Abstract
G protein-coupled receptor signaling does not result from sequential activation of a linear pathway of proteins/enzymes, but rather from complex interactions of multiple, branched signaling routes, i.e., signaling networks. In this work we present an exhaustive study of the cross-talk between H1 and H2 histamine receptors (H1R and H2R) in U937 cells and Chinese hamster ovary-transfected cells. By desensitization assays we demonstrated the existence of a crossdesensitization between both receptors independent of protein kinase A or C. H1R-agonist stimulation inhibited cell proliferation and induced apoptosis in U937 cells following treatment of 48 hours. H1R-induced antiproliferative and apoptotic response was inhibited by an H2R agonist suggesting that the cross-talk between both receptors modifies their function. Binding and confocal microscopy studies revealed cointernalization of both receptors upon treatment with the agonists. To evaluate potential heterodimerization of the receptors, sensitized emission fluorescence resonance energy transfer experiments were performed in human embryonic kidney 293T cells using H1R-cyan fluorescent protein and H2R-yellow fluorescent protein. To our knowledge these findings may represent the first demonstration of agonist-induced heterodimerization of the H1R and H2R. In addition, we also show that the inhibition of the internalization process did not prevent receptor crossdesensitization, which was mediated by G protein-coupled receptor kinase 2. Our study provides new insights into the complex signaling network mediated by histamine and further knowledge for the rational use of its ligands.
Introduction
Histamine [2-(4-imidazolyl)-ethylamine] is an important mediator of many physiologic and pathologic processes, including inflammation, gastric acid secretion, neuromodulation, regulation of immune function, and cell proliferation and differentiation. Histamine exerts its effects by binding to four different G protein-coupled receptors (GPCRs), namely histamine receptors 1, 2, 3, and 4 (H1R, H2R, H3R, H4R) (Parsons and Ganellin, 2006). Particularly, the H1R and H2R have been found to be coexpressed in most tissues and cell types such as neurons, airway, and vascular smooth muscle cells, endothelial cells, hepatocytes, epithelial cells, neutrophils, eosinophils, monocytes, dendritic cells, as well as T and B lymphocytes among others (Parsons and Ganellin, 2006; Jutel et al., 2009). In most tissues, H1R couples to Gαq/11 leading to the increase in phosphoinositide metabolism, whereas H2R couples to Gαs, triggering adenylyl cyclase (AC) activation and cAMP accumulation (Hill et al., 1997).
Several studies support a coordinated regulation between both receptors. For example, in guinea pig cerebral cortical slices, H1R stimulation augments cAMP responses to H2R stimulation via a mechanism which appears to involve the accumulation of inositol triphosphate and diacylglycerol (Selbie and Hill, 1998). In contrast, a reverse interaction can occur in bovine tracheal smooth muscle in which agents raising intracellular cAMP levels can inhibit H1R-mediated inositol phospholipid hydrolysis (Dickenson et al., 1993). In this case, the application of selective H1- and H2-receptor agonists and antagonists in the lung established the concept that the two receptor populations may mediate opposing physiologic and pharmacological effects in the pulmonary system. H1R mediates deleterious actions such as bronchoconstriction, vasoconstriction, and edema formation, while stimulation of pulmonary H2R plays a modulatory role, causing bronchodilation and inhibiting mediator release (Parsons and Ganellin, 2006).
The activity of GPCRs results from a coordinated balance among the diverse mechanisms that govern receptor signaling at different levels of signal propagation; in this way the final response of a cellular system results from the integration and weighting up of the different signals it receives.
An important adaptive response of the cell against multiple or sustained extracellular stimuli is receptor desensitization, which protects the cell from receptor overstimulation. The underlying mechanisms for turning off GPCR signalings are complex and may involve receptor phosphorylation, uncoupling from G proteins, internalization, and ultimately receptor down-regulation (Zhang et al., 1997). In some cases, the stimulation of one GPCR may lead to generalized desensitization of other unrelated GPCRs by a mechanism known as heterologous desensitization (Ferguson, 2001). This process, where nontargeted receptor signaling is inhibited by the activation of a different receptor, would represent a regulatory mechanism of coordination and balance of diverse signaling cascades.
In this context, the discovery of GPCRs’ homo- and hetero-oligomerization is revolutionizing the analysis of their pharmacology and signaling integration, thus leading to renewed interest in GPCRs as therapeutic targets and reinvigorating drug discovery and therapeutic strategies (Panetta and Greenwood, 2008). Receptor oligomerization is essential for receptor function in GABAB, taste and rhodopson receptors that form heterodimers/oligomeric structures in native disk membranes (Nelson et al., 2002; Filipek et al., 2004; Kniazeff et al., 2004; Pin et al., 2004). Oligomerization has also been shown to play a modulatory role. Furthermore, oligomerization can be constitutive or agonist-induced, and its functional relevance has been proved for many receptors (Breitwieser, 2004). In the case of H1R and H2R, both are known to be coexpressed and they have been even described as multimeric entities (Fukushima et al., 1997; Bakker et al., 2004). However, it is not known whether their coordinated regulation is mediated by the formation of heteromeric H1/H2R complexes. The purpose of the present work was to investigate the potential cross-regulation between H1R and H2R in U937 cells, in which both histamine receptors are endogenously expressed, and in Chinese hamster ovary (CHO)-transfected cells. Our findings showed that in both cell types treatment with H1R and H2R agonists induce crossdesensitization of both receptors by a mechanism independent of second messenger systems and their downstream kinases, kinases A (PKA) or C (PKC). Furthermore, the H1R agonist inhibited cell proliferation and induced apoptosis in U937 cells. Interestingly, when cells were exposed to both H1R and H2R agonists these responses were abolished. In transfected cells, colocalization and fluorescence resonance energy transfer (FRET) microscopy assays revealed the cointernalization of the receptors with the formation of heteromers in endosomes upon their activation. In addition, we also show that the inhibition of the internalization process did not prevent receptor crossdesensitization, which was mediated by GRK2.
These results indicate that there is a negative cross-regulation mechanism between H1 and H2 receptors, involving crossdesensitization, cointernalization, and heterodimerization, which is critical for the output response to histaminergic stimulation.
Materials and Methods
Materials
Cell culture medium, antibiotics, isobutylmethyl xanthine (IBMX), cAMP, prostaglandin E2 (PGE2), ATP, isoproterenol, histamine, forskolin, 2,3-trifluoromethylphenylhistamine (H1R agonist), myoinositol, bovine serum albumin (BSA), phosphoinositide-specific phospholipase C (PLC) inhibitor U73122, PKC inhibitor GF109203X, and PKA inhibitor KT5720 were obtained from Sigma Chemical Company (St. Louis, MO). Amthamine (H2R agonist), mepyramine, and tiotidine were acquired from Tocris Cookson Inc. (R&D Systems Inc., Minneapolis, MN). [3H]cAMP, [3H]mepyramine, [3H]tiotidine, and myo-[3H]inositol were purchased from Perkin Elmer Life Sciences (Boston, MA). Dowex AG-1X8 formate form resin was obtained from Bio-Rad (Hercules, CA). Other chemicals used were of analytical grade and obtained from standard sources.
Generation of cDNA Constructs
H2R-yellow fluorescent protein (-YFP) and H1R-cyan fluorescent protein (-CFP). To create expression plasmids encoding fusion proteins, the wild-type human H2R cDNA was amplified from pCEFL-H2R with a sense primer (5′GTAGAATTCCACCATGGCACCCAATGG3′) and an antisense primer (5′CGGGATCCTTCCTGTCTGTGG3′) and inserted into the EcoRI/BamHI sites of pEYFP-N1 (Clontech, Mountain View, CA). The wild-type human H1R cDNA was amplified from pCDNA3-H1R with a sense primer (5′GCTAGCACCATGAGCCTCCTA3′) and an antisense primer (5′GGCAAGCTTGGAGCGAATATGCAGAATTCTCT3′) and inserted into the NheI/HindIII sites of pECFP-N1 (Clontech).
Cell Culture and Transfections
U937 cells and derived clones (A2, DC6, and GB4) were cultured at 37°C in a humidified atmosphere of 5% CO2 in RPMI 1640 medium, CHO-H1R, CHO-H2R, and CHO-H1R-H2R cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 μM hypoxanthine, 16 μM thymidine, 0.8 mg/ml G418, and human embryonic kidney (HEK)293T were cultured in DMEM; all supplemented with 10% fetal calf serum and 5 μg/ml gentamicin. The stably expressing human H1R and H2R cell lines (CHO-H1R and CHO-H2R) were previously generated in our laboratory (Notcovich et al., 2010; Tubio et al., 2010). DC6, A2, and GB4 cell lines were obtained by U937 stable transfection with a dynamin dominant negative mutant, a G protein-coupled receptor kinase 2 (GRK2) antisense construct, and a GRK2 dominant negative mutant of the kinase site, and were previously characterized (Fernandez et al., 2002, 2008, 2011).
For transient transfection, cells were grown to 80–90% confluency and the cDNA constructs were transfected using LipofectAMINE 2000. The transfection protocol was optimized as recommended by the supplier (Invitrogen, Carlsbad, CA). Usually, assays were performed 48 hours after transfection.
cAMP Assay
For desensitization assays, cells were pretreated with agonists, antagonists, and/or inhibitors in the absence of IBMX at the times shown in the figures. Cells were then washed and resuspended in fresh medium containing 1 mM IBMX, incubated for 3 minutes, and exposed to 10 μM amthamine, 1 μM isoproterenol, or 1 μM PGE2 for 9 minutes to determine whether the system was able to generate cAMP. The reaction was stopped by ethanol addition followed by centrifugation at 2000g for 5 minutes. The ethanol phase was then dried and the residue resuspended in 50 mM Tris-HCl, pH 7.4, 0.1% BSA. cAMP content was determined by competition of [3H]cAMP for PKA, as previously described (Davio et al., 1995).
