Abstract
Peripheral arterial diseases, the major complication of diabetes, can result in lower limb amputation. Since endothelial progenitor cells (EPCs) are involved in neovascularization, the aim of this study was to examine whether EPCs isolated from Wharton's jelly (WJ-EPCs) of the umbilical cord, a rich source of mesenchymal stem cells, could reduce ischemia-induced hind limb injury in diabetic mice. We evaluated the effects of WJ-EPC transplantation on hind limb injury caused by femoral artery ligation in mice with streptozotocin (STZ)-induced diabetes. We found that the ischemic hind limb in mice with STZ-induced diabetes showed decreased blood flow and capillary density and increased cell apoptosis and that these effects were significantly inhibited by an injection of WJ-EPCs. In addition, hypoxia-inducible factor-1α (HIF-1α) and interleukin-8 (IL-8) were highly expressed in transplanted WJ-EPCs in the ischemic skeletal tissues and were present at high levels in hypoxia-treated cultured WJ-EPCs. Moreover, incubation of the NOR skeletal muscle cell line under hypoxic conditions in conditioned medium from EPCs cultured for 16 h under hypoxic conditions resulted in decreased expression of pro-apoptotic proteins and increased expression of anti-apoptotic proteins. The inhibition of HIF-1α or IL-8 expression by EPCs using HIF-1α siRNA or IL-8 siRNA, respectively, prevented this change in expression of apoptotic-related proteins. Wharton's jelly in the umbilical cord is a valuable source of EPCs, and transplantation of these EPCs represents an innovative therapeutic strategy for treating diabetic ischemic tissues. The HIF-1α/IL-8 signaling pathway plays a critical role in the protective effects of EPCs in the ischemic hind limb of diabetic mice.
Introduction
The peripheral vascular disease, the most common type of diabetic vasculopathy, is the main cause of morbidity and disability in diabetic subjects [1]. Microvascular complications in diabetes are typically associated with dysregulation of vascular remodeling and vascular growth, with decreased responsiveness to ischemic/hypoxic stimuli, impaired or abnormal neovascularisation, and a lack of endothelial regeneration [2]. Thus, there is a need for therapeutic interventions aimed at accelerating repair of dysfunctional endothelial cells and restoring blood flow, resulting in functional tissue regeneration. The use of endothelial progenitor cells (EPCs) to improve lower limb ischemia has been suggested as an effectively therapy for diabetic foot disease [3]. Accumulating evidence indicates that transplantation of various bone marrow-derived cells, including mononuclear cells, EPCs, mesenchymal stem cells (MSCs), and hematopoietic stem cells, can restore blood flow in ischemic diseases [4]. However, MSCs are not usually obtained from the bone marrow because of the pain and morbidity associated with bone marrow biopsy and the decline in the number and plasticity of these cells with aging and cardiovascular diseases, resulting in reduced neovascularization and reduced therapeutic potential [5]. New alternative sources of MSCs are being examined to improve EPC function and optimize cell-based therapy. A new option is the use of Wharton's jelly from the umbilical cord. In our previous study, we successfully induced MSCs from Wharton's jelly to differentiate into EPCs (WJ-EPCs) and found that transplantation of WJ-EPCs after vascular injury effectively re-established endothelial integrity and decreased neointimal formation [6].
Numerous factors are thought to be involved in neovascularization and apoptosis, the crucial steps in curing tissue ischemia [7]. Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that mediates adaptive responses under conditions of ischemia/hypoxia in vitro and in vivo [8,9]. HIF-1α expression in bone marrow-derived angiogenic cells has been shown to mediate a series of metabolic responses to hypoxia that maintain energy, pH, and redox homeostasis in ischemic tissue [10]. Adeno-associated virus transduction of a stabilized form of HIF-1α was superior to transduction with vascular endothelial growth factor (VEGF) in stimulating angiogenesis in skeletal muscle [11]. Under ischemic/hypoxic conditions, HIF-1α subunits accumulate, translocate to the nucleus, and bind to the HIF-1β subunit, and then, the complex binds to hypoxia response elements in the promoters of various genes, such as that coding for interleukin-8 (IL-8), a member of the CXC chemokine family, activating their transcription [12]. IL-8, a proinflammatory cytokine, is responsible for recruitment of neutrophils and also acts as an angiogenic factor because it increases endothelial cell proliferation, capillary tube organization, and matrix metalloproteinase production [13]. These observations suggest that HIF-1α and IL-8 may play critical roles in angiogenesis in ischemic/hypoxic tissues. However, whether WJ-EPCs are protective against hind limb ischemia in a diabetic mouse model and, if so, whether HIF-1α and IL-8 are involved is not known. In the present study, we evaluated the effects of WJ-EPC transplantation on hind limb injury caused by femoral artery ligation in mice with streptozotocin (STZ)-induced diabetes. We also examined the effects on neovascularization and apoptosis of conditioned medium produced by WJ-EPCs under hypoxic conditions. Our results showed that WJ-EPC transplantation effectively restored blood flow and prevented ischemia-induced apoptosis via activation of the HIF-1α/IL-8 pathway. In this study, we demonstrated, for the first time, to our knowledge, that HIF-1α and IL-8 play important roles in the regulation of vasculogenesis and apoptosis in the ischemic hind limb.
Materials and Methods
The expanded methods are provided as an online supplement (Supplementary Data are available online at www.liebertpub.com/scd).
Results
EPC transplantation effectively restores blood flow and function after femoral artery ligation of the hind limb of diabetic mice
To induce moderate diabetes, male ICR mice (Bltw:CD-1, 8-weeks old, n=120) were injected intraperitoneally with 150 mg/Kg of STZ in 0.9% sterile saline, daily for 3 days [14]. Blood glucose levels in diabetic mice were 121±7, 373±40, 480±18, and 502±20 mg/dL on days 0, 3, 5, and 11, respectively. Age-matched mice receiving no STZ served as controls. Blood glucose levels were increased 3.3±0.4-fold on day 3, 3.9±0.2-fold on day 5, and 4.3±0.2-fold on day 11 in diabetic mice compared with non-diabetic ones.
Supplementary Fig. S1 shows the endothelial phenotypes of EPCs derived from Wharton's jelly of the human umbilical cord evaluated for expression of CD31, CD34, kinase insert domain receptor (KDR), von Willebrand factor (vWF), thrombomodulin (TM) and CD45 by immunocytochemistry (A), flow cytometry (B), and western blotting (C). The majority of EPCs, but not MSCs, expressed the EPC markers CD34 and KDR and the mature endothelial cell markers CD31, vWF, and TM; while neither MSCs nor EPCs expressed the common leukocyte antigen CD45. The characteristics of these cells were similar to those described for EPCs in our previous report [6], have the capacity to differentiate into mature endothelial cells.
