INITIATION AND PROGRESSION OF PERIODONTAL DISEASES
Gingivitis and periodontitis are chronic inflammatory conditions that may affect as much as 80% of the adult population, making them one of the most prevalent diseases in humankind.1, 2 The disease process is initiated by the accumulation of bacteria along the gingival margin and in the interface between the gingival tissues and the teeth. Bacterial cells initially colonize the acquired salivary pellicle and if their accumulation is not disturbed by oral hygiene practices, bacteria will grow and proliferate, giving rise to a complex structure currently termed bacterial biofilm.3 As the biofilm develops and matures, bacterial succession occurs, which refers to the ability of other bacterial species than the ones initially colonizing the salivary pellicle to establish themselves within the extracellular polysaccharide matrix and the already attached bacteria.4, 5 Bacterial succession is responsible for a pathogenic shift in the gingival/periodontal flora, where the proportion of gram-negative anaerobes tends to increase as the biofilm matures.
Gingival tissues respond to the accumulation of bacteria and exposure to bacterial products with inflammation.6 The inflammatory changes are initially confined to the soft tissues and involve clinical changes in volume, color, shape, position and texture of the gingival tissues, and are often accompanied by bleeding upon probing. The clinical condition is termed gingivitis, which is associated neither with apical migration of the junctional epithelium nor with destruction of bone and periodontal ligament fibers. Gingivitis is a reversible condition that can usually be treated with professional biofilm removal and improvement in oral hygiene. Most, but not all, cases of long-standing gingivitis progress into periodontitis. Periodontitis is an inflammatory condition of the supporting structures of the teeth, and involves attachment and bone loss. If left untreated, periodontitis may lead to tooth loss.7 Periodontitis occurs because the inflammatory process migrates through the gingival tissues in the apical direction, following the paths of larger blood vessels. The inflammatory process contains numerous enzymes and cytokines, notably collagenases and prostaglandins, which degrade collagen and induce the activation of osteoclasts, resulting in attachment and bone loss.8, 9 Clinically, periodontitis shares signs and symptoms with gingivitis, but it differs from the latter by showing apical migration of the junctional epithelium from the cemento-enamel junction, which results in increased pocket depth (Figure 1). As pockets increase in depth, biofilm removal becomes more difficult, leading to the development of more inflammation and tissue destruction that contribute to the perpetuation of periodontitis.
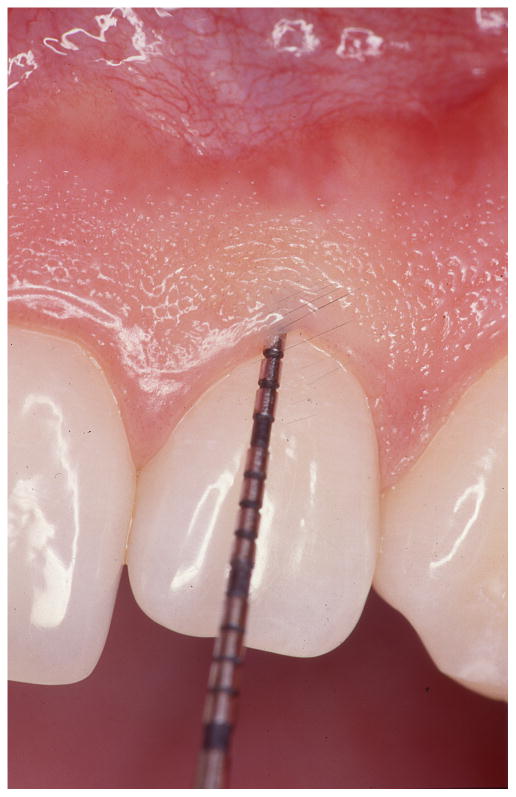
Figure 1. Bleeding on probing in the diagnosis of periodontal diseases.
