Abstract
Behavioral-economic studies have shown that differences between lean and obese Zuckers in food consumption depend on the response requirement for food. Since a response requirement inherently increases the delay to reinforcement, differences in sensitivity to delay may also be a relevant mechanism of food consumption in the obese Zucker rat. Furthermore, the endocannabinoid neurotransmitter system has been implicated in impulsivity, but studies that attempt to characterize the effects of cannabinoid drugs (e.g., rimonabant) on impulsive choice may be limited by floor effects. The present study aimed to characterize impulsive-choice patterns for sucrose using an adjusting-delay procedure in genetically lean and obese Zuckers. Ten lean and ten obese Zucker rats chose between one lever that resulted in one pellet after a standard delay (either 1 s or 5 s) and a second lever that resulted in two or three pellets after an adjusting delay. After behavior stabilized under baseline, rimonabant (0–10 mg/kg) was administered prior to some choice sessions in the two-pellet condition. Under baseline, obese Zuckers made more impulsive choices than leans in three of the four standard-delay/pellet conditions. Additionally, in the 2-pellet condition, rimonabant increased impulsive choice in lean rats in the 1-s standard-delay condition; however, rimonabant decreased impulsive choice in obese rats in the 1-s and 5-s standard-delay conditions. These data suggest that genetic factors that influence impulsive choice are stronger in some choice conditions than others, and that the endocannabinoid system may be a relevant neuromechanism.
Keywords: adjusting-delay procedure, cannabinoids, delay discounting, impulsive choice, obese Zucker rat, rimonabant
1. Obesity and food consumption
Obesity results from a combination of genetic and environmental factors that increase the likelihood of overeating. One model of obesity that has been used to study the genetic and neurochemical factors that contribute to food consumption and hyperphagia is the genetically obese Zucker rat [1–6]. The obese Zucker carries two homozygous fa “fatty” alleles that result in leptin insensitivity and are associated with increased levels of appetite-stimulating signals, such as neuropeptide Y [7, 8] and endocannabinoids [9, 10]. Moreover, obese Zucker rats have been shown to have a greater number of cannabinoid-1 receptors in brain regions related to feeding [11] and have shown increased sensitivity to cannabinoid drugs [5, 12–14] compared to lean rats. In many studies, both lean and obese Zuckers are placed in their respective homecages and allowed to free-feed where the amount of food consumed is used as an indicator of the reinforcing value of food. It is often the case that obese Zuckers consume more food than lean controls under free-feeding conditions [11, 15, 16] and it is then concluded that food is more reinforcing to obese rats compared to their lean counterparts.
Food consumption in environments that vary the response requirement of food shows a different pattern, however. Rasmussen et al. [1] found that obese Zucker rats consumed more food pellets than lean rats when food pellets were contingent upon 1–50 lever presses, but both obese and lean Zuckers consumed similar amounts of food when >50 lever presses were required for food. This pattern was replicated in a second study [17] suggesting that behavioral differences in food consumption between lean and obese Zucker rats may depend on whether food is effortful.
2. Delay discounting, response requirements, and food consumption
Experiments that employ behavioral-economic principles (i.e., those that examine food procurement across a range of response requirements) offer a fuller characterization of food’s reinforcing efficacy. It should be noted, however, that an increase in a response requirement to obtain food may inherently increase the delay to its receipt. This is apparent when one considers the time it would take a rat to press a lever 5 times as opposed to 50 times—as the response requirement increases, so does the delay to reinforcement. Thus, the finding that differences in food consumption between lean and obese Zuckers depend on the response requirement [1, 17] suggests that delay to reinforcement may also be a factor in determining choice for food, where obese Zuckers may be more sensitive to delays in some contexts than others.
Delay discounting, which describes how preference for an outcome, such as food, decreases as the delay to its receipt increases, is a procedure that can quantify sensitivity to delay. In the typical delay-discounting procedure, subjects are systematically presented with choices between a larger-later reinforcer (e.g., three food pellets delayed 15 s) and a smaller-sooner reinforcer (e.g., one food pellet immediately). A pattern of choosing the smaller-sooner over the larger-later reinforcer would be considered impulsive [18, 19]. Heightened sensitivity to delay has been shown to be a relevant mechanism of obesity in humans, where obese humans choose more immediate food- [20] and money-related outcomes [21, 22] over larger, delayed ones compared to healthy-weight humans [23–25]. No studies to our knowledge, however, have demonstrated that obesity in nonhumans (e.g., the obese Zucker rat) is associated with increased impulsive choice. As lean and obese Zuckers demonstrate both genetic and neurochemical differences that involve leptin and endocannabinoid signaling, examining impulsive choice in this strain may lead to insights in both the genetic and pharmacological factors involved in impulsive choice in the context of obesity.
3. Cannabinoids and delay discounting
The endocannabinoid neurotransmitter system is involved in many behavioral processes, including those related to food consumption. In free-feed environments, blocking the cannabinoid-1 (CB1) receptor using a CB1 antagonist and inverse agonist, such as rimonabant, reduces food intake in both standard laboratory and Zucker rat strains [5, 26–28]. In environments that vary in terms of response requirements for food, rimonabant (and other CB1 antagonists) attenuates the reinforcing properties of palatable food by reducing breakpoints under progressive ratio schedules of reinforcement [12, 29], suppressing food-reinforced behavior [30, 31], and increasing sensitivity to response requirements (i.e., elasticity of demand) for sucrose in Zucker rats [17]. Although the effects of rimonabant on food consumption in environments with both low and high response requirements have been characterized, the role of cannabinoids in impulsive choice is less understood.
Some studies have shown that CB1-specific drugs affect processes related to impulsivity in rats [32–34] and humans [35]. For example, Pattij et al. [32] found that rimonabant facilitated behavioral inhibition (another facet of impulsivity) on a 5-choice serial reaction time task and a stop-signal task. However, in this same study, rimonabant did not change sensitivity to delay in a procedure that examined choice between smaller-sooner versus larger-later reinforcers. It is possible though that the effects of rimonabant in the latter task may have been masked by a floor effect. Specifically, the delays used in the procedure suppressed baseline preference for the larger-later reinforcer to a degree that larger-later reinforcer choice could not decrease further (if rimonabant did indeed increase impulsive choice). A study that uses an adjusting delay may be able to rule out this potential floor effect.
4. The current study
The current study was designed to characterize impulsive-choice patterns in lean and obese Zucker rats using a delay-discounting procedure that titrated the delay to the larger-later reinforcer. Here, the delay to the larger-later reinforcer adjusts based on past choices until an indifference point (i.e., 50% preference for the larger-later outcome) is found. Therefore, the adjusting delay at which an indifference point is found is the dependent variable of interest, where longer indifference points would indicate self-controlled choice and shorter indifference points would indicate more impulsive choice. This study’s aim was to characterize impulsive-choice patterns in lean and obese Zucker rats and to determine the extent to which rimonabant affects these impulsive-choice patterns using the adjusting-delay procedure.
