Abstract
Taste sensitivity is assessed with various techniques, including absolute detection and quality recognition. For any stimulus, one might expect individual differences in sensitivity to be reflected in all measures, but they are often surprisingly independent. Here, we focus on sensitivity to sour and salty taste, in part because processing of these qualities is poorly understood relative to other tastes. In Study 1, we measured retest reliability for detection (modified, forced-choice staircase method) and recognition (modified Harris–Kalmus procedure) for both citric acid (CA) and sodium chloride (NaCl). Despite good retest reliability, individual differences in detection and recognition were weakly correlated, suggesting that detection and recognition of sour and salty stimuli may reflect different physiological processes. In Study 2, a subset of subjects returned to contribute full detection (psychometric) functions for CA and NaCl. Thresholds estimated from full detection functions correlated with both staircase and recognition thresholds, suggesting that both tasks may reflect absolute sensitivity to some extent. However, the ranges of individual differences were systematically compressed for staircase thresholds relative to those from full detection functions. Thus, individual differences in sensitivity appear to interact with different test methodologies in lawful ways. More work will be required to understand how different taste phenotypes relate to one another.
Key words: detection, salty, sensitivity, sour, taste phenotype
Introduction
Taste sensitivity is assessed with a variety of techniques. Broad categories include subjective ratings of intensity for suprathreshold stimuli, absolute detection thresholds (the minimum concentration subjects can reliably discriminate from water), recognition thresholds (the minimum concentration that gives rise to a characteristic quality, such as “salty” for sodium chloride [NaCl] or “sour” for acids), and differential thresholds (the minimum concentration difference necessary to determine that 2 suprathreshold stimuli are different) (Bartoshuk 1978). For a given stimulus, one might expect individual differences in sensitivity to be reflected in all these measures, but they are often surprisingly independent.
Yet, some psychophysical measures correlate with one another for selected taste stimuli. The bitter compound 6-n-propylthiouracil (PROP) is a notable example. Absolute detection, bitter recognition, and intensity ratings are all related to polymorphisms in the gene that encodes the bitter receptor protein T2R38 (Bufe et al. 2005; Galindo-Cuspinera et al. 2009). TAS2R38 was originally identified as related to PROP taste using only recognition threshold measures (Kim et al. 2003), but both detection and recognition have been widely used to characterize individual differences in PROP sensitivity (Galindo-Cuspinera et al. 2009).
More often, however, different measures of sensitivity do not correlate with one another (Bartoshuk 2000; Mojet et al. 2005; Keast and Roper 2007). For example, detection thresholds for sucrose do not correlate strongly with suprathreshold ratings of sweetness (Faurion 1987). One possible explanation is that the underlying physiological mechanisms for these perceptual traits are distinct despite their activation by the same stimulus (discussed for some bitter stimuli in Keast and Roper 2007). It is important, therefore, to understand the reliability of different taste phenotypes and how they relate to one another as well as to individual subject sensitivity.
Previous studies have examined the relationship between detection, recognition, and suprathreshold ratings for PROP (Galindo-Cuspinera et al. 2009), detection and suprathreshold rated intensity for several bitter stimuli (Keast and Roper 2007), and for detection and suprathreshold ratings for stimuli of various qualities (Bartoshuk 2000; Mojet et al. 2005). The current study extends these results by focusing on detection and recognition of citric acid (CA) and NaCl. Despite some promising leads in recent years (e.g., Huang et al. 2006; Chandrashekar et al. 2010; Breza and Contreras 2012), transduction of sour and salt taste is still not fully understood. Genetic analysis of variation in sensitivity has helped elucidate transduction mechanisms for sweet, bitter, and umami taste (Kim et al. 2004; Mombaerts 2004; Breslin and Huang 2006; Chen et al. 2009; Raliou et al. 2009; Shigemura et al. 2009). Similar approaches could be used for sour and salty taste, provided we first have a foundational understanding of measured phenotypes.
In measuring recognition thresholds for CA and NaCl (Wise et al. 2007), we noted that some subjects reported other taste qualities at lower concentrations (i.e., sweet or bitter for CA, or sweet for NaCl) before reaching concentrations that elicited the expected qualities of sour and salty (also see Cardello and Murphy 1977; Kim et al. 2004; Galindo-Cuspinera et al. 2009). This could imply that detection and recognition of these stimuli depend on different physiological mechanisms (Bartoshuk et al. 1978). Interestingly, twin studies on sensitivity to acids have found no evidence of heritable variation in absolute detection thresholds, but did find heritable variation in recognition thresholds (Kaplan et al. 1967; cf. Wise et al. 2007). It is unclear whether the inherent differences in dependent measures contributed to this difference.
The current study was conducted to understand the relationship between detection and recognition of sour and salty stimuli. Both detection (2-alternative, forced-choice staircase procedure) and recognition (modified Harris–Kalmus procedure; Harris and Kalmus 1949; Wise et al. 2007) thresholds were measured for CA and NaCl in the same subjects using the same sampling procedure (whole-mouth, sip-and-spit procedure). Correlations between dependent variables were assessed to determine whether the 2 tests characterize the same individual differences in perceptual sensitivity to these stimuli. Furthermore, to help better understand how psychophysical methods affect observed individual differences in absolute detection, the majority of subjects returned to contribute full psychometric (detection) functions (force-choiced, method of constant stimuli).
