Abstract
The bone marrow (BM) microenvironment has clearly been implicated in the pathogenesis of B-cell chronic lymphocytic leukemia (B-CLL). However, the potential involvement of BM stromal progenitors, the mesenchymal stem cells (MSCs), in the pathophysiology of the disease has not been extensively investigated. We expanded in vitro BM-MSCs from B-CLL patients (n=11) and healthy individuals (n=16) and comparatively assessed their reserves, proliferative potential, differentiation capacity, and immunoregulatory effects on T- and B-cells. We also evaluated the anti-apoptotic effect of patient-derived MSCs on leukemic cells and studied their cytogenetic characteristics in comparison to BM hematopoietic cells. B-CLL-derived BM MSCs exhibit a similar phenotype, differentiation potential, and ability to suppress T-cell proliferative responses as compared with MSCs from normal controls. Furthermore, they do not carry the cytogenetic abnormalities of the leukemic clone, and they exert a similar anti-apoptotic effect on leukemic cells and healthy donor-derived B-cells, as their normal counterparts. On the other hand, MSCs from B-CLL patients significantly promote normal B-cell proliferation and IgG production, in contrast to healthy-donor-derived MSCs. Furthermore, they have impaired reserves, defective cellular growth due to increased apoptotic cell death and exhibit aberrant production of stromal cell-derived factor 1, B-cell activating factor, a proliferation inducing ligand, and transforming growth factor β1, cytokines that are crucial for the survival/nourishing of the leukemic cells. We conclude that ex vivo expanded B-CLL-derived MSCs harbor intrinsic qualitative and quantitative abnormalities that may be implicated in disease development and/or progression.
Introduction
B-cell chronic lymphocytic leukemia (CLL), the most common type of leukemia in adults, is characterized by the neoplastic transformation of mature B-cells that express the typical B-cell markers CD19, CD20, and CD23 as well as the T-cell associated marker CD5 [1]. CLL is thought to develop in specialized niches in the bone marrow (BM) and secondary lymphoid organs, within which bi-directional interactions between CLL and nonmalignant accessory cells favor tumor growth and progression [2]. The dependence of leukemic B-cells on microenvironment signals is further highlighted by the observation that despite their prolonged survival in vivo [2], they undergo spontaneous apoptosis when cultured ex vivo [2]. Interestingly, a variety of stromal cell lines have been shown to enhance the survival of CLL cells in co-culture experiments [3–10] and even protect them from drug-induced apoptosis [9,11–14].
The BM microenvironment consists of a heterogeneous population of cells, of both mesenchymal and hematopoietic origin, which produce the extracellular matrix and soluble factors and also provide the cellular surface, collectively regulating the growth, proliferation, and differentiation of hematopoietic stem cells and their progeny throughout life [15]. The mesenchymal cell population originates from a rare population of multipotent progenitor cells, currently referred to as mesenchymal stem/stromal cells (MSCs)[15–17]. Given that MSCs have been shown to modulate not only normal B-cell proliferation and differentiation [18,19] but also the tumor growth [20], one could speculate that potential abnormalities in patient MSCs might contribute to the pathophysiology of CLL by providing malignant cells with aberrant growth/survival signals. Likewise, in view of the well-established immunoregulatory potential of BM MSCs on T-cells [18,19], it could be hypothesized that the common T-cell dysfunction in CLL [21,22] might be attributed to deregulated immunomodulatory effects exerted by BM MSCs.
Data on the possible role of human BM MSCs in the pathophysiology of CLL are rather limited [8,9,23,24], and the question as to whether patient MSCs differ from their normal counterparts has not been properly answered. In the current study, we investigate whether intrinsic defects might exist within the BM MSC compartment of CLL patients by evaluating the reserves and qualitative characteristics in terms of their proliferative potential, differentiation capacity, and immunoregulatory effects on B-cells and T-cell proliferation, in comparison to normal individuals. We have also explored the anti-apoptotic effect of patient-derived MSCs on leukemic cells, and we have finally studied their cytogenetic characteristics in comparison to BM hematopoietic cells.
Patients and Methods
Patients
We have studied 11 patients with CLL and 16 age- and sex-matched healthy individuals. Detailed patient characteristics are shown in Table 1. Institutional ethics committee approval was granted before the study, and informed consent according to the Helsinki Declaration was provided by all subjects.
Table 1.
Clinical Data of Chronic Lymphocytic Leukemia Patients
| UPN | Sex/age | Rai stage |
|---|---|---|
| 1 | F/70 | III |
| 2 | M/59 | III |
| 3 | M/69 | 0 |
| 4 | M/78 | II |
| 5 | M/68 | 0 |
| 6 | M/74 | 0 |
| 7 | F/83 | III |
| 8 | M/69 | II |
| 9 | M/65 | 0 |
| 10 | M/70 | 0 |
| 11 | M/80 | I |
UPN, unique patient number; F, female; M, male.
MSC cultures
BM cells from posterior iliac crest aspirates were diluted 1:1 in Dulbecco's modified Eagle's medium-Low Glucose (DMEM-LG; Gibco Invitrogen) supplemented with penicillin-streptomycin (PS; Gibco) and preservative-free heparin (Sigma). The BM mononuclear cells (BMMC) were obtained after gradient centrifugation on Histopaque-1077 (Sigma) and were cultured in DMEM-LG/10% fetal calf serum (FCS; Hyclone)/100 IU/mL PS (thereafter referred to as MSC medium) at a concentration of 2×105 cells/cm2 in 25 cm2 culture flasks at 37°C/5%CO2 fully humidified atmosphere. 1 to 2 days post seeding, nonadherent cells were removed, and thereafter, the medium was replaced twice a week. On 70%–90% confluency, cells were detached using 0.25% tryspin/0.1 mM EDTA (Gibco) and re-seeded at a concentration of 2×103 cells/cm2.
Cell-free supernatants were stored at −76°C for measurement of transforming growth factor beta 1 (TGFβ1), stromal cell-derived factor 1 (SDF-1), B-cell activating factor (BAFF), a proliferation inducing ligand (APRIL), vascular cell adhesion molecule-1 (VCAM-1), basic fibroblast factor growth factor (bFGF), and vascular endothelial growth factor (VEGF) by means of an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer. All ELISA kits were purchased from R&D Systems (Quantikine).
MSC differentiation assays
Trypsinized MSCs from passage (P)-2 were induced to differentiate into adipocytes and osteoblasts as previously described [25,26]. Briefly, for the adipogenic differentiation, cells were cultured for 3 weeks in MSC medium supplemented with 10% FCS, 0.5 mM 1-methyl-3-butylisoxanthine, 1 μM dexamethasone, 0.2 μM indomethacin, and 10 μg/mL insulin. Lipid vacuoles were revealed by Oil Red O staining [27] and were visualized under a microscope (Zeiss). For the osteogenic differentiation, cells were cultured in MSC medium supplemented with 0.1 μM dexamethasone, 0.15 mM ascorbate-2-phosphate, and 3 mM NaH2PO4. Osteogenesis was assessed by alkaline phospatase (ALP)/Von Kossa staining [27]. All reagents for adipogenic and osteogenic induction were purchased from Sigma.
Total RNA isolated from differentiated MSCs (RNeasy mini kit; QIAGEN, GmbH) was reverse transcribed (SUPERSCRIPT II; Gibco) and amplified by a reverse transcriptase polymerase chain reaction (RT-PCR) for the evaluation of specific, differentiation-associated gene expression, namely adipose fatty acid-binding protein (aP2) and peroxisome proliferator-activated receptor-g (PPARG) for adipocytes, ALP and runt-related transcription factor 2 (RUNX2) for osteocytes. Products were normalized according to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the ImageJ densitometry analysis system (http://rsb.info.nih.gov/ij/). Forward and reverse primer sequences for GAPDH were 5′-GCCCAATACGACCAAATCC-3′ and 5′-AGCCACATCGCTCAGACA-3′ respectively. All other primer sequences and RT-PCR conditions have been previously described [28].