[3H]-Inositol Phosphate Production
Total inositol phosphate production was assessed as previously described (Fitzsimons et al., 2004). Briefly, cells were incubated with myo-[3H]-inositol (5 μCi/ml) in DMEM with calf serum and cultured for 6 hours. Thereafter, the medium was replaced by DMEM without calf serum containing 10 mM LiCl and incubated for 10 minutes. Cells were pretreated 5 minutes with the different agonists and/or antagonists, washed, and cells were then stimulated for 20 minutes with H1R agonist or ATP in a final volume of 300 μl. In assays in the presence of inhibitor, PKA inhibitor was added 20 minutes before the agonist stimulation. The reaction was stopped by the addition of 2 ml of cold chloroform/methanol (1:2 v/v, freshly prepared), and phases were separated by adding 1 ml of water and 620 μl of chloroform. The mixture was then centrifuged at 1500g for 10 minutes, and the total water-soluble inositol phosphate fraction was purified by anion exchange chromatography. Radioactivity in the eluted fractions was measured using a Wallac 1410 liquid scintillation counter.
Intracellular Ca2+ Measurements
Intracellular Ca2+ measurement was assessed as previously described (Copsel et al., 2011). Briefly, U937 cells were resuspended and incubated in a buffered saline solution (BSS: 140 mM NaCl, 3.9 mM KCl, 0.7 mM KH2PO4, 0.5 mM Na2HPO4. 12 H2O, 1 mM CaCl2, 0.5 mM MgCl2 and 20 mM HEPES, 10 mM glucose, and 0.1% BSA, pH 7.5) in the presence of 2 mM Fura 2-AM for 30 minutes. Fluorescence was measured in a spectrofluorometer (Jasco, Tokyo, Japan) provided with the CA-61 accessory to measure Ca2+ with continuous stirring, with the thermostat adjusted to 37°C and an injection chamber. During 8 minutes intracellular Ca2+ ([Ca2+]i) levels were registered every second by exposure to alternating 340- and 380-nm light beams, and the intensity of light emission at 505 nm was measured. In this way, light intensities and their ratio (F340/F380) were tracked. Different agents were injected into the chamber as a 100-fold concentrated solution without interrupting recording. The preparation was calibrated by determining maximal fluorescence induced by 0.1% Triton X-100, and minimal fluorescence in the presence of 6 mM EGTA (pH 8.3). [Ca2+]i was calculated according to Grynkiewicz et al., 1985.
Cell Proliferation
U937 cells were seeded at 2 × 105 cells/ml and incubated for 1 or 2 days with different combinations of compounds. Cells were harvested, and their numbers were estimated using a hemocytometer chamber.
Determination of Apoptosis Markers
Cell Cycle Analysis.
U937 cells growing in exponential phase were treated as indicated for 48 hours. Then, cells were harvested and centrifuged at 1000 rpm for 5 minutes, resuspended in one volume of phosphate buffered saline (PBS) and fixed and permeabilized by vigorous addition of nine volumes of ice-cold 70% (v/v) ethanol and stored at –20°C for a minimum of 24 hours, prior to analysis. Cells at a density of approximately 106 were resuspended in 800 μl of propidium iodide (PI) staining solution (20 μg/ml PI and 200 μg/ml RNase A in PBS, pH 7.4) and incubated in the dark at room temperature for 30 minutes. The percentages of cells in the sub-G0/G1, G0/G1, S, and G2/M cell cycle phases were determined by a FACS Scan Flow Cytometer (Becton-Dickinson, Laguna Hills, CA). Data from at least three independent experiments were analyzed using Cyflogic free software (www.cyflogic.com).
Determination of Phosphatidylserine Exposure at the Cell Surface by Annexin V Binding Assay.
Cells growing in exponential phase were seeded at 3.5 × 105 cells/ml and treated with different compounds or 2% (v/v) dimethylsulfoxide (DMSO; positive control) for 48 hours. Following cold PBS washing, 2.0 × 105 cells were incubated with fluorescein isothiocyanate (FITC)-labeled annexin V and PI according to the manufacturer instructions (Invitrogen) and analyzed using a FACS Scan Flow Cytometer (Becton-Dickinson). The different cell subpopulations were identified according to the annexin V/PI staining pattern, as follows: cells labeled with annexin V only were considered to be at an early apoptotic stage, cells labeled with annexin V and PI were considered to be at a late apoptotic stage, and cells labeled with PI only were considered necrotic.
Determination of Caspase-3, and Poly(ADP-Ribose)Polymerase by Western Blot.
For Western blot assays cells were lysed in 50 mM Tris-HCl, pH 6.8, 2% SDS, 100 mM 2-mercaptoethanol, 10% glycerol, and 0.05% bromophenol blue and sonicated to shear DNA. Total cell lysates were resolved by 8% SDS-PAGE for poly(ADP-ribose)polymerase (PARP) detection or 15% SDS PAGE for caspase 3 detection, blotted, and incubated with the indicated primary antibodies (Santa Cruz Biotechnology, Dallas, TX), followed by horseradish peroxidase conjugated anti-rabbit (Santa Cruz Biotechnology), and developed by enhanced chemiluminesence (ECL) following the manufacturer’s instructions (Amersham, GE Healthcare Lifesciences, Pittsburgh, PA).
Radioligand Binding Assay.
Triplicate assays were performed in 50 mM Tris-HCl, pH 7.4. Saturation studies were performed by incubating 106 U937 cells/tube or 104 CHO-H1R, CHO-H2R, or CHO-H1R-H2R cells per 48 wells for 40 minutes at 4°C with increasing concentrations of [3H]tiotidine or [3H]mepyramine, ranging from 0.4 up to 240 nM in the absence or presence of 1 μM unlabeled tiotidine or mepyramine, respectively. The incubation was stopped by dilution with 3 ml of ice-cold 50 mM Tris-HCl, pH 7.4. For U937 cells, rapid filtration under reduced pressure onto Whatman GF/B glass-fiber filters, followed by three washes with 3 ml ice-cold buffer was performed. For CHO-transfected cells, after three washes with 3 ml ice-cold buffer the bound fraction was collected in 200 μl of ethanol. Experiments on intact cells were carried out at 4°C to avoid ligand internalization. The kinetic studies performed with 2 nM [3H]tiotidine or [3H]mepyramine at 4°C showed that the equilibrium was reached at 30 minutes and persisted for 4 hours (unpublished data).
Confocal Microscopy.
HEK293T cells were grown to 80–90% confluency in p35 glass-bottom dishes (MatTek, Ashland, MA) and transfected with the corresponding plasmids using LipofectAMINE 2000 reagent (Invitrogen). Assays were performed after 48 hours and cells were serum-starved for 2 hours prior stimulation. Live cell imaging was performed using a LSM 700 Zeiss confocal laser-scanning microscope with a Plan-Apochromat 63× 1.40 NA oil immersion objective and incubation at 37°C and 5% CO2. Cells were stimulated with 10 μM H1R or H2R agonist and images were acquired every 1–2 minutes over a period of 30 minutes. Excitation and filters were as follows: CFP, 445 nm excitation, band-pass (BP) 460–500-nm emission; YFP, 488 nm excitation, BP 520–600-nm emission. The image and statistical analysis was performed with the ImageJ software Colocalization Analysis plugin (NIH, Bethesda, MD).
FRET Measured by Confocal Microscopy.
HEK293T cells were grown and stimulated with H1R and H2R agonist as described before. To normalize FRET, the background given by the images from nontransfected cells was subtracted from the images from transfected cells, and the resulting images were processed by the FRET and Colocalization Analyzer plugin of ImageJ software. This software allows visualization of FRET images acquired by confocal sensitized emission, involving excitation of the donor fluorophore and detection of the energy transfer as an emission from the acceptor fluorophore into the FRET channel. The plugin calculates the Bleed-Through of the pair of fluorophores as constant values and substract them from the raw FRET channel Image using an equation similar to the one described in Youvan et al., 1997. This method reduces the interference of the user to a minimum by analyzing the entire image, pixel by pixel, and displays FRET images as a function of the colocalization of the two fluorescent partners.
Statistical Analysis.
Statistical analysis was performed from at least three independent experiments. Binding data, sigmoidal dose-response, and desensitization fittings were performed with GraphPad Prism 5.00 for Windows, GraphPad Software (San Diego, CA). One-way ANOVA followed by Dunnett’s post test was performed using GraphPad InStat version 3.01 (GraphPad Software). Specific binding was calculated by subtraction of nonspecific binding from total binding. Cellular proliferation and cell cycle statistical analysis were carried out by one-way ANOVA followed by Tukey’s multiple comparison post test.
Results
H1R and H2R Crossdesensitization.
