blocked by inhibition of PKC and down-regulation of GRK2, but not by down-regulation of GRK3. Furthermore, MORs lacking the GRK2 phosphorylation site, Ser375, were not phosphorylated or desensitized by acute N/OFQ pretreatment (Ozsoy et al., 2005). Therefore, acute exposure to N/OFQ enhanced MOR-mediated homologous desensitization in BE(2)-C human neuroblastoma cells. The mechanism(s) by which acute N/OFQ-desensitized MOR responses in CHO and HEK cells were not investigated (Hawes et al., 1998; Wang et al., 2005).
Although prolonged N/OFQ treatment reduced the ability of MOR agonists to inhibit cAMP accumulation in BE(2)-C and SH-SY5Y cells (Thakker and Standifer, 2002a), it did not alter the ability of MOR to activate ERK1/2 (Thakker and Standifer, 2002b). The desensitization of the cyclase response by N/OFQ involved up-regulation of GRK2 (SH-SY5Y) and GRK3 (BE(2)-C and SH-SY5Y) (Thakker and Standifer, 2002a); MOR desensitization was blocked in both cell lines by blocking up-regulation of the respective GRK. This is consistent with the mechanism of acute N/OFQ-mediated MOR desensitization. It would appear that MOR-mediated ERK1/2 activation is not sensitive to GRK up-regulation, but the role of GRK in DAMGO-stimulated ERK1/2 activation and desensitization has not been examined directly.
Acute N/OFQ prechallenge failed to desensitize DOR-mediated inhibition of cAMP accumulation in NG108-15 neuroblastoma cells (Ma et al., 1997). However, N/OFQ treatment also reduced MOR (Zhang et al., 2005) and KOR (Zhang et al., 1998) agonist-induced inhibition of calcium currents in freshly isolated rat DRG neurons. It is not clear whether the mechanism for this effect involves desensitization or competition for intracellular signaling components/G protein subunits or results from receptor internalization, as reported more recently (Evans et al., 2010). Indeed, N/OFQ induced internalization of MOR, KOR, and DOR when each was coexpressed with the NOP receptor (Evans et al., 2010). This issue is discussed further in the heterodimerization section below.
Of interest, MOR, KOR, or DOR receptor expression was unaltered in NOP receptor KO rats, compared with wild-type animals (Homberg et al., 2009), suggesting that endogenous N/OFQ tone was not important for basal expression of those receptors.
Cytokine Receptors.
The chemotactic effect mediated through CXCR4, an important HIV-1 coreceptor, was desensitized in primary leukocytes, U937 monocytes, and Jurkat T cells after N/OFQ treatment (Kaminsky and Rogers, 2011). This further supports a role for N/OFQ in immunomodulation.
Heterodimerization
Heterodimerization of the NOP receptor with other GPCRs was examined using coimmunoprecipitation (Pan et al., 2002; Wang et al., 2005; Evans et al., 2010) and immunofluorescence microscopy approaches (Evans et al., 2010). Heterodimerization also appears to play a role in opioid receptor regulation and function by altering receptor binding, functional activity, and trafficking. MOR/NOP receptor dimerization increased the affinity for MOR agonist binding (Pan et al., 2002), but had no effect on N/OFQ/NOP receptor–mediated inhibition of cAMP or activation of ERK1/2 in the absence of MOR agonist stimulation. In contrast, MOR/NOP receptor dimerization decreased MOR agonist potency in both signaling pathways in the absence of N/OFQ (Wang et al., 2005). Pretreatment with N/OFQ desensitized the MOR/NOP receptor heterodimers, similar to results described above with endogenously coexpressed MOR and NOP receptor. MOR/NOP receptor heterodimerization also required the C-terminal portions of both receptors (Pan et al., 2002; Wang et al., 2005), sites of GRK-mediated phosphorylation required for receptor desensitization and agonist-mediated internalization. The NOP receptor interacts with and regulates N-type calcium channels (Beedle et al., 2004); thus, regulation of the NOP receptor by MOR agonists may also modulate calcium channel activity. Of interest, MOR does not physically associate with N-type calcium channels, nor does the MOR agonist DAMGO induce calcium channel internalization unless coexpressed with NOP receptor. When all three molecules are coexpressed, DAMGO stimulates MOR/NOP receptor/calcium channel internalization. The ability of NOP receptor and MOR/NOP receptor heterodimers to regulate calcium currents and to internalize N-type calcium channels allows these opioid systems to regulate calcium influx (Evans et al., 2010) and, thus, to potentially regulate neurotransmission and nociceptive signaling. Although MOR/NOP receptor heterodimerization has yet to be demonstrated in the brain, MOR and NOP receptors are functionally coexpressed in neurons of the PAG, RVM, hypothalamus, dorsal root ganglion, trigeminal ganglion, locus coeruleus, and nucleus tractus solitarius (Connor et al., 1996a; Wagner et al., 1998; Borgland et al., 2001; Vaughan et al., 2001, 2003; Endoh, 2006; Murali et al., 2012). The potential for MOR/NOP receptor heterodimerization in these regions may provide an additional level of MOR and NOP receptor modulation of nociceptive processing and reproductive behavior. However, development of additional tools will be necessary to move this discussion past the speculative stage.