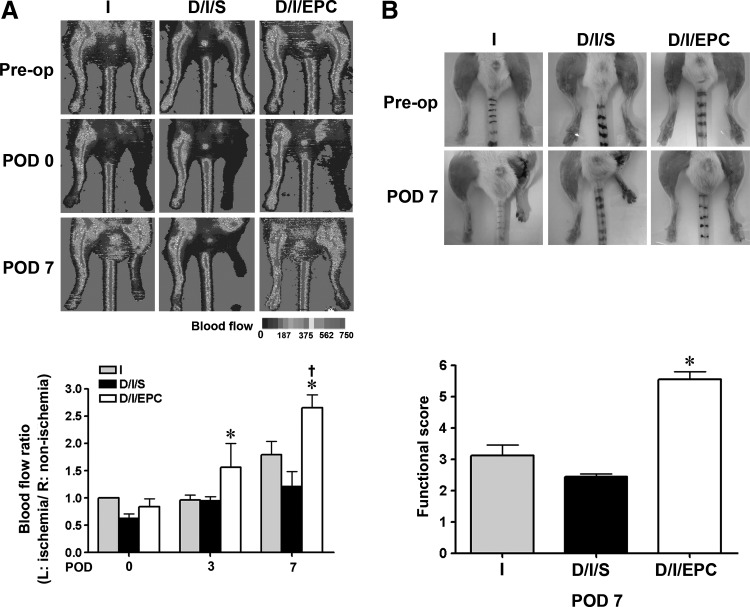
To examine whether EPCs had a protective effect on the ischemic limb of diabetic mice, STZ-treated mice that had undergone femoral artery ligation of the left hind limb at the end of 3 days of STZ initial injection were injected into the thigh muscle along the course of the femoral artery with 100 μL of saline (D/I/S group) or saline containing Q tracker-labeled EPCs (1×106) (D/I/EPC group), and blood flow was monitored over time by laser Doppler imaging; mice that had only undergone femoral artery ligation were also used as the ischemia control (group I). As shown in Fig. 1A, the laser Doppler perfusion images showed similar levels of blood flow in the 3 groups before ligation and a rapid drop in blood flow in the left hind limb after arterial ligation (POD 0), and blood flow remained low in the I and D/I/S groups at days 3 and 7 postligation, but was increased in the EPC-injected mice. An injection of EPCs significantly increased the ischemic (left)/non-ischemic (right) blood perfusion ratio by 2.3±0.5-fold compared with saline-injected diabetic ischemic mice at D7 after surgery. In addition, as shown in Fig. 1B, the morphology and the functional score of the left hind limb, measured by the Westvik method [14], showed that the ischemic leg in the saline-treated diabetic mice exhibited more severe gangrene and ulceration than the contralateral limb, but showed a significantly higher functional score in the EPC-treated group.
FIG. 1.
Endothelial progenitor cell (EPC) transplantation improves blood flow in hind-limb ischemia in diabetic mice. Control (I) or streptozotocin-injected diabetic mice (D/I) underwent left femoral artery ligation, and the diabetic mice were injected intramuscularly with saline (D/I/S) or EPC (D/I/EPC). (A) Laser Doppler perfusion images were taken before surgery (Pre-op) and immediately after surgery (POD 0) or at 7 days after surgery (POD 7). The upper panels show typical results, while the lower panel shows the mean blood flow rate expressed as the ratio of the value for the ischemic (left) hind limb to that of the contralateral (right) nonischemic hind limb compared with the ratio immediately after surgery (POD 0) in the I group. (B) Functional status of the ischemic hind limb at POD 7. The upper panels show representative photographs, and the lower panel depicts the statistical analysis of the functional score. n=8 per group, *P<0.05, compared with the D/I/S group. †, <0.05, compare with the D/I/EPC at POD 3 or at POD 0.
EPC transplantation improves muscular morphology in the ischemic hind limbs of diabetic mice
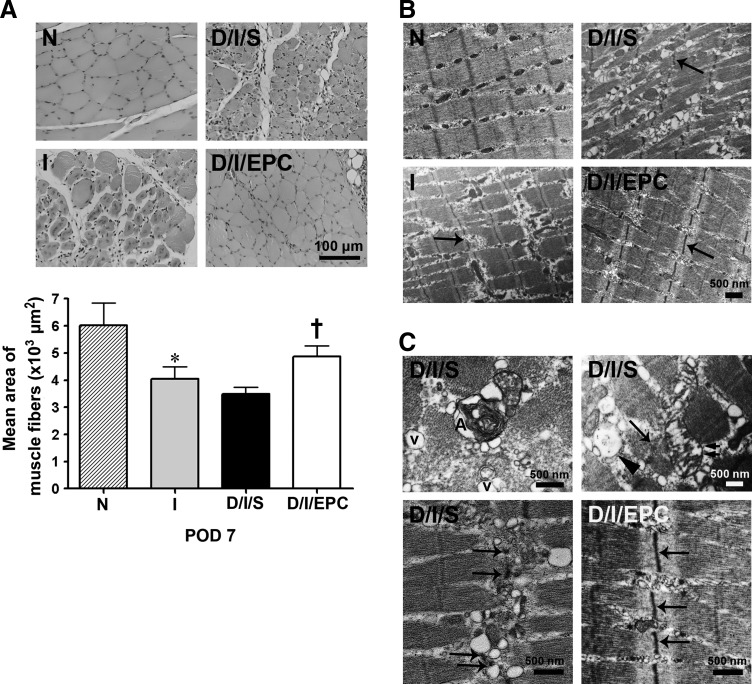
To determine what differences could account for the increased function of the hind limb after EPC treatment, we examined the morphology of the hind limb by hematoxylin-eosin staining and transmission electron microscopy. As shown in Fig. 2A, the ischemic diabetic limb showed a decreased mean muscle fiber area compared with the normal limb, and this effect was significantly decreased by EPC treatment. Ultrastructural analysis of skeletal muscle fibers by electron microscopy showed that the fibers in the ischemic group and D/I/S group were extensively disorganized compared with the normal group, and severe Z-disk damage associated with A-band disruption and misalignment of myofibrils was seen in both groups (Fig. 2B). At higher magnification, extensive dilation of the sarcoplasmic reticulum, disrupted mitochondria, large vacuoles between myofibrils and autophagosomes, and disrupted Z-disks were also observed (Fig. 2C). In the EPC-treated group, the ultrastructural morphology was improved compared with the ischemic diabetic group. These results suggest that EPC transplantation effectively reduced ischemia-induced hind limb injury in the diabetic mice.
FIG. 2.
