Abstract
Background
To determine whether botulinum toxin treatment history affected the outcomes of a study comparing the safety and efficacy of incobotulinumtoxinA with placebo in subjects with cervical dystonia (CD).
Methods
This was a prospective, double-blind, randomized, placebo-controlled, multicenter trial in botulinum toxin-treated or toxin-naïve CD subjects. Subjects received a fixed dose of either 120 U or 240 U of incobotulinumtoxinA or placebo. The primary outcome measure was change from baseline to Week 4 in the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) total score. Treatment-emergent adverse events (TEAEs) were also evaluated. This report represents a subgroup analysis of botulinum toxin-treated or toxin-naïve subjects.
Results
Participants (N = 233; 38.6% toxin-naïve) had a mean age of 52.8 years. IncobotulinumtoxinA significantly improved TWSTRS total scores from baseline to Week 4 in both dose groups versus placebo, and the improvement persisted through the end of the study (≤20 weeks). Both the previously toxin-treated and toxin-naïve subjects demonstrated significant improvements in TWSTRS total scores at Week 4 compared to baseline. The most frequent TEAEs in the incobotulinumtoxinA groups were dysphagia, neck pain, and muscular weakness, which were generally mild. TEAEs were more common in the 240 U group and toxin-naïve subjects.
Discussion
Overall, incobotulinumtoxinA was safe and effective in CD, regardless of toxin therapy history. A lower starting dose may be better tolerated among toxin-naïve subjects without sacrificing efficacy.
Keywords: Botulinum toxin type A, cervical dystonia, incobotulinumtoxinA, pretreated, treatment-naïve
Introduction
Cervical dystonia (CD), the most common focal form of dystonia,1 is marked by contractions of neck and shoulder musculature that affect head motion and posture.2,3 Up to three-fourths of patients experience pain, which is an important cause of disability.2,4 Class A evidence has established botulinum toxin treatment as an effective means of controlling CD symptoms.3 IncobotulinumtoxinA (marketed as XEOMIN® in the United States, Canada, and Europe; Merz Pharmaceuticals, GmbH, Frankfurt, Germany) is a botulinum toxin serotype A that differs from other available formulations in that the botulinum toxin complex is purified from the culture supernatant, and the active ingredient is then separated from accessory (complexing) proteins (hemagglutinins and non-hemagglutinins) through a series of steps, yielding only the active neurotoxin (molecular weight of 150 kDa).5,6
The specific aim of this report is to compare symptom improvement and tolerability associated with a single injection of incobotulinumtoxinA for CD in subjects with and without a history of botulinum toxin therapy. This report is a subgroup analysis of the previously published full clinical trial.7
Methods
This was a prospective, multicenter, double-blind, randomized, placebo-controlled study conducted at 37 sites in the United States. The primary outcome of this study has been previously reported by Comella and colleagues.7 Each site’s Institutional Review Board approved the study protocol and the informed consent process. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and is consistent with Good Clinical Practice and the applicable regulatory requirements. Prior to screening, all subjects provided written informed consent. The study was registered with clinicaltrials.gov (Identification number: NCT00407030).
