Abstract
Background
Dystonia is a hyperkinetic movement disorder characterized by involuntary, repetitive twisting movements. The anatomical structures and pathways implicated in its pathogenesis and their relationships to the neurophysiological paradigms of abnormal surround inhibition, maladaptive plasticity, and impaired sensorimotor integration remain unclear.
Objective
We review the use of high-resolution structural brain imaging using voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) techniques for evaluating brain changes in primary torsion dystonia and their relationships to the pathophysiology of this disorder.
Methods
A PubMed search was conducted to identify relevant literature.
Results
VBM and DTI studies produced somewhat conflicting results across different forms of primary dystonia and reported increases, decreases, or both in gray matter volume and white matter integrity. However, despite the discrepancies, these studies are consistent in revealing brain abnormalities in dystonia that extend beyond the basal ganglia and involve the sensorimotor cortex and cerebellum.
Discussion
Although limited to date, structural magnetic resonance imaging (MRI) studies combined with functional brain imaging and neurophysiological modalities begin to establish structural-functional relationships at different levels of the abnormal basal ganglia, cortical, and cerebellar networks and provide clues into the pathophysiological mechanisms that underlie primary dystonia. Cross-disciplinary studies are needed for further investigations of the interplay between structural-functional brain abnormalities and environmental and genetic risk factors in dystonia patients.
Keywords: Primary dystonia, voxel-based morphometry, diffusion tensor imaging, MRI
Introduction
Dystonia is a syndrome of involuntary, sustained muscle contractions, frequently causing twisting and repetitive movements or abnormal postures that tend to be triggered with action and abate during the rest or sleep. As one of the most common movement disorders after essential tremor and Parkinson's disease,1–4 dystonia has a prevalence of 15–30 per 100,000,5 with one study showing a prevalence of 732 per 100,000 in adults over 50 years old.6
Dystonia can be classified based on distribution, age of onset, and etiology (Table 1).7 It can be characterized as focal, segmental, generalized, or multifocal. Focal dystonia refers to one affected body part (e.g., eyes, neck, arm, larynx), whereas segmental dystonia affects contiguous body regions. Generalized dystonia involves at least one leg, the trunk, and another body part, whereas multifocal involvement, though rare, affects noncontiguous body parts.
Table 1. Dystonia Classification.
| Classification | |
|---|---|
| Body of distribution | Focal |
| Segmental | |
| Generalized | |
| Age of onset | Early onset (<26 years of age) |
| Late onset (∼30–50 years of age) | |
| Etiology | Primary torsion +/− tremor |
| Secondary | |
| Dystonia-plus syndrome | |
| Heredodegenerative | |
| Feature of another neurological condition |
Onset prior to 26 years old is termed early onset and is usually associated with a known gene carrier status (e.g., DYT1).8 Symptoms are more likely to begin in the lower extremities and have a tendency to be generalized. Late-onset dystonia begins at around 30–50 years of age and usually has focal distribution and a possible genetic component (e.g., GNAL, CIZ1).9,10
Dystonia etiologies are typically subdivided into five categories: 1) primary torsion with or without tremor, which is devoid of other movement disorders, muscle atrophy, spasticity, oculomotor abnormalities, cognitive impairment, and gross brain pathology; 2) secondary (e.g., due to brain injury, cerebrovascular disease); 3) dystonia-plus (e.g., dopa-responsive dystonia, myoclonus-dystonia, rapid-onset dystonia-parkinsonism); 4) heredodegenerative, including autosomal dominant (e.g., Huntington's disease, familial frontotemporal dementias), autosomal recessive (e.g., Wilson's disease, juvenile parkinsonism), X-linked (e.g., Lesch–Nyhan syndrome, McLeod's syndrome), and mitochondrial (Leigh's disease), and 5) a feature of another neurological condition (e.g., Parkinson's disease, progressive supranuclear palsy, multiple system atrophy).
In this review, we will focus on primary torsion dystonia (PTD), which may have early or late onset with focal, segmental, or generalized distribution and known (e.g., DYT1, DYT6, GNAL) or unknown genetic components. The exact etiology and pathophysiology of PTD remain unknown. Given the predilection for striatal lesions to trigger dystonia,11,12 basal ganglia dysfunction was historically considered the principal impetus associated with this disorder. However, over years of research and clinical observations, numerous studies have extended and modified this knowledge by suggesting the importance of other neurophysiological processes, such as loss of surround inhibition,13,14 abnormal cortical plasticity,15 altered sensorimotor integration,16,17 and impaired striato-cerebellar outflow involved in motor learning and coordination,12,18,19 in the PTD development. Here, we examine the contribution of high-resolution magnetic resonance imaging (MRI) with voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) in elucidating PTD anatomy and discuss the potential of neuroimaging in expanding our understanding of dystonia pathophysiology.
Findings from structural brain imaging
While conventional MRI does not typically reveal brain abnormalities in patients with PTD, the development of high-resolution imaging techniques opened new avenues for investigating microstructural cortical and subcortical gray and white matter changes in dystonia. Numerous structural imaging studies using VBM and DTI have been conducted on several forms of PTD, producing a vast array of information that arguably lays a foundation for identifying abnormal brain networks involved in dystonia pathophysiology.
VBM: results, interpretations, and limitations
Since its first use in 1995,20,21 VBM has evolved into a validated tool for investigating gray matter volumetric changes due to physiological and pathophysiological factors in healthy and diseased populations.22,23 Briefly, the VBM technique allows for the comparison of high-resolution T1-weighted MR images within and/or between groups based on statistical parametric mapping (SPM). Using a fully automated approach, the individual brain image is segmented into gray matter, white matter and cerebrospinal fluid (CSF).24,25 The segmented tissue is then registered into a standard atlas space (either Talairach–Tournoux or Montreal Neurology Institute (MNI)) and smoothed to reduce inter-subject variability and normalize the data for comparative analysis.