Study 1
Purpose
To compare 2 commonly used methods for phenotyping taste sensitivity to salty and sour stimuli, namely a modified, 2-alternative forced-choice detection threshold and a modified Harris–Kalmus recognition threshold.
Materials and methods
Subjects
Twenty-two (8 male, 14 female) healthy, nonsmoking adults (age range = 21–52, mean = 31.14, standard deviation [SD] = 8.58) participated. Subjects provided written informed consent using forms approved by an institutional review board (IRB) at the University of Pennsylvania before testing. All procedures were approved by the IRB and conducted in accordance with the guidelines of the Declaration of Helsinki. Most were employees of the Monell Chemical Senses Center. All were paid for participating. Most subjects had prior experience with psychophysical tests, including measurement of taste thresholds.
Taste materials
Subject received solutions of NaCl and CA (Sigma–Aldrich) in Millipore-filtered, deionized water. For the Harris–Kalmus recognition threshold, concentrations ranged from undetectable to clearly detectable for most people (6.10×10–4 to 5×100 mM for CA and 3.05×10–2 to 2.50×102 mM for NaCl) in fourteen, 2-fold steps. For modified staircase detection thresholds, concentrations ranged from 5.00×10–3 to 5.00×10–1 mM for CA and 2.00×10–1 to 2.00×101 mM for NaCl in seventeen, 0.125 log units. Stimuli were prepared less than 1 week in advance of use, stored under refrigeration in amber glass bottles, and warmed to room temperature before use. Water blanks consisted of Millipore-filtered deionized water stored and handled the same way.
Procedure for the modified Harris–Kalmus test
Recognition thresholds for sour and salty taste were measured using a modified Harris–Kalmus procedure (Harris and Kalmus 1949). During each experimental trial, subjects received a single, 10-mL sample presented in a medicine cup. Subjects attempted to identify the quality of the taste after holding the sample in their mouth for at least 5 s (whole-mouth, sip-and-spit procedure). Response options included “sweet,” “sour”, “bitter,” “salty,” or “water.” Subjects rinsed at least twice with deionized water between trials. A threshold run began with the lowest concentration. Subjects sampled each concentration once, in ascending order, until they identified the target quality: “sour” for CA and “salty” for NaCl (Wise et al. 2007). We do not suggest that no other qualities were perceived because these stimuli can elicit different tastes depending on concentration and subjects’ adaptation state. However, in this case, we were interested specifically in the recognition of the predominant suprathreshold quality.
To ensure that subjects experienced a reliable taste sensation, they completed a sorting task after reporting the “target” quality. Subjects received 3 samples at the concentration at which they identified the target quality intermixed with 3 blanks. The samples were presented at the same time, in random position. Subjects knew that exactly 3 cups contained taste solutions. Subjects were instructed to sort the cups into “tastes” and “waters.” If a subject could correctly sort the 6 samples in 2 consecutive trials, the threshold run ended. If a subject failed to sort correctly, the sorting task was repeated at the next higher concentration. The concentration that first allowed successive, correct sorts following quality recognition served as the taste quality recognition threshold. To confirm taste quality recognition, the next concentration higher than the determined threshold was presented and the quality was assessed. In all cases, subjects reported the same quality label from both concentrations. Thus, the lower of the 2 concentrations was accepted as the recognition threshold.
Procedure for the modified staircase
Absolute detection thresholds were measured using a modified staircase procedure (Wetherill and Levitt 1965; Levitt 1971). Stimulus sampling (whole-mouth, sip-and-spit procedure) matched that described above (see Procedure for the modified Harris–Kalmus test). However, during each trial, subjects received 1 taste solution and 1 blank in random order. Subjects were instructed to determine which sample tasted stronger. Starting with the best estimate of threshold, a 4-down, 1-up rule was used: concentration increased 1 step after each incorrect response and decreased 1 step after 4 consecutive correct responses at the same concentration. An increase in concentration that followed a decrease (or a decrease that followed an increase) was termed a reversal. Testing continued until subjects accrued 5 reversals. The threshold was calculated as the average of the concentrations at which the last 4 reversals occurred. In addition, the method was modified to lower the probability of false threshold estimates (McMahon et al. 2001). All reversals within the threshold run had to be within 5 concentration steps of each other (1.25 orders of magnitude). If this was exceeded, then counting the 5 reversals began again with the most recent reversal (collecting 5 more reversals).
Design and general procedures
All subjects participated in all conditions (complete within-subjects design). Subjects completed 8 threshold runs: 2 tasks (detection and recognition) × 2 stimuli (CA and NaCl) × 2 replicates. Measurements were completed in blocked random order: 2 tasks × 2 stimuli in random order, then again in random order. Subjects completed all threshold tests within 2–6 days (mean = 2.7 days, SD = 1.1). In all tests, subjects sampled taste stimuli using nose clips to help prevent volatiles (if present) from entering the nose either ortho- or retronasally.
Data analysis
Threshold values were log transformed before analyses because distributions tended to be positively skewed. Analyses were conducted using Statistica software (Version 8.0, Statsoft). Mean differences in thresholds were compared using analysis of variance (ANOVA). Test–retest reliability was assessed using both the intraclass correlation coefficient (Field 2005) and Pearson’s r. Correlations between measures were assessed using Pearson’s r. Differences between correlation coefficients were assessed using Steiger’s Z (Meng et al. 1992). The criterion of significance was P < 0.05.