5. Material and methods
5.1 Subjects
Male lean (n=10, Fa/Fa or Fa/fa) and obese (n=10, fa/fa) Zucker rats were obtained from Harlan (Livermore, CA, USA) at one month of age and housed in individual clear plexiglass cages on a 12 h light:dark cycle (lights on at 0700 h). Rats had ad libitum access to food and water for eight weeks, after which time training sessions began. During training, rats were deprived of food for 21 h before operant sessions to establish food as a reinforcer, and were allowed to free-feed for 2 h after operant sessions. At the time of experimental sessions, lean rats ranged in weight from 179.8 g to 208.5 g and obese rats from 234.3 g to 301.0 g. All procedures were approved by the Idaho State University Institutional Animal Care and Use Committee.
5.2 Apparatus
Seven Coulbourn® Habitest (Coulbourn Instruments, Whitehall, PA, USA) standard rat operant chambers were used for data collection. Chambers were equipped with two levers on the right side panel wall and were situated 5 cm from a grid floor. One or more 45-mg sucrose pellet(s) (95% sucrose; TestDiet®, Richmond, IN, USA) were delivered to a collection space centered between the two levers when response requirements were met. Chambers also had a 28-V house light situated 13 cm above the collection space and 28-V stimulus lights above each lever. White noise was generated from a speaker in the upper right corner of the left side wall panel and a 5 cm × 5 cm fan circulated air in the upper left corner of the left side wall panel. Each chamber was placed in a sound-attenuating cubicle and Graphic State® software (Coulbourn Instruments, Whitehall, PA, USA) on a Windows-based computer controlled all reinforcement contingencies and data collection within 0.01-s resolution. Computers and software were located in a room adjacent to the room containing the operant chambers. Sessions were conducted from 0900 h to 1500 h Monday through Friday at the same time every day (±15 min). The number of rats in each session was counterbalanced across group and time of day at which they were run.
5.3 Drug
Rimonabant (National Institute of Mental Health Chemical Synthesis and Drug Supply Program) was dissolved in a 1:1:18 solution of ethanol (Sigma), Cremaphor (Sigma), and saline (1 mL/kg). Acute injections of rimonabant (0–10 mg/kg) were administered via i.p. injection 1 h before experimental sessions.
5.4 Procedures
5.4.1 Lever-press training
To train lever-pressing, a rat was placed in an operant chamber for 3 h in which single responses on either lever were reinforced by single pellets throughout the session. When 60 reinforcers were earned from one lever within a session, that lever was considered trained. In subsequent sessions, the trained lever was inactive (i.e., no pellets were delivered after a lever-press) and the opposite lever remained active until 60 reinforcers were earned from that lever within a session. When at least 60 reinforcers were earned from both levers, a rat was considered lever-press trained. If a rat earned fewer than 60 reinforcers on either lever for more than seven sessions, lever-presses were handshaped, or reinforced by successive approximations. Of all rats, two lean Zuckers were handshaped.
5.4.2 Adjusting-delay procedure
After training, a two-lever choice procedure was implemented. In this procedure, a response on either lever resulted in the delivery of one or more sucrose pellets after a specified delay. One lever, the standard-delay lever, resulted in one sucrose pellet after a 1-s fixed delay. The opposite lever, the adjusting-delay lever, resulted in two pellets after a delay that systematically increased or decreased by 1 s within session (described below). In other conditions, the fixed delay was 5 s (instead of 1 s) and the larger-later reinforcer was three pellets (instead of two). All rats were exposed to both fixed delays and both pellet conditions, though the order was counterbalanced across subjects. An intertrial interval (ITI) was initiated after each pellet delivery to ensure that each choice trial would last 50 s regardless of which lever was chosen. The ITI was calculated on a trial-to-trial basis by subtracting the delay to the pellet(s) from 50 s. A response on a lever, the delay, the delivery of a pellet(s), and the ITI constituted one trial. The lever (right or left) on which the standard or adjusting delay was implemented was counterbalanced across subjects.
In an experimental session, repeating blocks of trials were arranged. A block consisted of two forced-choice trials followed by two free-choice trials. At the start of a forced-choice trial, the house light along with a stimulus light above a randomly (p=0.5) selected lever (e.g., the standard-delay lever) was illuminated, which signaled that a response on that lever resulted in one pellet after a fixed delay. When the lever was pressed, the stimulus light above the lever extinguished, and one pellet would be delivered after a fixed delay (either 1 or 5 s). The house light then extinguished after the delivery of the pellet and the remainder of the trial consisted of the ITI. For the duration of this first forced-choice trial, the opposite lever was deactivated and lever presses had no programmed consequences. After the first forced-choice trial was completed, the second forced-choice trial followed in a manner similar to the first. That is, the stimulus light above the opposite lever (e.g., the adjusting-delay lever) was illuminated, and a response on this lever would result in two (or three) pellets after an adjusting delay (described below). During this second forced-choice trial, the opposite lever was deactivated and responses had no programmed consequences. Two forced-choice trials were completed to ensure exposure to the contingencies associated with each lever.
After two forced-choice trials were completed, the remainder of a block consisted of two free-choice trials. Free-choice trials were similar in all respects to forced-choice trials except both stimulus lights were illuminated simultaneously, and a response on either lever resulted in delivery of the programmed pellet(s) after its respective delay. These four-trial blocks repeated throughout an experimental session until the session ended after 60 min. On average, lean rats completed 59 trials (SEM = 1.1) and obese rats completed 60 trials (SEM = 1.2) per session.
5.4.3 Adjusting delay
The adjusting delay to the larger reinforcer increased or decreased for the subsequent block of trials depending on choices made in the two free-choice trials of a block. If the standard-delay lever was chosen in both of the two free-choice trials of a block, the adjusting delay was decreased by 1 s in the next block of trials. If the adjusting-delay lever was chosen in both free-choice trials, the adjusting delay was increased by 1 s. If the adjusting-delay lever was chosen in one free-choice trial and then the standard-delay lever was chosen in the second free-choice trial (or vice versa), the adjusting delay remained the same for the following block of trials. At the start of a condition, the adjusting delay began at 0 s. In subsequent sessions, the adjusting delay began at the final delay from the last block of the previous session. The adjusting delay also was tracked across several sessions until stability occurred. Stability was determined using a modified version of Mazur’s [36] stability criteria. Each rat’s adjusting delay was sampled at every 10 min interval during a 60 min session. This resulted in six data points for every session. The average of these six adjusting delays then was computed and compared to the previous two averages of the last two sessions. An adjusting delay was considered stable when (1) the range of these three averages was no greater than 2 s, (2) there were no apparent trends in adjusting delays, and (3) at least three sessions had been conducted for the condition.
