Abstract
Background
The term middle ear myoclonus (MEM) has been invoked to explain symptoms of tinnitus presumably caused by the dysfunctional movement of either of the two muscles that insert in the middle ear: tensor tympani and stapedius. MEM has been characterized through heterogeneous case reports in the otolaryngology literature, where clinical presentation is variable, phenomenology is scarcely described, the pathogenic muscle is usually not specified, natural history is unknown, and the presumptive definitive treatment, tensor tympani or stapedius tendon lysis, is inconsistently effective. It is not surprising that no unique acoustogenic mechanism or pathophysiologic process has been identified to explain MEM, one of several descriptive diagnoses associated with the complicated disorders of myogenic tinnitus.
Methods
Here, we explore MEM from the neurologist’s perspective. Following the detailed descriptions of two informative cases from our clinic, we systematically evaluate the different mechanisms and movement disorder phenomena that could lead to a diagnosis of MEM.
Results
From a functional neuroanatomic perspective, we explain how tensor tympani MEM is best explained as a form of peritubal myogenic tinnitus, similar to the related disorder of essential palatal tremor. From a pathogenic perspective, we discuss how MEM symptomatology may reflect different mechanical and neurologic processes. We emphasize the diagnostic imperative to recognize when myogenic tinnitus is consistent with a psychogenic origin.
Discussion
Both individual patient care and further elucidation of MEM will rely on more detailed clinical characterization as well as multidisciplinary input from neurology, otolaryngology, and dentistry.
Keywords: Middle ear myoclonus, tinnitus, objective tinnitus, palatal tremor, Eustachian tube, psychogenic
Introduction
Middle ear myoclonus (MEM) is a rare diagnosis of tinnitus that is presumed secondary to abnormal movement of the tensor tympani or stapedius muscles. This diagnosis has been primarily used in case reports in the otolaryngology literature,1 where the phenomenology of the tinnitus, when described, is highly variable. MEM tinnitus is commonly characterized as clicking, suggested to be due the tensor tympani movement,2 or buzzing, suggested to be due to stapedius movement;3 however, it has also been described as throbbing, tapping, crackling like a grasshopper, bubbling, ticking, twitching, blowing, drum-like thumping, fluttering like a butterfly, whooshing or gushing.3–5 MEM tinnitus is usually characterized as rhythmic, being regular or irregular, continuous or intermittent, and unilateral or bilateral.1 The frequency, pitch, and intensity can vary within and among individuals.1 MEM tinnitus is often objective, being perceptible also to the examiner, but can be subjective, being perceptible only to the patient. While objective tinnitus clearly identifies a somatic rather than sensorineural etiology, subjective tinnitus does not delineate an acoustogenic mechanism. There is variable response to middle ear muscle tendon lysis, the presumptive definitive treatment of MEM tinnitus.1
Characterized as idiopathic and congenital, MEM has also been diagnosed in association with acoustic trauma, hemifacial spasm, and essential palatal tremor (EPT).1,6 Similar symptoms are also found in the setting of temporomandibular disorders (TMDs), bruxism, and a patulous Eustachian tube.1,4,7,8 Similar cases have been characterized as “otognatic syndrome,”9 “otomandibular syndrome,”10,11 and “tensor tympani syndrome.”12 To date, no unique pathophysiologic mechanism for this myogenic tinnitus has been identified.
Here, we describe two distinctly educational cases diagnosed as MEM and systematically discuss the symptom of myogenic tinnitus from a neurologic perspective. Because there is no single pathogenic basis for MEM and therapeutic studies are lacking, we do not make recommendations about management. A recent review by Bhimrao et al.1 provides a useful flowchart for the evaluation of patients with objective tinnitus, to which we would urge including a detailed assessment of tinnitus phenomenology. Decisions regarding treatment of myogenic tinnitus, such as middle ear muscle tendon lysis, botulinum toxin chemodenervation,2,13,14 Eustachian tube occlusion,15 oral medications,1,3,8 tinnitus masking devices,16 zygomatic pressure maneuvers,4 and/or psychotherapy,6,12 should follow comprehensive, individual patient assessment.
Case 1
A 10-year-old, right-handed girl with a history of mild learning disability, sensory integration difficulty, dysarthria, and clumsiness complained of 1 year of right ear clicking like “the sounds of a clock.” This symptom began when she “forgot to chew gum” upon descent in an airplane and became anxious that she may have damaged her ear, despite having no coincident pain or hearing loss. She grew increasingly worried when the unilateral clicking did not subside, and she occasionally noticed it in her left ear as well. The clicking was often present every day and had been heard by others. It became more prominent if people asked her about it, and is was reportedly absent if she spoke or ate, sat “quietly,” or was distracted by playing games, listening to music, or watching television. Occasionally the clicking became associated with mild, dull pain in the ear. She had been initially evaluated by her pediatrician and an otolaryngologist and been diagnosed with MEM. She underwent right tensor tympani and stapedius tendon lysis without any benefit. She underwent two courses of palatal botulinum toxin injections, as well as a trial of clonazepam and other medications, but she had only temporary relief with any of these interventions. Our examination revealed objective right-sided tinnitus that was audible through a stethoscope on the outer ear. The tinnitus was absent prior to the examiner mentioning listening for it, stopped during cognitively distracting tasks, and entrained to externally paced hand tapping. No palatal movements were observed upon inspection through the open mouth. Baseline features of dysarthria, slightly clumsy right hand, and symmetrically decreased arm swing were noted. Trace left ptosis was observed and did not seem abnormal to the parents. Based on the sudden onset in the setting of anxiety about potential ear injury, the nature of the self-reported variability of symptoms, and the clinically demonstrated suggestibility, distractibility, and entrainability of objective tinnitus, a “clinically established” diagnosis of psychogenic movement disorder (PMD) was made.17,18 Other examination findings were deemed unrelated and/or consistent with developmental dyspraxia and learning disability. The patient and her parents were educated about the diagnosis of functional tinnitus. She was referred for psychiatric evaluation and then biofeedback therapy, which led to progressive resolution of her symptoms.
Case 2
A 40-year-old, right-handed female with a remote history of postpartum depression complained of 1 year of distressful clicking tinnitus. The tinnitus began during a month when she also suffered intermittent and spontaneously resolved headaches, and the onset was otherwise not associated with any illness or evident stressful event. The symptom started in the left ear, had been present daily for the past year, and occasionally also involved the right ear. When both ears were involved, the bilateral clicking could be synchronous or asynchronous. The clicking was continuous, irregularly rhythmic, and could vary in both loudness and quality. It usually sounded like the “tick-tock of a clock,” but it could also sound like a “scratching” or “scraping.” It was never tonal. The clicking might stop for one or two beats upon opening the mouth but would then immediately return. It worsened during airplane flights, and she felt particularly anxious in warehouse stores, where the pressure in her ears felt “abnormal.” There was otherwise no change in symptoms with activity, position, or time of day. The clicking had been heard by her husband, reportedly even while she slept. The patient was evaluated by her primary care doctor and an otolaryngologist, had magnetic resonance imaging (MRI) and computed tomography scans of the brain that demonstrated only sinusitis, and was told there was a suspicion that “the bones in the ears were moving causing the clicking.” She underwent exploratory surgery of the left middle ear. She recalled cessation of left- but not right-sided clicking when the left stapedius tendon was isolated, but surgical notes documented no change in clicking. The tensor tympani was not explored because the surgery was stopped early secondary to nausea and vertigo. During the procedure, the patient also suffered pins and needles on the left side of the tongue, followed by 6 months of abnormal taste and sensation on the anterior left tongue, which resolved spontaneously. She suffered a persistent, continuous “shhhhhhh” sound and objective hearing loss in the left ear. She frequently suffered a sense of left ear fullness, persisting until she would “repressurize” by exhaling while holding her nose and mouth closed.