Study subjects. All subjects were outpatients age 18 to 75 years old with a clinical diagnosis of primary CD of predominantly rotational form, a Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS)8,9 total score ≥20 (out of 85), a TWSTRS severity subscore ≥10 (out of 35), a TWSTRS disability subscore ≥3 (out of 30), and a TWSTRS pain subscore ≥1 (out of 20). Subjects previously treated with botulinum toxin were eligible if the last two injection sessions were adequately documented, the therapeutic response had been stable, and at least 10 weeks had passed since the most recent session. Secondary non-responders to botulinum toxin type A or B were excluded, and subjects were excluded if they had been previously treated with incobotulinumtoxinA. Further details of the inclusion/exclusion criteria are provided in the full clinical trial.7
Randomization, study drug, and visits. Subjects were randomly allocated to equal-sized groups for a single intramuscular injection session of placebo or of a fixed total dosage of 120 U or 240 U incobotulinumtoxinA. (For additional details, see the full published clinical trial.7)
Efficacy assessments. The primary efficacy measure was the change in TWSTRS total score at 4 weeks compared with baseline. Secondary efficacy measures included change in TWSTRS total score at 8 weeks and the final visit; change in TWSTRS severity, disability, and pain subscores at 4 and 8 weeks after injection and at the final visit; and a global self-assessment of efficacy as rated by each subject on a nine-point scale10 at their final visit.7 (For additional details, see the full published clinical trial.7)
Safety assessments. Adverse events were evaluated during each visit and telephone contact. (For additional details, see the full published clinical trial.7)
Statistical analyses. A total of 222 subjects was planned, including at least 87 (or approximately 40%) who were naïve to botulinum toxin therapy. Previously reported comparisons between treatment groups were performed by using a fixed sequence test procedure (step downward) in the intent to treat (ITT) population. This procedure negates the need for type I error adjustment, and all tests of the fixed sequence test procedure were performed two-sided with a type-1 error of α = 0.05.7 (See the full published clinical trial for additional details.7)
For efficacy outcomes, subgroup analyses of previously treated versus toxin-naïve subjects were subjected to analysis of covariance (ANCOVA), log-rank or Mann-Whitney test, as appropriate. The analyses included in this manuscript were pre-planned and included in the statistical analysis plan; however, it is important to note that this study was not powered to detect differences between the two subgroups (i.e., previously treated versus toxin-naïve subjects).
Results
Baseline characteristics and subject disposition. Subjects’ baseline characteristics are summarized by treatment group and treatment history in Table 1. The median CD duration (time since first symptoms) and the median time since first diagnosis were shorter for toxin-naïve than for previously treated subjects. Across all treatment groups, toxin-naïve subjects were slightly younger than previously treated subjects.
Table 1. Subjects' Demographic and Baseline Cervical Dystonia Characteristics by Treatment Group and Treatment History (ITT Population).
| IncobotulinumtoxinA 120 U Group | IncobotulinumtoxinA 240 U Group | Placebo Group | ||||
|---|---|---|---|---|---|---|