In the late 1990s, Black et al.26 used MRI-based stereological volumetry to show a 10% increase of putaminal volume in 13 patients with either blepharospasm (BLS) or focal hand dystonia (FHD) compared to 12 age- and gender-matched controls. The results of this first imaging study on volumetric brain changes in PTD were in line with the assumptions that dystonia is a basal ganglia disorder and that intrinsic structural changes are present but may not be detectable with conventional MRI. Over the next 18 years, several studies employed VBM to attempt to verify these findings and further probe the striato-thalamo-cortical pathway in order to decipher gray matter changes in PTD. The results of the currently available VBM studies are summarized in Table 2. Some studies, however, tend to provide somewhat inconsistent findings within and across different forms of dystonia with regard to the direction of gray matter changes (i.e., increases, decreases, or both), as well as the brain structures affected.
Table 2. MRI with Voxel-Based Morphometry in Primary Dystonia.
| Primary Dystonia | Study | No. Cases | Average Age | Gray Matter Volume (GMV) | GMV Correlation | ||
|---|---|---|---|---|---|---|---|
| Increased | Decreased | Severity | Duration | ||||
| BLS | Black et al., 1998 | 5 | --- | Putamen | -- | -- | |
| Etgen et al., 2006 | 16 | 67.4 | Putamen | L. Inf. Parietal lobe | -- | NR | |
| Obermann et al., 2007 | 11 | 52.6 | Caud., CBL | Putamen, thalamus | -- | -- | |
| Horovitz et al., 2012 | 14 | 59.9 | L. mid-temporal gyrus, R. Postcentral gyrus, B/L precuneus | R. OFC, L. M1, L. IFG, R. OC, R. ACC | NR | Inv.- R occ. | |
| Martino et al., 2011 | 25 | 64.9 | R. middle frontal gyrus | L. Postcentral gyrus, L. STG | Inv. – R. middle frontal gyrus | NR | |
| CD | Draganski et al., 2003 | 10 | 44 | M1, CBL, R. GP | R. SMA, R. PFC, R. OC | --- | --- |
| Obermann et al., 2007 | 9 | 57.6 | Caud., L. CBL, STL, thalamus | Putamen, L. STL | --- | --- | |
| Egger et al., 2007 | 11 | 49.6 | R. GP, medial frontal cortex, L. SMA OFC, cingulate cortex | --- | --- | ||
| Draganski et al., 2009 | 29 | Putamen, GP | Inv.-Putamen | --- | |||
| Pantano et al., 2011 | 19 | 55.5 | L. Caud., putamen, SMC, PMC | NR | NR | ||
| FHD | Black et al., 1998 | 8 | --- | Putamen | --- | --- | |
| Delmaire et al., 2007 | 30 (RH) | 49.7 | L. M1, L. S1, B/L CBL, pulvinar | --- | --- | ||
| Granert et al., 2011 | 14 WC (RH) | 50.6 | L. M1 | --- | --- | ||
| Garraux et al., 2004 | 36 (31 WC, 5 MD) | 53 | B/L M1/S1 (Hand region) | --- | --- | ||
| Egger et al., 2007 | 11 (9 WC, 2 MD) | 41.9 | R. GP | --- | --- | ||
| Granert et al., 2011 | 11 MD (RH) | 43 | R>L putamen | Pos. - Putamen | --- | ||
| SD | Simonyan and Ludlow 2012 | 40 (25 AD, 15 AB) | 56.9 | L. M1/S1, supramarginal gyrus, putamen, R. IFG, B/L CBL | Pos.- R. IFG, L. CBL | NR | |
| HD | Egger et al., 2007 | 9 (DYT1) | 39.8 | R GP | --- | --- | |
| Draganski et al., 2009 | 22 DYT1 (11 MN, 11 NMN) | B/L putamen (NMN>MN) | Inv., putamen | ||||
In BLS, Etgen et al.27 showed an increase in putaminal gray matter volume (GMV) and a decrease in the left inferior parietal lobule in 16 affected patients compared to controls. In a separate study by Obermann et al.,28 11 BLS patients had increased GMV in the caudate nucleus and cerebellar hemispheres with reductions in the putamen and thalamus. In a cohort of 25 BLS patients, cortical GMV increases were observed in the right middle frontal gyrus, and decreased volumes were identified in the left postcentral and superior temporal gyri.29 It should, however, be noted that this study assessed 15 patients in whom BLS was part of segmental dystonia. Further wide-spread cortical volumetric abnormalities were demonstrated in a recent study of 14 BLS patients by Horovitz et al., who described GMV reductions in the left facial portion of the primary motor cortex, inferior frontal gyrus, right orbito-frontal cortex, occipital cortex, and anterior cingulate gyrus, as well as increased GMV in the left middle temporal gyrus, right postcentral gyrus, and bilateral precuneus.30 A negative correlation was found between BLS duration and right occipital lobe GMV in this group of patients.
There have been five VBM studies on cervical dystonia (CD). GMV increases were found in the globus pallidus,31,32,35 caudate nucleus,28 medial dorsal thalamus,28 primary motor cortex,31 cerebellum,28,31 left superior temporal lobe,28 left supplementary motor area (SMA), orbitofrontal cortex, cingulate cortex, and right medial frontal cortext.35 On the other hand, reduced GMV was also described in the putamen,28 as well as the right SMA, occipital cortex, and dorsolateral prefrontal cortex.31 In a longitudinal study of 20 CD patients, Pantano et al.33 initially showed reduced GMV in the putamen, left caudate nucleus, and bilateral premotor and primary sensorimotor cortices. Five years later, further reduction of GMV was found in the left somatosensory cortex. An inverse correlation between symptom severity and putamen volume in CD patients has also been described.32
With regard to FHD, two studies reported GMV increases in bilateral hand areas of the somatosensory cortices, primary motor cortex,34 and globus pallidus.35 Both studies examined patients with writer's cramp and musician's hand dystonia, though patients with writer's cramp were predominantly represented. In a separate study of 11 musicians (piano players) with dystonia only, the middle area of the putamen (right greater than left) was further shown to have a larger GMV increase, which correlated with disorder severity, measured as a temporal variability of piano playing.36 It should be noted that the primary focus of this study was on changes within the putamen only, making it difficult to relate the findings of this study to cortical GMV changes described in previous reports. Contrary to increased GMV observed in FHD, Delmaire et al.37 reported GMV reductions in the hand area of the left sensorimotor cortex, bilateral cerebellar hemispheres, and pulvinar in 30 patients with writer's cramp.