MSC quantification in the BM mononuclear cell fraction
A standard colony-forming-unit fibroblastic (CFU-F) assay was used to calculate MSC frequency within the BMMC fraction [29,30]. Briefly, day-0 BMMCs were seeded at 5 different concentrations (10×103–200×103 cells) in 60 mm culture dishes (Gibco) and expanded for 14 days. CFU-Fs were identified using Giemsa staining as previously described [27]. The number of CFU-F per 100×103 BMMCs was estimated on the basis of the linear regression analysis obtained from the 5 different initial cell concentrations [26,29,30].
Mesencyhymal stem cell immunophenotypic and survival characteristics
Trypsinized MSCs from P2, P3, and P4 were immunophenotypically characterized by flow cytometry, using anti-CD29 (4B4; Cyto-Stat/Beckman-Coulter), anti-CD44 (J173; Immunotech/Coulter), anti-CD73 (AD2; Pharmingen), anti-CD90 (F15.42; Immunotech/Coulter), anti-CD105 (SN6; Caltag), anti-CD45 (IMMU19.2; Immunotech/Coulter), anti-CD14 (RMO52; Immunotech/Coulter), and anti-CD34 (QBend10; Beckman-Coulter) monoclonal antibodies (mAb).
Apoptosis was studied by flow cytometry using 7-aminoactinomycin-D (7-AAD; Calbiochem-Novabiochem) as previously described [31]. Results were expressed as 7-AAD− (live), 7-AADdim (early apoptotic), and 7-AADbright (late apoptotic) cells. Flow cytometry data were processed in an Epics Elite model flow cytometer (Coulter). In a separate set of experiments, peripheral blood (PB) samples from CLL patients were centrifuged on Histopaque-1077 to obtain the mononuclear cells (PBMCs). The latter were cultured in RPMI-1640 medium (Gibco)/10% FCS in 96-well flat-bottomed culture plates in the absence or presence of allogeneic BM MSCs (P2) derived from CLL patients or healthy individuals at a 10:1 ratio (PBMCs:MSCs). In each experiment, the percentage of CD19+ cells in the PBMC fraction was >90%. Four days later, MSC apoptosis was quantified as the sum of 7AADdim and 7AADbright CD45− adherent cells.
Proliferative potential of in vitro expanded MSCs
MSC proliferative potential was evaluated by a Methyl Triazolyl Tetrazolium (MTT)-based assay in P2 cells and also by estimating the population doubling time through P2-P4 [26]. The formula 2n=Nx/N0 was used for calculation of population doublings (n) at each passage based on the number of cells counted in the flask after trypsinization (Nx) and the number of cells initially plated (N0).
T-cell proliferation assay
To obtain the CD3+ cell fraction, PBMCs from healthy individuals were fractionated by indirect magnetic labeling (magnetic-activated cell sorting; MACS isolation kit; Miltenyi Biotec GmbH). In each experiment, purity of CD3+ cells was >96% as estimated by flow cytometry.
To evaluate the capacity of MSCs from CLL patients and healthy subjects to suppress T-cell proliferative responses, we stimulated 5×104 immunomagnetically sorted normal CD3+ cells with phytohemagglutinin (PHA;2 μg/mL) or interleukin-2 (IL-2;500 IU/mL) in the presence or absence of 104 irradiated (30 Gy) allogeneic BM MSCs from patients or healthy controls in V-bottom 96-well culture plates for 5 days in 0.2 mL RPMI-1640 medium (Gibco)/10% FCS [32,33]. T-cell proliferation was measured on day 5 following a 18-h pulse with 1 μCi/well [3H]-thymidine (3H-TdR; Amersham). 3H-TdR incorporation was measured using a liquid scintillation counter (LS1701 beta counter-Beckman). The percentage of inhibition of T-cell proliferation by MSCs was calculated by dividing the difference of counts per minute (cpm) between cultures of T-cells with and without MSCs in the presence of the activator by the cpm obtained from the cultures of T-cells with the activator alone. Experiments were performed in triplicate.
Detection of immunoglobulin production
Peripheral blood B-cells were obtained from PBMCs derived from normal individuals by negative selection using an immune-magnetic bead B-cell isolation kit (B-cell isolation kit II, Miltenyi Biotec) according to the manufacturer's instructions. In each experiment, negatively isolated cells contained >95% CD19+ B cells, as evidenced by flow cytometry. Subsequently, purified B-cells from healthy individuals or patient-derived PBMCs (in which the percentage of CD19+ cells was >90%) were cultured in 96-well flat-bottomed culture plates in the absence or presence of allogeneic BM MSCs (P2) derived from healthy individuals or CLL patients, at a 10:1 ratio (B cells or PBMCs:MSCs), in the presence of 2.5 μg/mL CpG oligonucleotide 2006 (Invivogen) and 1000 U/mL IL-2 (R&D Systems) in a 0.2 mL RPMI-1640 medium/10% FCS per well. Five days later, supernatants were collected, and immunoglobulin (Ig) G levels were determined on a Dade Behring nephelometer BNII (Dade Behring).
B-cell proiliferation assay
Purified B-cells from healthy individuals or PBMCs from CLL patients were stained with carboxy fluorescein succinimidyl ester (CFSE; Invitrogen). In each experiment, the percentage of CD19+ cells in the PBMC fraction was >90%. Briefly, cells were washed twice with CFSE staining buffer (PBS/0.1% BSA). The cell concentration was adjusted to 105 cells per mL in CFSE staining buffer and labeled with CFSE at a final concentration of 5 μM. The cells were mixed well and incubated at 37°C for 10 min in the dark. The dye uptake was stopped by adding 5 volumes of ice-cold RPMI-1640 medium/10% FCS to the cells and incubating them for 5 min on ice. The cells were washed twice and were cultured for 4 days in the absence or presence of allogeneic BM MSCs (P2) derived from healthy individuals or CLL patients, at a 10:1 ratio (B cells or PBMCs:MSCs), in the presence of 2.5 μg/mL CpG oligonucleotide 2006 (Invivogen) and 1000 U/mL IL-2 (R&D Systems), as mentioned earlier.
Study of CLL B-cell apoptotic/survival characteristics
Freshly isolated patient-derived PBMCs were cultured in RPMI-1640 medium/10% FCS in 96-well flat-bottomed culture plates in the absence or presence of allogeneic BM MSCs (P2) derived from CLL patients or healthy individuals at a 10:1 ratio (PMBCs:MSCs). In each experiment, the percentage of CD19+ cells in the PBMC fraction was >90%. Four days later, nonadherent cells were collected and stained with anti-CD19 (J3-119; Beckman-Coulter) mAb and 7AAD as described earlier. Survival of CLL cells was assessed by quantifying the 7AAD− cells within the CD19+ fraction.
Fluorescence in situ hybridization analysis of hematopoietic cells and MSCs
Fluorescence In Situ Hybridization (FISH) was performed on BM slides and MSC cultures from P2 using commercially available locus-specific multi-color probe sets for tumor suppressor TP53 gene (17p13.1), ataxia-telangiectasia mutated (ATM) (11q22.3), chromosome 12 centromere, retinoblastoma (RB) gene (13q14.3), and IgH rearrangements following the protocol of the manufacturer (Abbott Laboratories). The signals of at least 100 interphase nuclei in each case were evaluated and documented (Zeiss Axioskop microscope, MetaSystems). The cut-off values were established at 2% for trisomy 12, 5% for ATM, TP53, and RB deletions, and <1% for IgH rearrangements.
Statistical analysis
Data were analyzed by means of the Mann–Whitney test and the 2-way analysis of variance (ANOVA) test (GraphPad Software). The chi-square test was used to determine differences between patients and controls in age and sex distribution as well as in TGFβ1 levels in culture supernatants. Grouped data were expressed as means±1standard error of the mean (SEM).