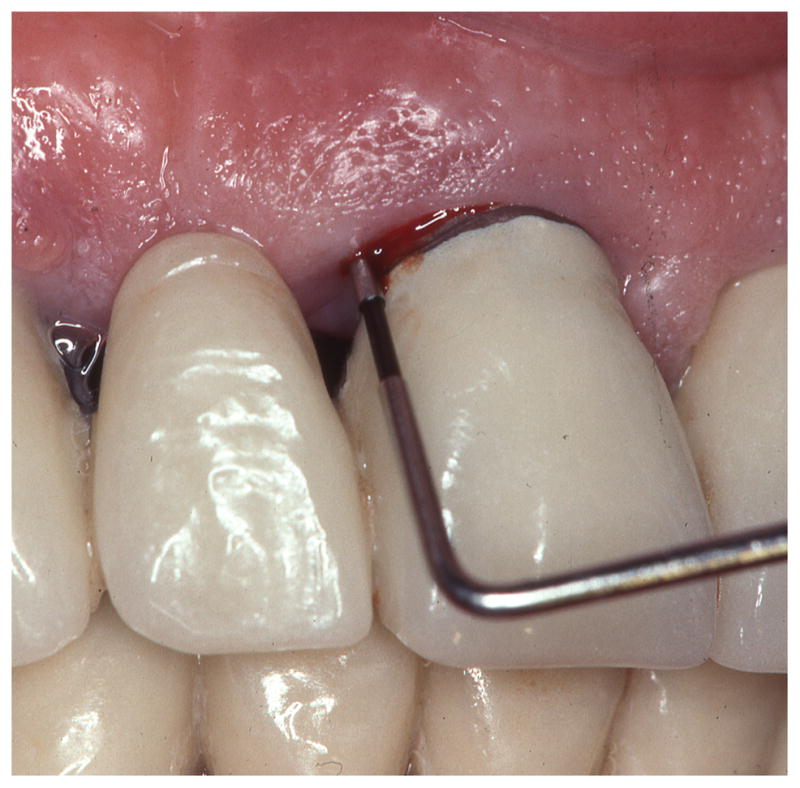
Clinical periodontal evaluation involves visual assessment of the gingival tissue and sulcus/pocket probing. The absence of bleeding upon probing is an excellent negative predictor of disease activity (left). The presence of bleeding on probing over-estimates the risk for further tissue breakdown (right) but it is still widely used in clinical practice because a better predictor has not been identified.
CURRENT STATUS OF THE DIAGNOSIS OF PERIODONTAL DISEASES
The diagnosis of periodontal diseases is usually made by a dental practitioner, and it includes visual inspection of the gingival tissues, the measurement of pocket depth and attachment loss, the observation of bleeding upon probing, and the evaluation of other clinical parameters such as gingival recession, tooth mobility and furcation invasions.10 Radiographs are used as supporting information to the clinical findings as they reveal bone levels around teeth, besides providing other information about teeth and mineralized tissues. Inflammation in gingivitis and in periodontitis presents several but not all cardinal signs and symptoms of inflammation in other areas of the body. Inflamed gingival tissues often appear swollen with rolled borders, which are present with soft textures and redness (as opposed to pink) in color. On the other hand, the presence of pain and an increase in body temperature (fever), which are often associated with the presence of inflammation in other areas of the human body, are usually missing from the clinical picture associated with gingivitis and periodontitis.11
The absence of pain in periodontal diseases is usually one of the main reasons why patients do not seek professional care in their early or even more advanced stages. This is particularly true for the segment of the population who does not receive dental care regularly in the form of periodic exams and preventive professional dental recalls. Patients may not be aware of and/or choose to ignore the signs and symptoms of gingival and periodontal inflammation such as red and bleeding gums, thus allowing the disease process to progress to a point at which it may require extensive periodontal treatment or where tooth loss can no longer be prevented.
THE FUTURE OF THE DIAGNOSIS OF PERIODONTAL DISEASES
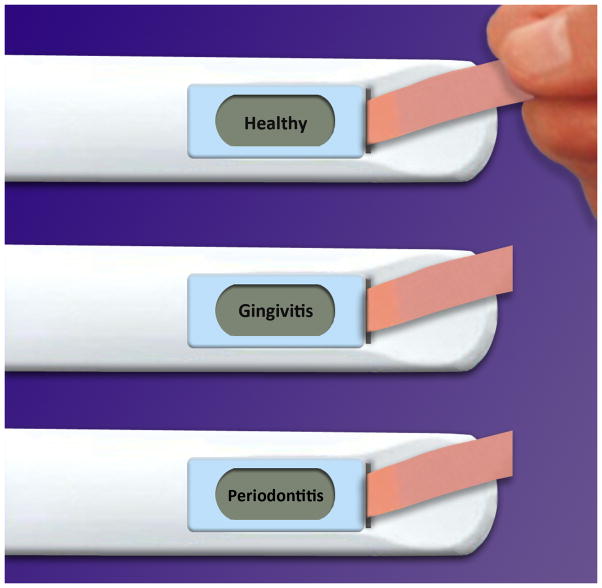
A self-administered home test that serves as a screening tool for periodontal diseases could play an important role in making individuals aware of the fact that a pathological process is occurring in their oral cavities and that a visit to a provider of dental services should be prioritized (Figure 2). An analogy to such screening test for periodontal diseases would be home pregnancy tests, where females who show positive results are encouraged to visit their physicians to confirm their pregnant statuses and receive appropriate care thereafter. In the case of periodontal diseases, saliva serves as an attractive vehicle on which a screening test could be conducted. Saliva is in close proximity with sites that are present with gingival and periodontal inflammation; therefore, it contains biological markers associated with these diseases. Moreover, saliva is an abundant fluid that is easy to collect and store, making it a convenient medium for conducting a high sensitivity screening test for periodontal diseases.