Results and discussion
Threshold values
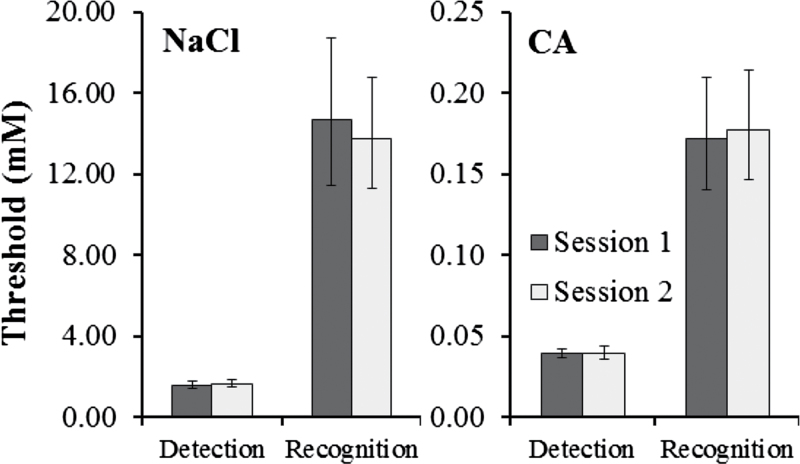
For both compounds, group threshold measurements appeared stable across sessions (Figure 1). Recognition threshold concentrations exceeded detection threshold concentrations by 4.4-fold for NaCl and 8.7-fold for CA. For NaCl, the main effect of psychophysical task reached significance, F(1,21) = 121.40, P < 0.0001, but the effect of session and the interaction did not (P > 0.50). Results for CA were parallel to those for NaCl. The main effect of psychophysical task reached significance, F(1,21) = 82.16, P < 0.0001, but the effect of session and the interaction did not (P > 0.80). Averaged across sessions, geometric mean (95% confidence interval [CI]) detection thresholds were 1.64 (1.29–2.07) mM for NaCl and 0.039 (0.033–0.047) mM for CA. Geometric mean recognition thresholds were 14.21 (9.29–21.73) mM for NaCl and 0.17 (0.12–0.26) mM for CA.
Figure 1 .
Geometric mean (across subjects) detection and recognition thresholds for NaCl (left) and CA (right) measured in 2 sessions (±SEM). Error bars are asymmetric due to conversion from log mM to mM.
Test–retest reliability
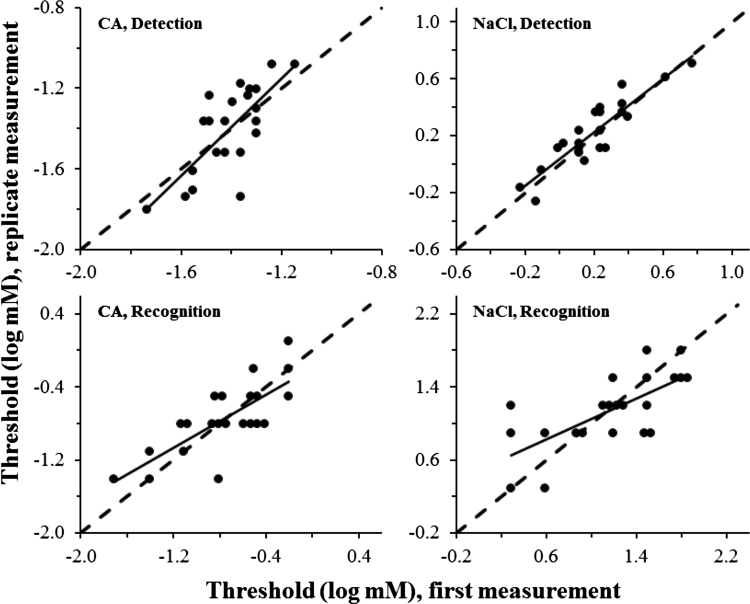
All measured thresholds proved reasonably reliable. For NaCl, the test–retest correlation reached significance for both detection, r(20) = 0.90 (intraclass correlation = 0.90), P < 0.0001, and recognition of saltiness, r(20) = 0.70 (intraclass correlation = 0.69), P < 0.001 (Figure 2). Similarly, for CA, the test–retest correlation reached significance for both detection, r(20) = 0.74 (intraclass correlation = 0.67, P < 0.0001), and recognition of sourness, r(20) = 0.77 (intraclass correlation = 0.78), P < 0.001 (Figure 2). Thus, retest correlations accounted for 50–80% of individual variance in measured thresholds.
Figure 2 .
Scatter plots illustrating test–retest reliability for a staircase method (detection thresholds) and a modified Harris–Kalmus recognition threshold (HK recognition) for 2 tastants. Dashed lines represent identity (perfect test–retest reliability). Solid lines represent least-squares linear fits. Reliability statistics appear in the text.
Correlation between detection and recognition
Correlations (Pearson’s r) between detection and recognition thresholds appear in Table 1. For both stimuli, all correlations were positive, suggesting that detection thresholds are weakly related to recognition thresholds. Correlations between detection and recognition only accounted for 1–20% (average = 12%) of variance across individuals.
Table 1.