Results
Reserves, morphologic and immunophenotypic properties of CLL-derived MSCs
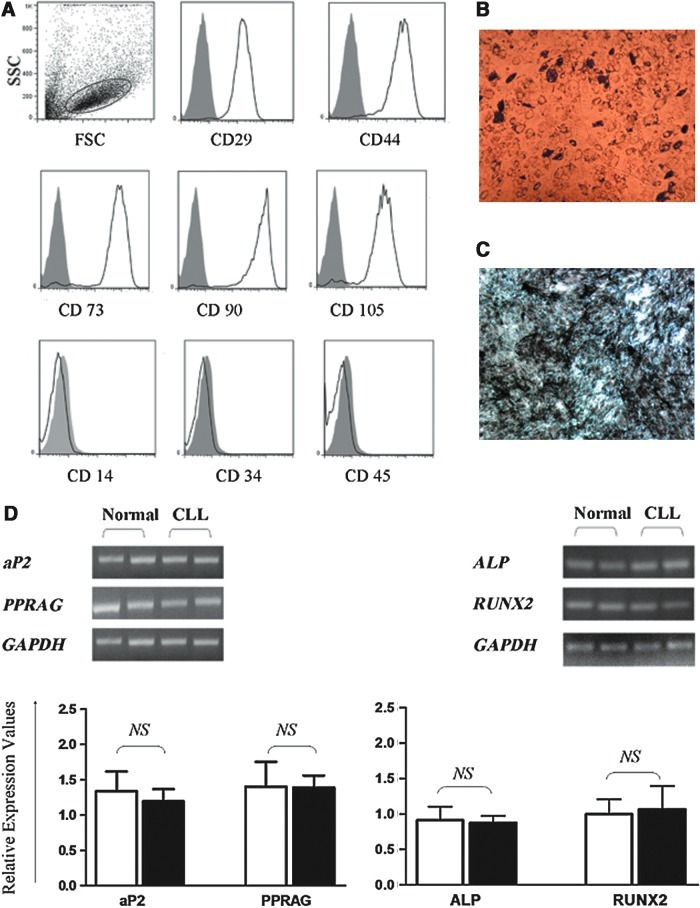
MSCs were successfully expanded from all patients and healthy controls included in the study. Adherent cells from both study groups displayed the typical spindle-shape morphology [34], and immunophenotypic analysis at the end of P2-P3-P4 demonstrated that cultures constituted a homogeneous cell population, typically expressing CD29, CD44, CD73, CD90, and CD105 while being negative for CD14, CD34, and CD45 (Fig. 1A). To calculate MSC frequency within the BMMC fraction, the CFU-F assay was used as a surrogate method [29,30]. As anticipated, the frequency of MSCs was significantly lower in patients (n=11) compared with healthy individuals (n=16) (3.279±0.92/105 BMMCs and 6.71±0.6/105 BMMCs, respectively; P=0.0039) apparently due to the predominance of the leukemic cell population within patient BMMCs.
FIG. 1.
Immunophenotypic characteristics and differentiation potential of chronic lymphocytic leukemia (CLL)-derived MSCs. (A) Representative flow cytometric characterization of P2 BM MSCs from a CLL patient. Open histograms depict the expression of CD29, CD44, CD73, CD90, and CD105 and the lack of CD14, CD34, and CD45. Gray-filled histograms depict isotypic control. (B) Adipogenic differentiation assessed by Oil Red O staining and (C) osteogenic differentiation evaluated by von Kossa staining of ex vivo expanded P2 BM MSCs from a representative CLL patient. (D) Individual and cumulative data from lineage-specific gene expression by P2 MSCs on differentiation. The upper panel shows aP2 and PPARG as well as ALP and RUNX2 mRNA expression indicating adipogenic and osteogenic differentiation, respectively, in a representative CLL patient and a normal subject. The lower panel depicts the cumulative data as a mean relative value (±SEM) of lineage-specific gene mRNA expression from CLL patients (n=11) and controls (n=16). Comparisons between patients (■) and controls (□) have been performed by means of the nonparametric Mann–Whitney test. SSC, side scatter; ALP, alkaline phosphate; aP2, adipose fatty acid-binding protein; PPARG, peroxisome proliferator-activated receptor-g; RUNX2, runt-related transcription factor 2; P, passage; SEM, standard error of the mean; CLL, chronic lymphocytic leukemia; MSCs, mesenchymal stem cells; BM, bone marrow; N.S. Nonsignificant difference. Color images available online at www.liebertpub.com/scd
B-cell CLL-derived MSC differentiation potentials
To evaluate the differentiation potential of culture-expanded MSCs from CLL patients, P2 cells from patients and controls were induced to differentiate into osteogenic and adipogenic lineages. Cytochemical staining revealed that CLL-derived MSCs (n=6) were not qualitatively different from normal MSCs (n=6) in terms of the capacity to differentiate into adipocytes or osteocytes (Fig. 1B, C). Similarly, the adipogenic and osteogenic capacity of CLL MSCs (n=11) was not quantitatively different from that of normal MSCs (n=16) as was demonstrated by the cumulative relative mRNA expression of aP2 and PPARG (adipogenic markers) and ALP and RUNX2 (osteogenic markers) (Fig. 1D). These data suggest that BM MSCs from CLL patients display normal differentiation potential, at least toward the 2 lineages tested.
Growth and survival characteristics of CLL-derived MSCs
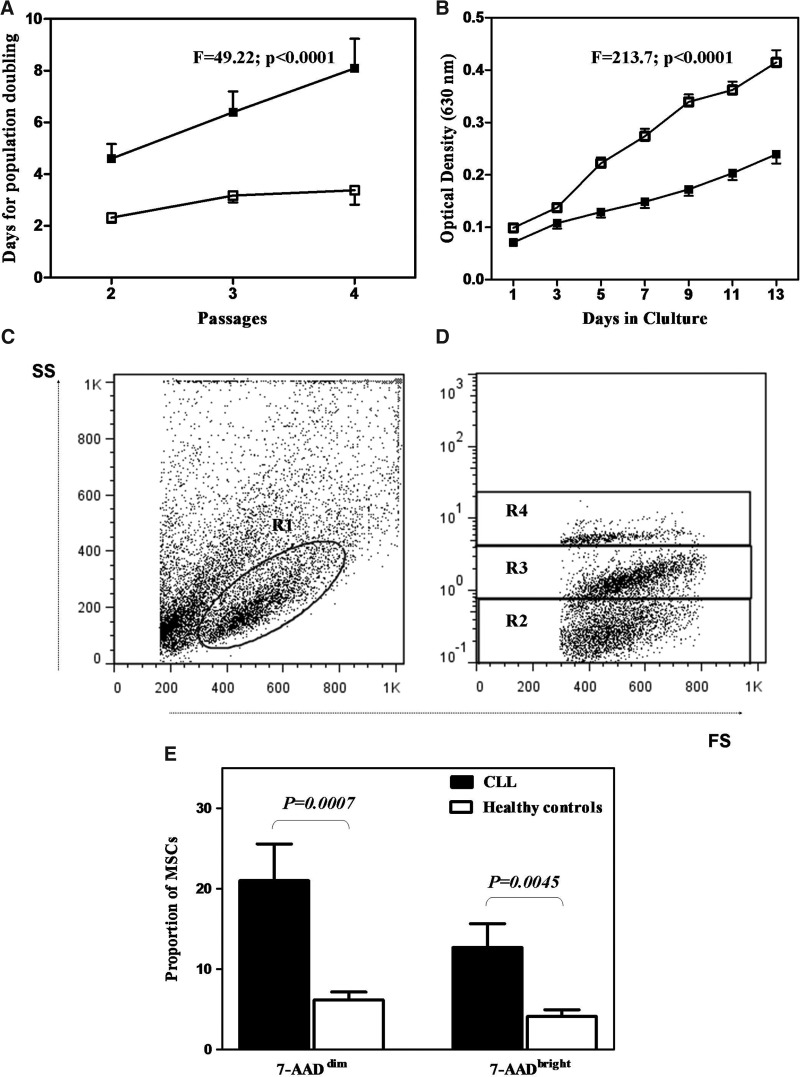
The growth properties of culture-expanded patient MSCs were initially evaluated by assessing their proliferative potential over time. As shown in Fig. 2A, patient MSCs (n=11) displayed significantly increased doubling time over P2-P4 compared with normal controls (n=16) (F=49.22, P<0.0001). Specifically, the MSC doubling time ranged from 4.603±0.558 (P2) to 8.094±1.141 (P4) days in patients and from 2.318±0.189 (P2) to 3.375±0.555 (P4) days in controls. These findings were further substantiated by the MTT assay, according to which the number of live cells, corresponding to the obtained optical density at a representative passage (P2), remained significantly lower in CLL patients (n=11) compared with controls (n=16) (F=213.7, P<0.0001) (Fig. 2B). All the data provided earlier suggest that BM MSCs of CLL patients display decreased proliferative potential compared with their normal counterparts.