The NOP receptor also heterodimerizes with KOR and DOR (Evans et al., 2010). In both cases, it resulted in N/OFQ- and U50,488- (KOR agonist), or deltorphin- (DOR agonist) mediated heterodimer internalization. Of interest, heterodimerization of NOP receptor with KOR or DOR reduced N/OFQ reduction of peak calcium current inhibition, especially with NOP receptor/DOR dimerization (Evans et al., 2010). This suggests that up-regulation of DOR or KOR in cells expressing the NOP receptor would be one means of reducing N/OFQ-mediated calcium channel modulation. Although autoradiographic and/or in situ hybridization studies suggest that KOR and NOP receptors are regionally colocalized in the descending analgesic reward pathways (Mansour et al., 1995; Berthele et al., 2003) and that DOR and NOP receptors are regionally colocalized in the cortex, nucleus accumbens, amygdala, and dorsal horn (Neal et al., 1999; Berthele et al., 2003), these methods do not confirm colocalization at a cellular level. However, functional evidence also supports cellular colocalization of KOR/NOP receptor in PAG and RVM, suggesting that KOR/NOP receptor heterodimerization may mediate nociceptive processing (Vaughan et al., 2001, 2003). DOR and NOP receptors colocalize at the cellular level in neurons in the PAG and the medial vestibular nucleus (Sulaiman et al., 1999; Vaughan et al., 2003), suggesting that heterodimers may modulate nociceptive processing and vestibular reflex.
NOP Receptor Function, Pain, and Inflammatory Response: The Role of NOP Receptor Regulation
One of the hallmarks of the opioid receptor family is regulation of pain. As indicated by its name, N/OFQ and the NOP receptor play a unique role in nociception and nociceptive processing. Depending on a number of factors, including species and strain, dose, time of testing after drug administration, or the testing paradigm itself, N/OFQ may produce a hyperalgesic or an analgesic response (Mogil and Pasternak, 2001). Although both hyperalgesic and analgesic actions of supraspinally administered N/OFQ are noted, spinally administered N/OFQ predominantly produces analgesia (Mogil and Pasternak, 2001). The biphasic effect (hyperalgesic versus analgesic) after supraspinal administration may be explained by NOP receptor desensitization, trafficking, and/or dimerization, but that is not yet clear.
The N/OFQ-NOP receptor system also modulates the actions of other opioids. Several reports document the anti-opioid activity of N/OFQ by demonstrating its ability to block supraspinal analgesia produced by selective MOR, DOR, and KOR agonists (Mogil et al., 1996a,b; Zhu et al., 1996). This effect is largely attributed to the expression of NOP receptors on cells regulating descending analgesia (Heinricher, 2005). However, it is possible that heterologous regulation of the other opioid receptors by the NOP receptor also contributes to the regulation of nociceptive transmission. N/OFQ treatment desensitizes the MOR in vitro (Hawes et al., 1998; Mandyam et al., 2000, 2002; Thakker and Standifer, 2002a; Ozsoy et al., 2005; Evans et al., 2010), raising the possibility that supraspinal NOP receptor agonist treatment attenuates MOR responsiveness in vivo, thus reducing the analgesic actions of MOR agonists.
As discussed above, the N/OFQ-NOP receptor system has an important immunomodulatory effect, although the mechanism(s) underlying this immunomodulation is somewhat unclear. N/OFQ and the NOP receptor are expressed on many different types of immune cells (Halford et al., 1995; Wick et al., 1995; Peluso et al., 1998; Arjomand et al., 2002) and modulate numerous processes within the immune and neuroimmune systems, including cytokine expression, antibody formation, neutrophil chemotaxis, and T cell function (Serhan et al., 2001; Fiset et al., 2003; Waits et al., 2004; Fu et al., 2007; Miller and Fulford, 2007; Anton et al., 2010). NOP receptor activation and up-regulation of transcription factors, including ATF-2, NFκB (Donica et al., 2011), activating protein-2 (Thakker and Standifer, 2002a), and Oct-2 (Thakker and Standifer, 2002b), provide potential molecular mechanisms for prolonged regulation of an inflammatory response. In addition to NOP receptor–mediated transcriptional regulation, the recent report of heterologous regulation of HIV-1 coreceptor CXCR4 by the NOP receptor (Kaminsky and Rogers, 2011) provides another mechanism for NOP receptor immunomodulation. Together, these studies demonstrate the breadth of NOP receptor signaling on immune system regulation and warrant a more thorough investigation to elucidate the potential mechanisms of this effect.