EPC transplantation improves muscular morphology in the ischemic hind limb of diabetic mice on day 7. (A) Hematoxylin and eosin staining. The upper panels show representative photomicrographs of muscle fiber morphology of normal (N), ischemic (I), ischemic-diabetic-saline (I/D/S), and ischemic-diabetic-EPC (I/D/EPC) mice, while the lower panel shows the mean area of the muscle fibers n=4 per group. *P<0.05 for I versus N mice. †P<0.05 for D/I/EPC versus D/I/S mice. Scale bar=100 μm. (B) Electron micrographs showing extensive disorganization of the muscle fibers in the ischemic or ischemic diabetic groups compared with the normal group and less disorganization in the EPC-treated group. The arrows indicate damage to the Z-disc. (C) Higher magnification electron micrographs showing Z disk damage (arrow), large vacuoles (v), autophagosomes (A), disrupted mitochondria (double arrows), dilation of the sarcoplasmic reticulum (arrowhead), and dis-alignment of myofibrils in D/I/S mice. The Z disks (arrows) in the D/I/EPC group were improved better than those in the D/I/S. Scale bar=500 nm.
EPC transplantation stimulates neovascularization after femoral artery ligation of the hind limb of diabetic mice
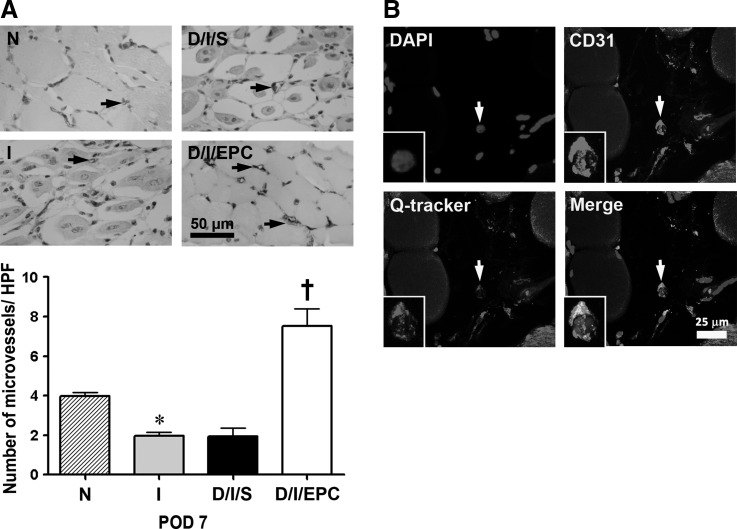
To determine whether the increased function after femoral limb ischemia in diabetic mice seen in EPC-transplanted mice reflected increased angiogenesis, we examined microvascular density in the ischemic limbs by immunostaining with anti-CD31 antibodies. As shown in Fig. 3A, after induction of limb ischemia, mice with or without diabetes showed a significant decrease in the number of microvessels compared with the normal group, whereas EPC transplantation resulted in an increase in numbers of ∼4-fold compared with those in the ischemic limb of saline-treated diabetic mice. Importantly, we found that EPCs were found adjacent to the muscle fibers and took part in capillary formation in the ischemic limb of the diabetic mice (Fig. 3B). These findings suggest that EPC transplantation increases neovascularization in the ischemic hind limb of diabetic mice.
FIG. 3.
EPC transplantation increases microvascular density on day 7 of hind-limb ischemia in diabetic mice. (A) The upper panels show representative photomicrographs of microvessel staining in normal (N), ischemic (I), diabetic, and ischemic saline-treated (D/I/S) and diabetic and ischemic EPC-treated (D/I/EPC) limbs on day 7. Limb muscles were fixed in 4% paraformaldehyde and embedded in paraffin; then, sections were incubated with anti-CD31 antibody to identify microvessels (arrows). The lower panels show the quantitative evaluation of microvascular density (number per high power field). Data are representative of 4 mice per group analyzing 5 fields from each tissue section per mouse. *P<0.05 for I versus N; †P<0.05 for D/I/EPC versus D/I/S. Scale bar=50 μm. (B) Confocal microscopy showing that Qtracker-labeled EPCs (CD31) are present in the skeletal muscle and form capillaries (arrow). Scale bar=25 μm.
EPC transplantation decreases cell apoptosis in the ischemic hind limb, and conditioned medium from hypoxia-treated EPCs increases expression of anti-apoptotic proteins in skeletal muscle cells subjected to hypoxia
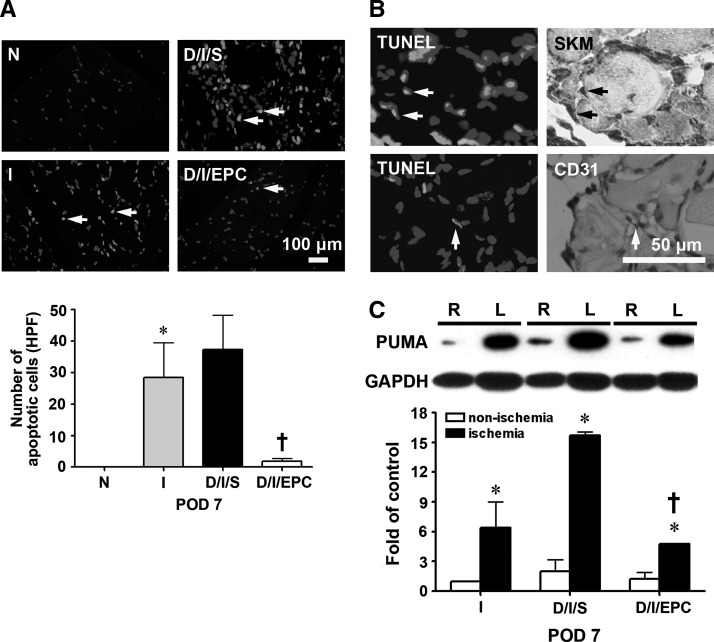
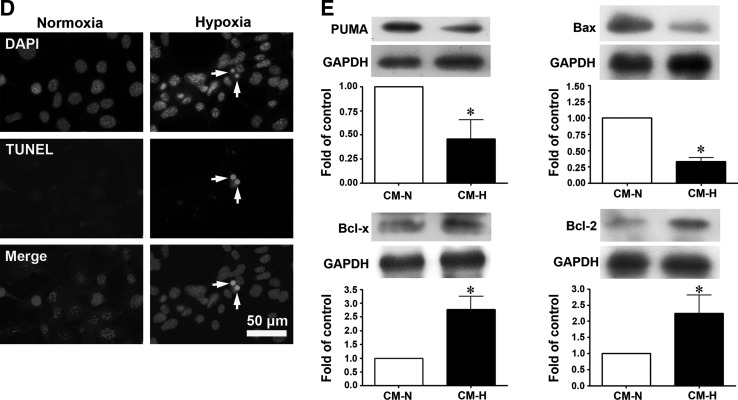
Since we found that EPC transplantation effectively reduced ischemia-induced hind limb injury in diabetic mice, we investigated whether this was due to decreased apoptosis of skeletal muscle cells. As shown in Fig. 4A, the number of terminal dUTP nick-end labelling (TUNEL)-positive cells per high power field in skeletal muscles was significantly increased in groups I and D/I/S compared with normal mice, and, as shown in Fig. 4B, on serial sections, the apoptotic cells colocalized with skeletal muscle cells stained with antibodies against skeletal muscle actin or with endothelial cells stained with anti-CD31 antibodies. EPC transplantation dramatically decreased the number of apoptotic cells in the ischemic hind limb of the diabetic mice (Fig. 4A). In addition, as shown in Fig. 4C, western blotting demonstrated that expression of p53 up-regulated modulator of apoptosis (PUMA), a pro-apoptotic protein, was significantly increased in the ischemic left hind limb in these 3 groups compared with the contralateral nonischemic limb, but the increase was much lower in the EPC-treated diabetic group than in the saline-treated diabetic mice.