FIG. 2.
MSC proliferative potential and survival characteristics. (A) Days (mean±SEM) for population doubling through P2-P4 as estimated by the cell yield at the end of each passage compared with cells initially plated. (B) Results of optical density (mean±SEM) corresponding to the number of live cells over a 13-day culture using the MTT assay in MSCs at P2. Comparison between patients (■) and controls (□) was performed using a 2-way analysis of variance (ANOVA), and the F and P values are depicted. Graphs C–E depict the survival characteristics of MSCs. MSCs from P2 were stained with 7-AAD and analyzed by flow cytometry for the study of apoptosis. Dot plot D shows the proportion of 7-AAD− (R2; live), 7-AADdim (R3; early apoptotic), and 7-AADbright (R4; late apoptotic) cells in the gate of R1 corresponding to P2 MSCs. (E) Cumulative data showing the mean (±SEM) proportion of early- and late-apoptotic MSCs in patient (■) and healthy control (□) cultures at P2. Comparisons were performed by means of the nonparametric Mann–Whitney test, and the P values are shown. D, days in culture; SEM, standard error of the mean; MTT: methyl triazolyl tetrazolium; 7-AAD, 7-aminoactinomycin-D; SS, side scatter; FS, forward scatter.
To get more insights into the mechanism underlying the defective proliferative potential of CLL-derived MSCs, we evaluated their survival characteristics at the representative P2 using flow cytometry and 7AAD staining (Fig. 2C–E). A statistically significant increase proportion of both early and late apoptotic cells was documented in CLL-derived MSCs (15.56%±1.959% and 9.973%±1.642%, respectively) compared with their normal counterparts (6.169%±0.99% and 4.1%±0.84%, P=0.0007 and P=0.0053 respectively). These data suggest that the impaired proliferative potential of patient-derived MSCs can be attributed, at least in part, to increased cell apoptosis.
Given that soluble factors from CLL cells have been shown to activate patient-derived MSCs and induce their proliferation [8,35], we investigated whether CLL cells night also influence MSC apoptosis. In this context, MSCs from (n=5) CLL patients (n=5) were cultured for 4 days in the presence or absence of (n=5) allogeneic patient-derived PBMCs (n=5). The proportion of apoptotic MSCs (defined as the sum of 7AADdim and 7AADbright CD45− adherent cells) did not differ in CLL-derived MSC cultures layered with patient PBMCs (25.48%±1.27%) as compared with MSCs cultured without PBMCs (22.52%±1.299%, P=0.222). Similarly, the addition of patient-derived PBMSCs had no effect on the apoptotic rate of MSCs obtained from (n=5) healthy individuals (10.74%±0.462% vs. 9.92%±0.483% in the absence of PBMCs; P=0.345).
Immunoregulatory functions of B-cell CLL-derived MSCs
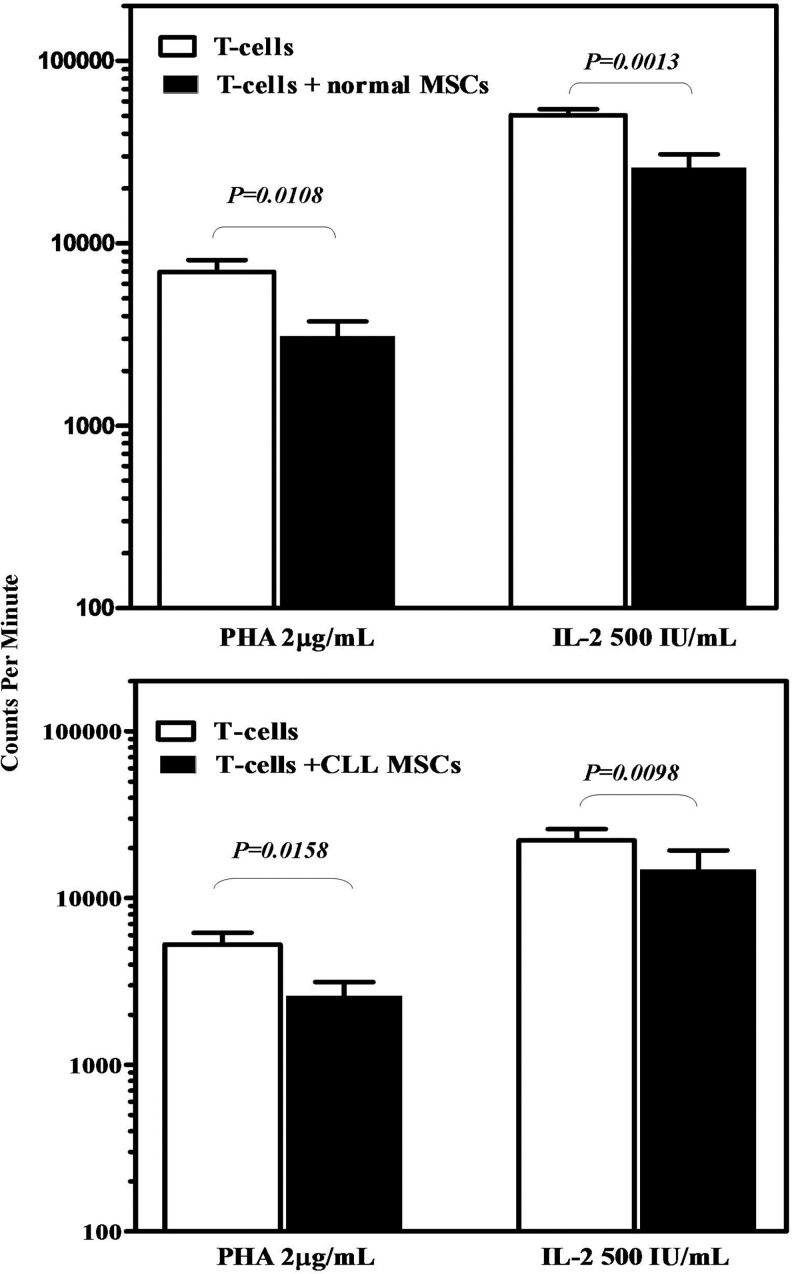
To determine whether patient MSCs could efficiently suppress T-cell proliferative responses induced by mitogenic stimuli, normal (n=5) or patient (n=5) MSCs were mixed with allogeneic normal CD3+ lymphocytes, in the absence or presence of PHA or IL-2, and 3H-TdR incorporation was measured 5 days later. Results are shown in Fig. 3. As expected, T-cell proliferation was significantly reduced when PHA- or IL-2-stimulated T-lymphocytes (7223±1178 cpm and 50366±4075 cpm, respectively) were co-cultured with MSCs from normal donors (3205±669 cpm and 25888±4870 cpm, respectively; P=0.0108 and P=0.0013, respectively). Similarly, T-cell proliferation after PHA or IL-2 stimulation (5275±916 cpm and 22160±3764 cpm respectively) was significantly suppressed in the presence of MSCs from CLL patients (2578±551 cpm and 14471±4569 cpm respectively; P=0.0158 and P=0.0098 respectively). Furthermore, the percentage of inhibition of PHA- or IL-2–induced T-cell proliferation by MSCs did not differ between patients (47.13%±7% and 58.67%±9.366%, respectively) and controls (53.08%±6.28% and 53.53%±7.845%, respectively; P=0.5441 and P=0.5796, respectively). These findings suggest that MSCs from CLL patients display normal immunosuppressive properties in terms of the capacity to suppress T-cell proliferative responses.