Numerous recent reviews have emphasized the importance of inflammatory factors, such as cytokines, chemokines, and infiltrating immune cells, and signaling molecules, such as substance P, calcitonin-related gene peptide, bradykinin, and prostaglandins, in developing, enhancing, and maintaining nociception (Fukuoka et al., 1994; Basbaum et al., 2009; Ren and Dubner, 2010; Kiguchi et al., 2012). NOP receptor activation has been suggested to play a role in the association between pain and inflammation (Mika et al., 2011). N/OFQ and NOP receptor expression are elevated in the dorsal root ganglion projections to the spinal dorsal horn, ventrolateral PAG, dorsal raphe nucleus, and the nucleus of raphe magnus in neuropathic or inflammatory pain models, consistent with N/OFQ-NOP receptor system involvement in neuropathic and inflammatory pain processing (Ma et al., 2005; Chen and Sommer, 2006). Intrathecal N/OFQ administration delays chronic constrictive nerve injury-induced hyperalgesia and abolishes sciatic nerve injury-induced pain (Yamamoto et al., 1997, 2000; Courteix et al., 2004). However, it also appears that N/OFQ exacerbates neuropathic pain states by inducing an influx of inflammatory mediators (Fu et al., 2007; Mika et al., 2011). It is possible that acute activation of the NOP receptor contributes partially to spinal analgesia by inhibiting proinflammatory cytokines, whereas persistent NOP receptor activation leads to NOP receptor desensitization and reduced ability of N/OFQ to inhibit proinflammatory cytokines. Therefore, NOP receptor desensitization would exacerbate neuropathic pain when NOP-mediated inhibition of inflammatory mediators is reduced. An inevitable limitation to the rising prospect of using NOP receptor agonists in the long-term management of several disorders, such as anxiety, drug abuse, asthma, and chronic neuropathic pain, is the development of tolerance to NOP receptor agonists. As discussed above, chronic N/OFQ exposure induces homologous desensitization, resulting in diminished cellular and behavioral function. Indeed, tolerance to the hyperalgesic (Kavaliers and Perrot-Sinal, 1996) and spinal analgesic actions of N/OFQ (Hao et al., 1997; Jhamandas et al., 1998) and its blockade of locomotor activity (Devine et al., 1996) and morphine-induced antinociception in vivo (Lutfy et al., 1999) have been reported. Although likely, it has yet to be reported whether the development of tolerance to chronic N/OFQ administration in vivo is the result of NOPr desensitization and down-regulation. N/OFQ tolerance, similar to that of other opioids (Nestler and Aghajanian, 1997), may possibly involve complex compensatory changes in the neuronal circuitry along with adaptations in intracellular signaling cascades and subsequent regulation of the NOP receptor that requires further investigation. As noted with NOP receptor internalization studies, the structure and efficacy of a ligand can alter its ability to regulate the receptor; examination of NOP receptor regulation by structurally distinct partial agonists also is warranted.
Conclusion
Almost two decades since the NOP receptor was cloned and its endogenous agonist, N/OFQ, identified, our understanding of NOP receptor cellular function and consequent regulation still has far to go. Activation of the NOP receptor initiates intracellular events similar to those initiated by the other opioid receptors, but NOP receptor–mediated actions oppose many antinociceptive actions of classic opioid agonists. Regulation of NOP receptor function also appears to follow a similar pattern of GPCR regulation, as observed for the other opioid receptors. Of note, however, a large percentage of NOP receptor signaling and regulation studies were performed using recombinant receptors overexpressed in cells with nonneuronal phenotypes. The significance of studying the receptor-effector coupling in a native environment has become increasingly evident with our understanding of how different cell types and levels of receptor expression impact receptor function (Kenakin, 1997, 2002). Therefore, a detailed examination of NOP receptor regulation in neuronal cells that endogenously express this receptor is an essential next step. Additional in vivo studies of NOP receptor regulation also is warranted to understand how the cellular actions of NOP receptor activation integrate with NOP receptor–mediated behavioral and physiologic outcomes.
Abbreviations
- ATF-2
activating transcription factor–2
- CHO
Chinese hamster ovary
- DOR
δ opioid receptor
- ERK
extracellular signal-regulated kinases
- GPCR
G protein–coupled receptor
- GRK
GPCR kinase
- HEK
human embryonic kidney
- KOR
κ opioid receptor
- KO
knockout
- MOR
µ opioid receptor
- NMDA
N-methyl-D-aspartate
- N/OFQ
nociceptin/orphanin FQ
- NOP receptor
nociceptin/orphanin FQ peptide receptor
- NFκB
nuclear factor kappa B
- PAG
periaqueductal gray
- PKC
protein kinase C
- Ro 64-6198
[(1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one]
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Donica, Awwad, Thakker, Standifer.
Footnotes
This work was supported by the Oklahoma Center for the Advancement of Science and Technology’s OHRS Award [Project HR08-152]; the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA017380]; and the Department of the Army [DMRDP W81XWH-11-2-0077].
The information and interpretations included in this article do not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
1Current affiliation: Medtronic Neuromodulation, Minneapolis, Minnesota.