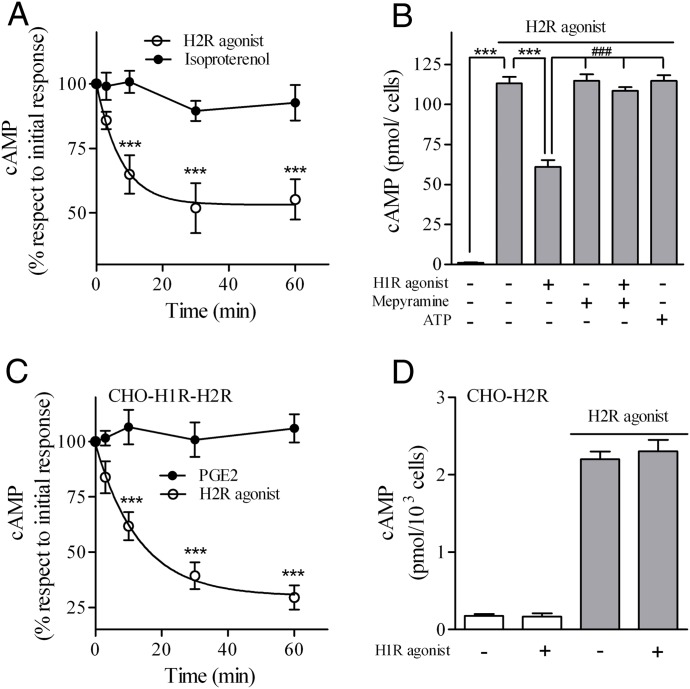
To evaluate the potential cross-regulation between H1 and H2 receptors we studied in U937 and in CHO-transfected cells the effect of 10 μM 2,3-trifluoromethylphenylhistamine (H1R agonist) pretreatment on H2R response and the effect of 10 μM amthamine (H2R agonist) pretreatment on H1R response. Cyclic AMP production following 10 μM H2R agonist stimulation decreased in U937 cells pretreated with H1R agonist at different time periods, whereas the response to 1 μM isoproterenol (β-adrenoreceptor agonist) was unaffected (Fig. 1A). The concentration used for each ligand corresponded to the maximum cAMP response in U937 cells (unpublished data). Furthermore, this crossdesensitization was abolished by 10 μM mepyramine (H1R antagonist), supporting that the response was mediated by H1R activation (Fig. 1B). To evaluate whether this effect resulted from modulation by second messengers, H1R response was evaluated following activation of another Gq-coupled receptor. Results showed that cAMP response to amthamine in ATP (purinergic-recepter agonist)-pretreated cells remained unchanged (Fig. 1B).We next addressed whether the cross-regulation observed in U937 was privative of this cell line by using CHO cells which do not express H1R or H2R membrane sites, as revealed by binding assays or the response to H1R or H2R agonists (unpublished data). We generated stably expressing human H1R CHO cells (CHO-H1R) (Notcovich et al., 2010) transiently transfected with H2R. In this model, amthamine but not PGE2 response was desensitized by pretreatment with 10 μM H1R agonist, in accordance with observations in U937 cells (Fig. 1C). To confirm that H1R agonist desensitized H2R response through H1R, the assay was performed in H2R-expressing CHO-H2R cells, which were previously characterized in our laboratory (Tubio et al., 2010). As shown in Fig. 1D, no crossdesensitization occurs in the absence of H1R. These findings clearly show that H1R stimulation induces crossdesensitization of the H2R response when endogenously expressed in U937 and in reconstituted CHO-H1R-H2R cells.
Fig. 1.
Effect of H1R agonist on H2R response. (A and C) H2R desensitization kinetic induced by 2,3-trifluoromethylphenylhistamine (H1R agonist). U937 (A) or CHO-H1R-H2R (C) cells were incubated with 10 μM H1R agonist at different time points and washed twice with PBS. cAMP response to 10 μM amthamine (H2R agonist), 1 μM isoproterenol, or 1 μM PGE2 was determined as detailed under Materials and Methods. Data represent mean ± S.E.M. (n = 3). ***P < 0.001 with respect to initial response. (B) U937 cells were exposed to 10 μM H1R agonist, 10 μM mepyramine, or 1 μM ATP, alone or in combination for 30 minutes, washed twice with PBS, and cAMP response to 10 μM amthamine determined (shaded bars). Data represent mean ± S.E.M. (n = 3). ***P < 0.001; ###P < 0.001 respect to H1R agonist pretreatment. (D) CHO-H2R cells were exposed to 10 μM H1 agonist for 30 minutes, washed, and cAMP response to 10 μM amthamine determined (shaded bars). Data represent mean ± S.E.M. (n = 3). cAMP basal levels were not significantly modified by H1R agonist preincubation.
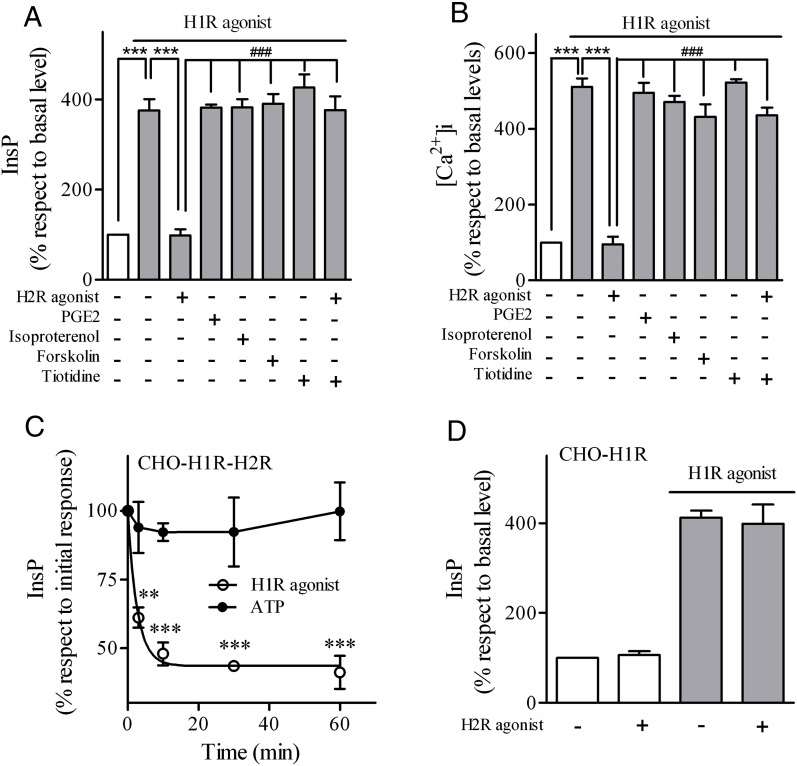
We next investigated whether H2R agonist induced crossdesensitization of H1R. As shown in Fig. 2, A and B, pretreatment of U937 cells with 10 μM H2R agonist decreased InsP production and Ca2+ accumulation in response to H1R stimulation. We then evaluated if H2R crossdesensitized H1R response. As shown in Fig. 2, A and B, U937 cells pretreated with 10 μM H2R agonist decreased InsP production and Ca2+ accumulation in response to H1R stimulation. This negative cross-talk was not evident when cells were pretreated with other ligands that increase cAMP levels, such as PGE2, isoproterenol, or forskolin (AC activator), and was inhibited by the H2R antagonist tiotidine. In CHO-H1R-H2R-transfected cells, pretreatment with the H2R agonist desensitized InsP-H1R response but not ATP response. Furthermore, in CHO-H1R cells crossdesensitization was not detected in the absence of H2R (Fig. 2D).
Fig. 2.
Effect of H2R agonist on H1R response. (A) U937 cells were exposed for 5 minutes to 10 μM H2R agonist, 1 μM PGE2, 1 μM isoproterenol, 75 μM forskolin, or 10 μM tiotidine as indicated, washed, and cells were then stimulated for 20 minutes with 10 μM H1R agonist (shaded bars). InsP production was measured as described under Materials and Methods. Data were calculated as the mean ± S.E.M. (n = 3). (B) U937 cells were exposed for 5 minutes to 10 μM H2R agonist, 1 μM PGE2, 1 μM isoproterenol, 75 μM forskolin, or 10 μM tiotidine as indicated and stimulated with 10 μM H1R agonist. [Ca2+]i was determined as described under Materials and Methods. Data were calculated as the mean ± S.E.M. (n = 3). ***P < 0.001; ###P < 0.001 with respect to H2R agonist pretreatment. (C) CHO-H1R-H2R cells were incubated with 10 μM H2R agonist at different time points and washed. InsP response to 10 μM H1 agonist or 1 μM ATP was determined as detailed under Materials and Methods. Data were calculated as the mean ± S.E.M. (n = 3). ***P < 0.001 with respect to initial response. (D) CHO-H1R cells were exposed to 10 μM H2R agonist for 30 minutes, washed, and InsP response to 10 μM H1 agonist was determined (shaded bars). Data were calculated as the mean ± S.E.M. (n = 3). InsP basal levels were not significantly modify by H2R-agonist preincubation.
Our findings demonstrate the existence of a specific and mutual crossdesensitization between H1R and H2R in two different cell lines.