FIG. 3.
MSC suppressive effect on T-cell proliferation. Proliferative responses expressed as cpm on 3H-TdR incorporation of unstimulated and activator-induced T-lymphocytes in the presence or absence of BM MSCs from allogeneic normal (upper panel) or patient (lower panel) MSCs. Data are expressed as mean (±SEM) of triplicates of 4 separate experiments. A comparison of cpm in the presence or absence of MSCs was performed by means of the nonparametric Mann–Whitney test, and the P values are depicted. cpm, counts per minute; SEM, standard error of the mean.
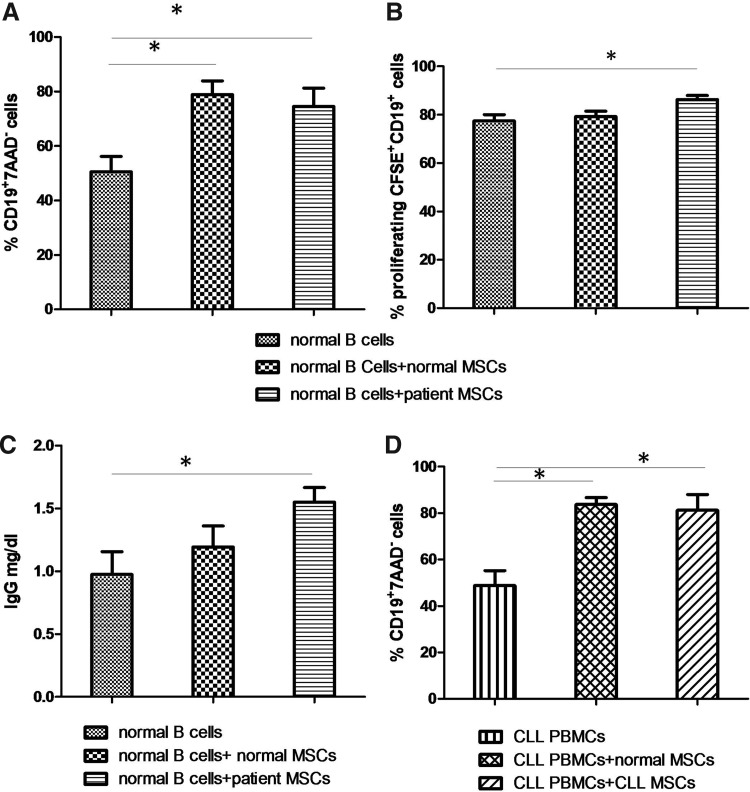
We then asked whether CLL-derived MSCs also display the same immunoregulatory effects on B cells, as compared with their normal counterparts. Four-day co-cultures of peripheral-blood-purified B-cells from (n=5) healthy individuals with (n=5) patient MSCs resulted in a significant increase in the number of viable (7AAD−) CD19+ cells, as compared with B-cells cultured in the absence of MSCs (74.5%±6.723% and 50.5%±5.7% respectively; P=0.0317; Fig. 4A). A similar effect was observed when B-lymphocytes were grown on a layer of MSCs derived from normal individuals (78.93%±4.964%; P=0.0357, n=5). Notably, the proportion of live CD19+ cells did not differ between cultures layered with MSCs from CLL or normal individuals (P=0.959).
FIG. 4.
MSC immunoregulatory effects on B cells. (A) Purified B-cells from healthy individuals were cultured in the absence or presence of allogeneic normal or patient-derived MSCs. Cumulative data depicting the mean (±SEM) percentage of viable (7AAD−) CD19+ cells cultured alone or co-cultured with MSCs is shown (B) CFSE-labeled B-cells from healthy individuals were cultured alone or in the presence of MSCs, as mentioned earlier, in the presence of CpG 2006 oligonucleotide and IL-2. Cumulative data showing the mean percentage (±SEM) of proliferating CFSE+CD19+ cells are depicted (C) Cumulative data indicating the mean (±SEM) IgG levels in supernatants of B-cells from healthy individuals cultured alone or in the presence of MSCs as previously mentioned, in the presence of CpG 2006 oligonucleotide and IL-2. (D) PBMCs from CLL patients were cultured in the absence or presence of allogeneic patient or normal-derived MSCs. Cumulative data depicting the mean (±SEM) percentage of viable (7AAD−) CD19+ cells are shown. PBMCs, peripheral blood mononuclear cells; CFSE, carboxy-fluorescein succinic-midyl ester; SEM, standard error of the mean; *P<0.05.
We also investigated the impact of CLL-derived MSCs on B-cell proliferation (Fig. 4B). In this regard, peripheral blood B-cells from (n=5) healthy individuals were labeled with CFSE and subsequently cultured, for 4 days, in the absence or presence of MSCs from (n=5) CLL patients or (n=5) normal donors in the presence of CpG oligonucleotide 2006 and IL-2. The presence of CLL MSCs resulted in a significant increase in B-cell proliferation (Fig. 4B) as shown by CFSE dilution within the CD19+ cell compartment (86.27%±1.642% proliferating CD19+ cells as compared with 77.48%±2.605% proliferating CD19+ cells cultured in the absence of CLL MSCs; P=0.0159). On the other hand, MSCs from healthy donors had no effect on B-cell proliferation (79.724%±2.221% proliferating CD19+ cells; P=0.69).
We next evaluated the effect of CLL MSCs on the production of IgG by peripheral blood B-cells obtained from healthy individuals (Fig. 4C). In this context (n=5), purified B-cells were cultured for 5 days in the absence or presence of (n=5) CLL-derived MSCs or (n=5) healthy donor-derived MSCs in the presence of CpG 2006 and IL-2. Supernatants of co-cultures of B-cells with CLL-derived MSCs contained significantly higher IgG levels (1.55±0.117 mg/dL) as compared with those in supernatants of B-cells cultured in the absence of MSCs (0.974±0.182 mg/dL; P=0.0317). On the contrary, normal MSCs had no effect on IgG production by B cells (1.194±0.147 mg/dL; P=0.4206).
MSC protective effects on CLL-derived B cells
To assess whether patient-derived MSCs might protect leukemic cells from spontaneous apoptosis, we evaluated the survival characteristics of (n=5) patient PBMCs after co-culture with allogeneic MSCs from (n=5) CLL patients or (n=5) healthy individuals (Fig. 4D). At the end of the culture period (day-4), we found that the proportion of CD19+ cells in the nonadherent cell fraction and the proportion of live (7AAD−) cells within the CD19+ cell compartment did not differ significantly between cultures layered with MSCs from CLL (81.32%±1.42% and 81.25%±6.72% respectively) and normal individuals (85.92%±1.82% and 83.7%±3.031% respectively, P=0.6857 and P=1 respectively). Collectively, these data suggest that CLL-derived MSCs do not confer a survival advantage over normal MSCs to the malignant clone.
To investigate whether BM MSCs from CLL patients might be a primary source of factors that promote the survival/nourishing of leukemic cells and/or their homing to the BM, we assessed the levels of BAFF, APRIL, TGFβ1, SDF-1, VCAM-1, FGF-2, and VEGF in P2 culture supernatants of CLL (n=11) and normal (n=16) MSCs (Table 2). VCAM-1, VEGF, and FGF2 levels did not differ significantly between patients and normal controls. Interestingly however, the levels of SDF-1 and BAFF were significantly lower in CLL patients compared with normal controls (P=0.002 and P=0.0036, respectively) and in contrast to normal cultures, TGFβ1 levels were undetectable in all patient cultures. On the other hand, APRIL levels were significantly increased in patient cultures (P=0.0002). The concentrations of SDF-1, BAFF, APRIL, and TGFβ1 were also assessed in P4 culture supernatants derived from (n=5) patients and (n=5) healthy individuals. No difference was observed in cytokine levels in P4 supernatants with regard to those in P2 supernatants (data not shown). Taken together, these data suggest an abnormal cytokine production by BM MSCs in CLL.