She investigated her symptoms on the internet, suspected “palatal myoclonus,” returned to the otolaryngologist, and was then found to have palate and throat tremor that occurred synchronously with the clicking. She was referred to a neurologist, who immediately diagnosed “palatal myoclonus.” Several unspecified medications were not helpful. Repeat MRI brain scan with thin cuts through the brainstem and basal ganglia was normal. Occasional twitches in the cheeks, lasting a few seconds every couple of days, started and were alleviated by clonazepam. About 1 year after the onset of clicking, she began to suffer rare episodes of choking sensation synchronous with the clicks and worsened by neck extension. The clicking remained the most troublesome of all her symptoms. She denied abnormal movements or postures of other body parts. She had no change in her vision, voice quality, or speech production. She denied any problems with focal or generalized power or sensation, imbalance or clumsiness, or gait. She tearfully described her mood as generally good but admitted having good and bad days. A sister had a congenital eye twitch, and there was no other family history of movement or other neurologic disorders.
Our examination demonstrated mild depression and anxiety. Abnormal movements of the palate and pharynx were observed upon direct inspection with the mouth open. Movements were inconsistent in character on two consecutive examinations performed within an hour of each other. At the first examination, there was rhythmic, 1–2 Hz, symmetric pharyngeal narrowing immediately preceding palate elevation. There was synchronous trace symmetric protrusion of the tongue. Clicking was perceived by the patient and was heard by the examiner near the patient’s left ear; however, temporal correlation between ear clicking and oropharyngeal movements was difficult to assess. Upon mouth opening, movements and clicks ceased only for a few seconds. At the second examination, palatal, pharyngeal, and lingual movements were more irregularly rhythmic, inconsistently synchronous, and slower than 1–2 Hz. During this examination when the mouth was open or closed, there were also semi-rhythmic elevations of the anterior neck, which temporally corresponded with the objective tinnitus. The clicking was much louder, being audible at least 10 feet (3 meters) across a room. During both examinations, all movements were somewhat entrainable to externally paced hand tapping and inconsistently distractible. The orifice of the right Eustachian tube was readily visualized, but the left Eustachian tube was not readily appreciated. The remainder of the motor as well as the cerebellar and gait examination was normal. The post-surgical decreased taste on the anterior left side of the tongue was present. There was decreased vibration in the distal bilateral lower extremities. Achilles reflexes were absent, but other appendicular reflexes were normal.
The patient was diagnosed with atypical palatal tremor, with symptomatic, essential, and functional components, and further testing was recommended. A sleep study revealed objective tinnitus throughout sleep, consistent with a symptomatic palatal tremor (SPT). Clinical neurophysiologic testing was consistent with a primarily “organic” palatal tremor, with limited entrainability and difficulty with paced tapping, features of a possible comorbid functional disorder. The variable craniocervical movement phenomenology, partial distractibility and entrainability, remote episode of depression, and clinically evident depression and anxiety remained consistent with a “probable” comorbid psychogenic diagnosis.17,18 Botulinum toxin treatment targeting the tensor veli palatini was initiated with moderate, early success. During management of the atypical SPT, attention to maintaining optimal mental health was encouraged. Nerve conduction and electromyographic study of left facial muscles and brainstem auditory evoked potentials to further assess presumed post-surgical facial nerve injury versus possible brainstem dysfunction were recommended, as was continued routine neurologic evaluation.
Discussion
MEM refers to a symptom of non-vascular, pulsatile tinnitus mediated through the middle ear. Tensor tympani or stapedius movement is the presumptive acoustogenic source; however, the etiology of MEM has not been well defined. Although the definitive treatment for MEM is considered middle ear muscle tendon lysis,1 the variable benefit following surgical management highlights the uncertainty of MEM tinnitus pathogenesis. Since the movement phenomenology of MEM tinnitus is rarely described and clinical neurophysiologic studies of the tensor tympani and stapedius muscles are impractical, it is unclear whether or when the phenomenologic designation “myoclonus” is accurate. Analogous to the tinnitus of EPT, MEM tinnitus likely reflects a variety of causes. While tensor tympani or stapedius myoclonus may explain some cases of MEM tinnitus, middle ear muscle movement may also represent a final common pathway for a variety of acoustogenic processes. A systematic exploration of the anatomy, physiology, and phenomenology associated with myogenic tinnitus clarifies some of the confounding factors that muddle MEM tinnitus and related disorders.
Anatomy and physiology of myogenic tinnitus
Tinnitus aurium refers to any audible symptom whose acoustic percept originates in the ear. As in this report, the term “tinnitus” is widely used to mean tinnitus aurium, as opposed to tinnitus cerebri, where the symptom originates in the brain.19 A diagnosis of tinnitus does not denote anything about the etiology of the sound. The most common etiologies of tinnitus are age-related sensorineural hearing loss and noise exposure, where damaged hair cells are believed to stimulate the misperception of a continuous tonotopic sound.20 Tinnitus may also be caused by mechanical perturbation of the vestibulocochlear nerve, such as with vascular malformations, which cause cardiogenic pulsatile tinnitus (and necessitate prompt angiographic evaluation) or acoustic neuromas, which cause non-pulsatile tinnitus.20–22 There is an increasing understanding of the central neurologic plastic changes and processes that support the establishment of and distress associated with tinnitus.23–27
A rare form of mechanically induced tinnitus is myogenic tinnitus, where movement of distinct, sometimes functionally related muscles in the head and neck generates a variety of different sounds. Somatosounds originate inside the body but outside the neural auditory pathways,22,28 and myogenic somatosounds are the essential clinical features of MEM tinnitus. Therefore, understanding the mechanics of MEM tinnitus requires a working knowledge of the complex peripheral auditory anatomy and physiology.