Correlations (Pearson’s r) between detection and recognition
| Detection | Recognition | |
|---|---|---|
| Session 1 | Session 2 | |
| NaCl | ||
| Session 1 | 0.34 | 0.12 |
| Session 2 | 0.45* | 0.29 |
| CA | ||
| Session 1 | 0.33 | 0.37 |
| Session 2 | 0.35 | 0.42* |
*Significant, P < 0.05.
Weak correlations between 2 psychophysical tests can be due to poor reliability within tests. However, cross-test correlations were significantly weaker than the test–retest correlations. For NaCl, the average cross-test correlation was significantly lower than the test–retest correlations for both detection, Z = 3.24, P = 0.001, and recognition, Z = 1.78, P = 0.04. Similarly, for CA, the average cross-test correlation was significantly lower than the test–retest correlations for both detection, Z = 1.88, P = 0.03, and recognition, Z = 2.11, P = 0.02. Thus, poor cross-test correlation is not due to poor test–retest correlation alone.
The detection thresholds measured in the current study are comparable to values in other reports (Bartoshuk et al. 1986; Stevens et al. 1995; Stevens 1996; Mojet et al. 2001) though our test–retest reliabilities were higher than previously observed (e.g., McMahon et al. 2001). Our recognition thresholds are also comparable to those in previous reports, both in terms of absolute values and test–retest correlations (Henkin and Christiansen 1967; Weiffenbach et al. 1983; Doty 1992; Ahne et al. 2000; Gudziol and Hummel 2007). Despite significant retest reliability for all measures, the relationship between detection and recognition thresholds was weak. This finding suggests that for these stimuli, detection and recognition, as measured in the current study, were partially independent phenotypes. If so, physiological and genetic analyses of individual differences in NaCl and CA taste should yield partially independent processes underlying detection thresholds and taste quality recognition thresholds. For example, if the perceptual qualities of salty or sour taste per se are of interest, absolute detection thresholds for NaCl and CA might not provide relevant phenotypic data.
Study 2
Purpose
Rapid sensitivity measures, such as the modified staircase procedure used in Study 1, are assumed to estimate a single (threshold) point on an underlying sensitivity function of proportion of trials correct versus stimulus concentration ranging in performance from chance to perfect detection (Wysocki and Wise 2003; Wise et al. 2008). The forced-choice method of constant stimuli (FC-MCS), in which proportion correct is measured over a broad range of concentrations, characterizes the detection (psychometric) function more thoroughly and directly than staircase estimations. However, detection thresholds assessed by the staircase method and the FC-MCS are typically not compared. Quantitatively, the up-down rule of a staircase procedure is believed to converge on a region of the psychometric function; that is, the more stringent the up-down rule (5 correct to decrease concentration on subsequent trials vs. 3 correct, etc.), the higher the level of convergence on the psychometric function (the greater the stimulus strength required for detection). However, this assumption has not been well characterized for taste detection. To further explore absolute detection of NaCl and CA, 19 of 22 subjects returned to contribute full psychometric functions measured with a classic method of constant stimuli.
Materials and methods
Subjects
Nineteen (7 male, 12 female) subjects (age range = 21–52, mean = 31.72, SD = 9.19) from Study 1 agreed to return for Study 2. Subjects provided written informed consent using forms approved by the Office of Regulatory Affairs at the University of Pennsylvania before testing.
Taste materials
Taste compounds were the same as used in Study 1, prepared and handled using similar methods. The full range of concentrations used for CA ranged from 8.22×10–4 to 1.98×100 mM in 28 steps of 0.125 log units. The full range for NaCl was 6.39×10–2 to 4.17×10mM in 24 steps of 0.125 log units. Each subject received 14 consecutive steps out of the full range. Concentrations were selected based on previous (staircase) threshold measurements and pilot work to include concentrations that subjects could not discriminate from blanks (chance level), concentrations that subjects could very easily discriminate from blanks, and intermediate concentrations.
Procedure
The procedure for stimulus sampling exactly matched that used for the staircase method in Study 1: whole-mouth, sip-and-spit, 2-alternative forced-choice procedure. The delay between finishing tests for Study 1 and beginning tests for Study 2 ranged from 6 to 81 days (mean = 46.11, SD = 19.93). Each subject contributed 40 trials per concentration (560 trials per subject for each compound). Concentrations were tested in blocks, that is, groups of 20 trials in which one concentration of a particular target was presented. Blocks occurred in random order, with the constraint that subjects who required the same concentration ranges were tested in batches to ease the burden of stimulus preparation. Subjects completed no more than 3 blocks on a given day (with rest periods between blocks). Subjects required between 14 and 40 days to complete all blocks (mean = 42.11, SD = 6.71).
Data analysis
For each concentration of both compounds, proportion correct was calculated and plotted versus log concentration. Cumulative Gaussian functions (a common sigmoidal function symmetric with respect to the point of inflection) were fit to the data of each subject using a maximum likelihood procedure described elsewhere (Harvey 1986). The procedure yields a P value associated with the quality of fit. Low P values correspond to poor curve fits (i.e., significant disagreement between data and model).
