Abstract
Glycyrrhizic acid (GA), the main component of radix glycyrrhizae, has a variety of pharmacological activities. In the present study, suspensions of GA nanoparticles with the average particle size about 200nm were prepared by a supercritical antisolvent (SAS) process. Comparative studies were undertaken using lipopolysaccardide(LPS)-stimulated mouse macrophages RAW 264.7 as in vitro inflammatory model. Several important inflammation mediators such as NO, PGE2, TNF-α and IL-6 were examined. These markers were highly stimulated by LPS and were inhibited both by nano-GA and unprocessed GA in a dose-dependent manner, especially PGE2 and TNF-α. However nano-GA and unprocessed GA inhibited NO only at a high concentration. In general, we found that GA nanoparticle suspensions exhibited much better anti-inflammatory activities compared to unprocessed GA.
Keywords: glycyrrhizic acid, nanoparticle, mouse macrophages RAW 264.7, inflammatory cytokines
Introduction
Inflammation is a natural biological response to injury or infection in the human body. Various factors, such as microbial infections, chemicals, and immunologic reactions can cause inflammation.1 Prolonged inflammation can be harmful, contributing to the pathogenesis of many diseases, including rheumatoid arthritis, obesity, cardiovascular diseases, neurodegenerative diseases, diabetes, and cancer.2,3
Macrophages are considered to play essential roles in inflammation. If activated by endotoxins, macrophages produce inflammatory cytokines, which in turn activate other macrophages and other nearby cells to promote, for example, inducible nitric oxide (NO) synthase gene expression.4 Much progress has been made in the delineation of cell signaling pathways, in which the inflammation initiates a cascade of events that result in the overproduction of certain inflammation-associated genes and proinflammatory cytokines.5
Inhibition of inflammatory cytokine and mediator production serves as a key mechanism in the control of inflammation. A number of anti-inflammatory molecules have already entered clinical trials for the treatment of inflammatory disorder such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), prostaglandin E2 (PGE2) or nitric oxide (NO).6 Drugs that suppress the expression of these inflammatory mediators have therefore attracted significant interest as potential therapeutics for the treatment of inflammatory diseases.3
Glycyrrhizic acid (GA), a terpenoid compound, is the main component of radix glycyrrhizae. GA has a variety of pharmacological activities such as antioxidative, anti-inflammatory, antiulcerous, antidotal, antiallergic, antiviral, immunomodulating, hepatoprotective, and cardioprotective properties.7–10 However, GA is slowly absorbed in vivo because of its poor water solubility. Large number of studies have shown that the reduction of the drug particle size to the nanometer scale considerably increases the activity and bioavailability of the drug.11 Supercritical antisolvent (SAS) process is an environmentally friendly technology developed in recent years. In this process, the drug is firstly dissolved in the solvent and then the drug solution is quickly sprayed into supercritical fluids (the antisolvent). Precipitation occurs immediately by a rapid recrystallization of the drug. In general, the SAS process can control the production of particle sizes within the nanometer range, and the solvent can be fully recovered. It is suitable to prepare nanocrystals of drugs or biologicaly active substance because of low temperature and inertia.
This study explored the anti-inflammatory activity and mechanisms of GA nanoparticles prepared by SAS in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages compared to unprocessed GA particles.
Materials and methods
Materials
GA (≥98%) for nanosuspension preparation was purchased from Xi’an Green-Life Natural Products Co, Ltd (Xi’an, People’s Republic of China). GA (99%) for quantitative analysis was purchased from Sigma-Aldrich (St Louis, MO, USA). Compound glycyrrhizin tablets were manufactured by Minophagen Pharmaceutical Co., Ltd (Tokyo, Japan). LPS was purchased from Sigma-Aldrich. Griess reagent kit was obtained from Biyuntian Biological Technology Co., Ltd (Shanghai, People’s Republic of China). PGE2, TNF-α, and IL-6 enzyme-linked immunosorbent assay kits were purchased from 4A Biotech Co., Ltd (Beijing, People’s Republic of China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich. High-performance liquid chromatography grade methanol was purchased from J&K Chemical Ltd (Beijing, People’s Republic of China). Purified water was obtained from a Milli-Q® system from EMD Millipore (Billerica, MA, USA). All other chemicals were of analytical reagent grade.
Preparation of GA nanosuspension
Two processes were performed to obtain the final GA nanoparticle suspension. First, the micronized GA was prepared with an SAS apparatus modified from a carbon dioxide supercritical extraction apparatus by using dimethyl sulfoxide (DMSO) as the solvent and carbon dioxide as the antisolvent. Under the optimum conditions, micronized GA with a particle size about 200 nm was obtained.