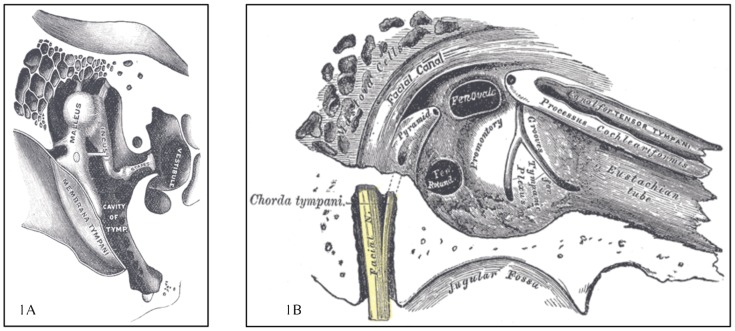
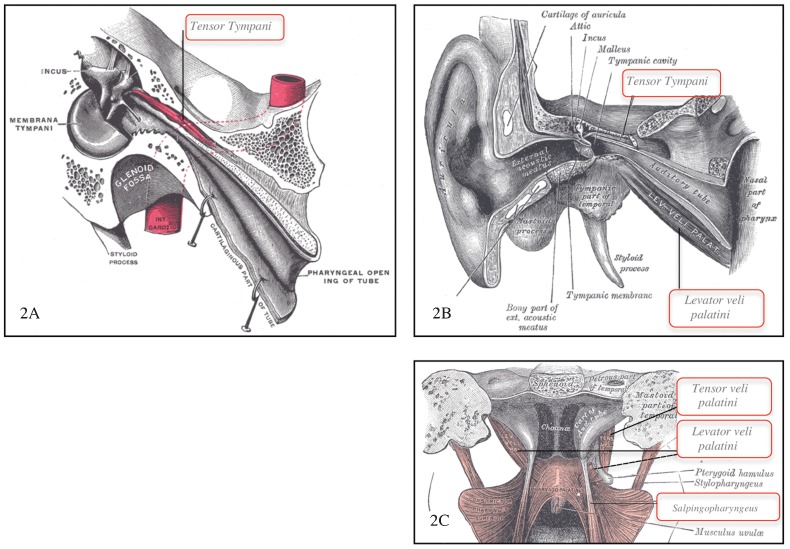
Sound originating outside the body is perceived when the sound waves travel through the outer ear to reach and vibrate the tympanic membrane (TM) (Figure 1). The vibrations travel through the middle ear ossicles—the malleus, incus, and stapes—are transmitted from the stapes footplate to the pliable round window of the cochlea, and are then converted to auditory nerve signals. Although not typically discussed in the context of normal sound perception, the cochlea also has a second, pliable window in the middle ear, the oval window, which if mechanically perturbed could also promote sound perception. Modulation of sound and maintenance of middle ear function is mediated by the stapedius, the Eustachian tube, and four peritubal muscles: tensor tympani, tensor veli palatini, levator veli palatini, and salpingopharyngeus (Figure 2).
FIGURE 1. Middle Ear Anatomy.
(A) Transverse view demonstrating tympanic membrane and middle ear ossicles. (B) Inner wall demonstrating cochlear windows, Eustachian tube, and tensor tympani. (Modified from Gray’s Anatomy, plates 919 and 91179).
FIGURE 2. Anatomy of Peritubal Muscles.
(A) Tensor tympani and (B) levator veli palatini parallel to Eustachian tube, (C) levator veli palatini, tensor veli palatini, and salpingopharyngeus as viewed from palate. (Modified from Gray’s Anatomy, plates 915, 907, 102879).
The stapedius is the only muscle lying solely within the middle ear and is innervated by the facial nerve. Contraction of this approximately 6-mm-long muscle pulls on the stapes to dampen round window vibrations, and also stabilizes the incus and malleus to indirectly dampen TM vibrations.1,29 Considered the essential middle ear muscle owing to its anatomic location and complex auditory physiology, stapedius both reflexively inhibits cochlear stimulation in response to loud external sounds and diminishes autophony associated with speaking.1 The brainstem-mediated acoustic reflex between the vestibulocochlear nerve, the superior olive, and the facial nerve is routinely evoked to indirectly measure stapedius function via tympanometry. Poorly understood descending cortical input supports some individuals’ ability to voluntarily contract this striated muscle.30
The tensor tympani is located largely outside the middle ear, is innervated by a terminal nerve originating in the mandibular branch of the trigeminal nerve (V3), and mediates several auditory-associated functions. Situated along the Eustachian tube (Figure 2), the approximately 20-mm-long tensor tympani is anchored to the sphenoid and the bony Eustachian tube and is also attached along the cartilaginous Eustachian tube.29 Although the distal tensor tympani tendon inserts on the malleus in the middle ear,29 the muscle location and major functions of the tensor tympani predominantly characterize a peritubal muscle. Repetitive contraction of the tensor tympani assists the tensor veli palatini and other peritubal muscles with opening and closing the tube during a variety of speech and swallowing activities.1 Within the middle ear, contraction of tensor tympani pulls on the malleus to dampen TM vibrations. This action is primarily described in the context of preventing autophony associated with the palatal actions of chewing and swallowing and is considered less important during vocalization.1,12 Although the tensor tympani and stapedius act in concert to mediate a variety of acoustic reflexes in other mammals, in humans the tensor tympani reflexively responds principally to external stimuli commonly associated with startle.12 It may contract upon a puff of air to the cornea, tactile stimulation around the eye or ear, and eye closure.31 Tensor tympani muscle spindles have also been described as having barometric properties, reflexively contracting in response to pressure changes.32 Independent voluntary control of the striated tensor tympani has been described;1 however, central nervous system networks specifically leading to tensor tympani activation are not well characterized.
The Eustachian tube directly connects the middle ear to the pharynx, where additional muscles that control Eustachian tube opening and closing play an integral role in three physiologic processes: equalizing middle ear and atmospheric pressure, promoting drainage of middle ear fluid, and decreasing autophony (Figure 2C). The tensor and levator veli palatini are innervated by V3 and a branch of the vagal nerve, respectively. Both muscles facilitate swallowing by elevating the palate. The salpingopharyngeus is innervated by a branch of the vagal nerve and assists swallowing by projecting food to the esophagus.
Both distinct and overlapping anatomic and physiologic properties of middle ear and peritubal muscles support mechanisms that could cause myogenic tinnitus. Because stapedius contraction can directly stimulate and tensor tympani contraction can indirectly stimulate the cochlea, tinnitus due to either middle ear muscle movement is readily appreciated. Following from Politzer’s 1878 description of objective tinnitus (a sound heard by an objective listener)6,33 and among the most commonly suggested sources of myogenic tinnitus, it is widely believed that a sound is produced upon changing the apposition of the mucosal surfaces of the lumen of the Eustachian tube during its opening and closing.1,2,34 The associated vibration through the Eustachian tube could then propagate to the middle ear, travel through the TM and/or the ossicles to reach the round window, or directly propagate to either of the cochlear windows to cause an audible percept. Since the tensor tympani connects to both the Eustachian tube and the malleus, any opening or closing of the Eustachian tube could also indirectly move the TM, either due to passive or stimulated reflexive change in tensor tympani tension. Whatever the acoustogenic route, myogenic tinnitus has been associated with abnormal movement of all of the peritubal muscles. It has also been proposed that independent of middle ear or peritubal muscle activity, twitching of craniocervical muscles in proximity to the middle ear could itself be audible, as characterized by phonomyography.1,35–38
Pathophysiology of peritubal myogenic tinnitus
Several mechanical, anatomical, neurologic, phylogenetic, and physiologic associations between the tensor tympani and tensor veli palatini muscles suggest that some cases of MEM and EPT tinnitus share a common pathogenesis. First, both of these muscles mediate Eustachian tube opening and closing, the widely proposed major mechanism of myogenic tinnitus. Second, an important gross anatomical study by Ramirez Aristeguieta et al.7,29 demonstrated that a tendon physically links the tensor veli palatini and tensor tympani, leading the authors to assert these are “nearly the same muscle by continuity.” Third, both of these muscles are innervated by the medial pterygoid nerve, a motor branch of V3 that mediates both voluntary and reflex contraction.29,39 Fourth, these muscles derive from the first branchial arch and belong to the phylogenetically associated group of V3-innervated masticatory muscles comprising the tensor veli palatini, tensor tympani, temporalis, masseter, lateral, and medial pterygoid, mylohyoid, and anterior diagastric.29 Finally, the tensor tympani and tensor veli palatini maintain a close functional relationship in a variety of oropharyngeal processes, both being activated during chewing, yawning, laughing, coughing, breathing, and speaking.7 Thus, tensor tympani-associated MEM tinnitus (and EPT tinnitus) might be better represented mechanistically as peritubal myogenic tinnitus.