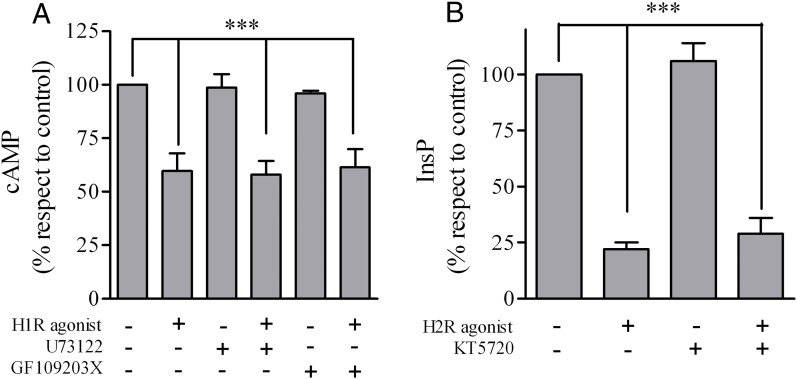
For a large number of related GPCRs, activation of one GPCR induces phosphorylation of C-terminal cytosolic tails of another GPCR by second messenger-mediated kinases, such as PKA or PKC. Phosphorylated receptors lose their capacity to couple to the downstream heterotrimeric G protein and therefore become insensitive to stimulation. With the aim to determine whether PKA and PKC was responsible for the crossdesensitization of H1R and H2R, assays in U937 cells were performed in the presence of pharmacological inhibitors of the kinases. As shown in Fig. 3A, GF109203X (PKC inhibitor) failed to modify the decrease in H2R-mediated cAMP response induced by crossdesensitization. In turn, KT5720 (PKA inhibitor) did not affect the negative cross-talk in the H1R inositol phosphates (InsP) response (Fig. 3B). We also explored whether inositol 1,4,5 triphosphate and 1,2-diacylglycerol (DAG) generation resulting from phosphatidylinositol 4,5-biphosphate hydrolysis mediated by H1R agonist was responsible for H2R desensitization. Fig. 3A shows that the crossdesensitization also occurs in the presence of a PLC inhibitor.
Fig. 3.
H1R and H2R crossdesensitization in the presence of PLC/PKC and PKA inhibitors. (A) U937 cells were exposed for 30 minutes to 10 μM H1R agonist, 10 μM U73122, or 20 μM GF109203X as indicated, washed, and cAMP response to 10 μM amthamine determined. (B) U937 cells were exposed for 5 minutes to 10 μM H2R agonist or 10 μM KT5720 as indicated and washed, and cells were then stimulated for 20 minutes with 10 μM H1R agonist. InsP production was measured as described under Materials and Methods. Data were calculated as the mean ± S.E.M. (n = 3). ***P < 0.001 with respect to untreated cells (control).
Our findings show that H1R and H2R crossdesensitization is independent of AC, PLC, PKA, and PKC activation.
H1 Agonist-Induced Apoptosis in U937 Cells Is Inhibited by an H2R Agonist.
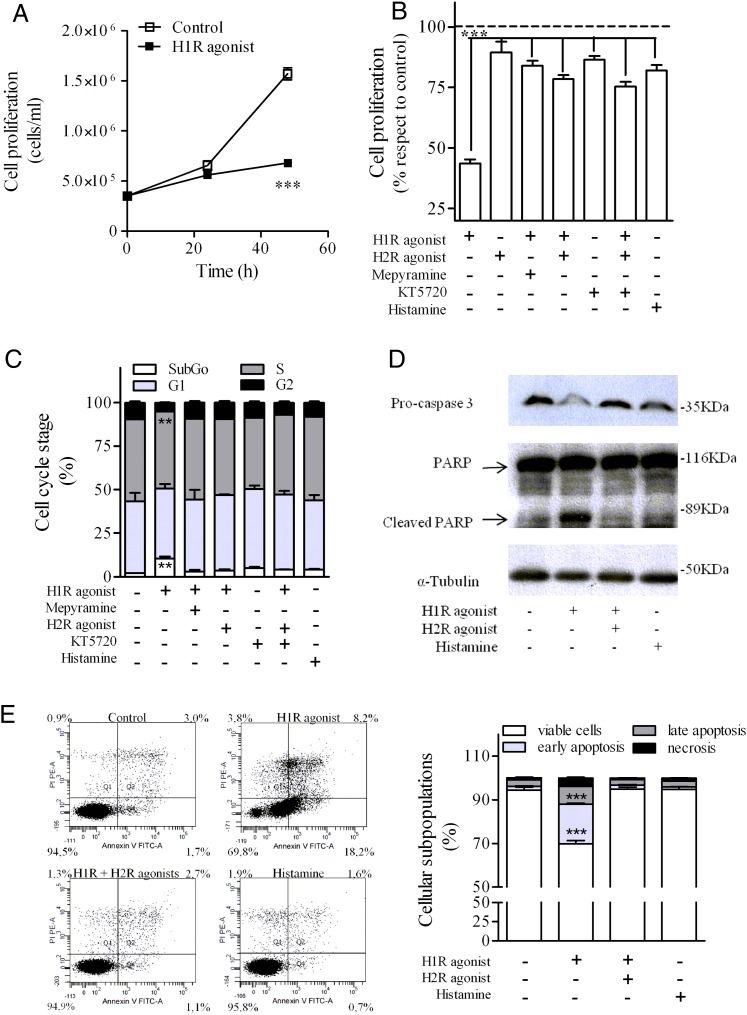
Histamine regulates cell proliferation in different cell types through H1R activation (Lázár-Molnár et al., 2002; Notcovich et al., 2010). Exposure of U937 cells to 10 μM H1R agonist for 48 hours inhibited cell proliferation by 56%, but the incubation with 10 μM H2R agonist did not affect it (Fig. 4A). To evaluate whether H1R and H2R crossdesensitization affected this response, cells were incubated for 48 hours with the H1R agonist alone or in combination with either H2R agonist or H1R antagonist mepyramine. The antiproliferative effect mediated by H1R was not only inhibited by H1R blockade (16%) but also by H2R stimulation (21%). Pretreatment of cells with a PKA inhibitor did not change H2R effect on H1R-mediated response (Fig. 4B). FACS studies showed that U937 cells incubated with the H1R agonist increased the number of cells in the sub-G0/G1 phase of the cell cycle (Fig. 4C). When cells were exposed to the H1R agonist and amthamine, cell cycle arrest was not achieved in accordance with the proliferative response observed. We next evaluated cleavage and activation of caspase 3, the key terminal effector of apoptosis by Western blot. The exposure of U937 cells to H1R agonist increased caspase-3 cleavage. Furthermore, the cleavage of caspase-3 downstream substrate and mediator of cell death PARP was also observed following 48-hour cell exposure to H1R agonist. However the activation of these proapoptotic proteins was inhibited in the presence of the H2R agonist (Fig. 4D). We also used annexin binding assay to evaluate apoptosis. Following U937 cells’ exposure to the H1R agonist, quantitative evaluation revealed a reduction in normal cells with an increase in early and late apoptotic subpopulations. The induction of apoptosis was blocked in the presence of H2R agonist (Fig. 4E). The observation that histamine failed to either inhibit cell proliferation or induce apoptosis may be explained in terms of the dual stimulation of both H1R and H2R by its natural ligand, leading to a sort of “neutral effect.”
Fig. 4.
Effect of H1R and H2R agonist on U937 cell proliferation and apoptosis. U937 cells were treated with 10 μM H1R agonist, 10 μM mepyramine, 10 μM H2R agonist, 10 μM KT5720, or 100 μM histamine alone or in combination for 24 and 48 hours (A) or 48 hours (B–E). After treatment, cell proliferation (A and B), cell cycle stage (C), pro-caspase 3 and PARP (D) and phosphatidylserine exposure at the cell surface by annexin V binding were evaluated as detailed in Materials and Methods. (A) Data were calculated as the means ± S.D. of assay triplicates. Similar results were obtained in at least three independent experiments. ***P ≤ 0.001 with respect to control cells. (B) Data represent mean ± S.E.M. (n = 3). ***P ≤ 0.001 with respect control cells. 100% corresponds to control cells. (C) Data were calculated as the means ± S.D. of assay triplicates. Similar results were obtained in at least three independent experiments. **P ≤ 0.01 with respect to control cells. (D) Equal amounts of protein were subjected to SDS-PAGE and analyzed by Western blot with anti-caspase-3, anti-PARP, and anti-tubulin antibodies. (E) Different cell subpopulations according to the annexin V/PI staining pattern: Cells labeled with annexin V only were considered to be at an early apoptotic stage, cells labeled with annexin V and PI were considered to be at a late apoptotic stage, and cells labeled with PI only were considered necrotic. Data were calculated as the means ± S.D. of assay triplicates. Similar results were obtained in at least three independent experiments. ***P ≤ 0.001 respect to control cells.
Our findings support that the cross-talk between H1R and H2R not only modulates receptor signaling but also determines the resulting final response.
H1R and H2R Cointernalization.