Results and discussion
A cumulative Gaussian curve fit the psychometric functions well, considering the relatively small number of trials (n = 40) per concentration step (see Figure 3 for representative fits). For CA, the fits were good (high P value, or no significant disagreement between data and model) for all but one subject. For NaCl, the fits were good (average P value = 0.46) for all but one other subject. Correlations between psychometric thresholds (points of inflection on the fitted cumulative Gaussian) and thresholds previously measured with the staircase method were modest but significant for CA, r(17) = 0.44, P = 0.03, and were stronger for NaCl (r(17) = 0.77, P = 0.000058). Regarding group thresholds, geometric mean (95% CI) detection thresholds were 1.00 (0.73–1.35) mM for NaCl and 0.022 (0.016–0.031) mM for CA. These values differed by less than 2-fold from corresponding values for the same 19 subjects measured using the modified staircase method, establishing a good correspondence between these 2 sensitivity measures overall.
Figure 3 .
Representative individual subject psychometric functions for both NaCl (top) and CA (bottom). Curves: cumulative Gaussian functions (fit using maximum likelihood estimation). Poor sensitivity is right shifted on the horizontal axis, and high sensitivity is left shifted on the horizontal axis.
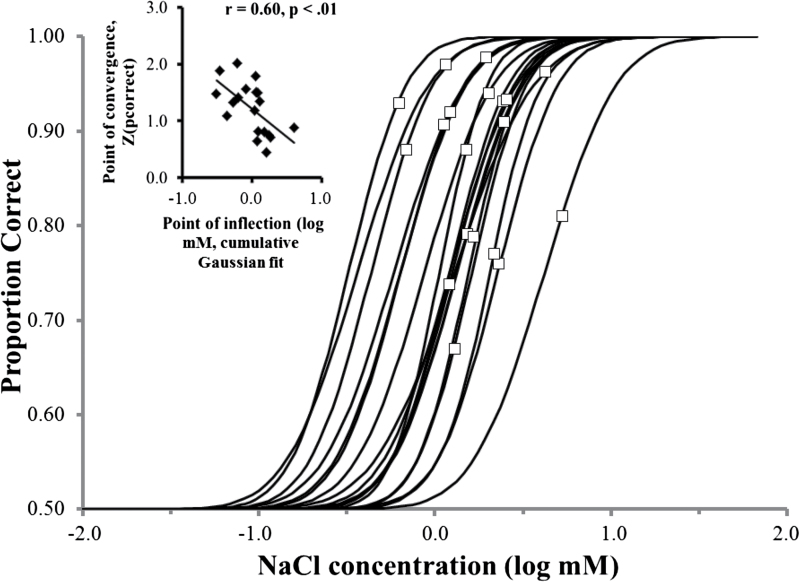
To further explore differences in how the 2 methods characterized individual differences, we conducted a post hoc analysis. According to the considerations of probability, a 4-down, 1-up staircase method should track, or converge on, a point on the psychometric function corresponding to 84% correct (Wetherill and Levitt 1965; Levitt 1971). The actual point of convergence varied widely but predictably among subjects for both compounds (Figures 4 and 5). Curiously, the point of convergence, that is, the proportion correct on the fitted psychometric function that corresponded to the concentration of the measured staircase threshold, was systematically related to the position of the fitted function on the concentration axis (inserts of Figures 4 and 5). That is, staircase runs converged onto a higher percent correct on the psychometric function for sensitive subjects and converged onto a lower percent correct for the less sensitive subjects. Accordingly, the range of individual differences measured using the staircase method was compressed relative to the range assessed by psychometric function measurement. For CA, staircase thresholds covered a 4.52-fold range (coefficient of variation [CV] = 0.37), whereas MCS thresholds covered an 18.74-fold range (CV = 0.71). For NaCl, the difference in ranges was nominally lower, but in the same direction: 8.66-fold range (CV = 0.59) for staircase thresholds and a 13.04-fold range (CV = 0.69) for MCS thresholds. In principle, point of convergence can be influenced by the slopes of psychometric functions (García-Pérez 2011). However, there was no significant correlation between threshold (position on the concentration axis) and slope for either CA (r = 0.01) or NaCl (r = 0.15).
Figure 4 .
Cumulative Gaussian fits for individual subject psychometric functions for NaCl (solid curves). Open squares represent the points on the functions corresponding to average (across 2 sessions) thresholds measured using the modified staircase method for the same subjects. Insert: Normal deviate transform of the proportion correct corresponding to the staircase thresholds versus log of thresholds estimated from psychometric functions (i.e., log of the concentrations corresponding to the points of inflection on the psychometric function).
Figure 5 .
Cumulative Gaussian fits for individual subject psychometric functions for CA (solid curves). Open squares represent the points on the functions corresponding to average (across 2 sessions) thresholds measured using the modified staircase method for the same subjects. Insert: Normal deviate transform of the proportion correct corresponding to the staircase thresholds versus log of thresholds estimated from psychometric functions (i.e., log of the concentrations corresponding to the point of inflection on the psychometric functions).
We also examined correlations between FC-MCS thresholds and recognition thresholds from Study 1. Correlations between FC-MCS and recognition thresholds were significant for both NaCl, r(17) = 0.56, P < 0.01, and CA, r(17) = 0.48, P < 0.02. The magnitude of these correlations was comparable to correlations between FC-MCS and modified staircase thresholds (not significantly different), suggesting that that FC-MCS thresholds were related to recognition thresholds and to staircase thresholds to a similar extent. Correlations between studies were modest compared with test–retest correlations in Study 1 though somewhat weaker correlations between studies would be expected given the average delay of 46 days between studies.