Table 2.
Cytokine Levels in Mesenchymal Stem Cell Supernatants
| CLL patients (n=11) | Normal controls (n=16) | P | |
|---|---|---|---|
| TGFβ1 (pg/mL) | undetectable | 14.13±4.531 | P<0.001 |
| SDF-1 (pg/mL) | 2171±262.8 | 3313±162.9 | 0.002 |
| BAFF (pg/mL) | 2.273±0.39 | 11.8±2.432 | 0.0036 |
| APRIL (pg/mL) | 2.078±0.247 | 0.7221±0.145 | 0.0002 |
| VCAM-1 (pg/mL) | 7914±1629 | 9762±1058 | NS |
| VEGF (pg/mL) | 3608±578.4 | 4816±546.7 | NS |
| FGF-2 (pg/mL) | 0.6411±0.098 | 1.097±0.207 | NS |
MSC, mesenchymal stem cells; TGFβ1, transforming growth factor β1; SDF-1, stromal-derived factor 1; BAFF, B-cell activating factor; APRIL, a proliferation-inducing ligand; VEGF, vascular endothelial growth factor; VCAM-1, vascular cell adhesion molecule-1; FGF-2, fibroblast growth factor 2; NS, nonsignificant.
Cytogenetic analysis of BM/PB hematopoietic cells and MSCs
FISH for TP53 (17p13.1), ATM (11q22.3), chromosome 12 centromere, RB (13q14.3), and IgH (14q32) rearrangements were performed on BM slides and P2 MSCs from the patients, and results are shown in Table 3. Chromosomal abnormalities in hematopoietic cells were, thus, detected in 8 out of 11 patients. More specifically, RB deletions were detected in 7 patients, TP53 deletions in one patient, ATM deletion in one patient, and trisomy 12 in 3 patients. None of the chromosome aberrations found in hematopoietic cells was detected in MSCs, suggesting that the latter cell population does not constitute a part of the malignant clone.
Table 3.
Fluorescence In Situ Hybridization Analysis of Bone Marrow Hematopoietic Cells and Mesenchymal Stem Cells from Chronic Lymphocytic Leukemia Patients
| UPN | Genetic aberrations in BM hematopoietic cells (%) | MSC passage | Genetic aberrations in BM MSCs |
|---|---|---|---|
| 1 | TP53-del (31%) trisomy 12 (36%) |
2 | No genetic abnormality was detected |
| 2 | ATM-del (51%) RB-del (58%) |
2 | No genetic abnormality was detected |
| 3 | RB-del (38%) trisomy 12 (34%) |
2 | No genetic abnormality was detected |
| 4 | RB-del (17%) trisomy 12 (27%) |
2 | No genetic abnormality was detected |
| 5 | Homozygous for RB-del (87%) | 2 | No genetic abnormality was detected |
| 6 | RB-del (30%) | 2 | No genetic abnormality was detected |
| 7 | RB-del (61%) | 2 | No genetic abnormality was detected |
| 8 | No genetic abnormality was detected | 2 | No genetic abnormality was detected |
| 9 | No genetic abnormality was detected | 2 | No genetic abnormality was detected |
| 10 | No genetic abnormality was detected | 2 | No genetic abnormality was detected |
| 11 | RB-del (50%) | 2 | No genetic abnormality was detected |
FISH, fluorescence in situ hybridization; BM, bone marrow; del, deletion; RB, retinoblastoma; ATM, ataxia telangiectasia mutated.
Discussion
There is available evidence suggesting that the microenvironment in both the BM and lymph nodes is critical for survival and accumulation, migration and homing of CLL cells to their specialized niches and disease progression [36]. In addition, different types of adherent accessory stromal cells have been shown to convey drug resistance to CLL cells [9,11–14]. In this context, elucidating the interactions between leukemic cells and their milieu emerges not only as an important issue for the proper understanding of disease biology, but also as a prerequisite for the development of targeted therapies that would modify the interactions between the clone and the supportive microenvironment. This is particularly important for the BM within which disease relapses preferentially occur [37].
The nonhematopoietic components of the BM microenvironment are thought to derive from MSCs [15–17]. This population has been shown to support hematopoiesis [15] and to affect B-cell proliferation and differentiation either positively or negatively [18,19], thereby providing the theoretical background for studying the MSC putative role in CLL pathogenesis. Notably, this particular field of research has not been extensively explored. In the present study, we sought to explore whether BM-derived MSCs from CLL patients harbor intrinsic abnormalities, which, in turn, might contribute to the pathophysiology of the disease. We have, thus, isolated and ex vivo expanded patient-derived MSCs and assessed their quantitative, functional, and cytogenetic characteristics.
MSCs were successfully expanded from all CLL patients included in the study. Their morphology and immunophenotype met the established criteria [34], and they were actually indistinguishable from MSCs derived from healthy individuals. These observations are in line with previously reported data [8]. However, in our study, even though MSC cultures could be established and serially replated from all CLL patients, their growth rate over passages was significantly reduced compared with cultures generated from normal individuals. Specifically, the population doubling time throughout passages was significantly prolonged in patients compared with controls and was associated with low cell proliferation rate in the MTT assay. Furthermore, our data on MSC survival characteristics suggest that the defective growth of patient MSCs could be attributed, at least in part, to increased apoptotic cell death. These abnormalities have not been reported so far. Although the underlying mechanisms for the impaired survival of the ex vivo expanded patient-derived BM MSCs remain elusive, we may speculate that the observed decreased constitutive production of SDF-1 in patient MSC cultures compared with normal individuals may have a role. In favor of this hypothesis is a recent study demonstrating that SDF-1 pretreatment significantly attenuates oxidative stress-induced MSC apoptosis in rats, thereby suggesting a critical role of SDF-1 in MSC survival [38]. Notably, SDF-1 interaction with its cognate receptor CXCR4 on CLL cells has also been reported to regulate the trafficking and homing of leukemic cells in the BM, where it actively protects them from spontaneous and drug-induced apoptosis [5,39]. Irrespective of the nature of the apoptotic stimulus, our findings may carry important clinical implications. For example, they would suggest that autologous MSC transplantation may not represent the best immunosuppressive treatment option for a CLL patient with refractory graft versus host disease after allogeneic transplantation.
Previous reports have shown that CLL cells can also influence MSCs within the BM and that reciprocal interactions eventually activate both cell populations [8,35]. In view of the established bi-directional cross-talk between CLL cells and their microenvironnment, we further investigated whether malignant B-cells might also affect patient-derived MSC survival. However, the addition of patient-derived PBMCs, to allogeneic MSCs obtained from CLL patients had no effect on MSC apoptotic rate. This finding suggests that the observed defect in CLL-derived MSC survival is most likely due to an intrinsic cell abnormality.
The frequency of MSCs within the BMMC fraction was estimated using the standard CFU-F assay [30]. As anticipated due to the BM infiltration by the malignant cells, MSC reserves were reduced in patients' BMMC fraction compared with healthy donors. The increased MSC apoptotic rate in CLL patients may also have a contributory role in the observed low frequency of CFU-F in CLL BM. With regard to the MSC differentiation capacity, we have shown normal osteogenic and adipogenic potential of CLL-derived MSCs. Our data are in line with a previous report [8] that also showed normal MSC differentiation potential with morphological and cytochemical assays. In our study, however, we have further substantiated the normal MSC differentiation potential in CLL by quantifying the osteogenesis- and adipogenesis-related specific gene expression.