After adjusting delays stabilized for the 2-pellet condition only (e.g., with the 1-s standard delay), acute injections of rimonabant (0–10 mg/kg) were administered before some sessions until the stability criteria were met under each dose. Sessions were not conducted between injection days, and each dose was administered at least three times and until adjusting delays stabilized (using the stability criteria described above). Injections were given in a pseudorandom fashion with at least two days separating doses, though vehicle injections separated higher doses of rimonabant (i.e., 3 and 10 mg/kg) to minimize carryover effects. For example, two days would separate each of the following injections—3 mg/kg rimonabant, vehicle, and 10 mg/kg rimonabant—such that at least four days would separate 3 and 10 mg/kg rimonabant injections. Table 1 shows the order of conditions and the number of sessions required to meet the stability criteria for each rat. Note that the number of sessions to meet the stability criteria under the vehicle and drug conditions is also the number of times that particular dose was injected.
Table 1.
Order of conditions (in bold) and number of sessions to meet stability criteria (in parentheses) for lean (“L”) and obese (“F”) Zucker rats in each pellet condition, standard-delay (1 s or 5 s) condition, and dose of rimonabant.
| Subject | 2 pellets
|
3 pellets
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 s | 5 s | 1 s | 5 s | |||||||||
|
|
|
|
||||||||||
| B | V | 1 m/k | 3 m/k | 10 m/k | B | V | 1 m/k | 3 m/k | 10 m/k | B | B | |
|
|
|
|
||||||||||
| L71 | 6 (10) | 9 (15) | 8 (6) | 7 (3) | 10 (4) | 1 (17) | 4 (32) | 3 (8) | 2 (3) | 5 (4) | 11 (6) | 12 (10) |
| L76 | 3 (5) | 6 (19) | 5 (7) | 4 (8) | 7 (9) | 8 (10) | 10 (4) | 12 (3) | 11 (7) | 9 (4) | 2 (49) | 1 (17) |
| L84 | 1 (13) | 4 (3) | 2 (4) | 3 (18) | 5 (3) | 6 (14) | 9 (11) | 8 (4) | 7 (4) | 10 (3) | 11 (16) | 12 (8) |
| L86 | 1 (15) | 4 (35) | 3 (4) | 2 (10) | 5 (5) | 6 (30) | 8 (15) | 9 (3) | 10 (4) | 7 (12) | 12 (4) | 11 (7) |
| L87 | 1 (22) | 4 (3) | 3 (14) | 2 (28) | 5 (8) | 6 (21) | 8 (14) | 10 (3) | 9 (3) | 7 (7) | 12 (5) | 11 (9) |
| L88 | 8 (22) | 10 (11) | 12 (14) | 11 (6) | 9 (7) | 3 (17) | 6 (5) | 4 (7) | 5 (8) | 7 (4) | 1 (13) | 2 (6) |
| L92 | 7 (5) | 9 (21) | 11 (12) | 10 (14) | 8 (5) | 1 (20) | 3 (6) | 5 (17) | 4 (4) | 2 (20) | 12 (12) | 6 (29) |
| L93 | 3 (17) | 6 (4) | 5 (8) | 4 (3) | 7 (6) | 8 (8) | 11 (3) | 9 (3) | 10 (5) | 12 (9) | 1 (22) | 2 (14) |
| L94 | 8 (5) | 11 (25) | 10 (4) | 9 (4) | 12 (4) | 3 (12) | 6 (20) | 5 (3) | 4 (13) | 7 (4) | 2 (30) | 1 (12) |
| L98 | 1 (25) | 3 (37) | 5 (11) | 2 (19) | 4 (3) | 6 (16) | 9 (8) | 7 (4) | 8 (10) | 10 (9) | 12 (10) | 11 (9) |
|
| ||||||||||||
| F73 | 1 (45) | 4 (12) | 2 (6) | 3 (4) | 5 (18) | 6 (4) | 9 (3) | 7 (3) | 8 (4) | 10 (13) | 11 (4) | 12 (10) |
| F75 | 3 (11) | 6 (9) | 5 (3) | 4 (5) | 7 (6) | 8 (6) | 11 (3) | 10 (3) | 9 (3) | 12 (6) | 2 (20) | 1 (15) |
| F78 | 8 (8) | 11 (5) | 9 (5) | 10 (4) | 12 (6) | 1 (20) | 4 (3) | 3 (15) | 2 (3) | 5 (14) | 6 (14) | 7 (6) |
| F80 | 8 (10) | 10 (5) | 12 (3) | 11 (15) | 9 (6) | 3 (13) | 6 (3) | 5 (3) | 4 (3) | 7 (9) | 1 (19) | 2 (12) |
| F82 | 1 (15) | 3 (44) | 4 (4) | 5 (7) | 2 (9) | 8 (16) | 10 (6) | 12 (3) | 11 (3) | 9 (6) | 6 (24) | 7 (6) |
| F83 | 8 (10) | 10 (7) | 12 (8) | 11 (4) | 9 (15) | 1 (12) | 4 (25) | 2 (4) | 3 (3) | 5 (4) | 6 (11) | 7 (10) |
| F85 | 8 (9) | 11 (3) | 10 (4) | 9 (3) | 12 (3) | 1 (12) | 3 (18) | 5 (5) | 4 (3) | 2 (10) | 6 (9) | 7 (13) |
| F89 | 6 (5) | 9 (5) | 8 (10) | 7 (28) | 10 (3) | 1 (25) | 3 (5) | 5 (3) | 4 (9) | 2 (4) | 11 (6) | 12 (5) |
| F95 | 6 (34) | 9 (5) | 7 (4) | 8 (6) | 10 (18) | 1 (13) | 4 (15) | 2 (3) | 3 (4) | 5 (5) | 11 (8) | 12 (20) |
| F99 | 3 (9) | 6 (5) | 5 (3) | 4 (4) | 7 (13) | 8 (11) | 10 (10) | 12 (3) | 11 (3) | 9 (6) | 1 (20) | 2 (18) |
5.5 Analysis
Baseline adjusting delays and extraneous responses during the delays (which were converted into responses per second to facilitate comparisons between delay periods) were analyzed using a 2 × 2 repeated measures ANOVA with genotype (lean vs. obese) as the between-subjects variable and standard delay (1 s vs. 5 s) as the within-subjects variables for each pellet condition separately. A 2 × 2 × 2 repeated measures ANOVA also was conducted to determine effects of pellet condition (2 vs. 3 pellets). When appropriate, independent samples t-tests were used to determine differences between groups at each standard delay in each pellet condition. Furthermore, paired samples t-tests were used to determine differences in adjusting delays within groups as a result of standard delay and pellet condition. When determining drug effects in the 2-pellet condition, adjusting delays and extraneous responses were transformed into percent of vehicle and were analyzed using a 2 × 2 repeated measures ANOVA with genotype as the between-subjects variable and drug dose as the within-subjects variable for each standard delay condition separately. In cases where response rate was 0 responses per second under vehicle, 0.01 was substituted in order to transform response rates into percent of vehicle.