The only study in patients with spasmodic dysphonia (SD) (25 with adductor type and 15 with abductor type) reported increased GMV in the left laryngeal primary sensorimotor cortex, supramarginal gyrus, putamen, right inferior frontal gyrus (IFG), and bilateral cerebellar hemispheres.38 Positive correlations were observed between GMV increases in the IFG and cerebellum and SD severity. Furthermore, significant relationships between GMV increases and abnormally increased functional brain activity during symptomatic syllable production were found in the IFG, superior temporal gyrus, and cerebellum in 15 SD patients from the same cohort.
VBM data on hereditary dystonia are sparse. Egger et al.35 reported an increase in GMV in the globus pallidus in nine patients with generalized dystonia, but DYT1 status was not known in this cohort. Interestingly, Draganski et al.32 observed a genotype-phenotype interaction: asymptomatic DYT1 mutation carriers and non-DYT1 PTD patients had significantly increased bilateral putamen volumes than healthy subjects and DYT1 symptomatic patients. Furthermore, a negative correlation was identified between dystonia severity and putamen volume in all DYT1 carriers.
Clearly, the VBM data are conflicting among the various forms of primary dystonia without a unifying pattern of gray matter changes (e.g., increases vs. decreases), which makes it difficult to interpret results. It is further complicated by the fact that, despite its wide use for characterizing gray matter organization in both healthy and diseased populations, the exact cellular mechanisms and causes of GMV changes remain unclear.39 Some hints about the underlying causes of GMV changes in dystonia come from recent studies showing that motor training and exercise in healthy subjects may lead to GMV increases in related brain regions.40–43 This may also be the case in some forms of task-specific primary dystonias (e.g., musician's dystonia and writer's cramp), which are known to be triggered by excessive motor training. Additional suggested mechanisms behind GMV changes may include the formation of new connections by dendritic spine growth and axonal remodeling, as well as strengthening of existing neural connections.44–47
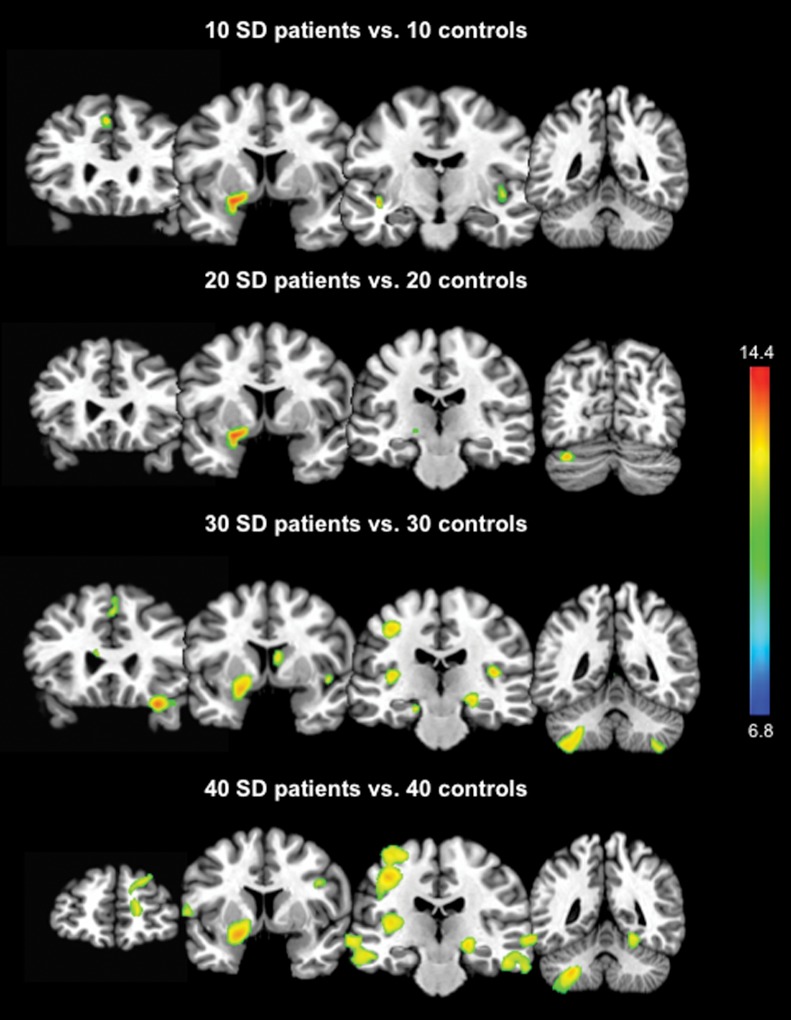
Discrepancies in GMV changes among different studies might also be a product of limitations in a number of factors contributing to data analyses, such as variability in patient populations, MRI sequences used, and statistical analyses. Population-based variability may result from the heterogeneous nature of the PTD, differences in diagnostic evaluations used by different research groups, patient treatment regimens with botulinum toxin, and the number of participants enrolled into the study, among others. The reported VBM studies in PTD included as few as nine patients28 and as many as 40 patients38, which may inherently impact the weight of statistical values and the study outcome. To demonstrate this, we re-examined GMV changes in the same group of 40 SD patients and 40 age- and gender-matched controls reported earlier38 while considering different group sizes (i.e., 10, 20, 30, or 40 subjects per group). When the VBM processing steps with the use of SPM8 software were held constant, an increase in the number of subjects resulted in the detection of more wide-spread GMV changes, including not only the basal ganglia and cerebellum, but also sensorimotor cortical regions at a family-wise error-corrected p = 0.05 (Figure 1). These data suggest that future VBM investigations may yield more insightful and perhaps less discrepant results if a larger number of dystonia patients are included.