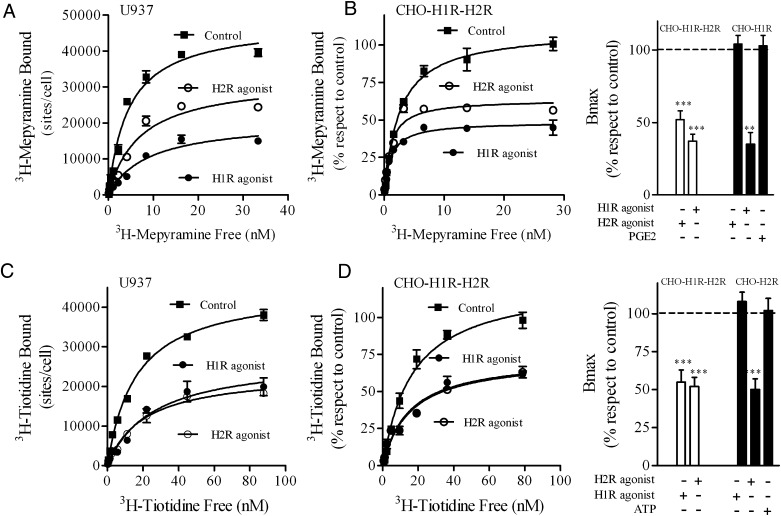
As the crossdesensitization was independent not only of second messengers but also of downstream second messenger-dependent protein kinases, a possible cross-regulation at the receptor level was evaluated. In a previous study performed in U937 cells endogenously expressing H2R as well as in COS7 and HEK293T cells transiently expressing H2R, we reported that the receptor is internalized following histamine or H2R-agonist stimulation as part of receptor trafficking (Fernandez et al., 2008). Furthermore, H1R is also internalized following histamine stimulation in H1R stably transfected CHO-K1 cells (Self et al., 2005; Hishinuma et al., 2010). Therefore internalization studies were performed in U937 and CHO-transfected cells to evaluate a putative reciprocal internalization of H1R and H2R. U937 cells exposed to either H1R or H2R agonist for 60 minutes showed membrane site internalization by 60% and 43%, respectively (Fig. 5A). Similar results were obtained in CHO-H1R-H2R cells (Fig. 5B). The H2R agonist failed to induce H1R internalization in CHO-H1R cells. Furthermore, PGE2, which increases cAMP, also failed to stimulate H1R internalization supporting the specificity of the cointernalization mechanism (Fig. 5B, right). In both cell types, U937 and CHO-H1R-H2 cells, H2R was also internalized following H2R stimulation (Fig. 5, C and D). In addition, H2R internalization did not occur in the absence of H1R in CHO-H2R cells or upon ATP stimulation as a control (Fig. 5D, right). These findings reveal a specific cross-regulation between H1R and H2R at the receptor level mediated by agonist-induced receptor cointernalization
Fig. 5.
Cointernalization of H1R and H2R. (A and C) [3H]Mepyramine (A) or [3H]tiotidine (C) saturation assays were performed in U937 cells: control (■), treated with 10 μM H2R agonist (○), or 10 μM H1R agonist (●) for 60 minutes. Data were calculated as the means ± S.D. of assay triplicates. Similar results were obtained in at least three independent experiments. (B and D) Left: [3H]mepyramine (B) or [3H]tiotidine (D) saturation assays were performed in CHO-H1R-H2R cells: control (■), treated with 10 μM H2R agonist (○), or 10 μM H1R agonist (●) for 60 minutes. Data were calculated as the means ± S.D. of assay triplicates. Similar results were obtained in at least three independent experiments. Right: Data represent the percentage Bmax value fitted by nonlinear regression of saturation assay, performed in CHO-H1R-H2R and CHO-H1R (B) or CHO-H1R-H2R and CHO-H2R cells (D) control, treated with 10 μM H1R agonist, 10 μM H2R agonist, 1 μM PGE2, or 1 μM ATP, calculated as the means ± S.E.M. (n = 3). 100% corresponds to untreated cells (control). ***P < 0.001; **P < 0.01 respect to control cells.
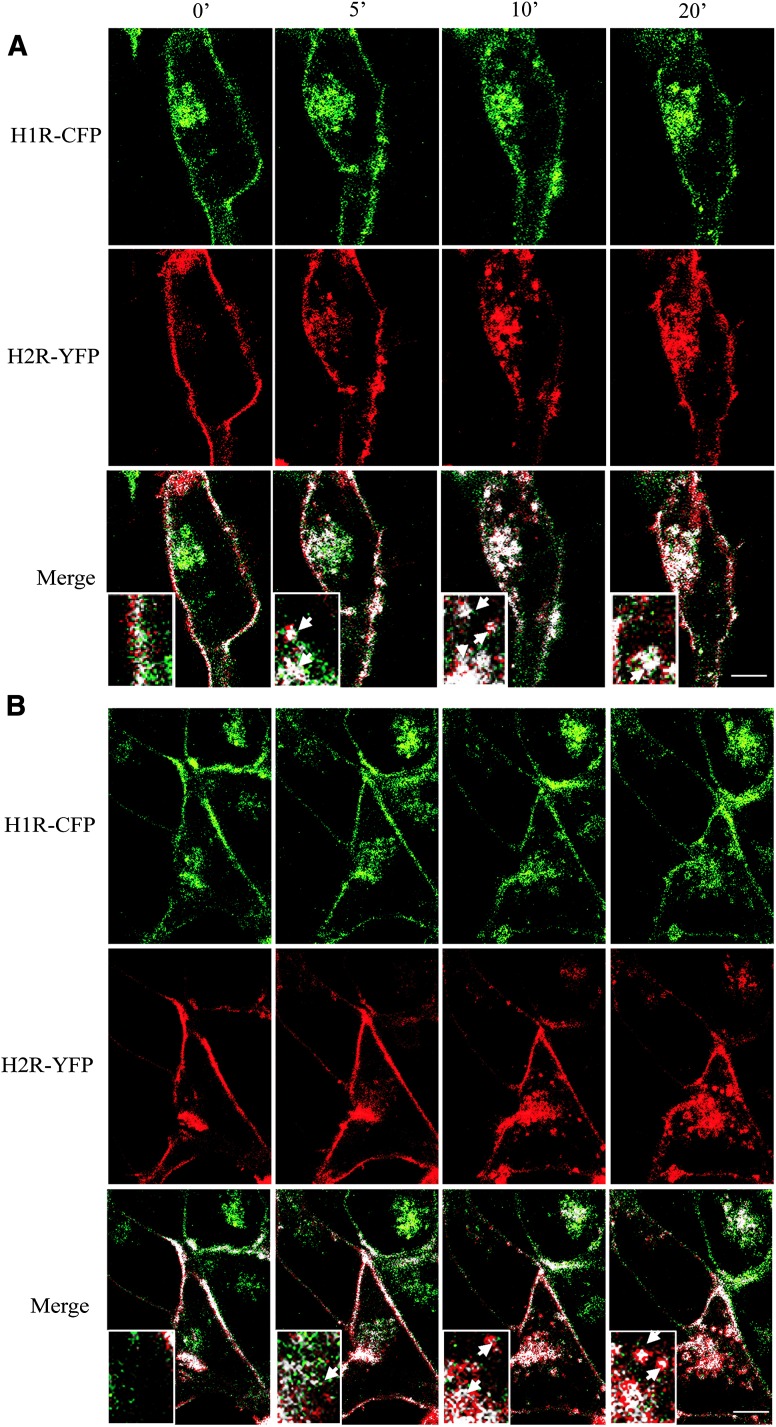
In an attempt to confirm these results, studies by confocal microscopy on HEK293T living cells were performed. Wild-type receptors were subcloned into pECFP-N1 and pEYFP-N1 vectors to obtain H1R-CFP and H2R-YFP, respectively. Binding assays were performed to evaluate the expression and internalization of the chimeric receptor (unpublished data). To visualize cointernalization, cells were transfected with both chimeric receptors, serum starved, and stimulated by 10 μM H1R agonist (Fig. 6A) or 10 μM H2R agonist (Fig. 6B). Ligand-induced internalization was monitored for 30 minutes. Images from three different experiments were analyzed with the plugin Colocalization Analyzer of ImageJ and representative pseudo-color images are shown. Cellular distribution analysis of the two receptors revealed partial colocalization in the plasma membrane before stimulation; mean Manders’ coefficients m1 (proportion of green signal that colocalize) and m2 (proportion of red signal that colocalize) were 0.597 and 0.582, respectively. As shown in Fig. 6, in the absence of agonist stimulation cells exhibited a basal receptor internalization due to the receptors over-expression and their constitute activity, but colocalization is visualized only in the plasma membrane.
Fig. 6.
Kinetics of ligand-induced H1R-CFP and H2R-YFP internalization. The cellular distribution of H1R-CFP (green) coexpressed with H2R-YFP (red) in HEK293T cells was videorecorded and displayed on a single confocal plane before (0 minutes) and following (5, 10, and 20 minutes) stimulation with 10 μM H1R agonist (A) or 10 μM H2R agonist (B). The corresponding colocalized points (H1R-H2R, white) are shown (Merge). Inset shows higher magnification of the area, arrows indicate intracellular endocytic vesicles. These images are representative of three independent experiments. Scale bars, 10 μM.
After 5 minutes treatment with H1R or H2R agonists, receptor cointernalization was observed as small bright spots within the cell, a characteristic distribution of receptors located in intracellular endocytic vesicles. The vesicular pattern reached a maximum of intensity at 10 minutes treatment with both ligands.
To analyze this colocalization, different regions of interest corresponding to the endocytic vesicles were used, where Manders’ coefficients values ranged from 0.91 to 0.96. These results not only confirmed receptor cointernalization but also showed the spatial dynamics of this phenomenon.