Table 2 shows adjusting delays for all rats where values in parentheses are standardized adjusting delays and represent standard deviations from the mean of the respective group. F95’s data ranged from −1.777 to 7.092 standard deviations away from the mean of obese rats’ adjusting delays across the various pellet and standard-delay conditions. Because of the skewness introduced when F95 was included in analyses, his data were excluded from analysis and data from 10 lean rats and 9 obese rats were used.
Table 2.
Adjusting delays (s) for lean (“L”) and obese (“F”) Zucker rats in each pellet and standard-delay (1 s or 5 s) condition.
| Subject | 2 pellets
|
3 pellets | ||
|---|---|---|---|---|
|
| ||||
| 1 s | 5 s | 1 s | 5 s | |
| L71 | 14.5 (0.865) | 23.3 (0.481) | 17.8 (0.321) | 35.8 (0.388) |
| L76 | 15.5 (1.240) | 19.7 (−0.333) | 14.5 (−0.436) | 24.6 (−1.180) |
| L84 | 10.1 (−0.560) | 14.0 (−1.911) | 13.8 (−0.593) | 25.6 (−1.020) |
| L86 | 9.1 (−0.924) | 13.9 (−1.953) | 15.1 (−0.295) | 37.7 (0.651) |
| L87 | 12.6 (0.219) | 25.4 (0.984) | 18.0 (0.359) | 33.3 (0.060) |
| L88 | 6.2 (−2.242) | 17.8 (−0.778) | 27.1 (4.080) | 28.4 (−0.600) |
| L92 | 16.3 (1.596) | 21.4 (0.053) | 19.5 (0.714) | 43.3 (1.558) |
| L93 | 13.7 (0.597) | 26.6 (1.304) | 10.7 (−1.437) | 20.9 (−1.882) |
| L94 | 12.3 (0.131) | 24.5 (0.759) | 15.8 (−0.144) | 34.8 (0.253) |
| L98 | 8.4 (−1.174) | 25.4 (0.984) | 11.8 (−1.100) | 44.4 (1.780) |
|
| ||||
| High: | 16.3 (1.596) | 26.6 (1.304) | 27.1 (4.080) | 44.4 (1.780) |
| Low: | 6.2 (−2.242) | 13.9 (−1.953) | 10.7 (−1.437) | 20.9 (−1.882) |
|
| ||||
| F73 | 3.6 (−1.131) | 14.4 (−0.950) | 8.1 (−0.817) | 21.8 (−0.792) |
| F75 | 8.9 (0.021) | 25.5 (0.989) | 16.2 (0.362) | 20.9 (−1.048) |
| F78 | 11.9 (0.624) | 15.6 (−0.732) | 12.1 (−0.218) | 31.6 (1.813) |
| F80 | 11.6 (0.553) | 16.2 (−0.632) | 10.3 (−0.473) | 22.8 (−0.551) |
| F82 | 7.1 (−0.360) | 30.0 (2.050) | 9.7 (−0.563) | 28.3 (0.786) |
| F83 | 7.2 (−0.326) | 25.4 (0.978) | 15.8 (0.299) | 21.9 (−0.763) |
| F85 | 10.3 (0.290) | 21.8 (0.329) | 11.7 (−0.274) | 27.0 (0.463) |
| F89 | 5.8 (−0.615) | 15.0 (−0.845) | 7.3 (−0.932) | 19.2 (−1.562) |
| F95 | 20.4 (3.664) | 10.7 (−1.777) | 33.2 (7.092) | 31.6 (1.792) |
| F99 | 1.7 (−1.638) | 24.0 (0.708) | 12.1 (−0.218) | 25.6 (0.116) |
|
| ||||
| High: | 20.4 (3.664) | 30.0 (2.050) | 33.2 (7.092) | 31.6 (1.813) |
| Low: | 1.7 (−1.638) | 10.7 (−1.777) | 7.3 (−0.932) | 19.2 (−1.562) |
Values in parentheses are standardized adjusting delays and represent standard deviations from the mean of the respective group. Data for F95 are bolded.
6. Results
6.1 Lever-press training
An independent samples t-test revealed that the mean number of sessions until lever-press trained was significantly lower for obese rats than lean rats [obese: M = 4.0, SEM = 0.39; lean: M = 6.1, SEM = 0.65; t(18) = 3.37, p < 0.01]. This includes the two hand-shaped lean rats, which were treated as if they completed eight sessions, since after seven they were hand shaped. When these two rats were excluded from the analysis, this difference was still significant [t(16) = 2.69, p = 0.02].
6.2 Baseline
6.2.1 Adjusting delays
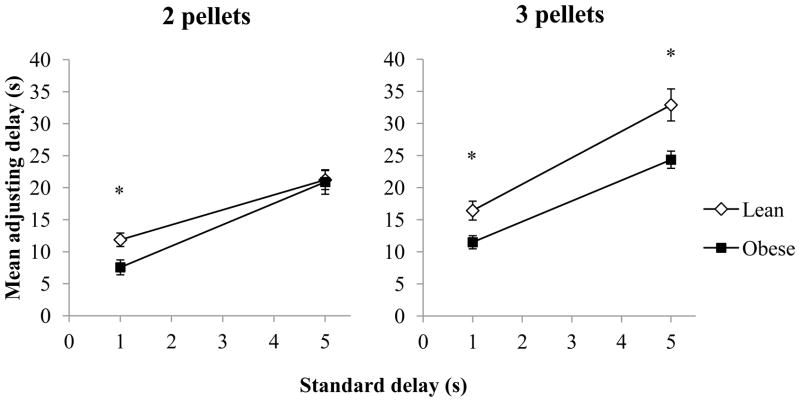
Figure 1 shows adjusting delays with each standard delay in the 2-pellet (left) and 3-pellet condition (right) for lean and obese Zucker rats. In the 2-pellet condition, there was a main effect of standard delay [F(1,17) = 69.75, p < 0.001, ηp2 = 0.80], as the 5-s fixed delay induced longer adjusting delays than the 1-s condition for both groups. There was neither a main effect of genotype nor a significant interaction; however, obese rats had significantly shorter adjusting delays compared to leans with the 1-s standard delay [t(17) = −2.74, p = 0.01]. In the 3-pellet condition, there was a main effect of standard delay [F(1,17) = 78.10, p < 0.001, ηp2 = 0.82], as the 5-s fixed delay again induced longer adjusting delays than the 1-s condition for both groups. There was also a main effect of genotype [F(1,17) = 14.12, p < 0.01, ηp2 = 0.45], in that the obese rats had significantly lower adjusting delays than leans in both standard delay conditions [1-s standard delay: t(17) = −2.69, p = 0.02; 5-s standard delay: t(17) = −2.915, p = 0.01]. There were no significant interactions. Analysis with both the 2- and 3-pellet conditions revealed a main effect of pellet condition [F(1,17) = 29.52, p < 0.001, ηp2 = 0.64], as the 3-pellet condition led to longer adjusting delays than the 2-pellet condition overall. Specifically, adjusting delays were significantly longer with 3 pellets than with 2 pellets in the 1-s standard delay conditions for both groups [lean: t(9) = −2.26, p = 0.05; obese: t(8) = −2.93, p = 0.02], but only for leans in the 5-s standard delay conditions [lean: t(9) = −4.29, p < 0.01]. There were no significant interactions involving pellet condition.