Figure 1. Differences in Increases in GMV in Patients with SD compared to Healthy Controls are Dependent on Sample Size. The color bar represents F values and reflects the significance of changes in patients compared to controls.
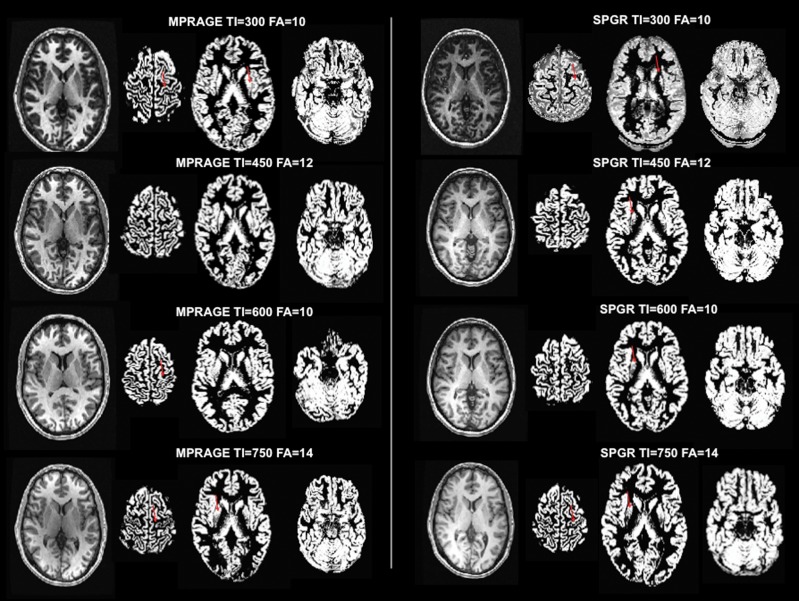
While VBM uses fully automatic procedures,21 the software version-based differences in pre-processing procedure for normalization, segmentation, and smoothing may introduce errors via misclassification of the tissue or displacement of brain structures due to misalignment of the template or anatomical variability (e.g., enlarged ventricles).23 Moreover, investigator-specified data smoothing to reduce variance between subjects may effectively impair the ability to detect subtle volumetric changes.23 The lack of a metric to adjudicate “goodness” of fit in the normalized space and scanner differences are other variables to be considered and factored in during data interpretation. Figure 2 demonstrates some of the disparities that can be introduced by choosing a specific MRI sequence for data collection. In five healthy subjects (two females, three males; ages 27–34), we acquired T1-weighted brain images using two commonly implemented sequences: MPRAGE (magnetization-prepared rapid acquisition with gradient echo) and SPGR (spoiled gradient recalled echo) while randomizing their inversion times (TI = 200, 450, 600, 750) and flip angles (FA = 10, 12, 14) (3-T GE Excite scanner equipped with an eight-channel head coil, Milwaukee, WI). Using SPM8 software, all brain images were segmented into gray matter, white matter, and CSF as routinely done within the VBM analysis pipeline without manual interference. When the parameters were held constant, the MPRAGE sequence produced somewhat better segmentation results compared to the SPGR sequence, particularly in the motor cortex and basal ganglia, which are the regions of interest in dystonia patients. Within-sequence comparisons showed that segmentation was not satisfactory in these brain regions at both higher and lower TIs (i.e., 750 and 300).
Figure 2. Impact of the Choice of T1-weighted Sequence on Brain Tissue Segmentation for VBM analysis. The segmented gray matter images were acquired using MPRAGE (magnetization-prepared rapid acquisition with gradient echo, left column) and SPGR (spoiled gradient recalled echo, right column) sequences. Fully automated segmentation into gray matter, white matter, and CSF was performed using SPM8 software. The red arrows indicate the over-segmented primary motor cortex and basal ganglia.
Taken together, these observations underscore the need for investigators to exercise extreme caution in interpreting their findings in the context of other studies and in making comparisons between patient populations recruited for the study and data acquisition and analysis techniques. Perhaps the development of standardized acquisition protocols along with standardized subject selection criteria might solve some of the technical issues leading to discrepant VBM findings. Efforts should also be made not to limit the number of subjects to the absolute minimum and to attempt comparative analyses between different PTD forms within the same research group. Our overall understanding of underlying causes for GMV changes would also be much enhanced if future VBM studies were coupled with evaluations of relationships between functional and structural changes, as well as brain pathology. While the former is reasonably achievable, the latter would be difficult to attain because of a limited availability of postmortem specimens from dystonia patients.
DTI: results, interpretations, and limitations
DTI is a form of diffusion-weighted imaging (DWI) that analyzes the microstructural integrity of white matter. It is based on the principal that water molecules differentially diffuse in parallel and perpendicular directions within brain tissue.48 The major index for this discrepant diffusion is fractional anisotropy (FA).49,50 Higher FA values correlate with more ordered tissue containing a larger number of aligned axons, such as in white matter, and thus reflect tissue integrity and coherence. Mean diffusivity (MD) is another frequently examined DTI measure that provides information about the organization of an extracellular space and intracellular water content. Therefore, high MD values typically reflect reduced cellularity.51 In addition, diffusion tensor tractography (DTT) allows for in vivo mapping of white matter pathways to study brain structural connectivity.