Figure 2. Oral fluid periodontal health test (home or office use).
An electrochemical device that can process transcriptomic biomarkers in saliva is shown. When a saliva-containing strip is inserted into the device, the outcome of the patient’s periodontal health is displayed on the screen. The device would come with follow-up instructions to consult with a dentist.
The conversion of gingivitis into periodontitis and the progression of attachment and bone loss in periodontitis are phenomena that are still not fully understood in the pathogenesis of periodontal diseases. It is evident that most cases of untreated and long-standing gingivitis will convert into some degree of periodontitis, and it is unequivocal that the presence of bacteria is necessary for such conversion.12 It is, however, clear that the bacterial biofilm, while necessary, is not sufficient for gingivitis to progress into periodontitis. Other factors, possibly related to the quality of an individual’s immune response, are likely to be determinant factors in the conversion of gingivitis into periodontitis.13 Once the periodontitis process is initiated, it is known that the destruction of periodontal tissues is neither linear nor predictable. It is mostly agreed upon that tissue destruction in periodontitis occurs in random bursts of activity, which takes place during relatively short periods of time.14 The main disease-causing factors still remain elusive in the field of Periodontology.
Attempts have been made to correlate periods of disease activity with microbiological indicators and the measurement of host-derived enzymes and other products, however, with limited success.15 Of all parameters evaluated, bleeding upon probing is an excellent negative predictor of periodontal disease activity (i.e., its absence very well predicts lack of periodontal tissue destruction), but an overly sensitive positive predictor of periodontal attachment loss.15 Yet, bleeding on probing is the standard of clinical science when it comes to risk factors associated with future tissue breakdown, despite the fact that its adoption as a predictor of periodontal tissue destruction leads to overtreatment. The periodontal scientific and clinical communities lack a more specific predictor, or combination of predictors, of periodontal disease activity.
It is currently accepted that analyzing multiple possible predictors of periodontal disease activity works better than examining each predictor individually.13 Therefore, this is an area of clinical periodontal practice where saliva could serve as an important and convenient vehicle to simultaneously evaluate a multitude of factors that could predict the bursts of activity known to occur in the progression of periodontitis. With this objective in mind, a chair-side saliva test administered by the dental professional could play a very important role in determining which patient and what specific sites are in need of periodontal therapy so that the tissue destruction process is prevented or arrested.
SALIVARY DIAGNOSTICS
Studies reveal a promising outlook for saliva as a key diagnostic medium for determining systemic diseases or health statuses of individuals.16 Because collecting saliva involves noninvasive methods and due to the fact that it is an abundant and easily accessible biofluid, saliva is attractive for diagnostic purposes greatly due to its highly enriched content of disease biomarkers that can be deciphered and analyzed. Biomarkers for the detection of specific diseases, such as Sjögren’s syndrome, pancreatic, breast, and oral cancer, or periodontal diseases can be detected in saliva.17–21 These properties of saliva open doors to a perfect method of exploring health and disease surveillance in clinical settings with just a minute amount of the oral fluid.