References
- Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, et al. (2006) ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci 9:31–40 [DOI] [PubMed] [Google Scholar]
- Anton B, Leff P, Meissler JJ, Calva JC, Acevedo R, Salazar A, Matus M, Flores A, Martinez M, Adler MW, et al. (2010) Nociceptin/orphanin FQ suppresses adaptive immune responses in vivo and at picomolar levels in vitro. J Neuroimmune Pharmacol 5:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjomand J, Cole S, Evans CJ. (2002) Novel orphanin FQ/nociceptin transcripts are expressed in human immune cells. J Neuroimmunol 130:100–108 [DOI] [PubMed] [Google Scholar]
- Barnes TA, McDonald J, Rowbotham DJ, Duarte TL, Lambert DG. (2007) Effects of receptor density on Nociceptin/OrphaninFQ peptide receptor desensitisation: studies using the ecdysone inducible expression system. Naunyn Schmiedebergs Arch Pharmacol 376:217–225 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, Hamid J, Nargeot J, Bourinet E, Zamponi GW. (2004) Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci 7:118–125 [DOI] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Büttner A, Assmus HP, Wurster K, Zieglgänsberger W, Conrad B, et al. (2003) [3H]-nociceptin ligand-binding and nociceptin opioid receptor mrna expression in the human brain. Neuroscience 121:629–640 [DOI] [PubMed] [Google Scholar]
- Bevan N, Scott S, Shaw PE, Lee MG, Marshall FH, Rees S. (1998) Nociception activates Elk-1 and Sap1a following expression of the ORL1 receptor in Chinese hamster ovary cells. Neuroreport 9:2703–2708 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Christie MJ. (2001) Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse. J Physiol 536:35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett 347:284–288 [DOI] [PubMed] [Google Scholar]
- Calò G, Rizzi A, Bogoni G, Neugebauer V, Salvadori S, Guerrini R, Bianchi C, Regoli D. (1996) The mouse vas deferens: a pharmacological preparation sensitive to nociceptin. Eur J Pharmacol 311:R3–R5 [DOI] [PubMed] [Google Scholar]
- Cannarsa R, Carretta D, Lattanzio F, Candeletti S, Romualdi P. (2012) ∆(9)-Tetrahydrocannabinol decreases NOP receptor density and mRNA levels in human SH-SY5Y cells. J Mol Neurosci 46:285–292 [DOI] [PubMed] [Google Scholar]
- Chan AS, Wong YH. (2000) Regulation of c-Jun N-terminal kinase by the ORL(1) receptor through multiple G proteins. J Pharmacol Exp Ther 295:1094–1100 [PubMed] [Google Scholar]
- Chan JS, Yung LY, Lee JW, Wu YL, Pei G, Wong YH. (1998) Pertussis toxin-insensitive signaling of the ORL1 receptor: coupling to Gz and G16 proteins. J Neurochem 71:2203–2210 [DOI] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. (1994) Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett 347:279–283 [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. (2006) Nociceptin and its receptor in rat dorsal root ganglion neurons in neuropathic and inflammatory pain models: implications on pain processing. J Peripher Nerv Syst 11:232–240 [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Fan GH, Zhao J, Zhang Z, Wu YL, Jiang LZ, Zhu Y, Pei G, Ma L. (1997) Endogenous opioid receptor-like receptor in human neuroblastoma SK-N-SH cells: activation of inhibitory G protein and homologous desensitization. Neuroreport 8:1913–1918 [DOI] [PubMed] [Google Scholar]
- Chiou LC, Chuang KC, Wichmann J, Adam G. (2004) Ro 64-6198 [(1S,3aS)-8-(2,3,3a,4,5,6-Hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one] acts differently from nociceptin/orphanin FQ in rat periaqueductal gray slices. J Pharmacol Exp Ther 311:645–651 [DOI] [PubMed] [Google Scholar]
- Civelli O. (2008) The orphanin FQ/nociceptin (OFQ/N) system. Results Probl Cell Differ 46:1–25 [DOI] [PubMed] [Google Scholar]
- Clarke S, Chen Z, Hsu MS, Hill RG, Pintar JE, Kitchen I. (2003) Nociceptin/orphanin FQ knockout mice display up-regulation of the opioid receptor-like 1 receptor and alterations in opioid receptor expression in the brain. Neuroscience 117:157–168 [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MJ. (1998) Modulation of Ca2+ channel currents of acutely dissociated rat periaqueductal grey neurons. J Physiol 509:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Chieng B, Christie MJ. (1996a) Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol 119:1614–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Yeo A, Henderson G. (1996b) The effect of nociceptin on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol 118:205–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbani M, Gonindard C, Meunier JC. (2004) Ligand-regulated internalization of the opioid receptor-like 1: a confocal study. Endocrinology 145:2876–2885 [DOI] [PubMed] [Google Scholar]
- Courteix C, Coudoré-Civiale MA, Privat AM, Pélissier T, Eschalier A, Fialip J. (2004) Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain 110:236–245 [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Wichmann J, Higelin J, Py-Lang G, Kratzeisen C, Malherbe P, Kilpatrick GJ, Jenck F. (2001) Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J Pharmacol Exp Ther 298:812–819 [PubMed] [Google Scholar]
- Devine DP, Taylor L, Reinscheid RK, Monsma FJ, Jr, Civelli O, Akil H. (1996) Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem Res 21:1387–1396 [DOI] [PubMed] [Google Scholar]