| Toxin-Naïve N = 31 | Previously Treated = 47 | Toxin-Naïve N = 31 | Previously Treated N = 50 | Toxin-Naïve N = 28 | Previously Treated N = 46 | |
| Sex, n (% of subgroup) | ||||||
| Females | 21 (67.7) | 30 (63.8) | 23 (74.2) | 31 (62.0) | 17 (60.7) | 32 (69.6) |
| Males | 10 (32.3) | 17 (36.2) | 8 (25.8) | 19 (38.0) | 11 (39.3) | 14 (30.4) |
| Age, mean (SD) | 50.9 (11.9) | 54.0 (11.2) | 50.9 (12.1) | 54.7 (12.1) | 51.5 (10.5) | 53.0 (11) |
| Race, n (% of subgroup) | ||||||
| White | 31 (100) | 43 (91.5) | 28 (90.3) | 46 (92.0) | 24 (85.7) | 41 (89.1) |
| Hispanic or Latino | 0 | 3 (6.4) | 2 (6.5) | 3 (6.0) | 2 (7.1) | 0 |
| Black | 0 | 0 | 1 (3.2) | 1 (2.0) | 2 (7.1) | 2 (4.3) |
| Other | 0 | 1 (2.1) | 0 | 0 | 0 | 3 (6.5) |
| Weight (lb), median | 161.9 | 191.0 | 158.1 | 161.0 | 165.0 | 164.0 |
| BMI (kg/m2), median | 24.7 | 28.7 | 24.7 | 24.3 | 27.4 | 26.8 |
| Estimated duration of CD (months), median | 72.0 | 92.0 | 60.0 | 102.0 | 60.0 | 122.5 |
| Toxin injections since first diagnosis, (number) mean (SD) | n/a | 13.3 (12.4) | n/a | 17.7 (17.1) | n/a | 15.5 (11.4) |
| Time since most recent toxin injection (months), median | n/a | 3.6 | n/a | 4.0 | n/a | 3.9 |
| Botulinum toxin type and mean doses of last injection prior to study entry | ||||||
| OnabotulinumtoxinA | ||||||
| Mean (SD) | 219.4 (74.4) | 222.8 (78.3) | 232.5 (72.4) | |||
| ≤ 120 U | n/a | 7 (17.5) | n/a | 6 (14.0) | n/a | 4 (10.5) |
| > 120 U to 180 U | n/a | 3 (7.5) | n/a | 3 (7.0) | n/a | 4 (10.5) |
| > 180 U | n/a | 30 (75.0) | n/a | 34 (79.1) | n/a | 30 (79.0) |
| RimabotulinumtoxinB | ||||||
| Mean (SD) | 12000 (500) | 10875 (2250.0) | 10200 (2049.4) | |||
| ≤ 6,000 U | n/a | 0 | n/a | 0 | n/a | 0 |
| > 6,000 U to 12,000 U | n/a | 2 (66.7) | n/a | 4 (100.0) | n/a | 5 (100.0) |
| > 12,000 U | n/a | 1 (33.3) | n/a | 0 | n/a | 0 |
| AbobotulinumtoxinA | ||||||
| Mean (SD) | 500 (0) | 550 (409.3) | – | |||
| ≤ 360 U | n/a | 0 | n/a | 1 (33.3) | n/a | 0 |
| > 360 U to 540 U | n/a | 2 (100) | n/a | 1 (33.3) | n/a | 0 |
| > 540 U | n/a | 0 | n/a | 1 (33.3) | n/a | 0 |
Frequencies and percentages are based on non-missing values.
Abbreviations: CD, cervical dystonia; ITT, intent to treat; N/n, total subject population/subset of total subject population; %, percentage; SD, standard deviation; U, Units.
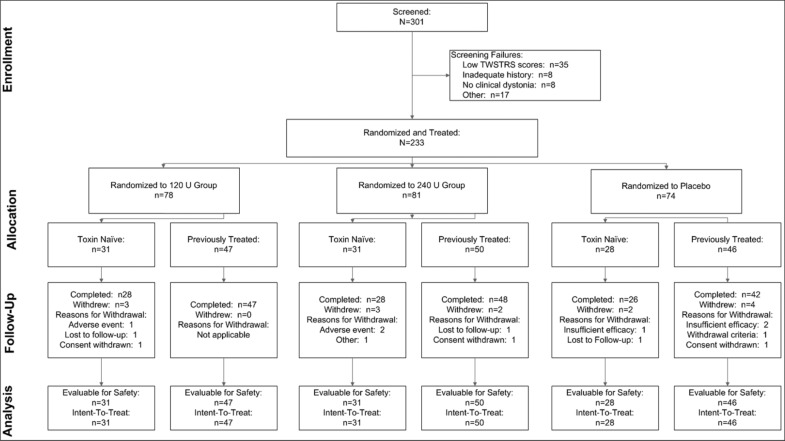
Of the 301 subjects screened, 233 were randomized to treatment (ITT population), including 90 subjects (38.6%) who were naïve to botulinum toxin treatment. In all, 81 (34.8%) subjects were injected with 240 U, 78 (33.5%) with 120 U, and 74 (31.8%) with placebo. Subject disposition throughout the study is diagrammed in Figure 1. Among ITT subjects, 219 (94.0%) completed the study. Among the 233 subjects in the ITT population, 18 subjects had one or more major protocol deviations, most often concerning study medication administration (seven subjects), leaving a treated-per-protocol (TPP) population of 215 subjects.
Figure 1. Subject Disposition.
Previously treated subjects at the most recent session had received a mean toxin dose of 225 U of onabotulinumtoxinA, 530 U of abobotulinumtoxinA, or 10,875 U of rimabotulinumtoxinB. A total of 85% had been previously treated with onabotulinumtoxinA. Most previously treated subjects (65.3%) had received high doses of botulinum toxin (>180 U of onabotulinumtoxinA, >12,000 U of rimabotulinumtoxinB, or >540 U of abobotulinumtoxinA) prior to entering the trial (Table 1).