The complicated case literature supports this nosology of MEM tinnitus while highlighting the diagnostic and clinical confusion surrounding myogenic tinnitus. For example, MEM and palatal myoclonus have been diagnosed in the same patient.40 In a case of bilateral, objective, clicking tinnitus, no palatal movement was detected, tensor tympani contraction synchronous with the objective tinnitus was documented, MEM was diagnosed, and bilateral tensor tympani tendons were sequentially lysed with only temporary improvement. The authors concluded that the tinnitus was likely due to non-visualized, pathogenic tensor veli palatini movement.6 These findings are reminiscent of the patient in Case 2, who suffered primarily unilateral objective tinnitus, underwent lysis of the tensor tympani tendon without symptomatic relief (but with neurologic complications), and was only later found to have abnormal palatal movement.
While tensor tympani movement may cause some cases of MEM tinnitus1,5,41 and tensor veli palatini is widely cited as the primary muscle involved in EPT tinnitus,2,42,43 movement of other palatal and pharyngeal muscles has also been associated with peritubal myogenic tinnitus.14,44 It should be noted that peritubal muscle movement does not always induce tinnitus. For example, vigorous levator veli palatini activity in SPT is typically not associated with tinnitus.43 Thus, peritubal myogenic tinnitus could be inappropriately diagnosed as MEM tinnitus due to similar symptomatology, unappreciated palatal movement, and non-specific positive impedance audiometry or tympanometry. When the pathogenic muscle is unclear, nasoendoscopic examination and electromyography (EMG) of the palate may be valuable.
Nosology of middle ear myoclonus
MEM shares clinical features with a variety of diagnoses that originated in different medical specialties, including otognatic syndrome,9 otomandibular syndrome,10,11 tensor tympani syndrome,12 and EPT.42 This diverse terminology probably does not distinguish unique disorders but reflects the clinical emphasis of the field in which the syndromes were described. Since objective neurophysiologic characterization of stapedius and tensor tympani activity are impractical and middle ear testing does not distinguish between these muscles, detailed phenomenologic descriptions of myogenic tinnitus are all the more important to elucidate the etiologies of MEM tinnitus.
Unfortunately, case literature provides limited and variable descriptions of MEM tinnitus phenomenology. Summarizing the clinical features in their recent review, Bhimrao et al1 suggested that MEM be diagnosed in the setting of “pulsatile” tinnitus, synchronous rhythmic movement of the TM, and associated changes in tympanic impedance, and they noted an inconsistently accompanying feature of cogwheel or saw-toothed pattern on tympanometry. In an effort to further define a specific disorder, they also proposed that MEM not be diagnosed in the setting of a patulous Eustachian tube or TMD. Although this exclusion may help direct treatment, such categorical distinctions have also likely contributed to the historically complicated terminology surrounding myogenic tinnitus, as recently articulated by Ramirez et al.:
For almost one hundred years several investigators have tried to outline possible cause-effect relationship between otic symptoms and TMD from mechanical, vascular, neurological, muscular and embryological viewpoints. As medicine and dentistry became more specialized the hearing organ and stomatognathic systems were abruptly separated; this situation has been discreetly corrected via specialization in otolaryngology and craniofacial pain which has attempted to close the breach in such thematic discussion which had been gaining the stigma of being a no man’s land.7
Bhimrao et al.1 also suggested that MEM not be diagnosed in the setting of “palatal tremor” due to associated neurologic lesions. This exclusion should be clarified to distinguish between SPT and EPT. Unlike EPT, SPT does not tend to cause tinnitus and typically does persist during sleep.45 EPT is not associated with any known neurologic lesions; however, SPT is associated with lesions in the dentato-rubro-olivary pathway and often inferior olivary hypertrophy. SPT lesions can be due to a variety of underlying pathologies such as stroke, multiple sclerosis, and tumors, as well as the less common sporadic olivopontocerebellar atrophy/multiple system atrophy type C, Alexander’s disease, progressive supranuclear palsy, spinocerebellar degeneration, progressive ataxia and palatal tremor syndrome, and neuroferritinopathy due to ferritin light chain.46,47 Complex cases of peritubal myogenic tinnitus, which may have symptoms of MEM, EPT, and/or SPT, as in Case 2, could be associated with this lengthy differential diagnoses and warrant appropriate evaluation and routine neurologic examination.
On the other hand, the functional anatomic, peripheral neurologic, and phylogenetic similarities between the peritubal tensor tympani and tensor veli palatini muscles actually suggest that the same hyperkinetic movements may contribute to tensor tympani MEM and EPT tinnitus. EPT is characterized by variably rhythmic clicking or other somatosounds, which are clinically associated with tremulous palatal movement.42,43 As with MEM tinnitus, EPT tinnitus is also synchronous with tympanometry recordings,48 demonstrating that this measurement does not specify the pathogenic muscle. Furthermore, the most often described acoustogenic mechanisms of tensor tympani MEM and EPT converge on the Eustachian tube. Reflecting the etiologic confusion about EPT, this diagnosis was previously described not only as essential palatal “myoclonus” but also by a variety of terms likely emphasizing the essential and idiopathic symptom of tinnitus.42 In 1998, the Movement Disorders Society decided that the disorder is best characterized as a form of “tremor.”49 Debate about EPT phenomenology and pathogenesis continues. While some have invoked a central tremor generator,50 others have maintained that EPT is a form of brainstem segmental myoclonus.46 Following their review of 103 cases, Zadikoff et al.43 concluded that the majority of cases of idiopathic myogenic tinnitus diagnosed as EPT were either psychogenic or a special skill.
Although the term “myoclonus” has also been invoked in MEM tinnitus, the inconsistent symptoms and their implied motor phenomenology similarly conform to no standard movement disorder classifications. It is likely that some of the clinical variability in MEM case literature reflects different underlying movements, which may include myoclonus, tremor, and dystonia, as well as different pathogenic processes ranging from nerve compression to conversion.
Middle ear myoclonus phenomenology and physiology: myoclonus
The presumptive phenomenon of MEM, myoclonus is characterized clinically as “sudden, brief, shock-like, involuntary movements.”46,51 It occurs in a variety of normal physiologic processes, neurologic disorders, and medical conditions, ranging from hiccoughs to juvenile myoclonic epilepsy to renal failure; and it is common among psychogenic movements.52 Myoclonus can be evaluated physiologically via EMG, electroencephalography (EEG), EMG–EEG back-averaging, and evoked potentials.53 Clinical neurophysiologic testing can further support classification of myoclonus by neuroanatomic localization, which may be cortical, cortical–subcortical, subcortical–supraspinal, spinal, and peripheral.46,51
Although the spare descriptions of MEM do not tend to include such terms as “shock-like” or “sudden,” cases of myogenic tinnitus that are largely irregular rather than rhythmic could reflect myoclonus of either middle ear muscle. While tympanometry demonstrating such irregularity may support a myoclonic tinnitus, further clinical neurophysiologic testing is not practical due the small size and anatomically restricted locations of the tensor tympani and stapedius muscles.