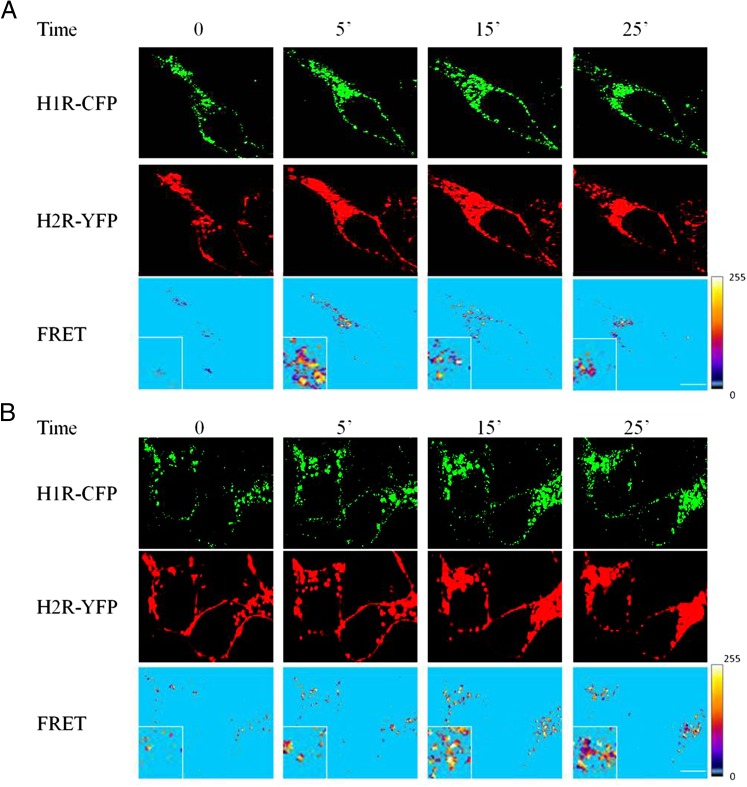
Since we detected colocalization of both receptors in the same subcellular compartment, we next analyzed the possible receptor heterodimerization by sensitized emission FRET experiments in HEK293T cells. Firstly, images with cells expressing only donor (H1R-CFP) or acceptor fluorophores (H2R-YFP) were carried out to determine the donor and acceptor Bleed-Through coefficients. FRET signals were measured in cells coexpressing both receptors. All images from three different experiments were analyzed with FRET and Colocalization Analyzer plugin of ImageJ and representative images are shown. The software generated a FRET image shown in Fig. 7 using the mean values of the donor and acceptor Bleed-Through coefficients. In these images, only the pixels that correspond to colocalization are shown. Surprisingly, FRET signals were not detected at the plasma membrane without stimulation, showing that no heterodimerization occurs in basal conditions. However, high FRET signals were observed in endocytic vescicles following H1R- or H2R-agonist stimulation, suggesting stimulus-induced heterodimerization when receptors are internalized. In Fig. 7, yellow areas indicate high values of FRET whereas sky blue areas indicate low values of FRET.
Fig. 7.
Kinetics of ligand-induced H1R-CFP and H2R-YFP heterodimerization using FRET. The cellular distribution of H1R-CFP (green) coexpressed with H2R-YFP (red) in HEK293T cells was videorecorded and is displayed on a single confocal plane before (0 minutes) and after (5, 15, and 25 minutes) stimulation with 10 μM H1R agonist (A) or 10 μM H2R agonist (B). The corresponding deduced FRET values are shown with yellow and sky blue colors corresponding to the maximum and minimum energy transfer, respectively. Inset shows higher magnification of the area. These images are representative of three independent experiments. Scale bars, 10 μM.
Overall, these results provide evidence that H1R and H2R not only cointernalize following stimulation with H1 or H2 agonists but they also colocalize forming heterodimers in endosomal vescicles.
H1R and H2R Crossdesensitization Is Not Dependent on Receptors’ Cointernalization.
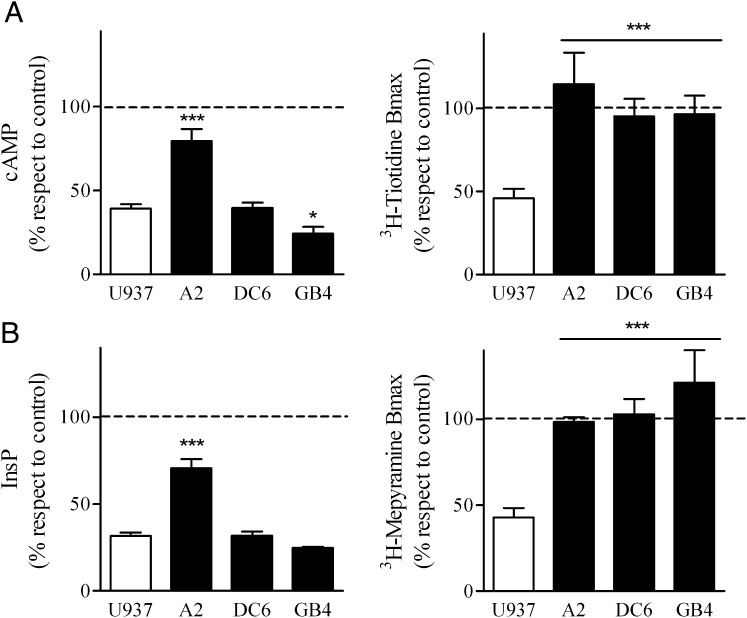
In an attempt to determine whether a direct cause-effect existed between receptor cointernalization and crossdesensitization we used U937 derived clones (DC6, A2, and GB4) previously characterized in our laboratory that exhibit blockade of H2R internalization by different mechanisms. DC6 cell line was obtained by stable transfection with a dynamin dominant negative mutant which proved to dampen agonist-induced H2R endocytosis (Fernandez et al., 2008). A2 cells have a GRK2 antisense construct that diminishes GRK2 expression levels and diminishes both H2R desensitization and internalization (Fernandez et al., 2002, 2011). GB4 expresses a GRK2 dominant negative mutant of the kinase site; in these cells H2R internalization which depends on GRK2 kinase activity was blocked but H2R desensitization proved to be enhanced since it is mediated by the regulator of G protein-signaling homology domain (RH) of GRK2 (Fernandez et al., 2011). As shown in Fig. 8A, although H2R internalization stimulated by the H1 agonist was abolished in all U937-derived clones, H2R crossdesensitization was observed in DC6 and GB4 cells. Similarly, pretreatment of cells with the H2 agonist did not induce H1R internalization in U937-derived clones but led to H1R crossdesensitization in DC6 and GB4 clones (Fig. 8B). These results clearly indicate that although both receptors cointernalize, the inhibition of the internalization process does not prevent receptor crossdesensitization.
Fig. 8.
Crossdesensitization and cross-internalization in U937 derived clones. (A) U937, A2, DC6, and GB4 cells were exposed (left) for 30 minutes to 10 μM H1R agonist, washed, and cAMP response to 10 μM amthamine determined, or (right) exposed for 60 minutes to 10 μM H1R agonist and [3H]tiotidine saturation assays performed. Data represent the percentage Bmax value fitted by nonlinear regression of saturation assay. Data were calculated as the mean ± S.E.M. (n = 3). *P ≤ 0.05; ***P ≤ 0.001 with respect to U937 cells. 100% corresponds to cAMP response or [3H]tiotidine Bmax of unpretreated cells (control). (B) U937, A2, DC6, and GB4 cells were exposed (left) for 10 minutes to 10 μM H2R agonist, washed, and cells then stimulated for 20 minutes with 10 μM H1R agonist. InsP production was measured as described under Materials and Methods. Alternatively, cells were exposed (right) for 60 minutes to 10 μM H2R agonist, and [3H]mepyramine saturation assays were performed. Data represent the percentage Bmax value fitted by nonlinear regression of saturation assay. Data were calculated as the mean ± S.E.M. (n = 3). ***P ≤ 0.001 with respect to U937 cells. 100% corresponds to InsP response or [3H]tiotidine Bmax of unpretreated cells (control).
On the other hand, crossdesensitization in GB4 cells was as effective as in U937 cells, suggesting that GRK2-mediated receptor phosphorylation is not necessary for H1R, and H2R crossdesensitization. However, GRK2 kinase activity is required for receptors cointernalization as well as in H2R internalization mediated by its agonist. It is important to point out that in the A2 clone the degree of both receptors’ crossdesensitization was significantly lower than in naïve U937 cells, suggesting that the RH domain of GRK2 is responsible for H1R and H2R crossdesensitization.
Discussion
Given 1) the wide in vivo coexpression of histamine H1 and H2 receptors, 2) the regulation between both receptors, and 3) the widespread prescription and over-the-counter sale of ligands acting at H1 and H2 histamine receptors, the full knowledge of the regulatory mechanisms underlying H1R and H2R activation and their interplay is of high interest for understanding current and designing novel therapeutic drugs.
Here, we present evidence of a H1R and H2R molecular cross-regulation in endogenous and heterologous expressing cells, which determines the ability of U937 cells to proliferate under histamine treatment. The cross-regulation is evidenced by a loss of responsiveness of H1R and H2R when cells were exposed to a prolonged stimulus with either H2R or H1R agonists, respectively.
It is generally accepted that heterologous desensitization results from signaling pathways cross-talk involving activity changes in GPCRs, G proteins, or effectors where second messenger-dependent protein kinases play a key role (Ferguson, 2001). However, in the present study, activation of AC, PLC, PKA or PKC was not involved in H1R or H2R heterologous desensitization in the cell types studied, but it was instead mediated by GRK2, which in turn triggered cointernalization of H1R/H2R complexes. GRK2 is a ubiquitous member of the G protein-coupled receptor kinase (GRK) family known to mediate GPCR homologous desensitization. The overall topology of the members of the GRK family consist of three well defined domains: 1) the conserved N-terminal region involved in receptor recognition, which exhibits sequence homology with RGS proteins (RH domain), 2) the central domain, which is responsible for the kinase activity itself, and 3) the poorly conserved C-terminal region that mediates membrane targeting of the kinases and in the case of GRK2 encodes a pleckstrin homology domain. This kinase mediates homologous desensitization of H1R and H2R as described by Iwata et al. (2005) and Fernandez et al. (2011). However, in recent years it has also been reported that GRK2 is involved in GPCRs heterologous desensitization (Heijink et al., 2005, Moulédous et al., 2012). In this way, cross-phosphorylation by GRK2 is responsible for GPCRs crossdesensitization. Remarkably, our findings show that in the case of H1R and H2R, GRK2-mediated receptor phosphorylation was not necessary for H1R and H2R crossdesensitization but it played a crucial role in receptor trafficking. On the other hand, considering that RH domain of GRK2 is responsible for H1R and H2R homologous desensitization and that the kinase activity of GRK2 is not necessary for receptor crossdesensitization, it would be expected that the RH domain of GRK2 mediates H1R and H2R crossdesensitization.