MSCs have been shown to possess diverse immunomodulatory properties such as inhibition of T-cell proliferation in response to alloantigens and nonspecific mitogens [15]. We have, therefore, investigated, for the first time, whether an impaired MSC-mediated immunoregulation might contribute to the T-cell dysfunction commonly found in CLL patients [21,22]. Our results, however, indicate that patient-derived MSCs sufficiently inhibit mitogen-induced T-cell proliferation, suggesting that abnormal MSC immunomodulatory properties seem unlikely to account for the abnormal T-cell responses in CLL.
BM-MSCs have also been reported to influence B-cell function, proliferation, and differentiation [18,19]. In this regard, we further examined the immuroregulatory effects on B-cells of CLL-derived MSCs as compared with their normal counterparts. Interestingly, the 2 MSC populations did not differ in their ability to promote B-cell survival. However, only patient-derived MSCs were able to significantly stimulate B-cell proliferation as well as IgG production. Notably, there have been some previous studies suggesting that normal MSCs can also promote B-cell growth and IgG secretion [40–42]. However, the experimental setting in those studies differs considerably from ours, and this probably explains the discrepancy in the reported results. Anyhow, the underlying mechanisms by which CLL-derived MSCs stimulate B-cells have not been explored thus far and need to be addressed. Based on our findings though, we may hypothesize that APRIL, a molecule known to induce B-cell proliferation and differentiation [43], may be implicated here. Interestingly, we have shown that this molecule is overexpressed in patient MSCs. We intend to test the validity of this hypothesis in a future work and to also investigate the ability of MSCs to influence cytokine production by B-lymphocytes with emphasis on those known to be associated with B-cell activation, such as IL-10, IL-6, IL-7, IL-14, and IFN-γ.
It has been convincingly demonstrated that CLL cells undergo spontaneous apoptosis in vitro, unless cultured on direct contact with marrow stroma [3–5,8,9]. In the present study, we have shown that ex vivo expanded patient MSCs can exert an anti-apoptotic effect on CLL cells; however, they provide a similar degree of protection with normal MSCs. Adhesion of CLL cells to stromal cells is thought to involve β1 (CD49d) and β2 (CD11a) integrin expression by leukemic cells and the expression of the respective ligands CD106 (VCAM-1) and CD54 (intracellular adhesion molecule-1, ICAM-1) by stroma [4]. To evaluate whether CLL MSCs might intrinsically favor leukemic adhesion, we assessed VCAM-1 levels in MSC culture supernatants from patients and healthy controls. However, our results indicate that CLL MSCs do not differ from their normal counterparts in terms of VCAM-1 secretion.
We also evaluated a number of stroma-derived cytokines known to have a role in B-cell differentiation, survival, and apoptosis. BAFF and ARIL, members of the TNF superfamily [43], have been shown to protect CLL cells from spontaneous and drug-induced apoptosis via both autocrine [44,45] and paracrine [46] pathways. Our results suggest that additional paracrine pathways involving interactions between MSCs and leukemic cells may be functional in CLL. More specifically, we provide evidence, for the first time, that BM MSCs from healthy donors and CLL patients produce both BAFF and APRIL. Interestingly, CLL MSC supernatants show higher levels of APRIL, as previously mentioned, and lower levels of BAFF as compared with supernatants from healthy donors. These findings are in accordance with plasma levels of the respective molecules in CLL patients [43,45,47,48]. Whether the observed differences in the production of BAFF, APRIL, and SDF-1, as previously mentioned, between patient-derived MSCs and healthy donor-derived MSCs, bear important functional consequences for CLL cells is an issue that still remains open. We intend to pursue this matter further in a future study.
TGFβ1 inhibits the growth and differentiation of normal B-cells [49] and induces apoptosis in resting B-lymphocytes as well as in normal and leukemic B-cell precursors [50]. It has been reported that CLL cells and patient-derived stromal cells secrete higher amounts of this cytokine as compared with their normal counterparts [51]. The exact source of the cytokine overproduction within patient stroma has not been identified. Interestingly, TGFβ1 levels in the supernatants of patient-derived MSCs were undetectable in contrast to their normal counterparts. To the best of our knowledge, this finding has not been previously reported, and it definitely merits further investigation in terms of the underlying mechanisms and the putative effects on patients' MSC biology and function.
To exclude the possibility that the observed MSC abnormalities in CLL might be due to a clonal origin, we performed FISH analysis to both BM hematopoietic and MSCs with a panel of probes for the most common chromosomal abnormalities in CLL [52]. Although hematopoietic cells in 8 out of 11 patients displayed genomic aberrations, MSCs from these patients did not share any of the cytogenetic abnormalities with CLL cells, thereby suggesting that they are not a part of the leukemic clone.
In conclusion, we have shown that CLL-derived ex vivo expanded BM MSCs exhibit normal phenotypic characteristics, differentiation potential, and T-cell immunoregulatory properties and do not harbor the same cytogenetic abnormalities as the leukemic clone. In addition, patient-derived MSCs exert a similar anti-apoptotic effect on CLL cells and B-cells from healthy donors, as compared with their normal counterparts. However, in contrast to the latter, CLL-derived MSCs significantly promote B-cell proliferation and IgG production. Finally, MSCs from patients have impaired reserves, defective cellular growth, and produce aberrant levels of factors that are crucial for the survival/nourishing of the leukemic cells. Interestingly, our results have been obtained in MSCs ex vivo expanded in the absence of leukemic cells, thereby indicating the presence of intrinsic qualitative and quantitative defects within this population. By extending existing knowledge on the CLL milieu, we anticipate that our observations will contribute to delineating CLL biology and will hopefully provide important clues for the design of appropriate microenvironment-targeted therapies.
Acknowledgment
This work was partially supported by the grant 09SYN-13-880 of the Greek Ministry of National Education and Religious Affairs to H.A.P.
Author Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Chiorazzi N. Rai KR. M M Ferrarini. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol. 2003;123:380–388. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 3.Panayiotidis P. Jones D. Ganeshaguru K. Foroni L. Hoffbrand AV. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1996;92:97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
- 4.Lagneaux L. Delforge A. Bron D. De Bruyn C. Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–2396. [PubMed] [Google Scholar]
- 5.Burger JA. Tsukada N. Burger M. Zvaifler NJ. Dell'Aquila M. Kipps TJ TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- 6.Pedersen IM. Kitada S. Leoni LM. Zapata JM. Karras JG. Tsukada N. Kipps TJ. Choi YS. Bennett F. Reed JC. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–1801. [PubMed] [Google Scholar]
- 7.Ringshausen I. Dechow T. Schneller F. Weick K. Oelsner M. Peschel C. Decker T. Constitutive activation of the MAPkinase p38 is critical for MMP-9 production and survival of B-CLL cells on bone marrow stromal cells. Leukemia. 2004;18:1964–1970. doi: 10.1038/sj.leu.2403544. [DOI] [PubMed] [Google Scholar]