General discussion
Relationship among the 3 measures of sensitivity
In total, we collected both staircase detection thresholds and recognition thresholds for 22 subjects and, after a delay, more rigorous detection thresholds (FC-MCS) for 19 of those subjects. The finding that staircase and recognition thresholds correlated only weakly despite good retest reliability for both measures (Study 1) suggested that detection and recognition sensitivity for these stimuli were partially independent phenotypes, perhaps related to variance in independent sets of underlying physiological mechanisms. However, the fact that FC-MCS thresholds in Study 2 correlated about as strongly with recognition thresholds (nominally a test of a different sensory ability) as they did with staircase thresholds (nominally another test of the same sensory ability) entangles detection and recognition measures somewhat. It seems that the particular task, apart from the sensory ability it purports to measure, affects characterization of individual differences to an extent.
Of course, comparisons between FC-MCS thresholds in Study 2 and both measures in Study 1 are complicated by the time delay between the 2 studies. Lower correlations might be expected because taste sensitivity can change over time. However, if variation in sensitivity over time was independent across subjects, then we might expect random variation in the staircase points of convergence onto the psychometric functions, appearing as a noisy band around the expected value of about 84% correct (in Figures 4 and 5). Instead, the staircase points of convergence onto the psychometric functions were systematically related to subject sensitivity: Point of convergence was negatively correlated with detection sensitivity (as measured by FC-MCS). Thus, we were able to account for some additional variance in the staircase method, though the meaning of the observed relationship between FC-MCS sensitivity and staircase threshold is not immediately clear.
In our staircase method, the first threshold run for a given subject began in the middle of the concentration range, a common practice. This would expose sensitive subjects to concentrations above their threshold, which could desensitize the system sufficiently to yield thresholds of relatively higher values, consistent with higher convergence points for sensitive people. Less sensitive subjects would experience the opposite, wherein their initial concentrations with the staircase method would be below their detection threshold and could converge on relatively lower concentrations. If this explanation is correct, longer intervals between trials or adaptive methods that employ ascending concentrations might yield a larger range of individual differences and would eliminate the correlation between points of convergence on the psychometric function and overall sensitivity of the subject.
The fact that the MCS was modified to include blocks of 20 trials at a fixed concentration might also be relevant. If relatively insensitive subjects were more prone to either adaptation or lapses of attention within a repetitive block of trials, this would tend to right shift their psychometric functions on the concentration axis making their staircase runs appear to converge at lower points on the function. Lack of adaptation or more sustained attention might not explain why psychometric functions for sensitive subjects appear left shifted. However, if more acute observers become progressively better at extracting sensory information within a repetitive block of trials, this would tend to left-shift psychometric functions and make staircase runs appear to converge at higher points on the function. These possibilities are not exhaustive and need not be mutually exclusive.
In short, subject characteristics, such as adaptation rates or attention, might interact with certain methods. Regardless, both measures tend to reflect average (group) sensitivity well and correlate with each other. Nevertheless, they differ systematically in their assessments of individual differences in sensitivity. Highly sensitive individuals tend to appear slightly less sensitive when tested with the staircase method, and less sensitive subjects tend to appear more sensitive when tested with the staircase method. The net result is a compressed range of observed individual differences for the staircase method relative to the modified method of constant stimuli.
Similar analyses on the points of the FC-MCS psychometric functions on which recognition thresholds fell (not shown) produced null correlations. Of course, recognition thresholds almost always fell on the flat (asymptotic) upper portion of psychometric functions: The concentration corresponding to the average (across subjects and compounds) recognition threshold corresponded to 99.9% correct (minimum = 92.1% correct), a region of the underlying sensitivity function inherently difficult to estimate. Regardless, we were unable to account for additional variance in recognition thresholds as we did for staircase detection thresholds, suggesting a somewhat different relationship between recognition and FC-MCS than between staircase detection thresholds and FC-MCS.
Additional considerations in comparisons between detection and recognition
Given published observations, it is plausible that detection and recognition, which typically occur at substantially different concentrations, engage different underlying physiological mechanisms for these particular stimuli. For example, there are reports that the taste quality of both stimuli can be concentration dependent (Cardello and Murphy 1977; Kim et al. 2004; Wise et al. 2007; Galindo-Cuspinera et al. 2009). In particular, the sweet taste of NaCl at low concentrations is consistent with the idea that weak salt stimuli may stimulate sweet receptors (Bartoshuk et al. 1978). In addition to the possibility of engaging multiple taste submodalities, acid is astringent and both NaCl and CA can evoke oral pain at high concentration (Green and Lawless 1991; Bajec and Pickering 2008). Recognition thresholds in the present study were below concentrations typically associated with significant somatosensory impact, but low levels of stimulation in these nongustatory systems remain possible. Thus, detection and recognition may depend on partially independent physiological mechanisms, at least as we have presently structured the recognition task. It is worth considering, for example, that detection thresholds for NaCl might be closer in concentration to and more tightly correlated with “sweetness” recognition thresholds for NaCl than with “saltiness” recognition thresholds for NaCl.