Efficacy.
Overall
The mean (SD) decrease (improvement) in TWSTRS total score from baseline to Week 4 in the ITT population was –10.9 (11.7) points in the 240 U group and –9.9 (10.4) in the 120 U group, versus –2.2 (7.3) in the placebo group (p < 0.001 for each comparison with placebo, and p = 0.447 for comparison of the two dose groups).7 For additional details, see the full published clinical trial.7
Toxin-Naïve versus Placebo
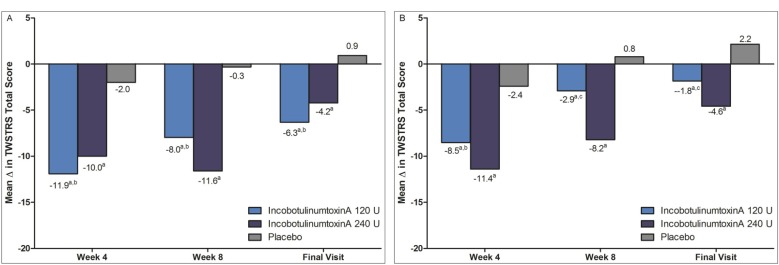
Among toxin-naïve subjects, the mean improvements in TWSTRS total score at Week 4, Week 8, and the final visit was superior to that for placebo at all time points for both dose groups (Table 2 and Figure 2a).
Table 2. Efficacy Assessments.
| IncobotulinumtoxinA 120 U Group | IncobotulinumtoxinA 240 U Group | Placebo Group | ||||
|---|---|---|---|---|---|---|
| Toxin-Naïve N = 31 | Previously Treated N = 47 | Toxin-Naïve N = 31 | Previously Treated N = 50 | Toxin-Naïve N = 28 | Previously Treated N = 46 | |
| TWSTRS Scores: Baseline Values and 4-Week Change by Treatment Group and Treatment History (ITT Population; missing values replaced by baseline values) | ||||||
| TWSTRS Total Score | ||||||
| Baseline, mean (SD) | 41.9 (9.7) | 43.1 (9.7) | 40.1 (9.2) | 43.4 (9.3) | 41.3 (6.5) | 42.0 (8.7) |
| Δ at Week 4, mean (SD) | −11.9 (11.1) | −8.5 (9.7) | −10.0 (9.2) | −11.4 (13.1) | −2.0 (6.0) | −2.4 (8.1) |
| p-value (ANCOVA) | ||||||
| Treatment vs. Placebo | <0.001 | 0.002 | <0.001 | <0.001 | n/a | n/a |
| 120 U vs. 240 U | 0.405 | 0.135 | n/a | n/a | n/a | n/a |
| TWSTRS Severity Score | ||||||
| Baseline, mean (SD) | 17.2 (4.8) | 18.6 (4.0) | 17.1 (3.8) | 19.6 (3.9) | 18.1 (3.3) | 19.3 (3.5) |
| Δ at Week 4, mean (SD) | −4.1 (4.3) | −3.7 (4.4) | −5.4 (5.5) | −5.6 (6.4) | −1.9 (4.5) | −1.9 (3.7) |
| p-value (ANCOVA) | ||||||
| Treatment vs. Placebo | 0.075 | 0.051 | 0.011 | <0.001 | n/a | n/a |
| 120 U vs. 240 U | 0.349 | 0.071 | n/a | n/a | n/a | n/a |
| TWSTRS Disability Score | ||||||
| Baseline, mean (SD) | 12.5 (5.2) | 13.4 (3.9) | 12.0 (4.6) | 12.8 (4.7) | 11.9 (3.1) | 11.7 (4.4) |
| Δ at Week 4, mean (SD) | −4.3 (5.9) | −2.6 (3.7) | −3.0 (4.0) | −3.0 (4.6) | 0.4 (3.0) | −0.2 (3.6) |
| p-value (ANCOVA) | ||||||
| Treatment vs. Placebo | <0.001 | 0.015 | 0.001 | 0.001 | n/a | n/a |
| 120 U vs. 240 U | 0.183 | 0.424 | n/a | n/a | n/a | n/a |
| TWSTRS Pain Score | ||||||
| Baseline, mean (SD) | 12.2 (3.8) | 11.1 (4.1) | 10.9 (4.4) | 11.0 (3.7) | 11.2 (3.8) | 11.0 (3.8) |
| Δ at Week 4, mean (SD) | −3.5 (4.0) | −2.2 (4.9) | −1.6 (4.6) | −2.9 (4.2) | −0.4 (2.8) | −0.3 (3.1) |
| p-value (ANCOVA) | ||||||
| Treatment vs. Placebo | 0.006 | 0.035 | 0.185 | <0.001 | n/a | n/a |