Surface morphology determined by scanning electron microscopy (SEM) of nanoparticles and unprocessed GA
The samples were fixed to an SEM stub and sputter coated with gold using SBC-12 ion sputter coater (KYKY Technology Development Ltd, Beijing, People’s Republic of China) to form a carbon conductive film. The surface morphology of the particles was then observed by SEM (Quanta™ 200; FEI Company, Hillsboro, OR, USA).
Cell culture
The RAW 264.7 cells, a murine macrophage-like cell line, were obtained from China Cell Line Bank (Beijing, People’s Republic of China). RAW 264.7 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37°C in a humidified atmosphere (5% carbon dioxide). The test compounds were diluted with 2% Dulbecco’s modified Eagle’s medium to the appropriate concentrations and added 1 hour before LPS treatment.
MTT assay for cell viability
The MTT assay was performed to measure cell viability. RAW 264.7 cells were mechanically scraped, seeded in 96-well plates, and incubated in a 37°C, 5% carbon dioxide incubator overnight. After 24 hours, cells were treated with different concentrations of GA nanoparticles and unprocessed GA or glycyrrhizin tablets for 24 hours. Subsequently, 20 μL of 5 mg/mL MTT in fetal bovine serum-free medium was added to each well, and the cells were incubated for 4 hours. MTT was removed and resolved with 150 μL/well dimethyl sulfoxide. The optical density was measured at 492 nm using a microplate reader (Tecan Infinite® M200, Tecan Group Ltd, Männedorf, Austria). Concentrations were determined for three wells of each sample, and this experiment was done in triplicate.
Analysis of NO production
Nitrite, a stable product of NO, was used to assess NO production based on the Griess reaction. RAW 264.7 cells were incubated with different concentrations of GA nanoparticles and unprocessed GA or glycyrrhizin tablets in the absence or presence of 1 μg/mL LPS. After 24 hours of incubation, the level of NO production was monitored by measuring the nitrite concentration in the supernatant of cultured medium using the Griess reagent assay. For the NO assay, 50 μL supernatant of cultured medium was mixed with the same volume of Griess reagent. The absorbance was measured at 540 nm on a microplate reader. The concentration of NO in the media of sample-treated cells was calculated using the standard curve obtained for sodium nitrite dissolved in Dulbecco’s modified Eagle’s medium. Concentrations were determined for three wells in each sample, and this experiment was done in triplicate.
Determination of PGE2, TNF-α, and IL-6 levels
To investigate the effect of GA nanoparticles and unprocessed GA or glycyrrhizin tablets on cytokine responses from LPS-treated cells, RAW 264.7 cells seeded on 96-well plates were treated with 303, 606, and 909 μM GA 1 hour before treatment with 1 μg/mL LPS for 24 hours in a 37°C, 5% carbon dioxide incubator. Cell-free supernatants were collected and stored at −20°C until assayed for cytokines. The concentrations of PGE2, TNF-α, and IL-6 in the culture supernatants of RAW 264.7 cell cultures were determined using an enzyme-linked immunosorbent assay kit. Concentrations were determined for three wells in each sample, and this experiment was done in triplicate.
Statistical analysis
The experimental values were represented as arithmetic mean ± standard deviation. The unpaired Student’s t-test was used to determine the statistical significance. Statistically, a P-value less than 0.05 was considered to be significant and a P-value less than 0.01 was considered to be very significant.
Results
Surface morphology of GA nanoparticles and unprocessed GA
Figure 1A and B show the particle size of unprocessed GA material and GA nanoparticle suspensions performed by scanning electron microscopy (SEM). The final GA nanosuspension prepared by SAS contained particles of about 200 nm, whereas GA raw materials exhibited an irregular shape and micrograde particle size.
Figure 1.
Scanning electron microscopy result of (A) unprocessed GA and (B) GA nanoparticles.
Abbreviation: GA, glycyrrhizic acid.
Cell viability and comparative effects of GA nanoparticles and unprocessed GA on LPS-induced NO production
Unprocessed GA, nano-GA and glycyrrhizin tablets up to 909 μM did not show cytotoxic effects (data not shown).