FIG. 4.
EPC transplantation decreases cell apoptosis in the ischemic hind limb of diabetic mice on day 7, and conditioned medium from hypoxia-treated EPCs increases the expression of anti-apoptotic proteins in NOR skeletal muscle cells under hypoxic conditions. (A) Detection of apoptosis by terminal dUTP nick-end labeling (TUNEL) staining in the normal, I, D/I/S, and D/I/EPC groups. The upper panels show a typical result, and the lower panel shows the number of apoptotic cells per high power field (HPF). n=4 per group. *P<0.05 for I versus N; †P<0.05 for D/I/EPC versus D/I/S. Scale bar=100 μm. (B) Overlap of TUNEL-positive cells and skeletal muscle cell or endothelial cell staining on serial sections. Scale bar=50 μm. (C) p53 up-regulated modulator of apoptosis (PUMA) expression in the ischemic (left, L) and non-ischemic (right, R) hind limb in the I, D/I/S, and D/I/EPC groups determined by western blotting. n=3 *P<0.5 for the ischemic limb versus the non-ischemic limb, †P<0.5 for D/I/EPC versus D/I/S. (D) NOR cells, a skeletal cell line, were exposed to normal conditions or hypoxia for 16 h; then, cell apoptosis (arrows) was examined by TUNEL staining; the results shown are representative of those seen in 3 experiments. Scale bar=50 μm. (E) NOR cells were incubated under hypoxic conditions for 16 h with conditioned medium collected from cultured EPCs subjected to 16 h of hypoxia (CM-H) or normoxia (CM-N); then, proteins were extracted from the cells; and the expression of pro-apoptotic proteins (PUMA and Bax) and anti-apoptotic proteins (Bcl-x and Bcl-2) was evaluated by western blotting. n=3 per group, *P<0.05 for CM-H versus CM-N.
To study the mechanisms involved in the protective effects induced by EPCs, NOR cells, a skeletal cell line, were used as an in vitro cell model. When NOR cells were exposed to hypoxia for 16 h, apoptosis was induced, as shown by TUNEL staining (Fig. 4D). When NOR cells were incubated under hypoxic conditions for 16 h with conditioned medium from EPCs subjected to hypoxia for 16 h (CM-H), expression of the pro-apoptotic proteins Bax and PUMA was decreased compared with that seen in NOR cells incubated with conditioned medium from EPCs incubated for 16 h in normoxic conditions (CM-N), whereas expression of the anti-apoptotic proteins Bcl-x and Bcl-2 was increased. These results suggest that cytokines released from hypoxia-treated EPCs protect skeletal muscle cells from undergoing apoptosis under hypoxic conditions.
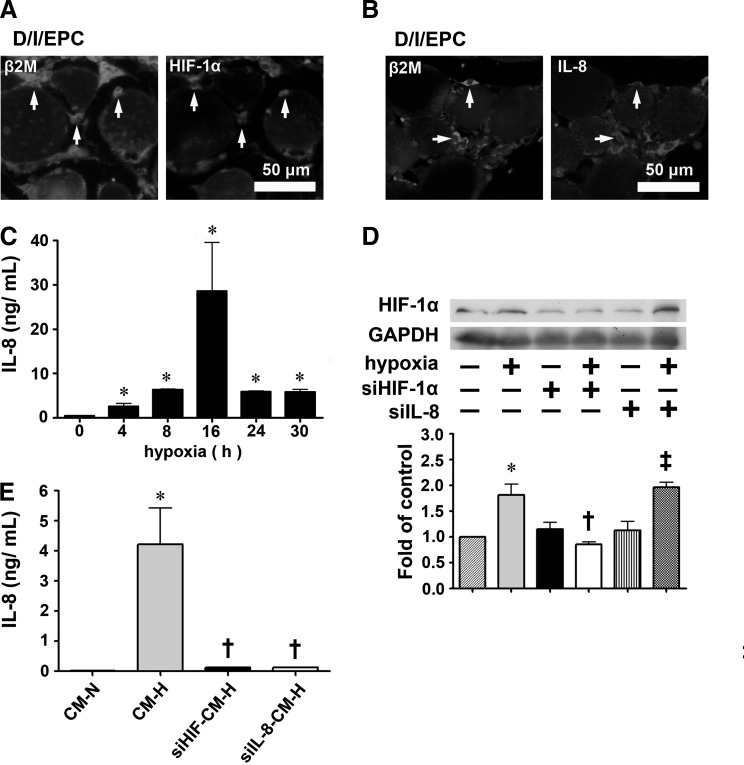
The reduced femoral artery ligation-induced injury of the hind limb in diabetic mice after EPC transplantation involves the HIF-1α/IL-8 pathway
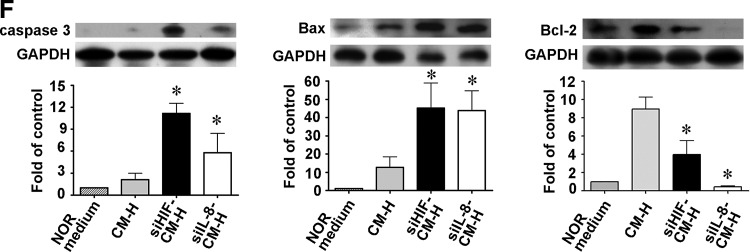
The HIF-1α/IL-8 pathway plays an important role in cell survival [10,13]. Immunostaining of EPCs in the injury site after femoral artery ligation of the hind limb of diabetic mice showed strong expression of HIF-1α (Fig. 5A) and IL-8 (Fig. 5B). Furthermore, the conditioned medium from EPCs cultured for 16 h under hypoxic conditions (CM-H) was found to contain large amounts of secreted IL-8, as shown in both a multiplex immunoassay (Fig. 5C) and ELISA (Fig. 5E), and these hypoxic conditions were shown by western blotting to significantly increase HIF-1α expression by EPCs (Fig. 5D). EPCs incubated under hypoxic conditions for 16 h showed significantly high levels of secreted IL-8 than those incubated under normoxic conditions (28.6±11.0 ng/mL vs. 0.05±0.01 ng/mL). In contrast, IL-8 levels in conditioned medium from human umbilical vein endothelial cells (HUVECs) under hypoxic conditions were slightly higher than those under normoxic conditions (1.80±0.15 ng/mL vs. 0.05±0.01 ng/mL). To examine whether the HIF-1α/IL-8 pathway mediated the protective effects of EPCs on skeletal muscles under hypoxic conditions, siRNA was used to knock down HIF-1α or IL-8 expression. When cells transfected with siHIF-1α RNA underwent hypoxia treatment, the cells showed decreased HIF-1α expression (Fig. 5D) and IL-8 secretion (Fig. 5E), whereas transfection with siIL-8 RNA followed by hypoxia treatment also resulted in decreased IL-8 secretion (Fig. 5E), but had no effect on HIF-1α expression (Fig. 5D). When NOR cells were incubated for 16 h under hypoxic conditions with conditioned medium from EPCs transfected with either siHIF-1α or siIL-8 RNA and subjected to hypoxia treatment for 16 h (siHIF-CM-H or siIL-8-CM-H), the cells showed increased expression of the pro-apoptotic proteins caspase 3 and Bax and decreased expression of the anti-apoptotic protein Bcl-2 compared with cells incubated with conditioned medium from normal EPCs subjected to hypoxia (CM-H) (Fig. 5F).