Figure 1.
Mean adjusting delay (±SEM) as a function of standard delay for lean and obese Zucker rats when the adjusting delay resulted in two pellets (left) or three pellets (right).
*p < 0.05 difference between lean and obese rats
6.2.2 Response rate
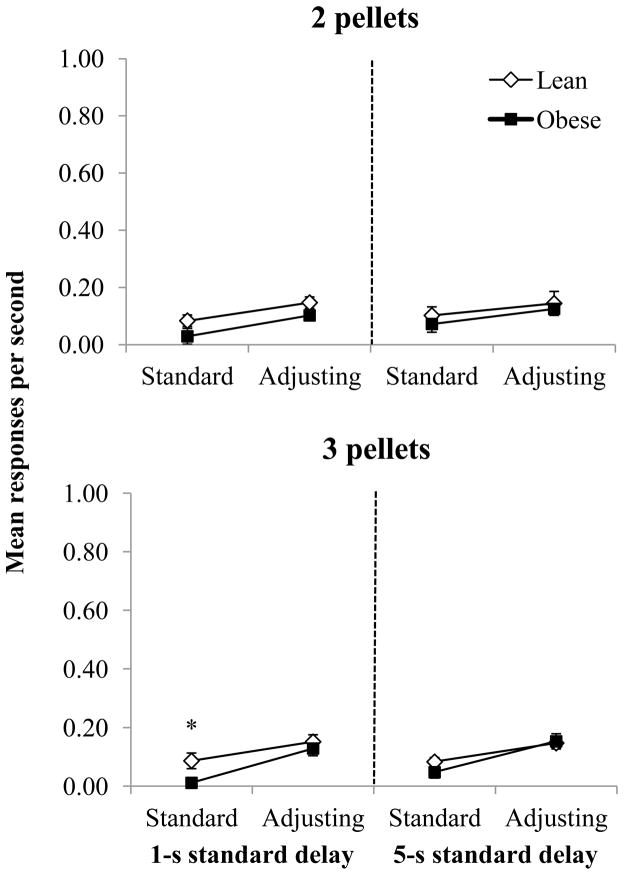
Figure 2 shows extraneous responses, converted into responses per second, during the standard and adjusting delays. Overall, response rates were very low (less than 0.18 response per second). In the 2-pellet condition, there was a main effect of delay [F(1,17) = 7.64, p = 0.01, ηp2 = 0.31], in that there were higher rates of responding during the adjusting delay compared to the standard delay. However, there were no other main effects of genotype or significant interactions. A similar pattern was observed in the 3-pellet condition in that there was a main effect of delay period [F(1,17) = 30.39, p < 0.01, ηp2 = 0.64], as response rates were higher in the adjusting delay compared to the standard delay, but there were no other main effects or significant interactions. Obese rats, however, had significantly lower response rates compared to leans during the 1-s standard delay [t(17) = −2.55, p = 0.02].
Figure 2.
Mean responses per second (±SEM) for lean and obese Zucker rats when the adjusting delay resulted in two pellets (top) or three pellets (bottom). Responses per second are separated into the standard and adjusting delay for the 1-s (left) and 5-s (right) standard-delay conditions.
*p < 0.05 difference between lean and obese rats
6.3 Drug
6.3.1 Adjusting delays
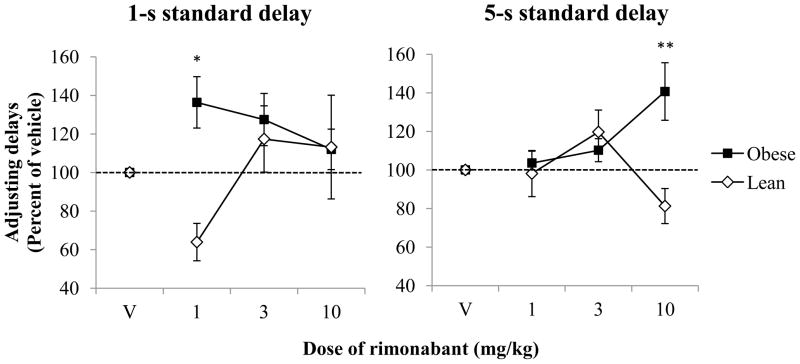
Figure 3 shows adjusting delays expressed as a mean percent of vehicle as a function of dose of rimonabant for lean and obese Zucker rats with the 1-s (left) and 5-s (right) standard delays in the 2-pellet condition. In the 1-s standard-delay condition, there were no main effects of genotype or drug (p’s > 0.14). However, there was a significant genotype X drug interaction [F(3,51) = 3.85, p = 0.02, ηp2 = 0.19], which was reflected in the lean and obese rats’ differential response to the 1 mg/kg dose of rimonabant. Contrasts revealed a significant decrease in adjusting delays expressed as percent of vehicle under the 1 mg/kg dose for lean rats [F(1,9) = 13.91, p = 0.01, ηp2 = 0.61], and a significant increase for obese rats [F(1,8) = 7.42, p = 0.03, ηp2 = 0.48]. In the 5-s standard-delay condition, a similar pattern was observed, though at the highest dose. Specifically, there was a significant genotype X drug interaction [F(3,51) = 8.73, p < 0.001, ηp2 = 0.34], with contrasts revealing that the 10 mg/kg dose increased adjusting delays expressed as percent of vehicle for obese rats [F(1,8) = 7.41, p = 0.03, ηp2 = 0.48], and marginally decreased adjusting delays expressed as percent of vehicle for leans [F(1,9) = 4.26, p = 0.069, ηp2 = 0.32].
Figure 3.
Adjusting delays expressed as a mean percent of vehicle (±SEM) as a function of dose of rimonabant for lean and obese Zucker rats with the 1-s (left) and 5-s (right) standard delays in the 2-pellet condition. The dotted lines represent 100% of vehicle (i.e., no change in adjusting-delay length). Note that symbols may obstruct error bars in some instances.