| 120 U vs. 240 U | 0.215 | 0.397 | n/a | n/a | n/a | n/a |
| Subject Self-Ratings of Global Response at Final Visit (ITT population; missing values replaced by no effect [value = 0 or unchanged]) | ||||||
| Toxin-Naïve N = 31 | Previously Treated N = 47 | Toxin-Naïve N = 31 | Previously Treated N = 50 | Toxin-Naïve N = 28 | Previously Treated N = 46 | |
| Global Response Rating | +2.0 | +0.9 | +1.4 | +1.3 | +0.3 | −0.4 |
| p-value (ANCOVA) | ||||||
| Treatment vs. Placebo | <0.001 | <0.001 | 0.009 | <0.001 | n/a | n/a |
| 120 U vs. 240 U | 0.101 | 0.228 | n/a | n/a | n/a | n/a |
Subject evaluation of global response scores: +4, complete abolishment of all signs and symptoms; +3, marked improvement; +2, moderate improvement; +1, slight improvement; 0, unchanged; −1, slight worsening; −2, moderate worsening; −3, marked worsening; −4, very marked worsening.
p-value: Analysis of Covariance (ANCOVA) based upon the Full Treatment Model.
Abbreviations: Δ, change; ITT, intent to treat; N/n, total subject population/subset of total subject population; %, percentage; SD, standard deviation; U, units.
Figure 2. Mean changes in TWSTRS Total Score, by Treatment Group and Treatment History.
Figure 2A. Toxin-Naïve Subjects. Figure 2B. Previously Toxin-Treated Subjects. ap<0.05 treatment group versus placebo; bp>0.05 120 U versus 240 U; cp<0.05 120 U versus 240 U; p-value: Analysis of Covariance (ANCOVA) – change from baseline in TWSTRS total score; missing values replaced by baseline value [full model].
Previously Treated versus Placebo
Among previously treated subjects, the mean improvements in TWSTRS total score at Week 4, Week 8, and the final visit was superior to that for placebo at all time points for both dose groups (Table 2 and Figure 2b).
Previously Treated versus Naïve
In the 120 U group, previously treated subjects showed a steeper, step-wise deterioration in their mean TWSTRS total score from baseline to month-to-month post-treatment (–8.5 points at Week 4, –3.8 at Week 8, and –1.8 at the final visit) compared to the treatment-naïve subjects, who showed a lesser degree of deterioration from month-to-month post-treatment (–11.9 and Week 4; −8.0 at Week 8, and −6.3 points at the final visit). Similarly for the 240 U group, the deterioration in the mean TWSTRS total score from baseline was gradual and step-wise from month-to-month for previously treated subjects ( –11.4 points at Week 4, –8.3 at Week 8, and –4.8 at the final visit), whereas deterioration in the mean TWSTRS total score among treatment-naïve subjects was noted only during the final visit and not in the first 2 months (–10.0 points at Week 4, –11.6 at Week 8, and –4.2 at the final visit).