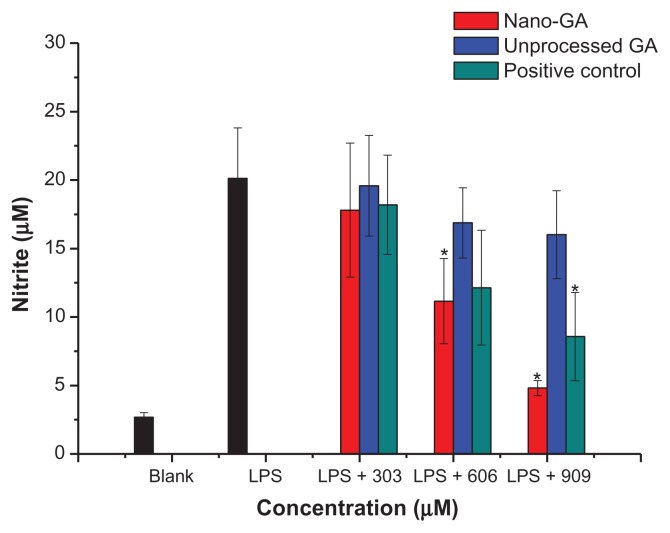
Nitric oxide (NO) is a short-living free radical that is produced from L-arginine by catalytic reactions of NO synthases in mammalian immune, cardiovascular and neural systems, where it functions as signaling molecule.12 To analyze potential anti-inflammatory properties of GA nanoparticles and unprocessed GA, we used murine RAW 264.7 macrophage cells, which produce NO upon stimulation with LPS. The amount of produced NO was determined by measurement of nitrite, a stable metabolite of NO. Cells were pretreated with GA nanoparticles or unprocessed GA, and then stimulated with 1 μg/mL LPS. The control group was untreated with both LPS and samples. After the cell culture media were collected, nitrite levels were determined. During the incubation time of 24 hours, RAW 264.7 macrophages produced 2.68 ± 0.34 μM nitrite in the resting state, whereas after LPS (1 μg/mL) stimulation, NO production dramatically increased to 20.12 ± 3.69 μM. Nano-GA and unprocessed GA inhibited nitrite production 24 hours after LPS stimulation in a dose-dependent manner (Figure 2). At 303 μM, nano-GA and unprocessed GA reduced NO production by 13.33% and 0.03%, respectively. At concentrations of 606 μM, nano-GA and unprocessed GA reduced NO production by 51.44% and 18.67%, respectively, whereas at 909 μM, nano-GA and unprocessed GA reduced NO production by 87.78% and 23.56%, respectively. Nano-GA inhibited NO generation much better than unprocessed GA, especially at high concentrations. When compared to the positive control (glycyrrhizin tablets), the inhibition rate of NO was: nano-GA > positive control > unprocessed GA.
Figure 2.
Effect of different concentrations of GA nanoparticles and unprocessed GA on LPS-induced nitric oxide.
Note: *P < 0.05, significantly different from the LPS-treated group by unpaired Student’s t-test.
Abbreviations: GA, glycyrrhizic acid; LPS, lipopolysaccharide.
Comparative effects of GA nanoparticles and unprocessed GA on LPS-induced PGE2 production
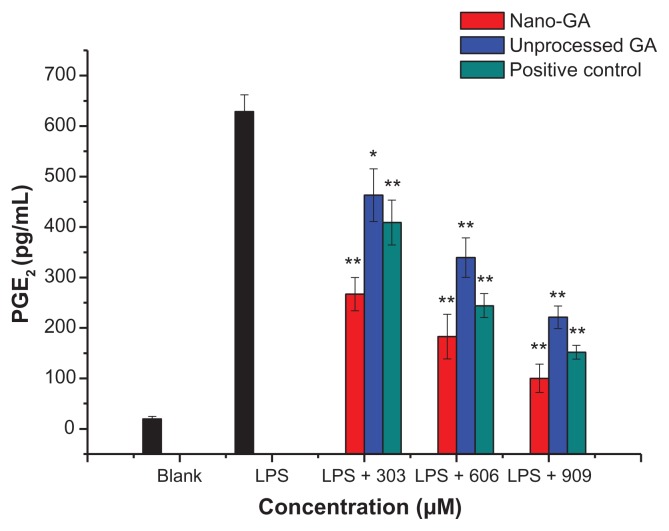
Levels of the pro-inflammatory lipid mediator PGE2 were analyzed upon LPS stimulation in RAW 264.7 cells. During the incubation for 24 hours, RAW 264.7 macrophages produced 19.75 ± 5.24 pg/mL PGE2 in the resting state, whereas after LPS stimulation, PEG2 production dramatically increased to 628.47 ± 33.59 pg/mL. Both GA nanoparticles and unprocessed GA reduced PEG2 production in a dose-dependent manner (Figure 3). At concentrations of 303 μM, GA nanoparticles and unprocessed GA reduced PGE2 production by 59.38% and 27.18%, respectively. Nano-GA exhibiting more than two-fold inhibition activity than unprocessed GA. At 606 μM, PGE2 production was reduced by 73.22% and 47.49%, respectively, whereas at 909 μM, nano-GA and unprocessed GA reduced PGE2 production by 86.78% and 66.90%, respectively. Nano-GA inhibited PGE2 generation much better than unprocessed GA. When compared to the positive control (glycyrrhizin tablets), the inhibition rate of PGE2 was: nano-GA > positive control > unprocessed GA.