*p < 0.05 difference compared to vehicle for both groups
**p < 0.05 difference compared to vehicle for obese rats; p = 0.069 for lean rats
6.3.2 Responses per trial
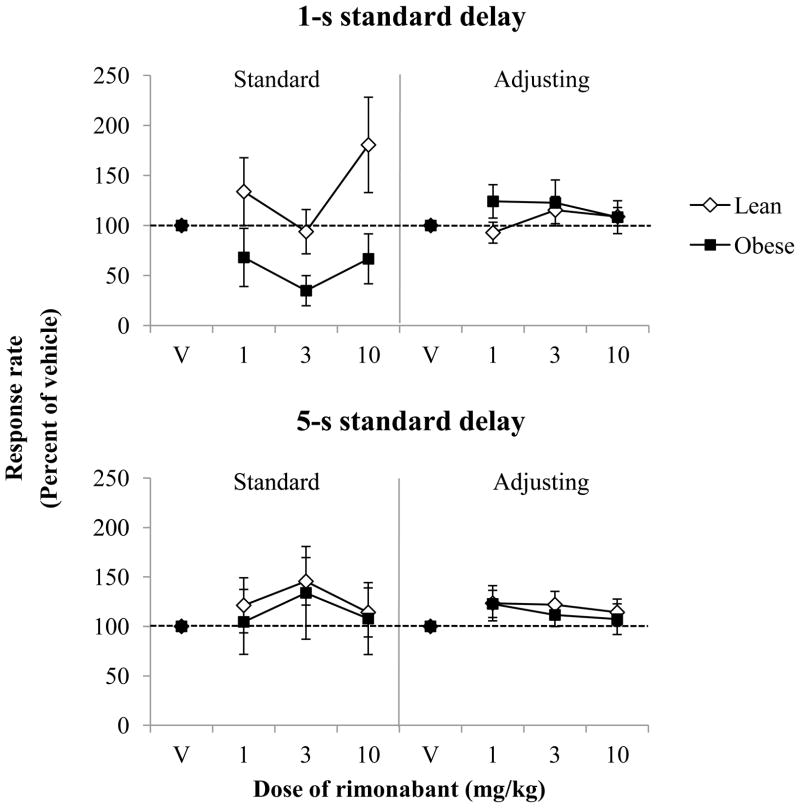
Figure 4 shows response rates expressed as a mean percent of vehicle in the delay period (standard delay, left; adjusting delay, right) as a function of dose of rimonabant for lean and obese Zucker rats with the 1-s (top) and 5-s (bottom) standard delays of the 2-pellet condition. In the 1-s standard-delay condition, there was a main effect of genotype for the standard-delay period [F(1,17) = 5.46, p = 0.03, ηp2 = 0.24], as obese rats had lower response rates than leans, but there were no other main effects or significant interactions for both the standard- and adjusting-delay period. Importantly, rimonabant did not alter the number of trials completed for either group of rats.
Figure 4.
Responses per second expressed as a mean percent of vehicle (±SEM) as a function of dose of rimonabant for lean and obese Zucker rats in the 1-s (top) and 5-s (bottom) standard-delay conditions when the adjusting delay resulted in 2 pellets. Response rates are divided into the standard and adjusting delays. Note that symbols may obstruct error bars in some instances.
7. Discussion
During training, obese rats acquired the lever-press response for sucrose in fewer sessions than lean rats, even when the two hand shaped lean rats were excluded from analysis. This finding replicates and extends those from a previous study that reported on single lever-press acquisition in the context of a progressive ratio schedule of reinforcement [12]. Although reasons why the obese Zuckers acquired the lever-press in fewer trials than lean rats in the current study (as well as other studies) are unclear, Rasmussen and Huskinson [12] posited that obese Zuckers might demonstrate increased sensitivity to sucrose reinforcement. This seems likely when one considers previous research showing obese Zuckers consume more reinforcers in environments with low response requirements [1, 17] and show greater sensitivity to food patches with higher densities of reinforcement than their lean counterparts [37]. It is plausible then that an increased sensitivity to sucrose reinforcement in the obese Zuckers contributed to the training effect in the current study.
Adjusting delays were examined across varying pellet amounts and standard-delay durations. Both groups of rats’ adjusting delays were significantly lengthened when the standard delay was longer (5 s compared to 1 s). That is, their choices became more self-controlled when the more immediate option required a 5-s waiting period. Lean rats also waited longer when the number of pellets available on the adjusting-delay lever was higher (3 instead of 2). These findings replicate previous studies that show adjusting delays increase when the standard delay and/or the amount of reinforcement on the adjusting-delay lever is increased in standard laboratory rats and pigeons [36, 38], but extend to the Zucker strain—a novel finding. Increasing the number of pellets on the adjusting-delay lever, however, only lengthened lean rats’ (compared to obese) adjusting delays when there was a 5-s standard delay in effect. This may be due to an interaction between genotype and choice condition but further investigation is needed.
In three out of the four conditions, we observed that obese Zucker rats had shorter adjusting delays than lean rats. Specifically, in the 3-pellet condition, obese rats had significantly shorter adjusting delays than lean rats both with the 1-s and 5-s standard delay (Figure 1). This was observed also in the 2-pellet condition with the 1-s standard delay. To our knowledge, this experiment is the first to not only characterize differences in impulsive-choice patterns between lean and obese Zucker rats, but also demonstrate that obesity in a nonhuman species is associated with increased impulsive choice. The current study also extends findings from experiments with humans using the delay-discounting procedure, which have shown that obese participants are more sensitive to hypothetical delayed food and monetary outcomes than healthy weight participants [20–25].
Extraneous responses, that is, responses that occurred during the standard and adjusting delays that did not contribute to a food choice, also were measured. Response rates overall were low in that one response occurred every 6–10 s (0.08 to 0.15 response per second). Rats on average tended to have higher response rates during the adjusting delay than the standard delay in all conditions. Furthermore, obese rats had significantly lower responses rates in the 1-s standard-delay period of the 3-pellet condition. Because of these findings, an argument could be made that there was a greater degree of effort in the form of a response requirement (though a response requirement was not part of the contingency) to obtain sucrose associated with the adjusting-delay lever compared to the standard-delay lever. Comparing the average response rates, however, shows that the difference in responding between the standard- and adjusting-delay periods across a 50-s interval (i.e., the length of one trial) would be approximately 3–4 responses at the greatest. Furthermore, previous research on behavioral-economic demand in obese Zuckers has demonstrated that sucrose is inelastic (i.e., does not reduce food consumption) at low response requirements [1, 17], such as those responses observed in the current study. Therefore, it is unlikely that strain differences or a sensitivity to response requirements accounted for the disparity in responding during the standard- and adjusting-delay periods.