Safety. Overall, AEs occurred in 56.8% of the 240 U group, 55.1% of the 120 U group, and 45.9% of the placebo group, making their incidence higher for active treatment than for placebo but with little difference between dosages. Among subjects receiving active treatment, the incidence of AEs was numerically higher in toxin-naïve than in previously treated subjects in the 240 U group (Table 3). The AE incidence in the 120 U group was lower in the toxin-naïve group than in the previously treated group. In toxin-naïve subjects, the incidence of AEs increased with dose. Muscular weakness and neck pain were more common in toxin-naïve than in previously treated subjects in the 240 U group. Dysphagia was the most common AE (active treatment > placebo, 240 U > 120 U, toxin-naïve > previously treated subjects). However, the investigators’ assessments of global treatment tolerability performed by IGAT at each subject’s final visit did not identify any significant differences among treatment groups.
Table 3. Treatment-Emergent Adverse Events (TEAEs) by Treatment Group and Treatment History (ITT Population).
| IncobotulinumtoxinA 120 U Group | IncobotulinumtoxinA 240 U Group | Placebo Group | ||||
|---|---|---|---|---|---|---|
| Toxin-Naïve N = 31 n (%) | Previously Treated N = 47 n (%) | Toxin-Naïve N = 31 n (%) | Previously Treated N = 50 n (%) | Toxin-Naïve N = 28 n (%) | Previously Treated N = 46 n (%) | |
| Any TEAE | 17 (54.8) | 26 (55.3) | 22 (71.0) | 24 (48.0) | 16 (5.71) | 18 (39.1) |
| Any adverse drug reaction | 12 (38.7) | 16 (34.0) | 18 (58.1) | 11 (22.0) | 7 (25.0) | 4 (8.7) |
| Any serious TEAE | 1 (3.2) | 3 (6.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any TEAE leading to discontinuation | 0 (0.0) | 1 (2.1) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Treatment Emergent AEs by MEDRA1 preferred term in ≥ 5% of subjects in any treatment group | ||||||
| Dysphagia2 | 5 (16.1) | 4 (8.5) | 7 (22.6) | 8 (16.0) | 0 (0.0) | 2 (4.3) |
| Neck pain | 1 (3.2) | 3 (6.4) | 7 (22.6) | 5 (10.0) | 1 (3.6) | 2 (4.3) |
| Muscular weakness | 2 (6.5) | 3 (6.4) | 7 (22.6) | 2 (4.0) | 1 (3.6) | 0 (0.0) |
| Injection site pain | 3 (9.7) | 4 (8.5) | 2 (6.5) | 1 (2.0) | 4 (14.3) | 0 (0.0) |
| Musculoskeletal pain | 3 (9.7) | 3 (6.4) | 2 (6.5) | 1 (2.0) | 0 (0.0) | 1 (2.2) |
| Headache | 0 (0.0) | 3 (6.4) | 2 (6.5) | 2 (4.0) | 3 (10.7) | 0 (0.0) |
| Nausea | 0 (0.0) | 2 (4.3) | 3 (9.7) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal stiffness | 1 (3.2) | 0 (0.0) | 3 (9.7) | 1 (2.0) | 1 (3.6) | 0 (0.0) |
| Sinusitis | 2 (6.5) | 0 (0.0) | 2 (6.5) | 1 (2.0) | 0 (0.0) | 2 (4.3) |
| Muscle spasms | 0 (0.0) | 1 (2.1) | 2 (6.5) | 1 (2.0) | 1 (3.6) | 1 (2.2) |
| Pharyngolaryngeal pain | 0 (0.0) | 2 (4.3) | 1 (3.2) | 1 (2.0) | 2 (7.1) | 0 (0.0) |
| Myalgia | 1 (3.2) | 0 (0.0) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nasopharyngitis | 1 (3.2) | 2 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (10.9) |
| Pain in extremity | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (6.0) | 0 (0.0) | 0 (0.0) |
| Paresthesia | 0 (0.0) | 0 (0.0) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Toothache | 2 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Medical Dictionary for Regulatory Activities, version 9.1. The AE types shown are those that affected ≥5% of any subgroup and are listed in descending order of frequency among all active-treatment recipients.