Stapedius or tensor tympani myoclonus could reflect irritability or aberrant activity of the facial or trigeminal nerves or nuclei, respectively. For example, several cases of MEM have been described in the setting of hemifacial spasm and following facial nerve injury,1 where pathologic peripheral nerve discharges and/or post-injury synkinesis could explain myoclonic activity of the stapedius. By analogy, irregular activity of the mandibular efferent branches of the trigeminal nerve, which is particularly susceptible to compression, could explain tensor tympani or tensor veli palatini myoclonus,54,55 akin to the sensory phenomenon of trigeminal neuralgia.55 Such nerve compression may also be associated with TMD and bruxism, which should be specifically sought in any case of myogenic tinnitus. Reports that physical manipulations, such as pressure on the zygomatic arch, can alleviate some MEM tinnitus symptoms provide further support of peripheral nerve compression-evoked tinnitus.4 Anatomic variability in the trigeminal and facial nerves may explain the tinnitus associated with usually unrelated, normal functions, such as tinnitus coincident with blinking.56
There is no imaging evidence of brainstem pathology to correlate with a source of segmental myoclonus in MEM tinnitus, and evoked potentials are not typically measured. Although it might be considered unusual for brainstem myoclonus to affect only one of the functionally related muscles supplied by the trigeminal or facial nerve nuclei, their topography supports this possibility. By analogy, it has been suggested that hemifacial spasm, which can be limited to individual muscles, may be mediated through aberrant facial nucleus activity.57,58 MEM tinnitus associated with focal brainstem myoclonus might be considered similar to proprospinal myoclonus, another hyperkinetic disorder of unclear, likely mixed, etiology.
The cortical and subcortical networks controlling hearing, swallowing, and speech that impinge on the middle ear muscles are complex and interrelated. There is no specific evidence of central myoclonic pathogenesis in MEM tinnitus; however, electroencephalograms are not typically performed. Auditory seizures causing central tinnitus should be considered a differential diagnosis of subjective tinnitus if symptoms are consistent with seizure semiology and there is no evidence of muscle or tympanic membrane movement. Epileptic tinnitus cerebri can be complex, including hearing songs or human voices, but has also been characterized as buzzing, hissing, or ringing, features similar to the somatosounds of myogenic tinnitus.59 Distinct from MEM tinnitus, auditory epileptic discharges usually spread, leading to olfactory, visual, language, and vertiginous symptoms, and tend to generalize.59 Auditory epilepsy is also often stimulus-sensitive, being provoked by sounds such as flowing water or telephone ring; however, reflex stimulation is common feature of psychogenic myoclonus and would not necessarily exclude a diagnosis of MEM.
Middle ear myoclonus phenomenology and physiology: tremor
There are a limited number of clinically and neurophysiologically distinct tremor types to be considered in the context of MEM tinnitus. The most common tremors are physiologic, essential, and parkinsonian.50 Rarer and poorly understood are palatal, dystonic, orthostatic, task- and position-specific, Holmes’, cerebellar, and neuropathic tremors.60 Among PMDs, tremor is the most common phenomenon.61 Tremors and their physiologic generators are characterized as mechanical, peripherally induced, or central according to the source of their oscillatory activity. Clinical neurophysiologic testing with EMG, accelerometry, and weighting helps make these distinctions.50 Because this objective testing is not readily applicable to middle ear muscles, any tremorogenic basis of MEM tinnitus must be derived from symptomatology and indirect physiologic testing, such as tympanometry.
The inconsistently reported rhythmicity and regularity of MEM tinnitus as well as the cogwheel and saw-toothed pattern on some tympanograms suggests that some cases of MEM tinnitus are due to tremor. The variable rhythmicity in EPT, which has been reported to range from 20 to 420 clicks per minute,42 is also suggestive of both physiologic and/or atypical tremor in this related disorder of peritubal myogenic tinnitus. Symptomatic treatment of MEM tinnitus with jaw position changes4 may suggest a position-specific tremor. The irregular, variable, and high-frequency tinnitus in both MEM and EPT is not suggestive of Parkinson or essential tremors, and clinical features do not correspond to orthostatic, task-specific, or Holmes’ tremors.
The mechanical oscillators that effect physiologic tremor may play an important role in some cases of MEM tinnitus. Mechanical systems such as a mass and spring oscillate with a frequency described by the formula
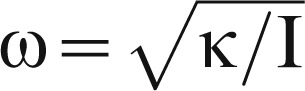 |
where ω is oscillatory frequency, κ is the stiffness of the spring, and Ι is the inertia of the mass. A muscle is a mechanical system analogous to a mass and spring, and when such a system is perturbed, it will oscillate at its resonant frequency. Such oscillation is a property of all muscles in the body and the cause of physiologic tremor. A common, frequent perturbation that can promote this mechanical tremor is the ballistocardiac impulse. Likewise, some cases of MEM tinnitus could be due to invoked physiologic tremor of the tensor tympani or stapedius muscles, which are located near the middle meningeal and internal carotid arteries, respectively, and may be particularly susceptible to this tremorogenic impulse due to their proximity to the rigid bony walls of middle ear. The parafunctional jaw movements of bruxism and common in TMD could also lead to evoked physiologic tremor in MEM tinnitus.
A clinically relevant feature of physiologic tremor, it is also subject to enhancement through peripheral and central nervous system-mediated reflex loops. Enhanced physiologic tremor may be stimulated by anxiety, stress, fatigue, medications, and metabolic derangements such as hyperthyroidism, hypoglycemia, and hypothermia,50 potentially useful variables to assess in the evaluation of patients with MEM. The phenomenon of enhanced physiologic tremor from exertion is particularly relevant to the specialists often diagnosing MEM: otolaryngologists are studying how to improve manual precision impaired by tremor during prolonged microsurgery.62–64
Middle ear myoclonus phenomenology and physiology: dystonia
Dystonia is not often discussed in the context of myogenic tinnitus;42 however, this poorly understood hyperkinetic movement disorder is appreciated among the oral motor disorders8 and could well be involved in MEM tinnitus. Dystonia is clinically characterized by abnormal movements and postures, which can be subtle to appreciate depending on the muscles involved.65 There is no standardized clinical testing for dystonia, and the pathophysiology is complex, with evidence suggesting dysfunctional peripheral sensory processing, increased central sensorimotor plasticity, and decreased central inhibition.65–67 In addition to the better known genetic, idiopathic, and drug-induced forms, dystonia has also been described following peripheral trauma,68 ranging from ulnar nerve overuse, task-specific dystonia in musicians69,70 to complex regional pain syndrome type II.71,72 Peripherally induced dystonia could be responsible for stapedius or tensor tympani MEM tinnitus following acoustic trauma, jaw trauma, bruxism, and/or TMD. Some forms of dystonia, which are often fixed and also frequently post trauma, are consistent with a psychogenic origin.72,73 Dystonia can be accompanied by tremor,74 which itself may cause myogenic tinnitus. Although the pathogenic relationship between dystonia and tremor is not well understood, it is not uncommon for dystonic tremor to be positional, as exemplified by tremor in cervical dystonia.