The impulsive choice patterns of lean and obese Zucker rats also were examined after acute administration of rimonabant in the 2-pellet condition. Rimonabant did not have main effects on behavior, which replicates Pattij et al. [32]. However, the drug did combine with some conditions to produce an interaction. When a 1-s standard delay was in effect, 1 mg/kg of rimonabant significantly shortened adjusting delays as a percent of vehicle for lean rats and lengthened adjusting delays as a percent of vehicle for obese rats (Figure 3). A similar pattern was observed with the 10 mg/kg dose in the 5-s standard-delay condition. Furthermore, rimonabant did not appear to have any systematic main effects or interactions on response rate (Figure 4) or the number of trials completed. Although reasons why the effective dose of rimonabant varied between standard-delay conditions are unclear, the prolonged drug testing regimen used in the current study may be a possible cause (as rats were injected at least three times with each dose of rimonabant; see Table 1). Regardless, no studies to date have shown that rimonabant interacts with delays to affect food reinforcement in lean and obese Zuckers, so this is a novel finding. This also suggests that with the doses that affected the adjusting delays, the effect was specifically related to sensitivity to delay (i.e., ability to wait) and not a psychomotor process.
The observation that some doses of rimonabant reduced impulsive choice (i.e., enhanced self-controlled choice) for obese rats, but had the opposite effect for lean rats is puzzling. Previous studies that examined the effects of rimonabant on food consumption and food-reinforced behavior have noted that obese Zuckers are typically more sensitive to rimonabant [5, 12, 13] and other cannabinoids [14] than leans. However, this increased sensitivity is usually in the same direction (not opposite). Another mechanism that may explain why rimonabant differentially affected obese and lean Zuckers’ behavior in the current study is the baseline rate of impulsive choice. A number of studies have shown that drug effects on impulsive choice are dependent on the initial rate of impulsive choice, that is, the rate before drug administrations [39–41]. For example, Perry et al. [41] found that administration of d-amphetamine increased impulsive choice in rats with higher baseline adjusting delays (i.e., rats who made more self-controlled choices under baseline), but decreased impulsive choice in rats with lower baseline adjusting delays (i.e., rats who made more impulsive choices under baseline). No studies to date have systematically shown baseline-dependent effects of rimonabant on impulsive choice, so further investigation is needed.
There were some limitations to the present study. First, a pattern of impulsive choice could occur from (1) a decreased sensitivity to the amount of the larger-later reinforcer or (2) an increased sensitivity to (or aversion towards) the delay to the larger-later reinforcer. While obese Zuckers responded more impulsively than lean rats, it is unclear whether these differences were due to changes in sensitivity to amount of reinforcement or delay to reinforcement. Future studies using an adjusting-delay procedure may consider adding some probe sessions in which the delay to both food outcomes is 0 s to help determine if amount sensitivity plays a role [41, 42]. Choice for the smaller, immediate outcome over the larger, immediate outcome in these sessions would reflect an insensitivity to amount. Second, and related to the concern of amount and delay sensitivity, the present study examined behavior under two levels of standard delays and pellet conditions. It may be useful to examine behavior under more than two levels of these variables as a fuller characterization of both may be established [36]. However, examining adjusting delays across many standard delays and pellet conditions as well as testing the effects of rimonabant on these adjusting delays would have significantly lengthened the study, which becomes a concern when using obese Zucker rats who are prone to health problems as they age [43, 44]. Finally, the present study may have benefited from additional levels of analysis, such as histological or physiological measures [e.g., 10, 11, 15]. However, we feel that the current study still provides some indirect evidence for neurochemical substrates. For example, impulsive-choice patterns were tested across several contexts (i.e., two standard-delay durations in each of two pellet conditions) using rats with different genetic polymorphisms that have been shown to directly influence leptin and endocannabinoid regulation [9, 10, 16]. We also reported differential behavioral effects of these two strains under varying doses of rimonabant, which also implicates the CB1 receptor. However, additional measures may bolster the present study’s primary conclusions. Future studies may be able to build on these observations by including central or peripheral measures of serum leptin levels, endocannabinoid levels, or perhaps measures of CB1 receptor densities in relevant brain regions to provide more direct support for the extent to which leptin or endocannabinoid-related activity is involved.
8. Conclusions
Despite these limitations, an adjusting-delay procedure was used to characterize differences in impulsive-choice patterns between lean and obese Zucker rats, both before and after acute injections of rimonabant. The results from the present study suggest that obese Zuckers behave more impulsively than lean Zuckers, and provide further support that differences in food consumption between lean and obese Zucker rats are largely dependent on the environmental arrangement of food. To our knowledge, this is the first study to characterize differences in impulsive-choice patterns in lean and obese Zucker rats. Because we controlled for floor effects by using the adjusting-delay procedure, the present study also provides some support that the CB1 receptor may be involved in impulsive choice depending on the specific genotypes and perhaps baseline rates of impulsive choice involved. Understanding the conditions under which genetic and pharmacological factors influence impulsive choice may prove helpful in designing effective treatments for obesity.
Highlights.
Obese Zucker rats made more impulsive choices for sucrose than lean rats.
Rimonabant decreased impulsive choice in obese Zucker rats;
Rimonabant increased impulsive choice in lean Zucker rats.
Effects of rimonabant were due to delay sensitivity, not psychomotor processes.