Regardless, weak correlations between detection and recognition could also reflect differences in response strategy or decision criteria. Both detection methods controlled potential response bias through the 2-alternative forced-choice task, which is very different than the multiple response alternatives in the recognition task. Behavioral performance in many psychophysical tasks decreases with the number of response alternatives (reviewed in van Maanen et al. 2012). In addition, the recognition response alternative of “water” to indicate lack of a clear taste did not force subjects guess when unsure, which might in turn have encouraged some subjects to adopt a strict criterion for reporting qualities. Thus, some subjects may have hesitated to attempt recognition until concentrations rose above those associated with probabilistic recognition (i.e., concentrations at which subjects recognized quality at above chance but imperfectly).
Correlations between detection and recognition in Study 1 might have been stronger if both methods had used a 2-alternative forced-choice task and specified a comparable criterion for proportion correct (Tanner 1956; also see Laska and Ringh 2010). We chose the detection and recognition methods in Study 1 to be representative of those commonly used in behavior genetics and in the chemosensory field in general. However, we emphasize here that poor correlations between tasks could depend on more than differences in underlying physiological mechanisms.
Conclusions
For both CA and NaCl, individual differences in modified staircase detection thresholds were at least partially independent from individual differences in modified Harris–Kalmus recognition thresholds. This result is consistent with the idea that absolute detection and quality recognition characterize partially independent sensory abilities with different underlying physiological mechanisms. However, the finding that recognition and modified staircase detection thresholds both correlated with more rigorous and direct measurements of absolute sensitivity (FC-MCS thresholds) suggests that modified Harris–Kalmus recognition thresholds and modified staircase detection thresholds might both reflect underlying variance in absolute sensitivity. This suggests that all 3 measures may relate to individuals’ absolute sensitivities to NaCl and CA to some extent. Regardless, comparisons between the absolute sensitivity measures show that even 2 tasks that purport to measure the same underlying sensory ability can return systematically different characterizations of individual differences in sensitivity. Thus, individual differences in sensitivity seem to interact with the methodological differences. We speculate that individual differences in adaptation rates and recovery might partially explain differences in absolute sensitivity between the 2 methods. In practical terms, the current results suggest that staircase methods might yield a compressed range of individual differences relative to MCS.
Funding
The work was supported in part by National Institutes of Health [DC 02995 and DC 011393 to P.A.S.B.].
Acknowledgements
We thank Mrs Anilet Tharp for excellent technical assistance. We thank Alan Spector, Larry Marks, and an anonymous reviewer for valuable comments.
References
- Ahne G, Erras A, Hummel T, Kobal G. 2000. Assessment of gustatory function by means of tasting tablets. Laryngoscope. 110:1396–1401 [DOI] [PubMed] [Google Scholar]
- Bajec MR, Pickering GJ. 2008. Astringency: mechanisms and perception. Crit Rev Food Sci Nutr. 48:858–875 [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. 1978. The psychophysics of taste. Am J Clin Nutr. 31:1068–1077 [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. 2000. Comparing sensory experiences across individuals: recent psychophysical advances illuminate genetic variation in taste perception. Chem Senses. 25:447–460 [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Murphy C, Cleveland CT. 1978. Sweet taste of dilute NaCl: psychophysical evidence for a sweet stimulus. Physiol Behav. 21:609–613 [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Rifkin B, Marks LE, Bars P. 1986. Taste and aging. J Gerontol. 41:51–57 [DOI] [PubMed] [Google Scholar]
- Breslin PA, Huang L. 2006. Human taste: peripheral anatomy, taste transduction, and coding. Adv Otorhinolaryngol. 63:152–190 [DOI] [PubMed] [Google Scholar]
- Breza JM, Contreras RJ. 2012. Anion size modulates salt taste in rats. J Neurophysiol. 107:1632–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W. 2005. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 15:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardello AV, Murphy C. 1977. Magnitude estimates of gustatory quality changes as a function of solution concentration of simple salts. Chem Sens Flav. 2:327–339 [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. 2010. The cells and peripheral representation of sodium taste in mice. Nature. 11:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QY, Alarcon S, Tharp A, Ahmed OM, Estrella NL, Greene TA, Rucker J, Breslin PA. 2009. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr. 90:770S–779S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. 1992. Diagnostic tests and assessment. J Head Trauma Rehabil. 7:47–65 [Google Scholar]
- Faurion A. 1987. MSG as one of the sensitivities within a continuous taste space: electrophysiological and psychophysical studies. In: Kawamura Y, Kare MR, editors. Umami a basic taste. New York (NY): Marcel Dekker; p. 387–408 [Google Scholar]