To date, several studies have examined DTI measures in different forms of PTD, and the results are described in Table 3. Similar to VBM studies, the results of DTI studies are contradictory, providing mixed information about white matter tract abnormalities within and across PTDs. In two studies,52,53 patients with CD were shown to have increased and decreased FA in the putamen and corpus callosum, respectively. Bonhila et al.54 reported additional FA reductions in the prefrontal cortex and thalamus. Both MD reductions in the caudate nucleus and putamen52 and increases in the prefrontal cortex, SMA, caudate nucleus, globus pallidus, and putamen were found in other studies.53,54 DTT in 12 CD patients revealed an asymmetry in left hemispheric connectivity between the ansa lenticularis and brainstem with concomitantly reduced FA in the left superior cerebellar peduncle.55
Table 3. Diffusion Tensor Imaging in Primary Dystonia.
| Primary Dystonia | Study | No. Cases | Average Age | DTI | DTI Correlations | ||
|---|---|---|---|---|---|---|---|
| Fractional Anisotropy (FA) | Mean diffusivity (MD) | Severity | Duration | ||||
| BLS | Fabbrini et al., 2008 | 16 | 66.5 | NS | NS | --- | --- |
| Horovitz et al., 2012 | 14 | 59.9 | Reduced connectivity | --- | --- | ||
| CD | Colosimo et al., 2005 | 15 | 51.9 | Inc. – Putamen | Dec. – L. GP, L. putamen, B/L Caud. | NR | NR |
| Dec. - genu CC | |||||||
| Bonilha et al., 2007 | 7 | 58 | Dec.-R. Thal., R. PFC | Inc.-B/L. GP, putamen, L. Caud. | --- | --- | |
| Fabbrini et al., 2008 | 18 | 55.5 | Inc. – Putamen | Inc.-B/L PFC, L.SMA | NR | NR | |
| Dec.- Body of CC | Dec.-R Caud | ||||||
| Blood et al., 2012 | 12 | 59.2 | Dec. GP-BS connectivity | --- | --- | ||
| FHD | Delmaire et al., 2009 | 26 | 42.8 | Inc.- IC, Thal., L. centrum semiovale | --- | --- | --- |
| SD | Simonyan et al., 2008 | 20 | 55 | Dec.- R. Genu IC | Inc.-CR, Genu & Posterior Limb IC, middle CBL peduncle, Thal., putamen | Pos. - MD in Thal. | --- |
| HD | Carbon et al., 2004 | 12 (DYT1) | 44.1 | Dec. SMC | --- | --- | |
| Carbon et al., 2008 | 7 (4 DYT1, 3 DYT6) | 29.3 | Dec. – Dorsal pontine BS, SMC, | --- | --- | --- | |
| Cheng et al., 2012 | 6 (DYT6) | Dec. - SMC | Inc. – SLF and CST | --- | --- | ||
| Van de Meer et al., 2012 | 16 (DYT11) | 43.5 | Inc. – R. STN, RN | Dec. –subgyral SMC; STN | --- | --- | |
Abbreviations: BS, brainstem; BLS, blepharospasm; Caud., caudate; CD, cervical dystonia; CC, corpus callosum; CR, corona radiata; Dec., decreased; FHD, focal hand dystonia; HD, hereditary dystonia; IC, internal capsule; Inc., increased; NR, no relationship; NS, no significance; Ped, peduncle; PFC, prefrontal cortex; RN, red nucleus; SD, spasmodic dysphonia; SMA–supplementary motor area; SMC, sensorimotor cortices; SLF, superior longitudinal fasciculus; STN, subthalamic nucleus; Thal., thalamus; (---), no data.
One study conducted in BLS patients reported no DTI abnormalities,53 although another demonstrated reduced left corticobulbar tract volume and connectivity.30 More research on white matter organization in BLS is needed to determine whether BLS is the only form of PTD without apparent white matter microstructural changes.
In 26 patients with FHD, Delmaire et al.56 observed increased FA in the posterior limb of the internal capsule (IC) and adjacent structures.
Simonyan et al.57 studied 20 SD patients and reported reduced FA in the genu of the right IC with increased MD within the corticobulbar/corticospinal tract, putamen, thalamus, and middle cerebellar peduncles. Abnormal MD measures in the thalamus were found to correlate with SD symptoms. These DTI findings were further substantiated by neuropathological evidence of decreased axonal density and myelin content in the right genu of the IC and accumulations of minerals in the parenchyma and vessel walls of the posterior limb of the IC, putamen, globus pallidus, and cerebellum in the postmortem tissue from one SD patient compared to three controls.
Among the hereditary dystonias, DYT1 patients (eight non-manifesting and four manifesting [two generalized, two segmental]) exhibited reduced FA in the subgyral white matter of the sensorimotor cortex compared to controls.58 This was present in both non-manifesting and manifesting carriers, but when controlled for age, manifesting carriers showed significantly greater changes in FA measures. Further studies found additional FA reductions in the bilateral sensorimotor cortices59,60 and dorsal pontine tegmentum near the left superior cerebellar peduncle59 in manifesting DYT1 and DYT6 carriers. In addition, DYT1 mutation carriers showed correlations among reduced cerebellar pathway integrity, decreased activation in the dentate nucleus, and increased premotor cortical activation during sequence learning but no relationship with abnormal striatal D2 receptor binding.61 Building on this knowledge, Argyelan et al.19 employed DTT to further demonstrate reduced integrity in cerebellar lobule VI adjacent to the dentate nucleus in 12 DYT1 and DYT6 manifesting carriers. Interestingly, non-manifesting carriers exhibited additionally reduced connectivity within the distal thalamo-cortical segment of the cerebello-thalamo-cortical tract, pointing to possible neural mechanisms underlying the differences in clinical symptoms of dystonia in manifesting vs. non-manifesting carriers of gene mutation(s). These results are in contrast with those of a recent study from Van de Meer et al.,62 in which 16 DYT11 patients had increased white matter volume and FA in the subthalamic area and red nucleus. Reduced MD was also found in the cortical sensorimotor cortices without changes in regional FA or white matter volume.