- Donica CL, Ramirez VI, Awwad HO, Zaveri NT, Toll L, Standifer KM. (2011) Orphanin FQ/nociceptin activates nuclear factor kappa B. J Neuroimmune Pharmacol 6:617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh T. (2006) Pharmacological characterization of inhibitory effects of postsynaptic opioid and cannabinoid receptors on calcium currents in neonatal rat nucleus tractus solitarius. Br J Pharmacol 147:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensor E, Kendall G, Allchorne A, Woolf CJ, Latchman DS. (1996) Induction of the Oct-2 transcription factor in primary sensory neurons during inflammation is nerve growth factor-dependent. Neurosci Lett 204:29–32 [DOI] [PubMed] [Google Scholar]
- Etoh S, Baba A, Iwata H. (1991) NMDA induces protein kinase C translocation in guinea pig cerebral synaptoneurosomes. Jpn J Pharmacol 56:287–296 [DOI] [PubMed] [Google Scholar]
- Evans RM, You H, Hameed S, Altier C, Mezghrani A, Bourinet E, Zamponi GW. (2010) Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J Biol Chem 285:1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Forssmann WG, Undem BJ. (1998) Nociceptin-induced inhibition of tachykinergic neurotransmission in guinea pig bronchus. J Pharmacol Exp Ther 285:902–907 [PubMed] [Google Scholar]
- Fiset ME, Gilbert C, Poubelle PE, Pouliot M. (2003) Human neutrophils as a source of nociceptin: a novel link between pain and inflammation. Biochemistry 42:10498–10505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin S, Suaudeau C, Meunier JC, Costentin J. (1996) Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur J Pharmacol 317:9–13 [DOI] [PubMed] [Google Scholar]
- Fu X, Zhu ZH, Wang YQ, Wu GC. (2007) Regulation of proinflammatory cytokines gene expression by nociceptin/orphanin FQ in the spinal cord and the cultured astrocytes. Neuroscience 144:275–285 [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T. (1994) cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett 343:42–46 [DOI] [PubMed] [Google Scholar]
- Fukuda K, Shoda T, Morikawa H, Kato S, Mima H, Mori K. (1998) Activation of phospholipase A2 by the nociceptin receptor expressed in Chinese hamster ovary cells. J Neurochem 71:2186–2192 [DOI] [PubMed] [Google Scholar]
- Fukuda K, Shoda T, Morikawa H, Kato S, Mori K. (1997) Activation of mitogen-activated protein kinase by the nociceptin receptor expressed in Chinese hamster ovary cells. FEBS Lett 412:290–294 [DOI] [PubMed] [Google Scholar]
- Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. (1994) Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res 657:133–140 [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27:107–144 [DOI] [PubMed] [Google Scholar]
- Gouardères C, Tafani JA, Meunier JC, Jhamandas K, Zajac JM. (1999) Nociceptin receptors in the rat spinal cord during morphine tolerance. Brain Res 838:85–94 [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJ. (1995) Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol 59:91–101 [DOI] [PubMed] [Google Scholar]
- Hao JX, Wiesenfeld-Hallin Z, Xu XJ. (1997) Lack of cross-tolerance between the antinociceptive effect of intrathecal orphanin FQ and morphine in the rat. Neurosci Lett 223:49–52 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Calo’ G, Guerrini R, Smith G, Lambert DG. (2002) Effects of chronic nociceptin/orphanin FQ exposure on cAMP accumulation and receptor density in Chinese hamster ovary cells expressing human nociceptin/orphanin FQ receptors. Eur J Pharmacol 449:17–22 [DOI] [PubMed] [Google Scholar]
- Hawes BE, Fried S, Yao X, Weig B, Graziano MP. (1998) Nociceptin (ORL-1) and mu-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem 71:1024–1033 [DOI] [PubMed] [Google Scholar]
- He X, Treacy MN, Simmons DM, Ingraham HA, Swanson LW, Rosenfeld MG. (1989) Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature 340:35–41 [DOI] [PubMed] [Google Scholar]
- Heinricher MM. (2005) Nociceptin/orphanin FQ: pain, stress and neural circuits. Life Sci 77:3127–3132 [DOI] [PubMed] [Google Scholar]
- Ho MK, New DC, Wong YH. (2002) Co-expressions of different opioid receptor types differentially modulate their signaling via G(16). Neurosignals 11:115–122 [DOI] [PubMed] [Google Scholar]
- Homberg JR, Mul JD, de Wit E, Cuppen E. (2009) Complete knockout of the nociceptin/orphanin FQ receptor in the rat does not induce compensatory changes in mu, delta and kappa opioid receptors. Neuroscience 163:308–315 [DOI] [PubMed] [Google Scholar]
- Humbert PO, Corcoran LM. (1997) oct-2 gene disruption eliminates the peritoneal B-1 lymphocyte lineage and attenuates B-2 cell maturation and function. J Immunol 159:5273–5284 [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, Nothacker HP, Civelli O. (1997) Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA 94:14854–14858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas KH, Sutak M, Henderson G. (1998) Antinociceptive and morphine modulatory actions of spinal orphanin FQ. Can J Physiol Pharmacol 76:314–324 [PubMed] [Google Scholar]