FIG. 5.
Wharton's jelly-derived endothelial progenitor cells (WJ-EPCs) in the ischemic hind limb strongly express hypoxia-inducible factor (HIF)-1α and interleukin-8 (IL-8) in vivo and in vitro, and these cytokines affect the expression of apoptosis-related proteins in NOR skeletal muscle cells incubated under hypoxic conditions. (A, B) Expression of HIF-1α (A) and IL-8 (B) co-localized with that of human-specific β2-microglobulin (β2M) in EPCs in the ischemic hind limb of diabetic mice after WJ-EPC injection, as shown by immunofluorescent staining of paraffin serial sections. Scale bar=50 μm. (C) IL-8 levels in conditioned medium from EPCs subjected to hypoxia for the indicated time evaluated by multiplex immunoassay. n=3 per group. *P<0.05 compared with the control group. Scale bar=50 μm. (D) EPCs (1×104 cells) were left untreated or were transfected with HIF-1α siRNA or IL-8 siRNA for 72 h, then subjected to normoxia or hypoxia for 16 h, and then levels of HIF-1α in the cell lysates were evaluated by western blotting. *P<0.05 for hypoxia versus normoxia; †P<0.05 for siHIF-hypoxia versus siHIF-normoxia; ‡P<0.05 for siIL-8-hypoxia versus siIL-8-normoxia. (E) EPCs (1×104 cells) were transfected with HIF-1α siRNA or IL-8 siRNA for 72 h, and were then subjected to hypoxia for 16 h, when IL-8 levels in the conditioned medium were evaluated by ELISA. The conditioned media from EPCs with normoxia treatment for 16 h were used as the control. Data are expressed as the mean±SEM, n=3 per group. *P<0.05 for hypoxia versus normoxia; †P<0.05 for siHIF-hypoxia or siIL8-hypoxia versus hypoxia. (F) NOR cells, a skeletal cell line, were exposed to hypoxia for 16 h in the presence of conditioned medium from EPCs subjected to hypoxia for 16 h (CM-H) or from transfected EPCs treated identically (siHIF-CM-H and siIL-8-CM-H) or were cultured in NOR medium under normoxic conditions for 16 h as a control; then, levels of apoptosis-related proteins in the cell lysates were evaluated by western blotting. n=3 per group. *P<0.05 for siHIF- or siIL8-hypoxia versus hypoxia. SEM, standard error of the mean.
HIF-1α and IL-8 increase migration and vascular tube formation of EPCs
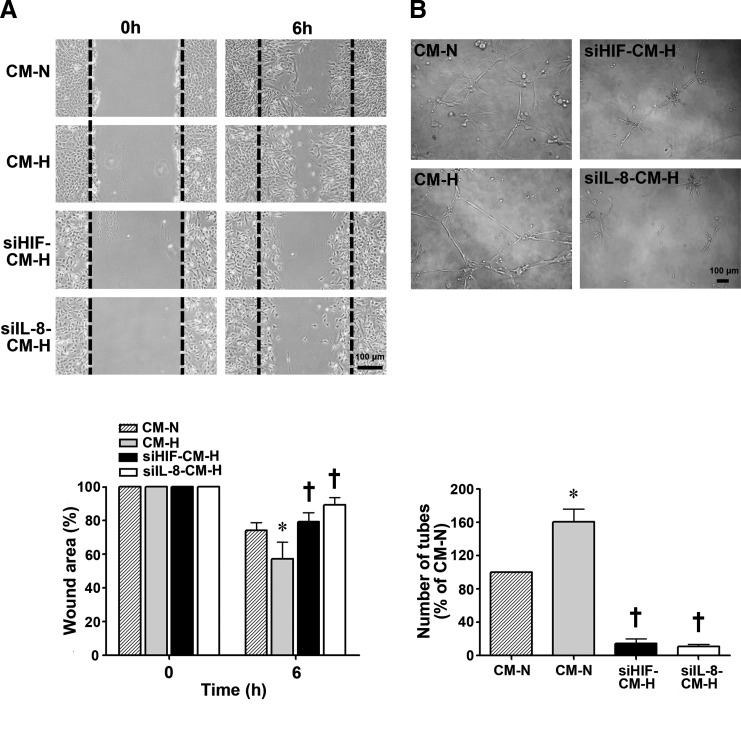
Previous studies have demonstrated that HIF-1α and IL-8 are chemoattractive factors for endothelial cells [15,16]. To examine in detail the role of HIF-1α and IL-8 in EPC migration to the injury site, an in vitro model of EPC wound repair was used in which a 350 μm wound was inflicted in an EPC monolayer using a sterile pipette tip. As shown in Fig. 6A, the number of EPCs that migrated into the wound area under normoxic conditions in 6 h was significantly increased by incubation in CM-H compared with incubation in CM-N. In contrast, incubation of EPCs with siHIF-CM-H or siIL-8-CM-H significantly reduced cell migration.
FIG. 6.
Effect of conditioned medium from hypoxia-treated EPCs on EPC migration and capillary-like structure formation in Matrigel. (A) The effect of different conditioned media on EPC migration was tested in a wound-healing assay in which a wound was produced in an EPC monolayer, and the cells were incubated for 6 h under normoxic conditions with CM-N, CM-H, siHIF-CM-H, or siIL-8-CM-H conditioned medium. The upper panels show a typical result, and the lower panel shows the statistical results for 4 different experiments. *P<0.05 for CM-H versus CM-N. †P<0.05 for siHIF-CM-H or siIL-8-CM-H versus CM-H. Scale bar=100 μm. (B) Effect of different conditioned media on the ability of EPCs to form capillary-like structures in Matrigel. EPCs in Matrigel were incubated under normoxic conditions for 16 h with CM-N, CM-H, siHIF-CM-H, or siIL-8-CM-H; then, the number of tubes per high power field was counted. n=3 per group, *P<0.05 for CM-H versus CM-N. †P<0.001 for siHIF-CM-H or siIL-8-CM-H versus CM-H. Scale bar=100 μm.