Subjects were specifically asked to report any swallowing difficulties using a 5-point dysphagia scale.
Abbreviations: N/n, total subject population/subset of total subject population; %, percentage; U, units.
Four serious AEs occurred in the 240 U group (three in toxin-naïve subjects and one in a previously treated subject). The events were staphylococcal infection, appendicitis, asthma, and chronic obstructive pulmonary disease, which were all judged to be unrelated to the trial medication. AEs leading to discontinuation occurred in three subjects, two of whom were toxin-naïve recipients of 240 U. One of them experienced musculoskeletal pain, neck pain, and muscle weakness, and the other experienced muscle weakness. The third was a previously treated recipient of 120 U who experienced nausea and dizziness.
Discussion
Regardless of toxin treatment history, an injection session of incobotulinumtoxinA was effective in subjects with CD in both dose groups. Based on the primary outcome measure (TWSTRS total score), both the 120 U and 240 U doses produced significant improvement compared with placebo as assessed at 4 weeks among previously treated and toxin-naïve subjects. Regardless of toxin treatment history, incobotulinumtoxinA was well tolerated. Although our study was not powered to detect a difference between the two active treatment groups, several themes emerge from these subgroup post-hoc analyses.
First, previously treated subjects – whose history included a mean of more than 15 injection sessions and a mean toxin dose, at the most recent session, of 224.7 U of onabotulinumtoxinA or 10,875 U of rimabotulinumtoxinB – tended to have a more pronounced response on most efficacy measures with the 240 U dose, whereas this trend was not apparent among toxin-naïve subjects. For example, the mean 4-week improvement among previously treated subjects was 11.4 points on the TWSTRS total score in the 240 U group and 8.5 points in the 120 U group. This result is not surprising given that many of the subjects randomized to the 120 U group received about half of their normal dose. In contrast, this trend was not noted among toxin-naïve subjects. By several measures – TWSTRS total score at all time points, disability and pain subscores at 4 weeks, and patient ratings of global improvement – the response was more pronounced at the lower dose. However, these differences did not reach statistical significance. Therefore, there was no added benefit of the higher dose among toxin-naïve subjects compared to previously treated subjects. These findings support current recommendations in clinical practice of initiating botulinum toxin treatment at lower dosages in naïve patients and then optimizing the dose during follow-up treatment.
Second, the superiority of 240 U over 120 U in improving the mean total TWSTRS score was mostly due to the severity subscale rather than the disability and pain subscale. It is therefore possible that the toxin dose required to reduce pain and disability may not be as high as the dose required to further improve the degree of neck, chin and shoulder displacement (as measured in the severity subscale).
Third, AEs were more common in the toxin-naïve subgroup compared to the previously treated subgroup, especially in incobotulinumtoxinA 240 U patients (71% versus 48%, respectively); whereas the overall AEs were similar in the incobotulinumtoxinA 120 U group patients (55% versus 55%, respectively). The explanation may lie in an increased tolerance among patients repeatedly treated with a medication, which in turn may be related to factors including changes in physiologic response. In this study, there was a double-blind extension in which the highest incidences of AEs were observed during the first injection interval.11 Incidences of AEs tended to decrease with subsequent injections or remained at constant levels throughout the double-blind extension, suggesting that there was no cumulative effect.
As alternative explanations to the development of tolerance, there might have been a treatment-group bias in attentiveness to events (i.e., the previously treated group may have become familiar with AEs and may no longer be concerned with their symptoms compared with the toxin-naïve group, thereby affecting reporting patterns). An additional bias, seen in the context of clinical trials, is that patients who have previously experienced adverse reactions to toxin treatments are less likely to volunteer as subjects.
In any case, no evidence of new, unexpected AEs observed. Overall, incobotulinumtoxinA was effective and safe in the treatment of CD, regardless of botulinum toxin treatment history, as assessed using the primary outcome of TWSTRS total score 4 weeks after injection.