Similar to the results of VBM studies, DTI findings lack consistency. Differences in patient cohorts and their clinical characteristics, as well as imaging acquisition protocols and data processing, may account for these discrepancies. As with the VBM studies, these inconsistencies may be better dealt with in multi-center studies that use standardized protocols for clinical evaluation and data acquisition and processing, as well as recruitment of larger cohorts of patients, which would ultimately allow for between-dystonia comparisons.
Contribution of high-resolution structural imaging to understanding dystonia pathophysiology
Despite the discrepancies in VBM and DTI results, some unifying conclusions across different PTDs can be drawn. First, gray matter changes are not restricted to the basal ganglia; rather, they extend to other cortical and subcortical regions, such as the sensorimotor cortices, thalamus, and cerebellum. Secondly, white matter aberrations along the cortico-striato-pallido-thalamic and cerebello-thalamo-cortical pathways appear to be present. Third, genotype (e.g., DYT1) and phenotype interactions may differentially impact brain network organization in dystonia, resulting in different manifestations of the disorder. Finally, non-task-specific PTD (e.g., BLS, CD) tend to demonstrate inverse correlations between symptom severity/duration and GMV changes compared to positive correlations observed in task-specific dystonias (e.g., SD and writer's cramp), which may suggest differential modulation of brain circuits depending on the motor programs involved (voluntary vs. involuntary). Taken together, we have strong evidence to assume that dystonia pathophysiology may be linked to microstructural changes in the gray and white matter. However, whether these changes are causative or compensatory remains unknown. Moreover, the recent progress in understanding dystonia brain anatomy raises new questions about the origins of this disorder, such as whether PTD begins exclusively in the basal ganglia (as historically proposed) with further involvement of sensorimotor brain regions or whether subtle abnormalities in different brain structures collectively contribute to brain network disorganization, which, in the presence of the environmental and genetic triggers, may lead to symptom manifestation.
As an attempt to provide a more comprehensive investigation into dystonia pathophysiology, some have employed a combined approach of functional and/or neurophysiological modalities with structural brain imaging. Abnormal sensory processing has long been postulated to play a prominent role as one of the underlying mechanisms of dystonia pathophysiology.16,63 Bradley et al.64 examined visual and tactile temporal discrimination thresholds (TDT) in 35 patients with adult-onset focal dystonias (17 familial, 28 sporadic), 42 first-degree relatives and 32 second-degree relatives. Among them, 86% of those affected with dystonia had abnormal TDTs compared to 52% of first-degree relatives and 50% of second-degree relatives, following an autosomal dominant pattern. Using VBM measurements, 13 unaffected relatives with abnormal TDTs exhibited increased GMV in the bilateral putamen compared to healthy controls. This study suggests that abnormal TDT may serve as a possible endophenotype for adult-onset PTD and that gray matter changes in the basal ganglia may precede symptom manifestation. Further studies including different types of dystonia are needed to confirm these findings and link them to disorder-specific clinico-behavioral characteristics, genetic status, and changes in brain activity and structure.
Repetitive activity causing abnormal motor cortical plasticity in some PTD patients (e.g., musician's dystonia) underlies the theory that excessive stimulation may trigger dystonia.15,65 Granert et al.43 recruited 14 patients with right-handed writer's cramp who underwent immobilization of the affected hand for 4 weeks followed by an 8-week motor retraining period. Follow-up VBM and transcranial magnetic stimulation (TMS) studies demonstrated a GMV reduction in the left hand region of the primary motor cortex and a decrease in cortico-motor excitability during the immobilization period. The retraining period reversed this effect: increased GMV was found in this region with concomitant cortico-motor hyperexcitabilty. Though only the left hand region of the primary motor cortex was targeted as a primary region of interest, these results reaffirm that motor learning is critical to neuroplasticity and that gray matter changes may be driven by cortico-motor excitability.
Basal ganglia dysfunction via the cortico-striato-pallido-thalamic pathway has commonly been associated with dystonia.12 Probing the interconnectivity of cerebellar outflow pathways with pallidal fibers and subsequent cortical activity, Uluğ et al.66 created a heterozygous knock-in mouse for torsinA (DYT1) mutation. Ex vivo DTI revealed reduced FA in the superior cerebellar tract, the white matter underlying the primary sensorimotor cortex, caudate nucleus, putamen, pons, pontomedullary junction, and superior colliculus, while tractography revealed fewer fibers in the cerebello-thalamic, thalamo-cortical, and thalamo-striatal pathways of mutants compared to controls. In addition, these microstructural abnormalities were correlated with increased metabolic activity in the sensorimotor cortex. These findings are consistent with the studies of human DYT1 manifesting/non-manifesting populations. Because the cerebello-thalamo-cortical tract facilitates intracortical inhibition via projections to interneurons in the sensorimotor cortex,67 these findings suggest that the intermediary pattern observed in non-manifesting carriers may act as a buffer against the aberrant outflow from the proximal part of this pathway.68 The negative correlation between cerebellar outflow tract breakdown and increased cerebral cortical activation further supported release of this inhibition.
In dystonias, the paucity of neuropathological correlations with neuroimaging findings in general and VBM and DTI results in particular limits our understanding of structural brain abnormalities. Neuropathological studies of dystonia are sparse, partly due to the low availability of postmortem tissue from these patients and the labor-intensive nature of exploratory neuropathological examination of the entire human brain. Only a few studies have examined neuropathological abnormalities in a handful of dystonic patients, providing limited information regarding the relationships between functional/structural brain changes and underlying neuropathology. Neuropathological case reports of patients with Meige's syndrome and cranial and cervical dystonias have described mild neural loss in the substantia nigra, locus coeruleus, dorsal raphe nucleus, tectum, and dentate nucleus, as well as infrequent Lewy bodies in the substantia nigra, nucleus basalis of Meynert, and nucleus ambiguus.69 To date, no studies have been conducted to examine the neuropathology underlying cortical structural changes despite the fact that a large body of neuroimaging literature clearly points to the presence of microstructural alterations in both cortical and subcortical regions in PTD. One way to overcome the challenges associated with neuropathological evaluation of postmortem brain tissue from PTD patients is to use brain imaging to identify regions with abnormal structure-function relationships and then target these regions with neuropathological examination of the corresponding brain tissue. Using a similar approach, a combined DTI and neuropathological study in one SD patient was able to reveal relationships between abnormal FA and MD measures and underlying brain pathology in the basal ganglia, thalamus, cerebellum, and related white matter pathways.57 However, limitations of this study included the sample size of one and unavailability of cortical regions of interest. Confirmation of these findings and detailed interpretation of other neuroimaging findings in SD and other forms of PTD requires access to larger samples of postmortem tissue through established brain banks and research collaborations.