Next, we examined the effect of EPCs on vascular tube formation and whether the effect was attributable to HIF-1α and IL-8 by incubating EPCs under normoxic conditions in the Matrigel assay with different conditioned medium. As shown in Fig. 6B, cells incubated with CM-H assembled into primitive vascular tube-like structures when plated on Matrigel plates, but far fewer tubes were seen using siHIF-CM-H or siIL-8-CM-H.
Together, these results show that HIF-1α and IL-8 are involved in the effects of EPCs on the migration and capillary-tube formation of EPCs under normoxic conditions.
Discussion
In the present study, we found that local transplantation of EPCs derived from Wharton's jelly (WJ-EPCs) improved various parameters of the ischemic hind limb in diabetic mice and rescued blood flow, recovered limb function, and decreased muscular injury and apoptosis. Transplantation of EPCs also increased microvascular density, and the EPCs were incorporated into vessel structures. Furthermore, the transplanted EPCs in the skeletal muscle strongly expressed HIF-1α and IL-8, and the cultured EPCs subjected to hypoxia for 16 h contained large amounts of both factors. The conditioned medium from hypoxia-treated EPCs was shown to alter the expression of apoptosis-related proteins on skeletal muscles and increased migration of, and tube formation by, EPCs through HIF-1α and IL-8 expression. Based on these findings, WJ-EPCs show great promise as a treatment for diabetic ischemia–induced vascular and skeletal muscle dysfunction.
The number and function of circulating EPCs are profoundly lowered in diabetic patients with peripheral arterial disease [17]. Although bone marrow and peripheral blood are the major natural sources of EPCs for the restoration of blood flow in ischemic diseases, the use of cells derived from these sources as therapy is not always possible for allogenic transplantation because of the invasive procedures used for aspiration, a high degree of viral infection, and the significant drop in cell numbers and proliferative/differentiation capacity with age [18]. However, cord blood and umbilical cord can be considered alternative sources of EPCs for experimental and clinical use. Although cord blood has many advantages, the number of nucleated cells is limited, and this is considered a seriously weak point in routine application for transplantation [19]. However, the stromal cells in Wharton's jelly have been shown to differentiate into diverse cell types under various culture conditions [20–22]. These cells show a higher proliferative potential and hypo-immunogenicity than do bone marrow-derived cells [23]. In our previous study, we described a simple method for the isolation and expansion of MSCs from Wharton's jelly and their differentiation into EPCs and demonstrated that transplantation of these cells not only accelerates re-endothelialisation, but also profoundly inhibits neointimal hyperplasia after vascular injury [6]. In the present study, our results demonstrated that blood flow in the ischemic hind limb of diabetic mice was improved by transplantation of EPCs, as in a previous report showing that WJ-EPCs can restore blood flow in a hind limb ischemia mouse model [24]. Moreover, our results demonstrated that skeletal muscle atrophy, ultrastructural changes, and apoptosis were reduced by EPC transplantation. These cells may, therefore, prove to be a new source of cells for cell therapies for the repair of stromal tissue and, potentially, the endothelium, thus avoiding the ethical and technical issues involved in the use of cells from other origins.
Therapeutic neovascularization is an important strategy for salvaging tissue from critical ischemia [25]. The present study demonstrated that EPC transplantation increased neovascularisation and blood flow in the ischemic hind limb of diabetic mice. Our results support those in a previous study by Kawamoto et al. [26], who showed that transplantation of EPCs derived from the peripheral blood of healthy human adults induces significant neovascularization in the heart during coronary artery ligation-induced myocardial ischemia. Our in vivo study further showed that transplanted WJ-EPCs were formed into capillaries and incorporated into the capillary networks in the preserved skeletal muscle cells in the ischemic limb of diabetic mice. In addition, MSCs from Wharton's jelly of the umbilical cord express more angiogenesis-related genes, such as the VEGF gene, than those from bone marrow [27]. Considering the fact that Wharton's jelly is a much more robust source of EPCs than adult peripheral blood, Wharton's jelly can serve as a novel and important source of EPCs.
The underlying mechanisms of the protective effects of WJ-EPCs on the ischemia limb of diabetic mice were then studied. Recent evidence suggests that neovascularization by stem cells does not depend solely on the homing and engraftment of the administered cells, but also involves paracrine effects and the local secretion of cytokines by these cells [28]. A previous study showed that conditioned medium from cultures of amniotic liquid-derived stem cells containing angiogenic factors, such as MCP-1, IL-8, SDF-1, and VEGF, can stimulate neo-arteriogenesis in a preclinical model of ischemic hind-limb mice [29]. The hypoxia-mediated increase in IL-8 gene expression in human microvascular endothelial cells involves HIF-1α expression, and the hypoxic response element has been identified in the IL-8 promoter [16]. Our immunofluorescent staining results directly demonstrated that the transplanted EPCs in the skeletal muscle in the ischemic limb of diabetic mice strongly expressed HIF-1α and IL-8. In addition, the conditioned medium collected from WJ-EPCs subjected to hypoxia for 16 h was found to contain large amounts of IL-8 as compared with that from cells grown under normal conditions. Importantly, the amount of IL-8 in the conditioned medium was significantly higher than that in conditioned medium from HUVECs with or without hypoxia treatment. Moreover, the hypoxia-induced IL-8 expression was blocked by siRNA for HIF-1α, indicating the role of HIF-1α in the regulation of IL-8 expression. These results show that EPCs incubated under hypoxic conditions express significantly high levels of HIF-1α and IL-8, that this is closely associated with neovascularisation, and that HIF-1α mediates the up-regulation of IL-8 in EPCs.