Virtually all GPCRs undergo ligand-induced internalization, a process originally considered as a mechanism tending to remove desensitized receptors from the cell surface. However, it is now well accepted that receptor endocytosis serves a variety of purposes, including receptor down-regulation, recycling, and relocalization of the cell signaling. H1R agonist-induced internalization was described as a mechanism involved in H1R regulation, although controversial evidence exists regarding the dependence on clathrin (Hishinuma et al., 2010) or caveolae/raft (Self et al., 2005). Concerning H2R, we have previously reported that internalization is part of receptor trafficking and crucial for the rapid recovery of H2R-mediated cAMP response. Furthermore, arrestin 3, dynamin, and clathrin are involved in both the internalization and resensitization of the H2R (Fernandez et al., 2008). Here, we evaluated as a mechanism of cross-regulation the cross-internalization of H1R and H2R in CHO-H1R-H2R and U937 cells based on the hypothesis that a cross-talk between H1R and H2R regulates their signaling capacity by modulating their internalization. Effectively, both ligands were able to induce the internalization of both receptors, as supported by radioligand binding assays (Fig. 5) and confocal microscopy in endogenously expressing and H1R/H2R-transfected cells (Fig. 6). Although cointernalization of H1R and H2R is not involved in their crossdesensitization, it may regulate diverse processes such as receptor down regulation, trafficking and recycling, and recruitment of scaffold proteins that can initiate alternative signaling.
G protein-coupled receptors have classically been assumed to exist and function as monomeric entities, and the paradigms of ligand binding and signal transduction were based on ligand/receptor/G protein 1:1:1 stoichiometry. However, a growing body of evidence indicates that some GPCRs can form both homodimers and heterodimers. Although their existence is now largely accepted (Angers et al., 2002; George et al., 2002) their functional importance remains more enigmatic and in some cases even controversial. Recent studies confirmed that monomeric signaling of GPCRs is possible and occurs with physiologic speed indicating that it is not necessary for GPCRs to dimerize to execute their basic function of transducing a signal from ligand binding to G protein activation (Whorton et al., 2008).
However, the possibility of GPCR dimer formation is now widely accepted, and concerning the functional consequences of GPCR oligomerization, important questions remain unresolved. In some cases, oligomerization has been shown to have a primary role in receptor maturation allowing the correct transport of receptors from the endoplasmic reticulum to the cell surface. Once at the plasma membrane, oligomers might become the target for dynamic regulation by ligand binding. It has been proposed that GPCR heterodimerization leads to both positive and negative ligand binding cooperativity as well as potentiating or attenuating signaling, or changing G protein selectivity (Breitwieser, 2004; Terrillon and Bouvier, 2004). Several studies have suggested that heterodimerization may affect agonist-promoted GPCR endocytosis. For many reported heterodimers, stimulation of only one of the receptors was sufficient to promote cointernalization of the entire dimer (Rocheville et al., 2000; Stanasila et al., 2003; Xu et al., 2003). Moreover, for SSTR2A somatostatin/opioid and for A2A adenosine/D2 dopamine receptors, the reported cointernalization was associated with signaling crossdesensitization (Hillion et al., 2002; Pfeiffer et al., 2002).
In our study using a heterologous expression system with receptors fused to fluorescent proteins we observed that both receptors colocalized approximately 60% on cell membrane and cointernalized when one is stimulated. Receptor dimerization was not detected on basal conditions, but there was a strong FRET signal when agonist-induced receptor cointernalization occurred. These results suggest that dimerization is not involved in receptor maturation or transport to the cell surface or alteration of ligand binding but it may play a role in the regulation of their trafficking and/or signaling within the cells after receptors are cointernalized. It is well established that once internalized, receptors can initiate additional, G protein-independent signaling pathways such as a β-arrestin-mediated coupling to mitogen-activated protein kinases (Kovacs et al., 2009). Therefore, further investigations will provide a better understanding of the role of H1R and H2R dimerization in their signaling. The disruption of dimerization would be an interesting tool to clarify the role and function of these heterodimers. In this context, in the last few years, great efforts have been made to develop small-molecule ligands specific for these complexes, as bivalent ligands (agonist or antagonist), monovalent “drug-like” heterodimer-specific compounds, and interface disrupting compounds (Filizola, 2010). Discovering small-molecule drugs that inhibit protein-protein interactions is an emerging but still very challenging area in drug design. On the other hand, several computational and mutagenesis strategies are developing to study GPCR interactions and have predicted TM1, TM4, and/or TM5 as likely dimerization/oligomerization contact interfaces of GPCRs. The study of the interacting domains of H1R and H2R would be of great interest for evaluating the functionality of the dimer formation in the signaling of both receptors and eventually for identifying novel pathways for drug discovery.
While these studies were performed in heterologous expression systems, these results were confirmed when the studies were carried out in U937 cells which express H1R and H2R endogenously. This cross-regulation has profound consequences on cell fate, since the exposure to H1-agonist ligand alone or in combination with specific H2 agonist, ultimately determines whether cells proliferate, arrest their cell cycle, or engage with apoptotic processes.
GPCR heterodimerization constitutes a new level of cross-talk that bears a priori unpredictable consequences, although with great potential clinical relevance. It has been suggested that impairment of dimers between angiotensin AT1 and bradykinin B2 receptors is related to preeclampsia and some forms of hypertension in experimental models (AbdAlla et al., 2005). Furthermore, dimerization of β2 adrenergic and PGE2 receptors decreases bronchodilating response of β2 agonists, diminishing the efficacy of asthma treatments (McGraw et al., 2006).
The fact that H1 and H2 receptors mutually desensitize each other, and cross-internalize forming heterodimers, provides now a key process determining cellular fate in response to H1R and H2R agonists. It can be speculated that in tissues where both receptors are coexpressed the regulatory mechanism described herein could have profound consequences on histamine response.
Acknowledgments
The authors thank Dr. Liliana Bianciotti, University of Buenos Aires, for critical reading and correction of the manuscript.
Abbreviations
- AC
adenyl cyclase
- amthamine
5-(2-aminoethyl)-4-methyl-1,3-thiazol-2-amine
- BSA
bovine serum albumin
- [Ca2+]i
intracellular Ca2+
- CFP
cyan fluorescent protein
- CHO
Chinese hamster ovary
- CHO-H1R
CHO-K1 cells stably expressing human H1R
- CHO-H2R
CHO-K1 cells stably expressing human H2R
- CHO-H1R-H2R
CHO-H1R cells expressing human H2R
- DMEM
Dulbecco’s modified Eagle’s medium
- forskolin
(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-3-vinyldodecahydro-1H-benzo[f]chromen-5-yl acetate
- FRET
fluorescence resonance energy transfer
- gentamicin
(3R,4R,5R)-2-{[(1S,2S,3R,4S,6R)-4,6-diamino-3-{[(2R,3R,6S)-3-amino-6-[(1R)-1-(methylamino)ethyl]oxan-2-yl]oxy}-2-hydroxycyclohexyl]oxy}-5-methyl-4-(methylamino)oxane-3,5-diol
- GF109203X
2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide
- GPCR
G protein-coupled receptor
- GRKs
G protein-coupled receptor kinases
- H1R
histamine receptor 1
- H2R
histamine receptor 2
- histamine
[2-(4-imidazolyl)-ethylamine]
- HEK293T
human embryonic kidney 293T cells
- hypoxanthine
1H-purin-6(9H)-one
- IBMX
isobutylmethyl xanthine (1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione)
- InsP
inositol phosphates
- isoproterenol
(RS)-4-[1-hydroxy-2-(isopropylamino)ethyl]benzene-1,2-diol
- KT5720
(5R,6S,8S)-hexyl 6-hydroxy-5-methyl-13-oxo-6,7,8,13,14,15-hexahydro-5H-16-oxa-4b,8a,14-triaza-5,8-methanodibenzo[b,h]cycloocta[jkl]cyclopenta[e]-as-indacene-6-carboxylate
- mepyramine
N-(4-methoxybenzyl)-N′,N′-dimethyl-N-pyridin-2-ylethane-1,2-diamine
- myoinositol
1,2,3,4,5,6-hexahydroxycyclohexane
- PARP
poly-(ADP-ribose)-polymerase
- PBS
phosphate buffered saline
- PGE2
prostaglandin E2 [(5Z,11α,13E,15S)-7-[3-hydroxy-2-(3-hydroxyoct-1-enyl)- 5-oxo-cyclopentyl] hept-5-enoic acid]
- PI
propidium iodide
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phosphoinositide-specific phospholipase C
- RH
regulator of G protein signaling (RGS)-homology domain
- U73122
1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione
- YFP
yellow fluorescent protein
Authorship Contributions
Participated in research design: Alonso, Fernandez, Davio, Shayo.