- Field AP. 2005. Intraclass correlation. In: Everitt BS, Howell DC, editors. Encyclopedia of statistics in behavioral sciences. Chichester (England): Wiley; [Google Scholar]
- Galindo-Cuspinera V, Waeber T, Antille N, Hartmann C, Stead N, Martin N. 2009. Reliability of threshold and suprathreshold methods for taste phenotyping: characterization with PROP and sodium chloride. Chemosens Percept. 2:214–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez MA. 2011. A cautionary note on the use of the adaptive up-down method. J Acoust Soc Am. 130:2098–2107 [DOI] [PubMed] [Google Scholar]
- Green BG, Lawless HT. 1991. The psychophysics of somatosensory chemoreception in the nose and mouth. In: Getchell TV, Doty RL, Bartoshuk LM, Snow J, editors. Smell and taste in health and disease. New York: Raven; p. 235–253 [Google Scholar]
- Gudziol H, Hummel T. 2007. Normative values for the assessment of gustatory function using liquid tastants. Acta Otolaryngol. 127:658–661 [DOI] [PubMed] [Google Scholar]
- Harris H, Kalmus H. 1949. The measurement of taste sensitivity to phenylthiourea (P.T.C.). Ann Hum Genet. 15:24–31 [DOI] [PubMed] [Google Scholar]
- Harvey LO. 1986. Efficient estimation of sensory thresholds. Behav Res Meth Instrum Comput. 18:623–632 [Google Scholar]
- Henkin RI, Christiansen RL. 1967. Taste localization on the tongue, palate, and pharynx of normal man. J Appl Physiol. 22:316–320 [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. 2006. The cells and logic for mammalian sour taste detection. Nature. 442:934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan AR, Fischer R, Karras A, Griffin F, Powell W, Marsters RW, Glanville EV. 1967. Taste thresholds in twins and siblings. Acta Genet Med Gemellol (Roma). 16:229–243 [DOI] [PubMed] [Google Scholar]
- Keast RSJ, Roper J. 2007. A complex relationship among chemical concentration, detection threshold, and suprathreshold intensity of bitter compounds. Chem Senses. 32:245–253 [DOI] [PubMed] [Google Scholar]
- Kim UK, Breslin PA, Reed D, Drayna D. 2004. Genetics of human taste perception. J Dent Res. 83:448–453 [DOI] [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. 2003. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 299:1221–1225 [DOI] [PubMed] [Google Scholar]
- Laska M, Ringh A. 2010. How big is the gap between olfactory detection and recognition of aliphatic aldehydes? Attention Percept Psychophys. 72:806–812 [DOI] [PubMed] [Google Scholar]
- Levitt H. 1971. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 49(Suppl 2):467–477 [PubMed] [Google Scholar]
- McMahon DB, Shikata H, Breslin PA. 2001. Are human taste thresholds similar on the right and left sides of the tongue? Chem Senses. 26:875–883 [DOI] [PubMed] [Google Scholar]
- Meng X-L, Rosenthal R, Rubin DB. 1992. Comparing correlated correlation coefficients. Psychol Bull. 111:172–175 [Google Scholar]
- Mojet J, Christ-Hazelhof E, Heidema J. 2001. Taste perception with age: generic or specific losses in threshold sensitivity to the five basic tastes? Chem Senses. 26:845–860 [DOI] [PubMed] [Google Scholar]
- Mojet J, Christ-Hazelhof E, Heidema J. 2005. Taste perception with age: pleasantness and its relationships with threshold sensitivity and suprathreshold intensity of five taste qualities. Food Qual Prefer. 16:413–423 [Google Scholar]
- Mombaerts P. 2004. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 5:263–278 [DOI] [PubMed] [Google Scholar]
- Raliou M, Wiencis A, Pillias AM, Planchais A, Eloit C, Boucher Y, Trotier D, Montmayeur JP, Faurion A. 2009. Nonsynonymous single nucleotide polymorphisms in human tas1r1, tas1r3, and mGluR1 and individual taste sensitivity to glutamate. Am J Clin Nutr. 90:789S–799S [DOI] [PubMed] [Google Scholar]
- Shigemura N, Shirosaki S, Sanematsu K, Yoshida R, Ninomiya Y. 2009. Genetic and molecular basis of individual differences in human umami taste perception. PLoS ONE. 4:e6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JC. 1996. Detection of tastes in mixture with other tastes: issues of masking and aging. Chem Senses. 21:211–221 [DOI] [PubMed] [Google Scholar]
- Stevens JC, Cruz LA, Hoffman JM, Patterson MQ. 1995. Taste sensitivity and aging: high incidence of decline revealed by repeated threshold measures. Chem Senses. 20:451–459 [DOI] [PubMed] [Google Scholar]
- Tanner WP. 1956. The theory of recognition. J Acoust Soc Am. 28:882–888 [Google Scholar]
- van Maanen L, Grasman RP, Forstmann BU, Keuken MC, Brown SD, Wagenmakers EJ. 2012. Similarity and number of alternatives in the random-dot motion paradigm. Attention Percept Psychophys. 74:739–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiffenbach JM, Wolf RO, Benheim AE, Folio CJ. 1983. Taste threshold assessment: a note on quality specific differences between methods. Chem Senses. 8:151–159 [Google Scholar]
- Wetherill GB, Levitt H. 1965. Sequential estimation of points on a psychometric function. Br J Math Stat Psychol. 18:1–10 [DOI] [PubMed] [Google Scholar]
- Wise PM, Bien N, Wysocki CJ. 2008. Two rapid odor threshold methods compared to a modified method of constant stimuli. Chemosens Percept. 1:16–23 [Google Scholar]
- Wise PM, Hansen JL, Reed DR, Breslin PA. 2007. Twin study of the heritability of recognition thresholds for sour and salty taste. Chem Senses. 32:749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CJ, Wise P. 2003. Methods, approaches, and caveats for functionally evaluating olfaction and chemesthesis. In: Deibler K, Delwiche JF, editors. Handbook of flavor characterization: sensory, chemical and physiological. New York: Marcel Dekker; p. 1–40 [Google Scholar]