Interestingly, voluntary physical manipulations, such as mouth opening or pressure on the zygomatic arch, can alleviate some cases of MEM tinnitus,4 as well as EPT tinnitus.42 These maneuvers do not clearly reflect any unique mechanism of action. They might mediate position-dependent resolution of dystonic tremor. They could also diminish myogenic tinnitus secondary to changes in muscle tone, Eustachian tube patency, or pathogenic nerve compression, independent of the underlying hyperkinetic movement. Finally, they could simply relieve a psychogenic movement through cognitive distraction or a competing motor program.
Middle ear myoclonus phenomenology and physiology: psychogenic
Several features of MEM tinnitus suggest a psychogenic origin, which may occur independent of or in conjunction with the other potential pathophysiologic processes described above. Functional disorders are often debilitating, misdiagnosed, inappropriately considered “diagnoses of exclusion,” and costly to the patient and society.75 The phenomenology of a PMD can appear similar to any classic movement disorder but can also be quite unusual52. PMDs reflect an intact yet dysfunctional neurologic system, which produces often bizarre and predictably variable symptoms. Clinical features of PMDs generally reflect incongruency with standard movement disorder phenomenology, such as inconsistency during cognitive distraction or competing motor task and entrainability to external pacing. The diagnosis of a PMD should be made according to the criteria proposed by Fahn and Williams.17,18
Unfortunately, most reports of MEM tinnitus do not describe sufficient history or examination findings to assess distractibility, entrainability, or suggestibility. Case 1 provides a useful example of both symptoms and signs that characterize a clinically established diagnosis of PMD and functional myogenic tinnitus. These clinical features should be systematically assessed in cases of idiopathic myogenic tinnitus, and demonstration of variable and irregular tympanometry may further support a psychogenic disorder. This objective audiometric sign of functional myogenic tinnitus was previously described by Klochoff12 in the disorder he called “tensor tympani syndrome,” which he attributed to emotional distress and anxiety. Pirio Richardson et al.76 also described the diagnostic role of clinical neurophysiologic testing in psychogenic palatal tremor.
PMDs are described primarily in the neurology literature, where they are considered to have a psychological origin, and the psychiatry literature, where they are classified as somatoform disorders, most often being conversion.77 Whether tinnitus begets affective disorders and/or affective disorders beget tinnitus, there is a strong comorbid relationship between tinnitus, depression, and anxiety.20 Biological evidence of this association has been elucidated in recent studies demonstrating such neuroanatomic relationships as correlated abnormal primary auditory and limbic function in patients with tinnitus.26,27 Stress is prominent in some cases of MEM tinnitus,3 and either a primary or comorbid functional disorder should be specifically considered in cases of myogenic tinnitus with atypical movement phenomenology. While the psychodynamic basis of PMDs remains a subject of study,78 there is increasing appreciation of related genetic and environmental influences as well as reversible functional neuroanatomic lesions.52 Therefore, an integrated biopsychosocial approach to the evaluation of patients with MEM or other forms of idiopathic myogenic tinnitus is warranted.78
Conclusion
MEM is a diagnosis invoked to explain myogenic tinnitus caused solely by dysfunctional tensor tympani or stapedius movement. Rather than representing a single disorder, MEM tinnitus likely reflects a variety of acoustogenic mechanisms, hyperkinetic movement disorders, and psychogenic etiologies. Tensor tympani MEM tinnitus and tensor veli palatini EPT tinnitus share several features and may reflect common pathogenic origins. Since myogenic tinnitus directly reflects the underlying pathologic movements, detailed characterization of symptom onset, evolution, and phenomenology is imperative to help distinguish among the differential diagnoses and to direct therapy. Identifying a pathogenic peritubal muscle may require nasopharyngeal inspection and/or EMG testing. Brainstem evoked potentials might identify a pathologic lesion in otherwise idiopathic cases. If no myogenic or other somatic cause of subjective tinnitus is identified and seizure semiology is suspected, EEG should be performed. Multidisciplinary evaluation should involve neurologists, otolaryngologists, and dentists with expertise in movement disorders, middle ear disorders, and parafunctional jaw movements, respectively. During history, examination, and middle ear physiology testing, features of variability, distractibility, and entrainability should be assessed. Working knowledge of functional symptoms and a biopsychosocial approach to evaluation will facilitate the important diagnosis of a primary or comorbid psychogenic disorder. Irrespective of the initial diagnosis for myogenic tinnitus, routine neurologic follow-up is recommended.
Acknowledgments
The authors thank Carmen Brewer, PhD, from the National Institute on Deafness and Other Communication Disorders, NIH for reading this manuscript and providing helpful suggestions.
Footnotes
Funding: NINDS Intramural Research Program.
Conflict of Interests: The authors report no conflicts of interest.
Financial disclosures: None.
References
- 1.Bhimrao SK, Masterson L, Baguley D. Systematic review of management strategies for middle ear myoclonus. Otolaryngol Head Neck Surg. 2012;146:698–706. doi: 10.1177/0194599811434504. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Lohle E, Heinen F, Lucking C. Ear click in palatal tremor: its origin and treatment with botulinum toxin. Neurology. 1991;41:1677–1679. doi: 10.1212/WNL.41.10.1677. [DOI] [PubMed] [Google Scholar]
- 3.Badia L, Parikh A, Brookes GB. Management of middle ear myoclonus. J Laryngol Otol. 1994;108:380–382. doi: 10.1017/s0022215100126866. [DOI] [PubMed] [Google Scholar]
- 4.Chan C, Palaniappan R. Middle ear myoclonus: a new technique for suppression of spontaneous clicking tinnitus. Int Tinnitus J. 2010;16:51–54. [PubMed] [Google Scholar]
- 5.Cohen D, Perez R. Bilateral myoclonus of the tensor tympani: a case report. Otolaryngol Head Neck Surg. 2003;128:441. doi: 10.1067/mhn.2003.6. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira CA, Negreiros Junior J, Cavalcante IC, Bahmad F, Jr, Venosa AR. Palatal and middle-ear myoclonus: a cause for objective tinnitus. Int Tinnitus J. 2003;9:37–41. [PubMed] [Google Scholar]