Conducted experiments: Alonso, Fernandez, Notcovich.
Contributed new reagents or analytic tools: Baldi, Simaan, Gutkind.
Performed data analysis: Alonso, Fernandez, Notcovich, Monczor, Davio, Shayo.
Wrote or contributed to the writing of the manuscript: Alonso, Fernandez, Monczor, Davio, Shayo.
Footnotes
This study was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas [PIP 0344]; and the Agencia Nacional de Promoción Científica y Tecnológica [PICT 2010-237] and [PICT 2010-1571]. This research was supported, in part, by the Intramural Research Program of the National Institutes of Health National Institute of Dental and Craniofacial Research [Grant Z01DE00551].
References
- AbdAlla S, Abdel-Baset A, Lother H, el Massiery A, Quitterer U. (2005) Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J Mol Neurosci 26:185–192 [DOI] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Bouvier M. (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42:409–435 [DOI] [PubMed] [Google Scholar]
- Bakker RA, Dees G, Carrillo JJ, Booth RG, López-Gimenez JF, Milligan G, Strange PG, Leurs R. (2004) Domain swapping in the human histamine H1 receptor. J Pharmacol Exp Ther 311:131–138 [DOI] [PubMed] [Google Scholar]
- Breitwieser GE. (2004) G protein-coupled receptor oligomerization: implications for G protein activation and cell signaling. Circ Res 94:17–27 [DOI] [PubMed] [Google Scholar]
- Copsel S, Garcia C, Diez F, Vermeulem M, Baldi A, Bianciotti LG, Russel FGM, Shayo C, Davio C. (2011) Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J Biol Chem 286:6979–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davio CA, Cricco GP, Bergoc RM, Rivera ES. (1995) H1 and H2 histamine receptors in N-nitroso-N-methylurea (NMU)-induced carcinomas with atypical coupling to signal transducers. Biochem Pharmacol 50:91–96 [DOI] [PubMed] [Google Scholar]
- Dickenson JM, White TE, Hill SJ. (1993) The effects of elevated cyclic AMP levels on histamine-H1-receptor-stimulated inositol phospholipid hydrolysis and calcium mobilization in the smooth-muscle cell line DDT1MF-2. Biochem J 292:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24 [PubMed] [Google Scholar]
- Fernández N, Monczor F, Lemos B, Notcovich C, Baldi A, Davio C, Shayo C. (2002) Reduction of G protein-coupled receptor kinase 2 expression in U-937 cells attenuates H2 histamine receptor desensitization and induces cell maturation. Mol Pharmacol 62:1506–1514 [DOI] [PubMed] [Google Scholar]
- Fernandez N, Monczor F, Baldi A, Davio C, Shayo C. (2008) Histamine H2 receptor trafficking: role of arrestin, dynamin, and clathrin in histamine H2 receptor internalization. Mol Pharmacol 74:1109–1118 [DOI] [PubMed] [Google Scholar]
- Fernandez N, Gottardo FL, Alonso MN, Monczor F, Shayo C, Davio C. (2011) Roles of phosphorylation-dependent and -independent mechanisms in the regulation of histamine H2 receptor by G protein-coupled receptor kinase 2. J Biol Chem 286:28697–28706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek S, Krzysko KA, Fotiadis D, Liang Y, Saperstein DA, Engel A, Palczewski K. (2004) A concept for G protein activation by G protein-coupled receptor dimers: the transducin/rhodopsin interface. Photochem Photobiol Sci 3:628–638 [DOI] [PubMed] [Google Scholar]
- Filizola M. (2010) Increasingly accurate dynamic molecular models of G-protein coupled receptor oligomers: Panacea or Pandora’s box for novel drug discovery? Life Sci 86:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons CP, Monczor F, Fernández N, Shayo C, Davio C. (2004) Mepyramine, a histamine H1 receptor inverse agonist, binds preferentially to a G protein-coupled form of the receptor and sequesters G protein. J Biol Chem 279:34431–34439 [DOI] [PubMed] [Google Scholar]
- Fukushima Y, Asano T, Saitoh T, Anai M, Funaki M, Ogihara T, Katagiri H, Matsuhashi N, Yazaki Y, Sugano K. (1997) Oligomer formation of histamine H2 receptors expressed in Sf9 and COS7 cells. FEBS Lett 409:283–286 [DOI] [PubMed] [Google Scholar]
- George SR, O’Dowd BF, Lee SP. (2002) G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov 1:808–820 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450 [PubMed] [Google Scholar]
- Heijink IH, Vellenga E, Oostendorp J, de Monchy JG, Postma DS, Kauffman HF. (2005) Exposure to TARC alters β2-adrenergic receptor signaling in human peripheral blood T lymphocytes. Am J Physiol Lung Cell Mol Physiol 289:L53–L59 [DOI] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL. (1997) International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev 49:253–278 [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, et al. (2002) Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 277:18091–18097 [DOI] [PubMed] [Google Scholar]
- Hishinuma S, Komazaki H, Fukui H, Shoji M. (2010) Ubiquitin/proteasome-dependent down-regulation following clathrin-mediated internalization of histamine H1-receptors in Chinese hamster ovary cells. J Neurochem 113:990–1001 [DOI] [PubMed] [Google Scholar]
- Iwata K, Luo J, Penn RB, Benovic JL. (2005) Bimodal regulation of the human H1 histamine receptor by G protein-coupled receptor kinase 2. J Biol Chem 280:2197–2204 [DOI] [PubMed] [Google Scholar]
- Jutel M, Akdis M, Akdis CA. (2009) Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy 39:1786–1800 [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prézeau L, Pin JP. (2004) Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol 11:706–713 [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. (2009) Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 17:443–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázár-Molnár E, Hegyesi H, Pállinger E, Kovács P, Tóth S, Fitzsimons C, Cricco G, Martin G, Bergoc R, Darvas Z, et al. (2002) Inhibition of human primary melanoma cell proliferation by histamine is enhanced by interleukin-6. Eur J Clin Invest 32:743–749 [DOI] [PubMed] [Google Scholar]
- McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, Liggett SB. (2006) Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest 116:1400–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulédous L, Froment C, Dauvillier S, Burlet-Schiltz O, Zajac JM, Mollereau C. (2012) GRK2 protein-mediated transphosphorylation contributes to loss of function of μ-opioid receptors induced by neuropeptide FF (NPFF2) receptors. J Biol Chem 287:12736–12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. (2002) An amino-acid taste receptor. Nature 416:199–202 [DOI] [PubMed] [Google Scholar]
- Notcovich C, Diez F, Tubio MR, Baldi A, Kazanietz MG, Davio C, Shayo C. (2010) Histamine acting on H1 receptor promotes inhibition of proliferation via PLC, RAC, and JNK-dependent pathways. Exp Cell Res 316:401–411 [DOI] [PubMed] [Google Scholar]
- Panetta R, Greenwood MT. (2008) Physiological relevance of GPCR oligomerization and its impact on drug discovery. Drug Discov Today 13:1059–1066 [DOI] [PubMed] [Google Scholar]
- Parsons ME, Ganellin CR. (2006) Histamine and its receptors. Br J Pharmacol 147 (Suppl 1):S127–S135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer M, Koch T, Schröder H, Laugsch M, Höllt V, Schulz S. (2002) Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem 277:19762–19772 [DOI] [PubMed] [Google Scholar]
- Pin JP, Kniazeff J, Binet V, Liu J, Maurel D, Galvez T, Duthey B, Havlickova M, Blahos J, Prézeau L, et al. (2004) Activation mechanism of the heterodimeric GABA(B) receptor. Biochem Pharmacol 68:1565–1572 [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. (2000) Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem 275:7862–7869 [DOI] [PubMed] [Google Scholar]
- Selbie LA, Hill SJ. (1998) G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci 19:87–93 [DOI] [PubMed] [Google Scholar]
- Self TJ, Oakley SM, Hill SJ. (2005) Clathrin-independent internalization of the human histamine H1-receptor in CHO-K1 cells. Br J Pharmacol 146:612–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanasila L, Perez JB, Vogel H, Cotecchia S. (2003) Oligomerization of the alpha 1a- and alpha 1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J Biol Chem 278:40239–40251 [DOI] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. (2004) Roles of G-protein-coupled receptor dimerization. EMBO Rep 5:30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubio MR, Fernandez N, Fitzsimons CP, Copsel S, Santiago S, Shayo C, Davio C, Monczor F. (2010) Expression of a G protein-coupled receptor (GPCR) leads to attenuation of signaling by other GPCRs: experimental evidence for a spontaneous GPCR constitutive inactive form. J Biol Chem 285:14990–14998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, Sunahara RK. (2008) Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem 283:4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. (2003) Heterodimerization of alpha 2A- and beta 1-adrenergic receptors. J Biol Chem 278:10770–10777 [DOI] [PubMed] [Google Scholar]
- Youvan DC, Silva CM, Bylina EJ, Coleman WJ, Dilworth MR, Yang MM. (1997) Calibration of Fluorescence Resonance Energy Transfer in Microscopy Using Genetically Engineered GFP Derivatives on Nickel Chelating Beads. Biotechnology et alia 3:1–18 [Google Scholar]
- Zhang J, Barak LS, Winkler KE, Caron MG, Ferguson SS. (1997) A central role for β-arrestins and clathrin-coated vesicle-mediated endocytosis in β2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem 272:27005–27014 [DOI] [PubMed] [Google Scholar]