Figure 3.
Effect of different concentrations of GA nanoparticles and unprocessed GA on LPS-induced PGE2.
Notes: *P < 0.05; **P < 0.01, significantly different from the LPS-treated group by unpaired Student’s t-test.
Abbreviations: GA, glycyrrhizic acid; LPS, lipopolysaccharide; PGE2, prostaglandin E2.
Comparative effects of GA nanoparticles and unprocessed GA on LPS-induced TNF-α production
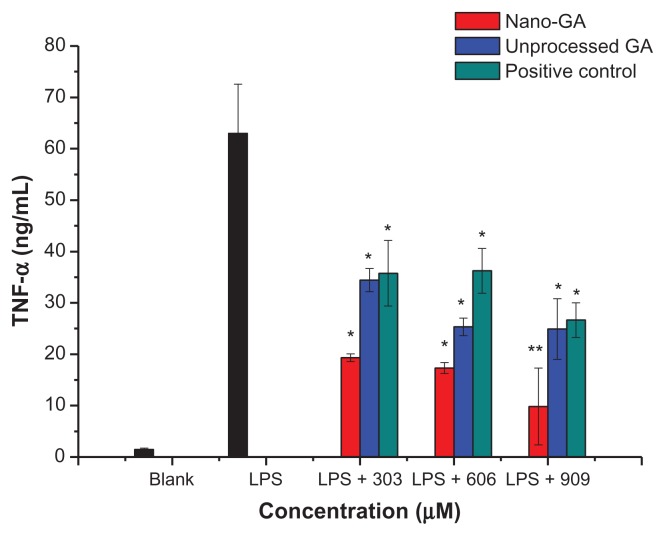
TNF-α is one of the most important cytokines and is required for the induction of NO synthesis in LPS-stimulated macrophages. TNF-α elicits a number of physiological effects, such as septic shock, inflammation, cachexia, and cytotoxicity.13 TNF-α concentrations in the culture supernatants of RAW 264.7 cells were measured by enzyme-linked immunosorbent assay (Figure 4). RAW 264.7 cells treated with LPS produced significant amounts of TNF-α. The concentration of TNF-α increased from 1.47 ± 0.27 ng/mL to 62.99 ± 9.56 ng/mL after LPS stimulation. However, the concentrations of TNF-α in the supernatant of cells treated with GA nanoparticles and unprocessed GA were significantly decreased compared to the LPS control group. At concentrations of 303 μM, GA nanoparticles and unprocessed GA reduced TNF-α production by 76.94% and 57.01%, respectively. At 606 μM, TNF-α production was reduced by 79.61% and 69.02%, respectively, whereas at 909 μM, TNF-α production was reduced by 89.51% and 69.58%, respectively. Both nano-GA and unprocessed GA strongly inhibited TNF-α generation. When compared to the positive control (glycyrrhizin tablets), the reduction rate of TNF-α was: nano-GA > unprocessed GA > positive control.
Figure 4.
Effect of different concentrations of GA nanoparticles and unprocessed GA on LPS-induced TNF-α.
Notes: *P < 0.05; **P < 0.01, significantly different from the LPS-treated group by unpaired Student’s t-test.