- Kaminsky DE, Rogers TJ. (2011) Nociceptin/orphanin FQ receptor-driven heterologous desensitization of the major HIV-1 co-receptor CXCR4. J Neuroimmune Pharmacol 6:546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Tsang W, Doll S, Scherle P, Ko HS, Tran AC, Lenardo MJ, Staudt LM. (1992) Induction of the POU domain transcription factor Oct-2 during T-cell activation by cognate antigen. Mol Cell Biol 12:3149–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. (1997) Diuretic and antinatriuretic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ). Life Sci 60:PL15–PL21 [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Perrot-Sinal TS. (1996) Pronociceptive effects of the neuropeptide, nociceptin, in the land snail, Cepaea nemoralis. Peptides 17:763–768 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (1997) Differences between natural and recombinant G protein-coupled receptor systems with varying receptor/G protein stoichiometry. Trends Pharmacol Sci 18:456–464 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2002) Recombinant roulette versus the apparent virtues of ‘natural’ cell receptor systems: receptor genotypes versus phenotypes. Trends Pharmacol Sci 23:403–404 [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Kobayashi Y, Kishioka S. (2012) Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol 12:55–61 [DOI] [PubMed] [Google Scholar]
- Knoflach F, Reinscheid RK, Civelli O, Kemp JA. (1996) Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J Neurosci 16:6657–6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. (1998) The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 38:289–319 [DOI] [PubMed] [Google Scholar]
- Lou LG, Ma L, Pei G. (1997) Nociceptin/orphanin FQ activates protein kinase C, and this effect is mediated through phospholipase C/Ca2+ pathway. Biochem Biophys Res Commun 240:304–308 [DOI] [PubMed] [Google Scholar]
- Lü N, Han M, Yang ZL, Wang YQ, Wu GC, Zhang YQ. (2010) Nociceptin/Orphanin FQ in PAG modulates the release of amino acids, serotonin and norepinephrine in the rostral ventromedial medulla and spinal cord in rats. Pain 148:414–425 [DOI] [PubMed] [Google Scholar]
- Lutfy K, Sharza SA, Maidment NT. (1999) Tolerance develops to the inhibitory effect of orphanin FQ on morphine-induced antinociception in the rat. Neuroreport 10:103–106 [DOI] [PubMed] [Google Scholar]
- Ma F, Xie H, Dong ZQ, Wang YQ, Wu GC. (2005) Expression of ORL1 mRNA in some brain nuclei in neuropathic pain rats. Brain Res 1043:214–217 [DOI] [PubMed] [Google Scholar]
- Ma L, Cheng ZJ, Fan GH, Cai YC, Jiang LZ, Pei G. (1997) Functional expression, activation and desensitization of opioid receptor-like receptor ORL1 in neuroblastoma x glioma NG108-15 hybrid cells. FEBS Lett 403:91–94 [DOI] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. (1995) Possible therapeutic application of GABAB receptor agonists and antagonists. Clin Neuropharmacol 18:285–305 [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Altememi GF, Standifer KM. (2000) beta-Funaltrexamine inactivates ORL1 receptors in BE(2)-C human neuroblastoma cells. Eur J Pharmacol 402:R1–R37 [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Christensen JL, Standifer KM. (2002) Orphanin FQ/nociceptin-mediated desensitization of opioid receptor-like 1 receptor and mu opioid receptors involves protein kinase C: a molecular mechanism for heterologous cross-talk. J Pharmacol Exp Ther 302:502–509 [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Standifer KM. (2003) Mu-opioid-induced desensitization of opioid receptor-like 1 and mu-opioid receptors: differential intracellular signaling determines receptor sensitivity. J Pharmacol Exp Ther 306:965–972 [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. (1995) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18:22–29 [DOI] [PubMed] [Google Scholar]
- McDonald J, Barnes TA, Okawa H, Williams J, Calo’ G, Rowbotham DJ, Lambert DG. (2003) Partial agonist behaviour depends upon the level of nociceptin/orphanin FQ receptor expression: studies using the ecdysone-inducible mammalian expression system. Br J Pharmacol 140:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535 [DOI] [PubMed] [Google Scholar]
- Mika J, Obara I, Przewlocka B. (2011) The role of nociceptin and dynorphin in chronic pain: implications of neuro-glial interaction. Neuropeptides 45:247–261 [DOI] [PubMed] [Google Scholar]
- Miller TR, Fulford AJ. (2007) Regulation of nociceptin/orphaninFQ secretion by immune cells and functional modulation of interleukin-2. Peptides 28:2243–2252 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK. (1996a) Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci Lett 214:131–134 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. (2001) The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev 53:381–415 [PubMed] [Google Scholar]
- Mogil JS, Sternberg WF, Balian H, Liebeskind JC, Sadowski B. (1996b) Opioid and nonopioid swim stress-induced analgesia: a parametric analysis in mice. Physiol Behav 59:123–132 [DOI] [PubMed] [Google Scholar]
- Mollereau C, Mouledous L. (2000) Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides 21:907–917 [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341:33–38 [DOI] [PubMed] [Google Scholar]
- Morikawa H, Fukuda K, Mima H, Shoda T, Kato S, Mori K. (1998) Nociceptin receptor-mediated Ca2+ channel inhibition and its desensitization in NG108-15 cells. Eur J Pharmacol 351:247–252 [DOI] [PubMed] [Google Scholar]
- Murali SS, Napier IA, Rycroft BK, Christie MJ. (2012) Opioid-related (ORL1) receptors are enriched in a subpopulation of sensory neurons and prolonged activation produces no functional loss of surface N-type calcium channels. J Physiol 590:1655–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr (1999) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 412:563–605 [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. (1997) Molecular and cellular basis of addiction. Science 278:58–63 [DOI] [PubMed] [Google Scholar]