- 7.Ramirez LM, Ballesteros LE, Sandoval GP. Tensor tympani muscle: strange chewing muscle. Med Oral Patol Oral Cir Bucal. 2007;12:E96–100. [PubMed] [Google Scholar]
- 8.Clark GT, Ram S. Four oral motor disorders: bruxism, dystonia, dyskinesia and drug-induced dystonic extrapyramidal reactions. Dent Clin North Am. 2007;51:225–243. viii–ix. doi: 10.1016/j.cden.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Myrhaug H. The incidence of ear symptoms in cases of malocclusion and temporo-mandibular joint disturbances. Br J Oral Surg. 1964;2:28–32. doi: 10.1016/S0007-117X(64)80004-4. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein JM, Mohl ND, Spiller H. Temporomandibular joint dysfunction masquerading as disease of ear, nose, and throat. Trans Am Acad Ophthalmol Otolaryngol. 1969;73:1208–1217. [PubMed] [Google Scholar]
- 11.Arlen H. The otomandibular syndrome: a new concept. Ear Nose Throat J. 1977;56:60–62. [PubMed] [Google Scholar]
- 12.Klochoff I. Impedance fluctuation and a “Tensor Tympani Syndrome”. In: Penha RL, Pizarro W, editors. Universidade Nova de Lisboa; Lisbon: 1981. pp. p 69–76. Proceedings of the Fourth International Symposium on Acoustic Impedance Measurement. [Google Scholar]
- 13.Liu HB, Fan JP, Lin SZ, Zhao SW, Lin Z. Botox transient treatment of tinnitus due to stapedius myoclonus: case report. Clin Neurol Neurosurg. 2011;113:57–58. doi: 10.1016/j.clineuro.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Chien HF, Sanchez TG, Sennes LU, Barbosa ER. Endonasal approach of salpingopharyngeus muscle for the treatment of ear click related to palatal tremor. Parkinsonism Relat Disord. 2007;13:254–256. doi: 10.1016/j.parkreldis.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Ensink RJ, Vingerhoets HM, Schmidt CW, Cremers CW. Treatment for severe palatoclonus by occlusion of the Eustachian tube. Otol Neurotol. 2003;24:714–716. doi: 10.1097/00129492-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 16.East CA, Hazell JW. The suppression of palatal (or intra-tympanic) myoclonus by tinnitus masking devices. A preliminary report. J Laryngol Otol. 1987;101:1230–1234. doi: 10.1017/S0022215100103573. [DOI] [PubMed] [Google Scholar]
- 17.Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol. 1988;50:431–455. [PubMed] [Google Scholar]
- 18.Williams DT, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol. 1995;65:231–257. [PubMed] [Google Scholar]
- 19.Ropper AH, Brown RH, editors. New York: McGraw-Hill; 2005. Deafness, dizziness, and disorders of dysequilibrium; p. p 251. [Google Scholar]
- 20.Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206:200–226. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Bartels H, Staal MJ, Albers FW. Tinnitus and neural plasticity of the brain. Otol Neurotol. 2007;28:178–184. doi: 10.1097/MAO.0b013e31802b3248. [DOI] [PubMed] [Google Scholar]
- 22.Bender KJ, Trussell LO. Synaptic plasticity in inhibitory neurons of the auditory brainstem. Neuropharmacology. 2011;60:774–779. doi: 10.1016/j.neuropharm.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sismanis A. Tinnitus. Curr Neurol Neurosci Rep. 2001;1:492–499. doi: 10.1007/s11910-001-0112-9. [DOI] [PubMed] [Google Scholar]
- 26.Han BI, Lee HW, Kim TY, Lim JS, Shin KS. Tinnitus: characteristics, causes, mechanisms, and treatments. J Clin Neurol. 2009;5:11–19. doi: 10.3988/jcn.2009.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herraiz C, Aparicio JM. Diagnostic clues in pulsatile tinnitus (somatosounds) Acta Otorrinolaringol Esp. 2007;58:426–433. doi: 10.1016/S0001-6519(07)74960-9. [DOI] [PubMed] [Google Scholar]
- 28.Hazell JW. Tinnitus. II: Surgical management of conditions associated with tinnitus and somatosounds. J Otolaryngol. 1990;19:6–10. [PubMed] [Google Scholar]
- 29.Ramirez Aristeguieta LM, Ballesteros Acuna LE, Sandoval Ortiz GP. Tensor veli palatini and tensor tympani muscles: anatomical, functional and symptomatic links. Acta Otorrinolaringol Esp. 2009;61:26–33. doi: 10.1016/j.otorri.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Mukerji S, Windsor AM, Lee DJ. Auditory brainstem circuits that mediate the middle ear muscle reflex. Trends Amplif. 2010;14:170–191. doi: 10.1177/1084713810381771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moller A. Function of the middle ear. In: Keidel WD, Neff WD, editors. New York: Springer-Verlag; 1974. Handbook of sensory physiology: auditory system. [Google Scholar]
- 32.Malkin DP. The role of TMJ dysfunction in the etiology of middle ear disease. Int J Orthod. 1987;25:20–21. [PubMed] [Google Scholar]
- 33.Politzer A. IF Enke: Stuttgart; 1878. Lehrbuch der Ohrenhelkunde. [Google Scholar]
- 34.Slack RW, Soucek SO, Wong K. Sonotubometry in the investigation of objective tinnitus and palatal myoclonus: a demonstration of eustachian tube opening. J Laryngol Otol. 1986;100:529–531. doi: 10.1017/S002221510009962X. [DOI] [PubMed] [Google Scholar]
- 35.Yetiser S, Kazkayasi M, Civitci D. Acoustic impedance study of peritubal myoclonus. Acta Otolaryngol. 2002;122:504–509. doi: 10.1080/00016480260092309. [DOI] [PubMed] [Google Scholar]
- 36.Barry DT, Cole NM. Muscle sounds are emitted at the resonant frequencies of skeletal muscle. IEEE Trans Biomed Eng. 1990;37:525–531. doi: 10.1109/10.55644. [DOI] [PubMed] [Google Scholar]
- 37.Barry DT. Muscle sounds from evoked twitches in the hand. Arch Phys Med Rehabil. 1991;72:573–575. [PubMed] [Google Scholar]
- 38.Hemmerling TM, Donati F, Beaulieu P, Babin D. Phonomyography of the corrugator supercilii muscle: signal characteristics, best recording site and comparison with acceleromyography. Br J Anaesth. 2002;88:389–393. doi: 10.1093/bja/88.3.389. [DOI] [PubMed] [Google Scholar]
- 39.Walker HK. Cranial nerve V: the trigeminal nerve. In: Walker HK, Hall WD, Hurst JW, editors. Boston: Butterworths; 1990. pp. p 318–321. Clinical methods: the history, physical, and laboratory examinations. 3rd edition. [PubMed] [Google Scholar]
- 40.Kim YS, Lee IH, Park JH, Lee BD. One case of objective tinnitus due to palatal and middle ear myoclonus. Korean J Otorhinolaryngol-Head Neck Surg. 2010;53:578–581. doi: 10.3342/kjorl-hns.2010.53.9.578. [DOI] [Google Scholar]
- 41.Howsam GD, Sharma A, Lambden SP, Fitzgerald J, Prinsley PR. Bilateral objective tinnitus secondary to congenital middle-ear myoclonus. J Laryngol Otol. 2005;119:489–491. doi: 10.1258/0022215054273223. [DOI] [PubMed] [Google Scholar]
- 42.Zadikoff C, Lang AE, Klein C. The ‘essentials’ of essential palatal tremor: a reappraisal of the nosology. Brain. 2006;129:832–840. doi: 10.1093/brain/awh684. [DOI] [PubMed] [Google Scholar]