Abbreviations: GA, glycyrrhizic acid; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
Comparative effects of GA nanoparticles and unprocessed GA on LPS-induced IL-6 production
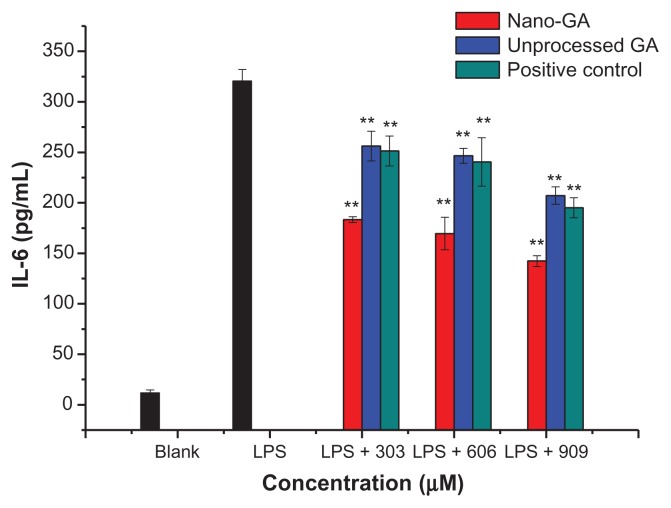
IL-6 represents an endogenous mediator of LPS-induced fever. RAW 264.7 cells treated with LPS produced significant amounts of IL-6, increasing from 11.67 ± 3.06 pg/mL to 320.33 ± 11.59 pg/mL after LPS stimulation (Figure 5). IL-6 in the supernatant of cells treated with GA nanoparticles and unprocessed GA did not significantly change compared to the LPS control group. At concentrations of 303 μM, GA nanoparticles and unprocessed GA reduced IL-6 production by 44.39% and 20.79%, respectively. At 606 μM, IL-6 production was reduced by 48.85% and 23.92%, respectively, whereas at 909 μM, IL-6 production was reduced by 57.67% and 36.66%, respectively. When compared to the positive control (glycyrrhizin tablets), the reduction rate of IL-6 was: nano-GA > positive control > unprocessed GA. Nano-GA inhibited IL-6 generation much better than unprocessed GA.
Figure 5.
Effect of different concentrations of GA nanoparticles and unprocessed GA on LPS-induced IL-6.
Note: **P < 0.01, significantly different from the LPS-treated group by unpaired Student’s t-test.
Abbreviations: GA, glycyrrhizic acid; LPS, lipopolysaccharide;IL-6, interleukin-6.
Discussion
Inflammation is the primary response of the immune system against infection or irritation, and macrophages play a crucial role during the inflammatory process.14 In the presence of stimuli such as LPS, macrophages are activated and produce various cytokines such as TNF-α, IL-1β, IL-6, and IL-10, as well as inflammatory mediators such as NO and PGE2.3,15,16 Therefore, LPS-activated macrophages have typically been used to evaluate the anti-inflammatory effects of various materials. The production of these cytokines and mediators may result in the systemic inflammatory response syndrome, severe tissue damage, and septic shock.17 Therefore, agents that regulate cytokines and inflammatory mediators may have therapeutic effects.
Glycyrrhiza uralensis (Leguminosae) has long been used throughout the world as a sweetener and in folk medicine because of its antioxidative, anti-inflammatory, antibacterial, antiangiogenic, and antiallergenic properties.1 Its main components are considered to be the triterpene saponins glycyrrhizin and GA.18–20
Nanomaterials are important materials due to their unique physical and chemical properties.21 Due to accumulation of nanoparticles in the cell by enhanced permeability and retention effect, nanoparticles achieve new biological properties.22,23 In the current report, solid dispersion microparticles of poorly water-soluble GA were prepared by using SAS method. This technique is a feasible and efficient way to enhance the solubility of poorly water-soluble GA with high dissolution rate.
In order to compare the anti-inflammatory activity of GA nanoparticles and unprocessed GA in LPS-stimulated RAW 264.7 macrophages, several important inflammation mediators were examined. We found that the release of NO, PGE2, TNF-α and IL-6 release, which was highly stimulated by LPS, was inhibited by both nano-GA and unprocessed GA in a dose-dependent manner. In particular, GA suspensions suspension, compared with the unprocessed GA significantly enhanced the reduction of PGE2 and TNF-α even at low concentrations, but did not significantly affect NO production at low concentrations. Nano-GA exhibited much better inhibition activities compared to unprocessed GA. This is due to the fact that nanoparticles have a smaller particle sizes compared to unprocessed drug particles, which leads to an increase of the interfacial surface area and consequently improvement of water solubility, allowing for smaller dosages and more rapid and direct usage of otherwise poorly water-soluble drugs.24 When compared to the positive control (glycyrrhizin tablets) the reduction rate of NO, PGE2, and IL-6 was: nano-GA > positive control > unprocessed GA, whereas for TNF-α it was: GA nanoparticles > unprocessed GA. positive control.