Acknowledgments
This research was supported by funding from the Idaho INBRE Program, NIH grant nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences) as well as Faculty Research Committee and Undergraduate Research Committee grants at Idaho State University. We thank Starlie Belnap, Megan Brinton, Jessica Buckley, Aarica Burke, Kory Farley, Bradley Gossett, and Aaron Miller for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rasmussen EB, Reilly W, Hillman C. Demand for sucrose in the genetically obese Zucker (fa/fa) rat. Behav Processes. 2010;85(2):191–7. doi: 10.1016/j.beproc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Saitoh Y, Liu R, Ueno H, Mizuta M, Nakazato M. Oral pioglitazone administration increases food intake through ghrelin-independent pathway in Zucker fatty rat. Diabetes Res Clin Pract. 2007;77(3):351–6. doi: 10.1016/j.diabres.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Simler N, Grosfeld A, Peinnequin A, Guerre-Millo M, Bigard A. Leptin receptor-deficient obese Zucker rats reduce their food intake in response to hypobaric hypoxia. Am J Physiol Endocrinol Metab. 2005;290(3):E591–7. doi: 10.1152/ajpendo.00289.2005. [DOI] [PubMed] [Google Scholar]
- 4.Thanos PK, Ramalhete RC, Michaelides M, Piyis YK, Wang G, Volkow ND. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse. 2008;62(9):637–42. doi: 10.1002/syn.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickers S, Webster L, Wyatt A, Dourish C, Kennett G. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003;167:103–11. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- 6.White CL, Purpera MN, Ballard K, Morrison CD. Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiol Behav. 2010;100(4):408–16. doi: 10.1016/j.physbeh.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck B. Neuropeptides and obesity. Nutrition. 2000;16(10):916–23. doi: 10.1016/s0899-9007(00)00410-x. [DOI] [PubMed] [Google Scholar]
- 8.Dryden S, Pickavance L, Frankish HM, Williams G. Increased neuropeptide Y secretion in the hypothalamic paraventricular nucleus of obese (fa/fa) Zucker rats. Brain Res. 1995;690(2):185–8. doi: 10.1016/0006-8993(95)00628-4. [DOI] [PubMed] [Google Scholar]
- 9.DiMarzo V, Goparaju SKI, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 10.Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, et al. Peripheral endocannabinoid dysregulation in obesity: Relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol. 2009;158(2):451–61. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thanos PK, Ramalhete RC, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Leptin receptor deficiency is associated with upregulation of cannabinoid-1 receptors in limbic brain regions. Synapse. 2008;62(9):637–42. doi: 10.1002/syn.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen EB, Huskinson SL. Effects of rimonabant on behavior maintained by progressive ratio schedules of sucrose reinforcement in obese Zucker (fa/fa) rats. Behav Pharmacol. 2008;19(7):735–42. doi: 10.1097/FBP.0b013e3283123cc2. [DOI] [PubMed] [Google Scholar]
- 13.Serrano A, del Arco I, Javier Pavón F, Macías M, Perez-Valero V, Rodríguez de Fonseca F. The cannabinoid CB1 receptor antagonist SR141716A (Rimonabant) enhances the metabolic benefits of long-term treatment with oleoylethanolamide in Zucker rats. Neuropharmacology. 2008;54(1):226–34. doi: 10.1016/j.neuropharm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Smith S, Rasmussen EB. Effects of 2-AG on the reinforcing properties of exercise in lean and obese Zucker rats. Behav Pharmacol. 2010;21:292–300. doi: 10.1097/FBP.0b013e32833aec4d. [DOI] [PubMed] [Google Scholar]
- 15.Thanos PK, Michaelides M, Piyis YK, Wang G, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo μPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62(1):50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- 16.Zucker L, Zucker T. Fatty, a new mutation in the rat. Journal Hered. 1962;52:275–8. [Google Scholar]
- 17.Rasmussen EB, Reilly W, Buckley J, Boomhower SR. Rimonabant reduces the essential value of food in the genetically obese Zucker rat: An exponential demand analysis. Physiol Behav. 2012;105(3):734–41. doi: 10.1016/j.physbeh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82(4):463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 19.Rachlin H. Self-control: Beyond commitment. Behav Brain Sci. 1995;18:109–59. [Google Scholar]
- 20.Rasmussen EB, Lawyer SR, Reilly W. Percent body fat is related to delay and probability discounting for food in humans. Behav Processes. 2010;83(1):23–30. doi: 10.1016/j.beproc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Weller RE, Cook EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51(3):563–9. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Fields SA, Sabet M, Peal A, Reynolds B. Relationship between weight status and delay discounting in a sample of adolescent cigarette smokers. Behav Pharmacol. 2011;22(3):266–8. doi: 10.1097/FBP.0b013e328345c855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appelhans BM, Waring ME, Schneider KL, Pagoto SL, DeBiasse MA, Whited MC, et al. Delay discounting and intake of ready-to-eat and away-from-home foods in overweight and obese women. Appetite. 2012;59(2):576–84. doi: 10.1016/j.appet.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity. 2011;19(11):2175–82. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, et al. Behavioral economic predictors of overweight children’s weight loss. J Consult Clin Psychol. 2012 doi: 10.1037/a0029827. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carai MAM, Colombo G, Maccioni P, Gessa GL. Efficacy of rimonabant and other cannabinoid CB1 receptor antagonists in reducing food intake and body weight: Preclinical and clinical data. CNS Drug Rev. 2006;12(2):91–9. doi: 10.1111/j.1527-3458.2006.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63(8):PL113–7. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 28.Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology (Berl) 2004;179(2):452–60. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- 29.Droste SM, Saland SK, Schlitter EK, Rodefer JS. AM 251 differentially effects food-maintained responding depending on food palatability. Pharmacol Biochem Behav. 2010;95(4):443–8. doi: 10.1016/j.pbb.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Sink KS, McLaughlin PJ, Wood JAT, Brown C, Fan P, Vemuri VK, et al. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2007;33(4):946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14(8):583–8. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Pattij T, Janssen MCW, Schepers I, González-Cuevas G, Vries TJ, Schoffelmeer ANM. Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology (Berl) 2007;193(1):85–96. doi: 10.1007/s00213-007-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiskerke J, Stoop N, Schetters D, Schoffelmeer ANM, Pattij T. Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PLoS ONE. 2011;6(10):e25856. doi: 10.1371/journal.pone.0025856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: Evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27(7):639–51. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 35.McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28(7):1356–65. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- 36.Mazur JE, Biondi DR. Delay-amount tradeoffs in choices by pigeons and rats: hyperbolic versus exponential discounting. J Exp Anal Behav. 2009;91(2):197–211. doi: 10.1901/jeab.2009.91-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckley J, Rasmussen EB. Obese and lean Zucker rats demonstrate differential sensitivity to rates of food reinforcement in a choice procedure. Physiol Behav. 2012;108:19–27. doi: 10.1016/j.physbeh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazur J. Rats’ choices between one and two delayed reinforcers. Learn Behav. 2007;35:169–76. doi: 10.3758/bf03193052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbelivien A, Billy E, Lazarus C, Kelche C, Majchrzak M. Rats with different profiles of impulsive choice behavior exhibit differences in responses to caffeine and d-amphetamine and in medial prefrontal cortex 5-HT utilization. Behav Brain Res. 2008;187(2):273–83. doi: 10.1016/j.bbr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Huskinson SL, Krebs CA, Anderson KG. Strain differences in delay discounting between Lewis and Fischer 344 rats at baseline and following acute and chronic administration of d-amphetamine. Pharmacol Biochem Behav. 2012;101(3):403–16. doi: 10.1016/j.pbb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193(1):48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128(2):161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 43.Azain M, Broderson J, Martin R. Effects of long-term somatotropin treatment on body composition and life span in aging Zucker rats. Exp Biol Med (Maywood) 2006;231:76–83. doi: 10.1177/153537020623100109. [DOI] [PubMed] [Google Scholar]
- 44.Johnson P, Stern J, Horwitz B, Harris R, Greene S. Longevity in obese and lean male and female rats of the Zucker strain: prevention of hyperphagia. Am J Clin Nutr. 1997;66(4):890–903. doi: 10.1093/ajcn/66.4.890. [DOI] [PubMed] [Google Scholar]