- 43.Deuschl G, Toro C, Hallett M. Symptomatic and essential palatal tremor. 2. Differences of palatal movements. Mov Disord. 1994;9:676–678. doi: 10.1002/mds.870090615. [DOI] [PubMed] [Google Scholar]
- 44.Jamieson DR, Mann C, O’Reilly B, Thomas AM. Ear clicks in palatal tremor caused by activity of the levator veli palatini. Neurology. 1996;46:1168–1169. doi: 10.1212/WNL.46.4.1168. [DOI] [PubMed] [Google Scholar]
- 45.Deuschl G, Toro C, Valls-Sole J, Zeffiro T, Zee DS, Hallett M. Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain. 1994;117:775–788. doi: 10.1093/brain/117.4.775. [DOI] [PubMed] [Google Scholar]
- 46.Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4:47–62. doi: 10.1177/1756285610395653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulkarni PK, Muthane UB, Taly AB, Jayakumar PN, Shetty R, Swamy HS. Palatal tremor, progressive multiple cranial nerve palsies, and cerebellar ataxia: a case report and review of literature of palatal tremors in neurodegenerative disease. Mov Disord. 1999;14:689–693. doi: 10.1002/1531-8257(199907)14:4<689::AID-MDS1022>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Krause E, Heinen F, Gurkov R. Difference in outcome of botulinum toxin treatment of essential palatal tremor in children and adults. Am J Otolaryngol. 2008;31:91–95. doi: 10.1016/j.amjoto.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13:2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 50.Hallett M. Overview of human tremor physiology. Mov Disord. 1998;13:43–48. doi: 10.1002/mds.870131308. [DOI] [PubMed] [Google Scholar]
- 51.Caviness JN, Brown P. Myoclonus: current concepts and recent advances. Lancet Neurol. 2004;3:598–607. doi: 10.1016/S1474-4422(04)00880-4. [DOI] [PubMed] [Google Scholar]
- 52.Ellenstein A, Kranick SM, Hallett M. An update on psychogenic movement disorders. Curr Neurol Neurosci Rep. 2011;11:396–403. doi: 10.1007/s11910-011-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toro C, Hallett M. Pathophysiology of myoclonic disorders. In: Watts RL, Standaert D, Obeso JA, editors. New York: McGraw Hill; 2011. pp. p 761–779. Movement disorders. 3rd edition. [Google Scholar]
- 54.Piagkou M, Demesticha T, Skandalakis P, Johnson EO. Functional anatomy of the mandibular nerve: consequences of nerve injury and entrapment. Clin Anat. 2011;24:143–150. doi: 10.1002/ca.21089. [DOI] [PubMed] [Google Scholar]
- 55.Hunt K, Patwardhan R. Trigeminal neuralgia: a modern-day review. Int Rev Neurobiol. 2007;79:621–631. doi: 10.1016/S0074-7742(07)79027-X. [DOI] [PubMed] [Google Scholar]
- 56.Ohki M, Kato H. Idiopathic tinnitus concomitant with eye closure. Otol Neurotol. 2012;33:267–269. doi: 10.1097/MAO.0b013e318241c25d. [DOI] [PubMed] [Google Scholar]
- 57.Ferguson JH. Hemifacial spasm and the facial nucleus. Ann Neurol. 1978;4:97–103. doi: 10.1002/ana.410040202. [DOI] [PubMed] [Google Scholar]
- 58.Esteban A, Molina-Negro P. Primary hemifacial spasm: a neurophysiological study. J Neurol Neurosurg Psychiatry. 1986;49:58–63. doi: 10.1136/jnnp.49.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nobile C, Michelucci R, Andreazza S, Pasini E, Tosatto SC, Striano P. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum Mutat. 2009;30:530–536. doi: 10.1002/humu.20925. [DOI] [PubMed] [Google Scholar]
- 60.Lucking CH, Hellwig B. Uncommon tremors. In: Hallett M, editor. New York: Elsevier; –2003.pp. p 261–279. editor. Handbook of clinical neurophysiology. Vol. 1. [Google Scholar]
- 61.Bhatia KP, Schneider SA. Psychogenic tremor and related disorders. J Neurol. 2007;254:569–574. doi: 10.1007/s00415-006-0348-z. [DOI] [PubMed] [Google Scholar]
- 62.Slack PS, Coulson CJ, Ma X, Pracy P, Parmar S, Webster K. The effect of operating time on surgeon’s hand tremor. Eur Arch Otorhinolaryngol. 2009;266:137–141. doi: 10.1007/s00405-008-0714-9. [DOI] [PubMed] [Google Scholar]
- 63.Slack PS, Ma X. Time dependency assessment of muscular fatigue index and hand tremor under operating conditions. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:4822–4825. doi: 10.1109/IEMBS.2007.4353419. [DOI] [PubMed] [Google Scholar]
- 64.Coulson CJ, Slack PS, Ma X. The effect of supporting a surgeon's wrist on their hand tremor. Microsurgery. 2010;30:565–568. doi: 10.1002/micr.20776. [DOI] [PubMed] [Google Scholar]
- 65.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 66.Hallett M, Rothwell J. Milestones in clinical neurophysiology. Mov Disord. 2011;26:958–967. doi: 10.1002/mds.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Rooijen DE, Geraedts EJ, Marinus J, Jankovic J, van Hilten JJ. Peripheral trauma and movement disorders: a systematic review of reported cases. J Neurol Neurosurg Psychiatry. 2011;82:892–898. doi: 10.1136/jnnp.2010.232504. [DOI] [PubMed] [Google Scholar]
- 69.Charness ME, Ross MH, Shefner JM. Ulnar neuropathy and dystonic flexion of the fourth and fifth digits: clinical correlation in musicians. Muscle Nerve. 1996;19:431–437. doi: 10.1002/mus.880190403. [DOI] [PubMed] [Google Scholar]
- 70.Ross MH, Charness ME, Lee D, Logigian EL. Does ulnar neuropathy predispose to focal dystonia? Muscle Nerve. 1995;18:606–611. doi: 10.1002/mus.880180607. [DOI] [PubMed] [Google Scholar]
- 71.Maihofner C, Birklein F. Complex regional pain syndromes: new aspects on pathophysiology and therapy. Fortschr Neurol Psychiatr. 2007;75:331–342. doi: 10.1055/s-2006-944310. [DOI] [PubMed] [Google Scholar]
- 72.Schrag A, Trimble M, Quinn N, Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain. 2004;127:2360–2372. doi: 10.1093/brain/awh262. [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim NM, Martino D, van de Warrenburg BP, et al. The prognosis of fixed dystonia: a follow-up study. Parkinsonism Relat Disord. 2009;15:592–597. doi: 10.1016/j.parkreldis.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 74.Deuschl G. Dystonic tremor. Rev Neurol. 2003;159:900–905. [PubMed] [Google Scholar]
- 75.Hallett M. Psychogenic movement disorders: a crisis for neurology. Curr Neurol Neurosci Rep. 2006;6:269–271. doi: 10.1007/s11910-006-0015-x. [DOI] [PubMed] [Google Scholar]
- 76.Pirio Richardson S, Mari Z, Matsuhashi M, Hallett M. Psychogenic palatal tremor. Mov Disord. 2006;21:274–276. doi: 10.1002/mds.20731. [DOI] [PubMed] [Google Scholar]
- 77.Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109–116. doi: 10.1016/j.psym.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kranick SM, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Mov Disord. 2011;26:1844–1850. doi: 10.1002/mds.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gray H. Philadelphia: Lea & Febiger; 1918. Anatomy of the human body. www.bartleby.com/107/ [Google Scholar]