It should be noted that subjects received fixed doses of incobotulinumtoxinA in order to meet the regulatory authority-specified requirements. Thus, the study design does not reflect the doses typically used in clinical practice. Botulinum toxin dosing should be individualized to meet a patient’s needs while also taking into account the patient’s botulinum toxin treatment history.
Acknowledgements
The authors would like to thank the subjects and their families who participated in this study. The authors would also like to thank the investigators who participated in this study (Appendix 1) and acknowledge the technical writing contributions of Linnea Elliott of The Curry Rockefeller Group and Starr L. Grundy, B.Sc. Pharm., of SD Scientific, Inc., funded by Merz Pharmaceuticals, LLC.
*Appendix 1 (Investigators):
U.S. XEOMIN Cervical Dystonia Study Group: R. Barbano, A. Brashear, M. Brodsky, M. Chehrenama, C. Comella, P. Cullis, A. Dalvi, F. Danisi, R. Dubinsky, A. Ellenbogen, M. Evatt, V. Evidente, H. Fernandez, S. Goldstein, S. Gollomp, D. Greeley, P. Hanna, R. Hauser, N. Hermanowicz, Z. Huang, B. Jabbari, U. J. Kang, M. LeDoux, K. Levin, P. LeWitt, A. Nicholas, R. Rodnitzky, A. Sahay, H. Schwartz, B. Scott, K. Sethi, J. Shahed, D. Silver, C. Singer, L. Struck, W. Sunter, D. Truong, A. Vasquez, M. Watts.
Footnotes
Funding: This study was funded by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany. Technical writing was performed by SD Scientific, Inc. and funded by Merz Pharmaceuticals, LLC.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflicts of interest.
References
- 1.Nutt JG, Muenter MD, Aronson A, et al. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord. 1988;3:188–194. doi: 10.1002/mds.870030302. [DOI] [PubMed] [Google Scholar]
- 2.Crowner BE. Cervical dystonia: disease profile and clinical management. Phys Ther. 2007;87:1511–1526. doi: 10.2522/ptj.20060272. [DOI] [PubMed] [Google Scholar]
- 3.Albanese A, Asmus F, Bhatia KP, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 4.Cano SJ, Hobart JC, Edwards M, et al. CDIP-58 can measure the impact of botulinum toxin treatment in cervical dystonia. Neurology. 2006;67:2230–2232. doi: 10.1212/01.wnl.0000249310.25427.f2. [DOI] [PubMed] [Google Scholar]
- 5.Frevert J. Xeomin is free from complexing proteins. Toxicon. 2009;54:697–701. doi: 10.1016/j.toxicon.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Merz Pharmaceuticals, LLC. XEOMIN® (incobotulinumtoxinA) for injection US Prescribing Information . 2011:Version 07/2011. [Google Scholar]
- 7.Comella CL, Jankovic J, Truong DD, et al. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN(R), botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J Neurol Sci. 2011;308:103–109. doi: 10.1016/j.jns.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Consky E, Basinski A, Belle L, et al. The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS): assessment of validity and inter-rater reliability (Abstract 1199P) Neurology. . 1990;40 [Google Scholar]
- 9.Consky ES, Lang AE. New York, NY: Marcel Dekker, Inc.; 1994. Clinical assessments of patients with cervical dystonia; Therapy with Botulinum Toxin; pp. 211–237. [Google Scholar]
- 10.Wissel J, Muller J, Dressnandt J, et al. Management of spasticity associated pain with botulinum toxin A. J Pain Symptom Manage. 2000;20:44–49. doi: 10.1016/S0885-3924(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 11.Evidente VG, Fernandez HH, LeDoux MS, et al. Mov Disord. 2012. Efficacy and safety of repeated incobotulinumtoxinA (Xeomin) injections in subjects with cervical dystonia: a randomized, double-blind extension study. under consideration. [Google Scholar]