CURRENT SALIVARY DIANOSTIC TESTS FOR PERIODONTAL DISEASES
There are currently two salivary diagnostic tests available in the American market for the detection of periodontal diseases.22 These tests enable clinicians to collect saliva and send the samples to a laboratory, where DNA-Polymerase Chain Reaction analysis is used to complete the diagnostic tests and devise comprehensive risk assessment reports. One test identifies the type and concentration of specific periodontal pathogenic microorganisms in patients’ saliva samples. The laboratory report provides the clinician with pathogenic properties of the detected pathogen(s), which allows the clinician to determine the most appropriate antimicrobial therapy, and therefore, the ability to customize a treatment plan. Another salivary test claims to detect genetic susceptibility to periodontitis in individuals, which allows the clinician to identify patients who are at a greater risk for the development of severe periodontal destruction. The test analyzes genetic variation in individuals that affects the production of the inflammatory cytokines interleukin-1 a and b; therefore, the test detects the patient’s ability to over-express such molecules within his/her inflammatory responses.22 Both salivary tests state that they can potentially detect the periodontal diseases early and evaluate the susceptibility to periodontitis in individuals. Although the tests claim to be quick and easily administered at chair-side, four to five days are required for the laboratory results to be returned to the clinician. These tests identify general risk factors for the development of periodontitis, but lack the ability to determine when periodontal destruction will occur, thereby not being able to specifically predict periods of disease activity.
A novel Salivary Occult Blood Test (SOBT), which has been proposed as a periodontal status screening method, is currently available in Japan.23 This method has been reported to detect individuals with poor periodontal health, which is defined as bleeding on probing in ≥20% of teeth or the presence of probing pocket depth ≥6 mm plus bleeding on probing in ≥1 teeth. A proprietary paper strip containing gold-labeled anti-human hemoglobin monoclonal antibody is dipped into the saliva sample. Upon forming an immune complex with hemoglobin, the immune complex travels up the paper strip by capillary action until it is immobilized. This results into a magenta line, indicating a positive test result for the manufacturer’s reference concentration of ≥2 μg/ml human hemoglobin. A study of 1,998 subjects in a suburb of the Fukuoka metropolitan area in southern Japan reported that sensitivity and specificity of the SOBT in screening for poor periodontal status were 0.72 and 0.52, respectively.23 The investigators involved in this study suggested that these values were not very high; however, SOBT can be utilized as a simple screening method at a low cost for identifying periodontal status of a patients and increasing their oral health awareness.
The salivary diagnostic tests mentioned above claim to detect periodontal diseases based on purely microbial or single inflammatory-based information. They could be labeled as first-generation tests involving saliva in the diagnosis and prognosis of patients with periodontal diseases, and they represent the beginning of an era where disease-associated agents are detected and analyzed in saliva. Giannobile and coworkers are leading the field by combining microbial biomarkers from periodontal pathogens and salivary biomarkers from host-response changes to better understand the multi-factorial nature of periodontal diseases.24, 25 Since the existing tests do not allow for a direct correlation between the presence of specific bacteria and isolated inflammatory markers with the predictive value of periodontal attachment and bone loss, the next generation of salivary tests is presumed to be enhanced by enabling the understanding of these relationships in the diagnosis and prognosis of periodontal diseases.
FUTURE OF PERIODONTAL DISEASES DIAGNOSIS USING SALIVA
Even though the diagnostic value of saliva has been recognized and several potential biomarkers of periodontal diseases identified, most of the work conducted to date came short of providing clinically reliable and useful information for practitioners in terms of developing a more precise periodontal diagnosis and subsequent treatment planning.26–28 Three major challenges to current periodontal disease diagnosis using saliva are (1) the current tests are based on a small number of potential biomarkers, (2) the tests are not “true” real-time assessments, and (3) the tests are heavily based on general microbial and inflammatory cytokines that may or may not be disease-specific. In order for the salivary diagnostics for periodontal diseases to be clinically relevant, the appropriate bioinformatics have to augment biomarker discovery so that validated biomarkers have disease discriminatory power. The test also needs to be real-time, where the patient’s periodontal status can be immediately evaluated while he/she is in the dental office. Lastly, biomarkers should not only diagnose the disease but also predict the risk of future disease activity by simple and affordable means.