- Okawa H, Nicol B, Bigoni R, Hirst RA, Calo G, Guerrini R, Rowbotham DJ, Smart D, McKnight AT, Lambert DG. (1999) Comparison of the effects of [Phe1psi(CH2-NH)Gly2]nociceptin(1-13)NH2 in rat brain, rat vas deferens and CHO cells expressing recombinant human nociceptin receptors. Br J Pharmacol 127:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy HZ, Thakker DR, Standifer KM. (2005) Orphanin FQ/nociceptin potentiates [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin-Induced mu-opioid receptor phosphorylation. Mol Pharmacol 68:447–456 [DOI] [PubMed] [Google Scholar]
- Pan YX, Bolan E, Pasternak GW. (2002) Dimerization of morphine and orphanin FQ/nociceptin receptors: generation of a novel opioid receptor subtype. Biochem Biophys Res Commun 297:659–663 [DOI] [PubMed] [Google Scholar]
- Pan YX, Cheng J, Xu J, Rossi G, Jacobson E, Ryan-Moro J, Brooks AI, Dean GE, Standifer KM, Pasternak GW. (1995) Cloning and functional characterization through antisense mapping of a kappa 3-related opioid receptor. Mol Pharmacol 47:1180–1188 [PubMed] [Google Scholar]
- Pan YX, Xu J, Wan BL, Zuckerman A, Pasternak GW. (1998) Identification and differential regional expression of KOR-3/ORL-1 gene splice variants in mouse brain. FEBS Lett 435:65–68 [DOI] [PubMed] [Google Scholar]
- Pei G, Ling K, Pu L, Cunningham MD, Ma L. (1997) Nociceptin/orphanin FQ stimulates extracellular acidification and desensitization of the response involves protein kinase C. FEBS Lett 412:253–256 [DOI] [PubMed] [Google Scholar]
- Peluso J, Gavériaux-Ruff C, Matthes HW, Filliol D, Kieffer BL. (2001) Orphanin FQ/nociceptin binds to functionally coupled ORL1 receptors on human immune cell lines and alters peripheral blood mononuclear cell proliferation. Brain Res Bull 54:655–660 [DOI] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gavériaux-Ruff C. (1998) Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol 81:184–192 [DOI] [PubMed] [Google Scholar]
- Pertwee RG. (2008) Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol 13:147–159 [DOI] [PubMed] [Google Scholar]
- Pomonis JD, Billington CJ, Levine AS. (1996) Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport 8:369–371 [DOI] [PubMed] [Google Scholar]
- Pu L, Bao GB, Ma L, Pei G. (1999) Acute desensitization of nociceptin/orphanin FQ inhibition of voltage-gated calcium channels in freshly dissociated hippocampal neurons. Eur J Neurosci 11:3610–3616 [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270:792–794 [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. (2010) Interactions between the immune and nervous systems in pain. Nat Med 16:1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi D, Bigoni R, Rizzi A, Jenck F, Wichmann J, Guerrini R, Regoli D, Calo G. (2001) Effects of Ro 64-6198 in nociceptin/orphanin FQ-sensitive isolated tissues. Naunyn Schmiedebergs Arch Pharmacol 363:551–555 [DOI] [PubMed] [Google Scholar]
- Sandin J, Georgieva J, Schött PA, Ogren SO, Terenius L. (1997) Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci 9:194–197 [DOI] [PubMed] [Google Scholar]
- Seong KH, Maekawa T and Ishii S (2012) Inheritance and memory of stress-induced epigenome change: roles played by the ATF-2 family of transcription factors. Genes Cells 17:249–263. [DOI] [PMC free article] [PubMed]
- Serhan CN, Fierro IM, Chiang N, Pouliot M. (2001) Cutting edge: nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered-15-epi-lipoxin A4. J Immunol 166:3650–3654 [DOI] [PubMed] [Google Scholar]
- Shah S, Page CP, Spina D. (1998) Nociceptin inhibits non-adrenergic non-cholinergic contraction in guinea-pig airway. Br J Pharmacol 125:510–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowe SJ, Clarke S, Lena I, Goody RJ, Lattanzi R, Negri L, Simonin F, Matthes HW, Filliol D, Kieffer BL, et al. (2001) Autoradiographic mapping of the opioid receptor-like 1 (ORL1) receptor in the brains of mu-, delta- or kappa-opioid receptor knockout mice. Neuroscience 106:469–480 [DOI] [PubMed] [Google Scholar]
- Spampinato S, Baiula M. (2006) Agonist-regulated endocytosis and desensitization of the human nociceptin receptor. Neuroreport 17:173–177 [DOI] [PubMed] [Google Scholar]
- Spampinato S, Di Toro R, Alessandri M, Murari G. (2002) Agonist-induced internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Cell Mol Life Sci 59:2172–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato S, Di Toro R, Qasem AR. (2001) Nociceptin-induced internalization of the ORL1 receptor in human neuroblastoma cells. Neuroreport 12:3159–3163 [DOI] [PubMed] [Google Scholar]
- Sulaiman MR, Niklasson M, Tham R, Dutia MB. (1999) Modulation of vestibular function by nociceptin/orphanin FQ: an in vivo and in vitro study. Brain Res 828:74–82 [DOI] [PubMed] [Google Scholar]
- Thakker DR, Altememi GF, Mandyam CD, Ibrahim H, Ramirez VI, Standifer KM. (2007) Naloxone benzoylhydrazone activates extracellular signal-regulated protein kinases and modulates nociceptin opioid peptide receptor activity. Am J Pharmacol Toxicol 2:51–59 [Google Scholar]
- Thakker DR, Standifer KM. (2002a) Induction of G protein-coupled receptor kinases 2 and 3 contributes to the cross-talk between mu and ORL1 receptors following prolonged agonist exposure. Neuropharmacology 43:979–990 [DOI] [PubMed] [Google Scholar]
- Thakker DR, Standifer KM. (2002b) Orphanin FQ/nociceptin blocks chronic morphine-induced tyrosine hydroxylase upregulation. Brain Res Mol Brain Res 105:38–46 [DOI] [PubMed] [Google Scholar]