- 8.Ding W. Nowakowski GS. Knox TR. Boysen JC. Maas ML. Schwager SM. Wu W. Wellik LE. Dietz AB, et al. Bi-directional activation between mesenchymal stem cells and CLL B-cells: implication for CLL disease progression. Br J Haematol. 2009;147:471–483. doi: 10.1111/j.1365-2141.2009.07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtova AV. Balakrishnan K. Chen R. Ding W. Schnabl S. Quiroga MP. Sivina M. Wierda WG. Estrov Z, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 114:4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz A. Toedt G. Zenz T. Stilgenbauer S. Lichter P. Seiffert M. Inflammatory cytokines and signaling pathways are associated with survival of primary chronic lymphocytic leukemia cells in vitro: a dominant role of CCL2. Haematologica. 2011;96:408–416. doi: 10.3324/haematol.2010.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay NE. Shanafelt TD. Strege AK. Lee YK. Bone ND. Raza A. Bone biopsy derived marrow stromal elements rescue chronic lymphocytic leukemia B-cells from spontaneous and drug induced cell death and facilitates an “angiogenic switch”. Leuk Res. 2007;31:899–906. doi: 10.1016/j.leukres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balakrishnan K. Burger JA. Quiroga MP. Henneberg M. Ayres ML. Wierda WG. Gandhi V. Influence of bone marrow stromal microenvironment on forodesine-induced responses in CLL primary cells. Blood. 2010;116:1083–1091. doi: 10.1182/blood-2009-10-246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fecteau JF. Bharati IS. O'hayre M. Handel TM. Kipps TJ. Messmer D. Sorafenib-induced apoptosis of chronic lymphocytic leukemia cells is associated with downregulation of RAF and Mcl-1. Mol Med. 2012;18:19–28. doi: 10.2119/molmed.2011.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoellenriegel J. Meadows SA. Sivina M. Wierda WG. Kantarjian H. Keating MJ. Giese N. O'Brien S. Yu A, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pontikoglou C. Deschaseaux F. Sensebe L. Papadaki HA. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011;7:569–589. doi: 10.1007/s12015-011-9228-8. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 17.Bianco P. Robey PG. Saggio I. Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 2010;21:1057–1066. doi: 10.1089/hum.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao S. Wehner R. Bornhauser M. Wassmuth R. Bachmann M. Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19:607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- 19.English K. Mahon BP. Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem. 2011;112:1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- 20.Klopp AH. Gupta A. Spaeth E. Andreeff M. Marini F., III Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scrivener S. Goddard RV. Kami ER. Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma. 2003;44:383–389. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- 22.Riches JC. Ramsay AG. Gribben JG. T-cell function in chronic lymphocytic leukaemia. Semin Cancer Biol. 2010;20:431–438. doi: 10.1016/j.semcancer.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Giannoni P. Scaglione S. Quarto R. Narcisi R. Parodi M. Balleari E. Barbieri F. Pattarozzi A. Florio T, et al. An interaction between hepatocyte growth factor and its receptor (c-MET) prolongs the survival of chronic lymphocytic leukemic cells through STAT3 phosphorylation: a potential role of mesenchymal cells in the disease. Haematologica. 2011;96:1015–1023. doi: 10.3324/haematol.2010.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamatopoulos B. Meuleman N. De Bruyn C. Pieters K. Mineur P. Le Roy C. Saint-Georges S. Varin-Blank N. Cymbalista F. Bron D. Lagneaux L. AMD3100 disrupts the cross-talk between chronic lymphocytic leukemia cells and a mesenchymal stromal or nurse-like cell-based microenvironment: preclinical evidence for its association with chronic lymphocytic leukemia treatments. Haematologica haematol. 2011 doi: 10.3324/haematol.2011.052779. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delorme B. Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. In: Hause H, editor; Fussenegger M, editor. Methods in Molecular Medicine. 2nd. Humana Press, Inc.; Totowa, NJ: 2007. pp. 67–82. Tissue engineering. [DOI] [PubMed] [Google Scholar]
- 26.Kastrinaki MC. Sidiropoulos P. Roche S. Ringe J. Lehmann S. Kritikos H. Vlahava VM. Delorme B. Eliopoulos GD, et al. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann Rheum Dis. 2008;67:741–749. doi: 10.1136/ard.2007.076174. [DOI] [PubMed] [Google Scholar]
- 27.Gronthos S. Graves SE. Simmons PJ. Isolation, purification and in vitro manipulation of human bone marrow stromal precursor cells. In: Beresford JN, editor; Owen M, editor. Marrow Stromal Cell Culture. Cambridge University Press; Cambridge: 1998. pp. 26–42. [Google Scholar]
- 28.Kastrinaki MC. Andreakou I. Charbord P. Papadaki HA. Isolation of human bone marrow mesenchymal stem cells using different membrane markers: comparison of colony/cloning efficiency, differentiation potential, and molecular profile. Tissue Eng Part C Methods. 2008;14:333–339. doi: 10.1089/ten.tec.2008.0173. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Malaspina H. Gay RE. Resnick G. Kapoor N. Meyers P. Chiarieri D. McKenzie S. Broxmeyer HE. Moore MA. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 30.Wexler SA. Donaldson C. Denning-Kendall P. Rice C. Bradley B. Hows JM. Adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 31.Philpott NJ. Turner AJ. Scopes J. Westby M. Marsh JC. Gordon-Smith EC. Dalgleish AG. Gibson FM. The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood. 1996;87:2244–2251. [PubMed] [Google Scholar]
- 32.Di Nicola M. Carlo-Stella C. Magni M. Milanesi M. Longoni PD. Matteucci P. Grisanti S. Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 33.Klyushnenkova E. Mosca JD. Zernetkina V. Majumdar MK. Beggs KJ. Simonetti DW. Deans RJ. McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 34.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop D. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 35.Ding W. Knox TR. Tschumper RC. Wu W. Schwager SM. Boysen JC. Jelinek DF. Kay NE. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116:2984–2993. doi: 10.1182/blood-2010-02-269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burger JA. Ghia P. Rosenwald A. Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meads MB. Hazlehurst LA. Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 38.Liu X. Duan B. Cheng Z. Jia X. Mao L. Fu H. Che Y. Ou L. Liu L. Kong D. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2:845–854. doi: 10.1007/s13238-011-1097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger M. Hartmann T. Krome M. Rawluk J. Tamamura H. Fujii N. Kipps TJ. Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 40.Rasmusson I. Le Blanc K. Sundberg B. Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 41.Tabera S. Perez-Simon JA. Diez-Campelo M. Sanchez-Abarca LI. Blanco B. Lopez A. Benito A. Ocio E. Sanchez-Guijo FM. Canizo C. San Miguel JF. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93:1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- 42.Traggiai E. Volpi S. Schena F. Gattorno M. Ferlito F. Moretta L. Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- 43.Haiat S. Billard C. Quiney C. Ajchenbaum-Cymbalista F. Kolb JP. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukaemia. Immunology. 2006;118:281–292. doi: 10.1111/j.1365-2567.2006.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novak AJ. Bram RJ. Kay NE. Jelinek DF. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100:2973–2979. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 45.Kern C. Cornuel JF. Billard C. Tang R. Rouillard D. Stenou V. Defrance T. Ajchenbaum-Cymbalista F. Simonin PY. Feldblum S. Kolb JP. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–688. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 46.Nishio M. Endo T. Tsukada N. Ohata J. Kitada S. Reed JC. Zvaifler NJ. Kipps TJ. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106:1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Planelles L. Castillo-Gutierrez S. Medema JP. Morales-Luque A. Merle-Beral H. Hahne M. APRIL but not BLyS serum levels are increased in chronic lymphocytic leukemia: prognostic relevance of APRIL for survival. Haematologica. 2007;92:1284–1285. doi: 10.3324/haematol.10317. [DOI] [PubMed] [Google Scholar]
- 48.Bojarska-Junak A. Hus I. Chocholska S. Wasik-Szczepanek E. Sieklucka M. Dmoszynska A. Rolinski J. BAFF and APRIL expression in B-cell chronic lymphocytic leukemia: correlation with biological and clinical features. Leuk Res. 2009;33:1319–1327. doi: 10.1016/j.leukres.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 49.Kehrl JH. Roberts AB. Wakefield LM. Jakowlew S. Sporn MB. Fauci AS. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986;137:3855–3860. [PubMed] [Google Scholar]
- 50.Buske C. Becker D. Feuring-Buske M. Hannig H. Wulf G. Schafer C. Hiddemann W. Worman B. TGF-beta inhibits growth and induces apoptosis in leukemic B cell precursors. Leukemia. 1997;11:386–392. doi: 10.1038/sj.leu.2400586. [DOI] [PubMed] [Google Scholar]
- 51.Lagneaux L. Delforge A. Bron D. Bosmans E. Stryckmans P. Comparative analysis of cytokines released by bone marrow stromal cells from normal donors and B-cell chronic lymphocytic leukemic patients. Leuk Lymphoma. 1995;17:127–133. doi: 10.3109/10428199509051712. [DOI] [PubMed] [Google Scholar]
- 52.Dohner H. Stilgenbauer S. Benner A. Leupolt E. Krober A. Bullinger L. Dohner K. Bentz M. Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]