Conclusion
Dystonia was historically thought to be a basal ganglia disorder. While conventional MRI usually does not reveal any gross brain abnormalities, high-resolution MRI and DTI have been able to identify microstructural gray and white matter changes in PTD patients. Structural brain imaging studies have shown that not only the basal ganglia but also the sensorimotor cortical regions and cerebellum may be instrumental in the pathophysiology of this disorder. While multimodal studies combining structural and functional imaging with clinico-behavioral evaluations, genetic analysis, and postmortem neuropathology are still rare, they have a tremendous potential to subvert the data discrepancies and provide a more coherent understanding of dystonia pathophysiology.
Footnotes
Funding: This work was supported by the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (R01DC0118085 to K.S.).
Financial disclosures: None.
Conflict of Interests: None.
References
- 1.Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–1193. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- 2.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 3.Geyer HL, Bressman SB. The diagnosis of dystonia. Lancet Neurol. 2006;5:780–790. doi: 10.1016/S1474-4422(06)70547-6. [DOI] [PubMed] [Google Scholar]
- 4.Tarsy D, Simon DK. Dystonia. N Engl J Med. 2006;355:818–829. doi: 10.1056/NEJMra055549. [DOI] [PubMed] [Google Scholar]
- 5.Nutt JG, Muenter MD, Melton LJ, Aronson A, Kurland LT. Epidemiology of dystonia in Rochester, Minnesota. Adv Neurol. 1988;50:361–365. [PubMed] [Google Scholar]
- 6.Muller J, Kiechl S, Wenning GK, et al. The prevalence of primary dystonia in the general community. Neurology. 2002;59:941–943. doi: 10.1212/01.WNL.0000026474.12594.0D. [DOI] [PubMed] [Google Scholar]
- 7.Fahn S. Classification of movement disorders. Mov Disord. 2011;26:947–957. doi: 10.1002/mds.23759. [DOI] [PubMed] [Google Scholar]
- 8.de Carvalho Aguiar PM, Ozelius LJ. Classification and genetics of dystonia. Lancet Neurol. 2002;1:316–325. doi: 10.1016/S1474-4422(02)00137-0. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45:88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao J, Uitti RJ, Zhao Y, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71:458–469. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108:463–483. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 12.Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord. 2002;17:S49–S62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]
- 13.Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004;56:595–599. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- 14.Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quartarone A, Bagnato S, Rizzo V, et al. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- 16.Tinazzi M, Rosso T, Fiaschi A. Role of the somatosensory system in primary dystonia. Mov Disord. 2003;18:605–622. doi: 10.1002/mds.10398. [DOI] [PubMed] [Google Scholar]
- 17.Carbon M, Argyelan M, Habeck C, et al. Increased sensorimotor network activity in DYT1 dystonia: A functional imaging study. Brain. 2010;133:690–700. doi: 10.1093/brain/awq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phukan J, Albanese A, Gasser T, Warner T. Primary dystonia and dystonia-plus syndromes: Clinical characteristics, diagnosis, and pathogenesis. Lancet Neurol. 2011;10:1074–1085. doi: 10.1016/S1474-4422(11)70232-0. [DOI] [PubMed] [Google Scholar]
- 19.Argyelan M, Carbon M, Niethammer M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright IC, McGuire PK, Poline JB, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. NeuroImage. 1995;2:244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Voxel-based morphometry – the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 22.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 23.Gitelman DR, Ashburner J, Friston KJ, Tyler LK, Price CJ. Voxel-based morphometry of herpes simplex encephalitis. NeuroImage. 2001;13:623–631. doi: 10.1006/nimg.2000.0734. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Ashburner J, Friston K. Multimodal image coregistration and partitioning—a unified framework. NeuroImage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 26.Black KJ, Ongur D, Perlmutter JS. Putamen volume in idiopathic focal dystonia. Neurology. 1998;51:819–824. doi: 10.1212/WNL.51.3.819. [DOI] [PubMed] [Google Scholar]
- 27.Etgen T, Muhlau M, Gaser C, Sander D. Bilateral grey-matter increase in the putamen in primary blepharospasm. J Neurol Neurosurg Psychiatry. 2006;77:1017–1020. doi: 10.1136/jnnp.2005.087148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obermann M, Yaldizli O, De Greiff A, et al. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. 2007;22:1117–1123. doi: 10.1002/mds.21495. [DOI] [PubMed] [Google Scholar]
- 29.Martino D, Di Giorgio A, D'Ambrosio E, et al. Cortical gray matter changes in primary blepharospasm: A voxel-based morphometry study. Mov Disord. 2011;26:1907–1912. doi: 10.1002/mds.23724. [DOI] [PubMed] [Google Scholar]
- 30.Horovitz SG, Ford A, Najee-Ullah MA, Ostuni JL, Hallett M. Anatomical correlates of blepharospasm. Transl Neurodegener. 2012;1:12. doi: 10.1186/2047-9158-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. “Motorcircuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–1231. doi: 10.1212/01.WNL.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- 32.Draganski B, Schneider SA, Fiorio M, et al. Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. NeuroImage. 2009;47:1141–1147. doi: 10.1016/j.neuroimage.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantano P, Totaro P, Fabbrini G, et al. A transverse and longitudinal MR imaging voxel-based morphometry study in patients with primary cervical dystonia. AJNR Am J Neuroradiol. 2011;32:81–84. doi: 10.3174/ajnr.A2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. 2004;55:736–739. doi: 10.1002/ana.20113. [DOI] [PubMed] [Google Scholar]