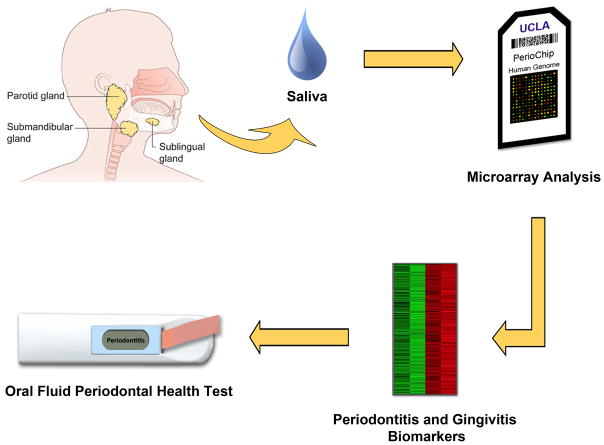
Recent advances in transcriptomic high-throughput technologies are shedding new light on salivary biomarker discovery, which can elevate salivary diagnosis of periodontal diseases to a higher level. The salivary transcriptome refers to a collection of transcripts, deoxyribonucleic acid (DNA) that is transcribed into ribonucleic acid (RNA), within saliva. By analyzing the transcriptome, one can assess which genes are turned on or off and if there is a difference in gene expression between saliva from healthy and periodontal diseased patients. David Wong and his team at UCLA believe that biomarkers found in saliva may actually predict bursts of periodontal disease activity (Figure 3). They are in the process of discovering gene signatures in patients by performing multiplex transcriptomic analysis of messenger RNA (mRNA) in human saliva (healthy vs. gingivitis vs. periodontitis). The basis for this investigation in the field of periodontal diseases is derived from a similar approach that has been successfully applied to the detection of cancer-associated biomarkers in saliva.
Figure 3. Salivary biomarker discovery by transcriptomic high-throughput technologies.
mRNA is isolated from saliva. Transcriptomes of healthy and patients with periodontal diseases are profiled by a microarray. Disease-specific (gingivitis and periodontitis) and significant biomarkers are found and validated. The oral fluid periodontal health test uses combination of validated markers to analyze and display the patient’s periodontal status. 31
In fact, it has been proved that saliva contains disease discriminatory mRNAs in oral cancer. Using high-density oligonucleotide microarrays (54,000 probe sets representing approximately 38,500 genes), saliva mRNAs from healthy and oral cancer patients have been thoroughly profiled and statically compared. They discovered four mRNA biomarkers for oral cancer with a sensitivity of 91% and specificity of 91% to distinguish cancer from the normal.29, 30 The same discovery platform can be used to profile healthy and periodontal transcriptomes and to find the most significant candidate genes for the onset and progression of periodontal diseases. Those discriminatory candidate genes must be validated for their sensitivity and specificity as saliva biomarkers.
In addition to technological advances, in order for salivary biomarkers for periodontal diseases to have the intended clinical context, study design and clinical trials must reflect the ultimate goal of obtaining approval from the Food and Drug Administration (FDA) agency. Recently, FDA approved the oral fluid-based Human Immunodeficiency Virus (HIV) antibody test. This milestone achievement in salivary diagnostics proved that saliva can be a disease-discriminatory biofluid much like the “gold-standard” serum. Secondly, it reminded the research community that the end goal of future salivary biomarker discovery is the application of novel treatment and therapeutics to the real-world by translating the knowledge from the laboratory bench to a medical or dental setting. Thus, the study design and clinical trials for the biomarker discovery should minimize bias and maximize clinically relevance. Clinical trial design such as the Prospective Randomized Open Blinded End-Point (PROBE) design maximizes similarity to standard clinical practice, makes the research results more easily applicable in routine dental settings, and aids in the translation step of future biomarker discovery.
The main goals of mRNA salivary biomarker discovery by PROBE design would be (1) to detect periodontal diseases early, (2) to provide oral health professionals with an accurate chair-side periodontal disease diagnostic and prognostic tool, (3) to provide an easy self-test which the public can use in the comfort of the home, and (4) to improve and encourage access to dental care and reduce health disparities across the U.S. and around the world.
In conclusion, periodontal disease diagnosis and follow-up care will greatly advance in the near future via the discovery of disease-specific salivary biomarkers. Prototype electrochemical devices such as the oral fluid periodontal health test (UCLA) may provide accurate and real-time assessments of periodontal diseases for the general public either at home or at the dental office. Although challenges remain ahead, using saliva to gauge periodontal health appears bright for future application to aid in the diagnosis of periodontal diseases and the prediction of periodontal treatment outcomes.