- Ueda H, Inoue M, Takeshima H, Iwasawa Y. (2000) Enhanced spinal nociceptin receptor expression develops morphine tolerance and dependence. J Neurosci 20:7640–7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Bagley EE, Drew GM, Schuller A, Pintar JE, Hack SP, Christie MJ. (2003) Cellular actions of opioids on periaqueductal grey neurons from C57B16/J mice and mutant mice lacking MOR-1. Br J Pharmacol 139:362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. (1996) Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol 117:1609–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Connor M, Jennings EA, Marinelli S, Allen RG, Christie MJ. (2001) Actions of nociceptin/orphanin FQ and other prepronociceptin products on rat rostral ventromedial medulla neurons in vitro. J Physiol 534:849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Christie MJ. (1997) Actions of the ORL1 receptor ligand nociceptin on membrane properties of rat periaqueductal gray neurons in vitro. J Neurosci 17:996–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Grandy DK, Kelly MJ. (1998) The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology 67:73–82 [DOI] [PubMed] [Google Scholar]
- Waits PS, Purcell WM, Fulford AJ, McLeod JD. (2004) Nociceptin/orphanin FQ modulates human T cell function in vitro. J Neuroimmunol 149:110–120 [DOI] [PubMed] [Google Scholar]
- Wang HL, Hsu CY, Huang PC, Kuo YL, Li AH, Yeh TH, Tso AS, Chen YL. (2005) Heterodimerization of opioid receptor-like 1 and mu-opioid receptors impairs the potency of micro receptor agonist. J Neurochem 92:1285–1294 [DOI] [PubMed] [Google Scholar]
- Wang HL, Kuo YL, Hsu CY, Huang PC, Li AH, Chou AH, Yeh TH, Chen YL. (2006) Two C-terminal amino acids, Ser(334) and Ser(335), are required for homologous desensitization and agonist-induced phosphorylation of opioid receptor-like 1 receptors. Cell Signal 18:670–678 [DOI] [PubMed] [Google Scholar]
- Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR. (1994) cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett 348:75–79 [DOI] [PubMed] [Google Scholar]
- Wick MJ, Minnerath SR, Lin X, Elde R, Law PY, Loh HH. (1994) Isolation of a novel cDNA encoding a putative membrane receptor with high homology to the cloned mu, delta, and kappa opioid receptors. Brain Res Mol Brain Res 27:37–44 [DOI] [PubMed] [Google Scholar]
- Wick MJ, Minnerath SR, Roy S, Ramakrishnan S, Loh HH. (1995) Expression of alternate forms of brain opioid ‘orphan’ receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Brain Res Mol Brain Res 32:342–347 [DOI] [PubMed] [Google Scholar]
- Wu EH, Lo RK, Wong YH. (2003) Regulation of STAT3 activity by G16-coupled receptors. Biochem Biophys Res Commun 303:920–925 [DOI] [PubMed] [Google Scholar]
- Xie GX, Meuser T, Pietruck C, Sharma M, Palmer PP. (1999) Presence of opioid receptor-like (ORL1) receptor mRNA splice variants in peripheral sensory and sympathetic neuronal ganglia. Life Sci 64:2029–2037 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. (1997) Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience 81:249–254 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ohtori S, Chiba T. (2000) Effects of pre-emptively administered nociceptin on the development of thermal hyperalgesia induced by two models of experimental mononeuropathy in the rat. Brain Res 871:192–200 [DOI] [PubMed] [Google Scholar]
- Yu TP, Fein J, Phan T, Evans CJ, Xie CW. (1997) Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus 7:88–94 [DOI] [PubMed] [Google Scholar]
- Zhang J, Salojin KV, Gao JX, Cameron MJ, Bergerot I, Delovitch TL. (1999a) p38 mitogen-activated protein kinase mediates signal integration of TCR/CD28 costimulation in primary murine T cells. J Immunol 162:3819–3829 [PubMed] [Google Scholar]
- Zhang M, Wang XM, Zhang DB, Xu GH, Dong HW, Yu YX, Han JS. (2005) Orphanin FQ antagonizes the inhibition of Ca(2+) currents induced by mu-opioid receptors. J Mol Neurosci 25:21–27 [DOI] [PubMed] [Google Scholar]
- Zhang NR, Planer W, Siuda ER, Zhao HC, Stickler L, Chang SD, Baird MA, Cao YQ, Bruchas MR. (2012a) Serine 363 is required for nociceptin/orphanin FQ opioid receptor (NOPR) desensitization, internalization, and arrestin signaling. J Biol Chem 287:42019–42030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Tong Y, Tian M, Dehaven RN, Cortesburgos L, Mansson E, Simonin F, Kieffer B, Yu L. (1998) Dynorphin A as a potential endogenous ligand for four members of the opioid receptor gene family. J Pharmacol Exp Ther 286:136–141 [PubMed] [Google Scholar]
- Zhang Y, Donica CL, Standifer KM. (2012b) Sex differences in the Nociceptin/Orphanin FQ system in rat spinal cord following chronic morphine treatment. Neuropharmacology 63:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang Y, Rishi AK, Sheikh MS, Shroot B, Reichert U, Dawson M, Poirer G, Fontana JA. (1999b) Activation of the p38 and JNK/SAPK mitogen-activated protein kinase pathways during apoptosis is mediated by a novel retinoid. Exp Cell Res 247:233–240 [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang Y, Xin SM, Ma L, Pei G. (1998) Attenuation of nociceptin/orphanin FQ-induced signaling by N-methyl-D-aspartate in neuronal cells. Neuroreport 9:631–636 [DOI] [PubMed] [Google Scholar]
- Zheng F, Grandy DK, Johnson SW. (2002) Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharmacol 136:1065–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Xu SF, Cao XD, Wu GC, Zhang XL, Li MY, Cui DF, Chi CW. (1996) Antagonistic action of orphanin FQ on acupuncture analgesia in rat brain. Acupunct Electrother Res 21:199–205 [DOI] [PubMed] [Google Scholar]