- 35.Egger K, Mueller J, Schocke M, et al. Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord. 2007;22:1538–1542. doi: 10.1002/mds.21619. [DOI] [PubMed] [Google Scholar]
- 36.Granert O, Peller M, Jabusch HC, Altenmuller E, Siebner HR. Sensorimotor skills and focal dystonia are linked to putaminal grey-matter volume in pianists. J Neurol Neurosurg Psychiatry. 2011;82:1225–1231. doi: 10.1136/jnnp.2011.245811. [DOI] [PubMed] [Google Scholar]
- 37.Delmaire C, Vidailhet M, Elbaz A, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology. 2007;69:376–380. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- 38.Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. 2012;22:417–425. doi: 10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandettini PA. What's new in neuroimaging methods? Ann NY Acad Sci. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 41.Ceccarelli A, Rocca MA, Pagani E, Falini A, Comi G, Filippi M. Cognitive learning is associated with gray matter changes in healthy human individuals: A tensor-based morphometry study. NeuroImage. 2009;48:585–589. doi: 10.1016/j.neuroimage.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Quallo MM, Price CJ, Ueno K, et al. Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc Natl Acad U S A. 2009;106:18379–18384. doi: 10.1073/pnas.0909751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granert O, Peller M, Gaser C, et al. Manual activity shapes structure and function in contralateral human motor hand area. NeuroImage. 2011;54:32–41. doi: 10.1016/j.neuroimage.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Chklovskii DB. Synaptic connectivity and neuronal morphology: Two sides of the same coin. Neuron. 2004;43:609–617. doi: 10.1016/j.neuron.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 46.Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 47.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 48.Moseley ME, Cohen Y, Kucharczyk J, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- 49.Stieltjes B, Kaufmann WE, van Zijl PC, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. NeuroImage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- 50.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 51.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colosimo C, Pantano P, Calistri V, Totaro P, Fabbrini G, Berardelli A. Diffusion tensor imaging in primary cervical dystonia. J Neurol Neurosurg Psychiatry. 2005;76:1591–1593. doi: 10.1136/jnnp.2004.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fabbrini G, Pantano P, Totaro P, et al. Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. Eur J Neurol. 2008;15:185–189. doi: 10.1111/j.1468-1331.2007.02034.x. [DOI] [PubMed] [Google Scholar]
- 54.Bonilha L, de Vries PM, Vincent DJ, et al. Structural white matter abnormalities in patients with idiopathic dystonia. Mov Disord. 2007;22:1110–1116. doi: 10.1002/mds.21295. [DOI] [PubMed] [Google Scholar]
- 55.Blood AJ, Kuster JK, Woodman SC, et al. Evidence for altered basal ganglia-brainstem connections in cervical dystonia. PLOS ONE. 2012;7:e31654. doi: 10.1371/journal.pone.0031654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delmaire C, Vidailhet M, Wassermann D, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer's cramp. Arch Neurol. 2009;66:502–508. doi: 10.1001/archneurol.2009.8. [DOI] [PubMed] [Google Scholar]
- 57.Simonyan K, Tovar-Moll F, Ostuni J, et al. Focal white matter changes in spasmodic dysphonia: A combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carbon M, Kingsley PB, Su S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol. 2004;56:283–286. doi: 10.1002/ana.20177. [DOI] [PubMed] [Google Scholar]
- 59.Carbon M, Kingsley PB, Tang C, Bressman S, Eidelberg D. Microstructural white matter changes in primary torsion dystonia. Mov Disord. 2008;23:234–239. doi: 10.1002/mds.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng FB, Wan XH, Feng JC, et al. Subcellular distribution of THAP1 and alterations in the microstructure of brain white matter in DYT6 dystonia. Parkinsonism Relat Disord. 2012;18:978–982. doi: 10.1016/j.parkreldis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Carbon M, Argyelan M, Ghilardi MF, et al. Impaired sequence learning in dystonia mutation carriers: A genotypic effect. Brain. 2011;134:1416–1427. doi: 10.1093/brain/awr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Meer JN, Beukers RJ, van der Salm SM, Caan MW, Tijssen MA, Nederveen AJ. White matter abnormalities in gene-positive myoclonus-dystonia. Mov Disord. 2012;27:1666–1672. doi: 10.1002/mds.25128. [DOI] [PubMed] [Google Scholar]
- 63.Hallett M. Is dystonia a sensory disorder? Ann Neurol. 1995;38:139–140. doi: 10.1002/ana.410380203. [DOI] [PubMed] [Google Scholar]
- 64.Bradley D, Whelan R, Walsh R, et al. Temporal discrimination threshold: VBM evidence for an endophenotype in adult onset primary torsion dystonia. Brain. 2009;132:2327–2335. doi: 10.1093/brain/awp156. [DOI] [PubMed] [Google Scholar]
- 65.Quartarone A, Sant'Angelo A, Battaglia F, et al. Enhanced long-term potentiation-like plasticity of the trigeminal blink reflex circuit in blepharospasm. J Neuroscience. 2006;26:716–721. doi: 10.1523/JNEUROSCI.3948-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uluğ AM, Vo A, Argyelan M, et al. Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proc Nat Acad U S A. 2011;108:6638–6643. doi: 10.1073/pnas.1016445108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molinari M, Filippini V, Leggio MG. Neuronal plasticity of interrelated cerebellar and cortical networks. Neuroscience. 2002;111:863–870. doi: 10.1016/S0306-4522(02)00024-6. [DOI] [PubMed] [Google Scholar]
- 68.Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2011;42:202–209. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Standaert DG. Update on the pathology of dystonia. Neurobiol Dis. 2011;42:148–151. doi: 10.1016/j.nbd.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]