Neovascularization is a multistep process including endothelial cell proliferation and migration and capillary tube formation that are mediated by angiogenic factors, such as IL-8 [29,30]. Recombinant human IL-8 has been shown to induce endothelial cell proliferation and capillary tube organization, and neutralization of IL-8 with anti-IL-8 antibody blocks IL-8-mediated capillary tube organization in vitro [13]. In addition, transcription factor HIF-1α, which regulates the expression of the IL-8 gene, is involved in bone marrow-derived angiogenic cell migration to ischemic sites [9]. HIF-1α expression plays a master role in many crucial physiological processes, such as angiogenesis, cell migration, growth, and apoptosis [7,15]. Recent studies have demonstrated that ischemia-induced HIF-1α activation and the subsequent recruitment of bone marrow-derived angiogenic cells are impaired by aging and diabetes, and that this impairment can be overcome by local intramuscular injection of AdCA5, a recombinant adenovirus encoding a constitutively active form of the HIF-1α subunit, resulting in improved recovery of blood flow and prevention of tissue damage after artery ligation of young and middle-aged mice [31,32]. Combined HIF-1-based gene and bone marrow-derived angiogenic cells therapy was found to reduce tissue necrosis [10]. In addition, destabilization of HIF-1 is most likely the event that transduces hyperglycemia into the loss of the cellular response to hypoxia in most diabetic complications [33]. Local transfer of HIF constructs demonstrated that stabilization of HIF-1α is necessary and sufficient for promoting wound healing in diabetic mice [34]. In the present study, we observed that conditioned medium obtained from hypoxia-treated EPCs stimulated tubular formation and migration of EPCs and that these effects were not seen with conditioned medium from EPCs transfected with siHIF-1α or siIL-8 RNA, suggesting that HIF-1α and IL-8 promote vascularization by directly interacting with endothelial cells. Since angiogenesis is essential for the therapy of ischemia in a diabetic environment, these data suggest that HIF-1α and IL-8 from WJ-EPCs play an autocrine/paracrine role in angiogenesis.
A recent report suggested that, in addition to vascularization, cell survival is a novel therapeutic target for ischemia [35]. The effects of bone marrow-derived MSCs in protecting cardiomyocytes against ischemic/hypoxic injury might be related to a paracrine mechanism involving the release of a wide range of cytokines [36]. Bone marrow-derived stromal cells expressing an adenovirus-born active form of HIF-1α protect ischemic cardiomyocytes against CoCl2-induced apoptosis [36]. Treatment of bone marrow-derived angiogenic cells with dimethyloxalylglycine, an α-ketoglutarate antagonist that induces HIF-1α activity, has significant survival advantage under conditions of low O2 and low pH ex vivo and in ischemic tissues [10]. IL-8 and its receptors, CXCR1 and CXCR2, are expressed on microvascular endothelial cells and have been shown to play a role in endothelial cell survival and proliferation [37,38]. CXCR2 was also found in human skeletal muscles [39]. A previous study demonstrated that addition of IL-8 to cultured HUVECs significantly enhances survival and inhibits cell apoptosis by increasing the expression of anti-apoptotic genes [13]. The present study demonstrated that incubation of NOR skeletal muscle cells under hypoxic conditions with conditioned medium from hypoxia-treated EPCs decreased expression of the pro-apoptotic proteins Bax and PUMA and increased expression of the anti-apoptotic proteins Bcl-x and Bcl-2 compared with values in NOR cells incubated with conditioned medium from normoxia-treated EPCs. In contrast, conditioned medium from EPCs transfected with siHIF-1α or siIL-8 RNA subjected to hypoxia attenuated Bcl-2 expression and increased caspase 3 and Bax expression in NOR skeletal muscle cells under hypoxic conditions. These data suggest that the protective effects of WJ-EPCs against hypoxia/ischemia-induced apoptosis of skeletal muscle cells are related to HIF-1α/IL-8 expression and that these factors regulate the expression of apoptosis-related proteins. Since cell survival is essential for the therapy of ischemia, these data suggest that HIF-1 and IL-8 from EPCs play a master role in cell survival during hypoxia/ischemia and that HIF-1α/IL-8 might regulate angiogenesis by modulating the endothelial cell anti-apoptosis pathway. Whether HIF-1α/IL-8 from EPC affected the anti-necrosis pathway requires further study.
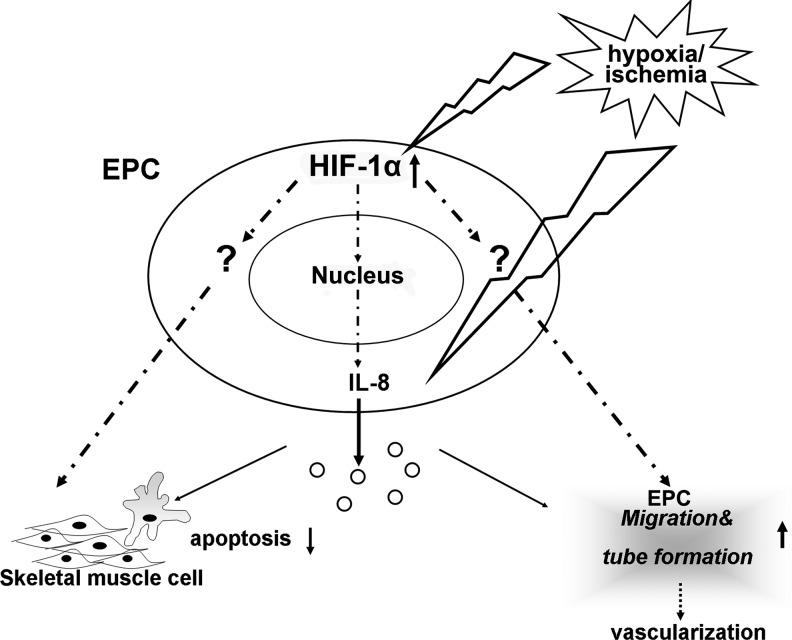
In conclusion, an intramuscular injection of EPCs is an effective route for cell transplantation for promoting revascularization and functional recovery of the ischemic limb. At the molecular level, this effect is due, at least in part, to HIF-1α and L-8 expressed by EPCs, which enhance the migration of EPCs and capillary formation and reduce cell apoptosis. Based on reports in the literature and our findings, Fig. 7 depicts a model for the mechanisms underlying the protective effects of EPCs from Wharton's jelly of the umbilical cord on the ischemic hind limb of diabetic mice. HIF-1α and IL-8 released from EPCs play an important role in inhibiting apoptosis and promoting angiogenesis in hind-limb injury and may represent a new and promising strategy for clinical application designed to re-vascularize ischemic tissues.
FIG. 7.
Schematic diagram showing the involvement of the HIF-1α/IL-8 pathway in the protective effects of WJ-EPCs on ischemic limb via neovascularization and inhibition of apoptosis.
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Science Council (NSC 99-2320-13-002-022-MY3) and from the National Taiwan University Hospital (UN101-025).
Authors Disclosure Statement
None to declare.