References
- 1.Miller AJBJ, Carlos JP, Brown LJ, Loe H. The national survey of oral health in US employed adults and seniors: 1985–86. US Department of Health and Human Services, NIH Publication; Bethesda, MD: National Institute of Dental Research; 1987. Oral health in United States adults: National findings. [Google Scholar]
- 2.Third National Health and Nutrition Examination Survey. Hyattsville, MD: Centers of Disease Control. CDC; 1997. No. 7–0627. [Google Scholar]
- 3.Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmur R, et al. Oral biofilm architecture on natural teeth. PLoS One. 2010;5(2):e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Y, Berg EA, Costello CE, Troxler RF, Oppenheim FG. Identification of protein components in human acquired enamel pellicle and whole saliva using novel proteomics approaches. J Biol Chem. 2003;278(7):5300–8. doi: 10.1074/jbc.M206333200. [DOI] [PubMed] [Google Scholar]
- 5.Kolenbrander PE, Palmer RJ, Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 6.Loe H, Theilade E, Jensen SB. Experimental Gingivitis in Man. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 7.Reddy MS, Geurs NC, Jeffcoat RL, Proskin H, Jeffcoat MK. Periodontal disease progression. J Periodontol. 2000;71(10):1583–90. doi: 10.1902/jop.2000.71.10.1583. [DOI] [PubMed] [Google Scholar]
- 8.Reinhardt RA, Stoner JA, Golub LM, Lee HM, Nummikoski PV, Sorsa T, et al. Association of gingival crevicular fluid biomarkers during periodontal maintenance with subsequent progressive periodontitis. J Periodontol. 2010;81(2):251–9. doi: 10.1902/jop.2009.090374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noguchi K, Ishikawa I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontol 2000. 2007;43:85–101. doi: 10.1111/j.1600-0757.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 10.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 11.Newman MGTH, Carranza FA. Carranza’s Clinical Periodontology. Saunders; 2002. [Google Scholar]
- 12.Loe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol. 1986;13(5):431–45. doi: 10.1111/j.1600-051x.1986.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 13.Burt B. Position paper: epidemiology of periodontal diseases. J Periodontol. 2005;76(8):1406–19. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 14.Goodson JM. Clinical measurements of periodontitis. J Clin Periodontol. 1986;13(5):446–60. doi: 10.1111/j.1600-051x.1986.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 15.Armitage GC. Periodontal diseases: diagnosis. Ann Periodontol. 1996;1(1):37–215. doi: 10.1902/annals.1996.1.1.37. [DOI] [PubMed] [Google Scholar]
- 16.Wong DT. Salivary diagnostics for oral cancer. J Calif Dent Assoc. 2006;34(4):303–8. [PubMed] [Google Scholar]
- 17.Hu S, Wong DT. Oral cancer proteomics. Curr Opin Mol Ther. 2007;9(5):467–76. [PubMed] [Google Scholar]
- 18.Hu S, Gao K, Pollard R, Arellano-Garcia M, Zhou H, Zhang L, et al. Preclinical validation of salivary biomarkers for primary Sjogren’s syndrome. Arthritis Care Res (Hoboken) 2010;62(11):1633–8. doi: 10.1002/acr.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Farrell JJ, Zhou H, Elashoff D, Akin D, Park NH, et al. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 2010;138(3):949–57. e1–7. doi: 10.1053/j.gastro.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Xiao H, Karlan S, Zhou H, Gross J, Elashoff D, et al. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS One. 2010;5(12):e15573. doi: 10.1371/journal.pone.0015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabors TW, McGlennen RC, Thompson D. Salivary testing for periodontal disease diagnosis and treatment. Dent Today. 2010;29(6):53–4. 56, 58–60. quiz 61. [PubMed] [Google Scholar]
- 23.Shimazaki Y, Akifusa S, Takeshita T, Shibata Y, Doi Y, Hata J, et al. Effectiveness of the salivary occult blood test as a screening method for periodontal status. J Periodontol. 2011;82(4):581–7. doi: 10.1902/jop.2010.100304. [DOI] [PubMed] [Google Scholar]
- 24.Giannobile WV. Salivary diagnostics for periodontal diseases. J Am Dent Assoc. 2012;143(10 Suppl):6S–11S. doi: 10.14219/jada.archive.2012.0341. [DOI] [PubMed] [Google Scholar]
- 25.Kinney JS, Morelli T, Braun T, Ramseier CA, Herr AE, Sugai JV, et al. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011;90(6):752–8. doi: 10.1177/0022034511399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci. 2007;1098:230–51. doi: 10.1196/annals.1384.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. Salivary biomarkers associated with alveolar bone loss. Ann N Y Acad Sci. 2007;1098:496–7. doi: 10.1196/annals.1384.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49(3):551–71. vi. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Zhou X, St John MA, Wong DT. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 2004;83(3):199–203. doi: 10.1177/154405910408300303. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, St John MA, Zhou X, Kim Y, Sinha U, Jordan RC, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10(24):8442–50. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 31.About salivary gland cancer - A quick guide. Cancer Research UK; 2011. http://cancerhelp.cancerresearchuk.org. [Google Scholar]