References
- 1.Miettinen H. Salomaa V. Diabetes and macrovascular disease. Coron Artery Dis. 1996;7:708–714. doi: 10.1097/00019501-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Liu ZJ. Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869–1882. doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgescu A. Alexandru N. Constantinescu A. Titorencu I. Popov D. The promise of EPC-based therapies on vascular dysfunction in diabetes. Eur J Pharmacol. 2011;669:1–6. doi: 10.1016/j.ejphar.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Shintani S. Murohara T. Ikeda H. Ueno T. Sasaki K. Duan J. Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 5.Ballard VL. Edelberg JM. Stem cells and the regeneration of the aging cardiovascular system. Circ Res. 2007;100:1116–1127. doi: 10.1161/01.RES.0000261964.19115.e3. [DOI] [PubMed] [Google Scholar]
- 6.Wang SH. Lin SJ. Chen YH. Lin FY. Shih JC. Wu CC. Wu HL. Chen YL. Late outgrowth endothelial cells derived from Wharton jelly in human umbilical cord reduce neointimal formation after vascular injury: involvement of pigment epithelium-derived factor. Arterioscler Thromb Vasc Biol. 2009;29:816–822. doi: 10.1161/ATVBAHA.109.184739. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P. Dor Y. Herbert JM. Fukumura D. Brusselmans K. Dewerchin M. Neeman M. Bono F. Abramovitch R, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A. Brixius K. Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–136. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- 9.Rey S. Lee K. Wang CJ. Gupta K. Chen S. McMillan A. Bhise N. Levchenko A. Semenza GL. Synergistic effect of HIF-1α gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci U S A. 2009;106:20399–20404. doi: 10.1073/pnas.0911921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rey S. Luo W. Shimoda LA. Semenza GL. Metabolic reprogramming by HIF-1 promotes the survival of bone marrow-derived angiogenic cells in ischemic tissue. Blood. 2011;117:4988–4998. doi: 10.1182/blood-2010-11-321190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajusola K. Künnapuu J. Vuorikoski S. Soronen J. André H. Pereira T. Korpisalo P. Ylä-Herttuala S. Poellinger L. Alitalo K. Stabilized HIF-1α is superior to VEGF for angiogenesis in skeletal muscle via adeno-associated virus gene transfer. FASEB J. 2005;19:1365–1367. doi: 10.1096/fj.05-3720fje. [DOI] [PubMed] [Google Scholar]
- 12.Fang HY. Hughes R. Murdoch C. Coffelt SB. Biswas SK. Harris AL. Johnson RS. Imityaz HZ. Simon MC, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li A. Dubey S. Varney ML. Dave BJ. Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 14.Westvik TS. Fitzgerald TN. Muto A. Maloney SP. Pimiento JM. Fancher TT. Magri D. Westvik HH. Nishibe T. Velazquez OC. Dardik A. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg. 2009;49:464–473. doi: 10.1016/j.jvs.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsythe JA. Jiang BH. Iyer NV. Agani F. Leung SW. Koos RD. Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KS. Rajagopal V. Gonsalves C. Johnson C. Kalra VK. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J Immunol. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP. Sartore S. Schiavon M. Albiero M. Baesso I. Cabrelle A. Agostini C. Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006;49:3075–3084. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- 18.Scheubel RJ. Zorn H. Silber RE. Kuss O. Morawietz H. Holtz J. Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Tse W. Laughlin M. Cord blood transplantation in adult patients. Cytotherapy. 2005;7:228–242. doi: 10.1080/14653240510027154. [DOI] [PubMed] [Google Scholar]
- 20.Romanov YA. Svintsitskaya VA. Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 21.Wang HS. Hung SC. Peng ST. Huang CC. Wei HM. Guo YJ. Fu YS. Lai MC. Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 22.Fu YS. Cheng YC. Lin MY. Cheng H. Chu PM. Chou SC. Shih YH. Ko MH. Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 23.Anzalone R. Lo Iacono M. Corrao S. Magno F. Loria T. Cappello F. Zummo G. Farina F. La Rocca G. New emerging potentials for human Wharton's jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010;19:423–438. doi: 10.1089/scd.2009.0299. [DOI] [PubMed] [Google Scholar]
- 24.Wu KH. Zhou B. Lu SH. Feng B. Yang SG. Du WT. Gu DS. Han ZC. Liu YL. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608–616. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- 25.Yanagisawa-Miwa A. Uchida Y. Nakamura F. Tomaru T. Kido H. Kamijo T. Sugimoto T. Kaji K. Utsuyama M. Kurashima C. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science. 1992;257:1401–1403. doi: 10.1126/science.1382313. [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto A. Gwon HC. Iwaguro H. Yamaguchi JI. Uchida S. Masuda H. Silver M. Ma H. Kearney M. Isner JM. Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh JY. Fu YS. Chang SJ. Tsuang YH. Wang HW. Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton's jelly of umbilical cord. Stem Cells Dev. 2010;19:1895–1910. doi: 10.1089/scd.2009.0485. [DOI] [PubMed] [Google Scholar]
- 28.Gnecchi M. Zhang Z. Ni A. Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teodelinda M. Michele C. Sebastiano C. Ranieri C. Chiara G. Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischemic model. Biomaterials. 2011;32:3689–3699. doi: 10.1016/j.biomaterials.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 30.Kebir A. Harhouri K. Guillet B. Liu JW. Foucault-Bertaud A. Lamy E. Kaspi E. Elganfoud N. Vely F, et al. CD146 short isoform increases the proangiogenic potential of endothelial progenitor cells in vitro and in vivo. Circ Res. 2010;107:66–75. doi: 10.1161/CIRCRESAHA.109.213827. [DOI] [PubMed] [Google Scholar]
- 31.Bosch-Marce M. Okuyama H. Wesley JB. Sarkar K. Kimura H. Liu YV. Zhang H. Strazza M. Rey S, et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar K. Fox-Talbot K. Steenbergen C. Bosch-Marcé M. Semenza GL. Adenoviral transfer of HIF-1α enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci U S A. 2009;106:18769–18774. doi: 10.1073/pnas.0910561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bento CF. Pereira P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia. 2011;54:1946–1956. doi: 10.1007/s00125-011-2191-8. [DOI] [PubMed] [Google Scholar]
- 34.Botusan IR. Sunkari VG. Savu O. Catrina AI. Grünler J. Lindberg S. Pereira T. Ylä-Herttuala S. Poellinger L. Brismar K. Catrina SB. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z. von Ballmoos MW. Faessler D. Voelzmann J. Ortmann J. Diehm N. Kalka-Moll W. Baumgartner I. Di Santo S. Kalka C. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211:103–109. doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y. Sun A. Xue J. Feng C. Li J. Wu J. Bone marrow derived stromal cells modified by adenovirus-mediated HIF-1α double mutant protect cardiac myocytes against CoCl2-induced apoptosis. Toxicol In Vitro. 2009;23:1069–1075. doi: 10.1016/j.tiv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Koch AE. Polverini PJ. Kunkel SL. Harlow LA. DiPietro LA. Elner VM. Elner SG. Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch C. Monk PN. Finn A. Cxc chemokine receptor expression on human endothelial cells. Cytokine. 1999;11:704–712. doi: 10.1006/cyto.1998.0465. [DOI] [PubMed] [Google Scholar]
- 39.Frydelund-Larsen L. Penkowa M. Akerstrom T. Zankari A. Nielsen S. Pedersen BK. Exercise induces interleukin-8 receptor (CXCR2) expression in human skeletal muscle. Exp Physiol. 2007;92:233–240. doi: 10.1113/expphysiol.2006.034769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.