Conclusion
In summary, the findings presented here suggest that GA nanoparticles prepared by the SAS method performed much better inhibition activity for LPS-induced NO, PGE2, TNF-α, and IL-6 production in macrophage cells than unprocessed GA. Thus, GA nanoparticles may have therapeutic potential for the modulation and regulation of macrophage activation, and may provide safe and effective treatment options for a variety of inflammation-mediated diseases.
Acknowledgements
The authors gratefully acknowledge the financial supports by special scientific fund for non-profit public industry (201204601) and postdoctoral science-research developmental foundation of Heilongjiang province (LBH-Q11183).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shin EM, Zhou HY, Guo LY, et al. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264.7 macrophages. Int Immunopharmacol. 2008;8(11):1524–1532. doi: 10.1016/j.intimp.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995;35:655–677. doi: 10.1146/annurev.pa.35.040195.003255. [DOI] [PubMed] [Google Scholar]
- 3.Park YD, Jin CH, Choi DS, Byun MW, Jeong IY. Biological evaluation of isoegomaketone isolated from Perilla frutescens and its synthetic derivatives as anti-inflammatory agents. Arch Pharm Res. 2011;34(8):1277–1282. doi: 10.1007/s12272-011-0806-8. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6(12):3051–3064. [PubMed] [Google Scholar]
- 5.O’Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5(7):549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- 6.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29(Suppl 7):S121–S125. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- 7.Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46(3):167–192. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Hennell JR, Lee S, Khoo CS, Gray MJ, Bensoussan A. The determination of glycyrrhizic acid in Glycyrrhiza uralensis Fisch. ex DC. (Zhi Gan Cao) root and the dried aqueous extract by LC–DAD. J Pharm Biomed Anal. 2008;47(3):494–500. doi: 10.1016/j.jpba.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Kimura M, Moro T, Motegi H, et al. In vivo glycyrrhizin accelerates liver regeneration and rapidly lowers serum transaminase activities in 70% partially hepatectomized rats. Eur J Pharmacol. 2008;579(1–3):357–364. doi: 10.1016/j.ejphar.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 10.Kurisu S, Inoue I, Kawagoe T, et al. Clinical profile of patients with symptomatic glycyrrhizin-induced hypokalemia. J Am Geriatr Soc. 2008;56(8):1579–1581. doi: 10.1111/j.1532-5415.2008.01781.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Wei W, Zu YG, Yang L, Zhao XH, Zu BS. Comparative study on effects of GA nanoparticles suspension and unprocessed GA solution on oral bioavailability, hepatic function and liver antioxidant enzymes activities in carbon tetrachloride-induced liver damage rats. Mater Sci Forum. 2011;694:423–429. [Google Scholar]
- 12.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269(19):13725–13728. [PubMed] [Google Scholar]
- 13.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7(2):93–124. [PubMed] [Google Scholar]
- 14.Ialenti A, Moncada S, Di Rosa M. Modulation of adjuvant arthritis by endogenous nitric oxide. Br J Pharmacol. 1993;110(2):701–706. doi: 10.1111/j.1476-5381.1993.tb13868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhule A, Navarro S, Smith JR, Shepherd DM. Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. J Ethnopharmacol. 2006;106(1):121–128. doi: 10.1016/j.jep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Ci X, Ren R, Xu K, et al. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-κB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation. 2010;33(2):126–136. doi: 10.1007/s10753-009-9166-7. [DOI] [PubMed] [Google Scholar]
- 17.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189(11):1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akamatsu H, Komura J, Asada Y, Niwa Y. Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991;57(2):119–121. doi: 10.1055/s-2006-960045. [DOI] [PubMed] [Google Scholar]
- 19.Matsui S, Matsumoto H, Sonoda Y, et al. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 2004;4(13):1633–1644. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin YW, Jung HA, Liu Y, et al. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra) J Agric Food Chem. 2007;55(12):4691–4697. doi: 10.1021/jf0703553. [DOI] [PubMed] [Google Scholar]
- 21.Venkatasubbu GD, Ramasamy S, Avadhani GS, Palanikumar L, Kumar J. Size-mediated cytotoxicity of nanocrystalline titanium dioxide, pure and zinc-doped hydroxyapatite nanoparticles in human hepatoma cells. J Nanopart Res. 2012;14:819–836. [Google Scholar]
- 22.Kasemets K, Ivask A, Dubourguier HC, Kahru A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol in Vitro. 2009;23(6):1116–1122. doi: 10.1016/j.tiv.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Lee BS, Park K, Park S, et al. Tumor targeting efficiency of bare nanoparticles does not mean the efficacy of loaded anticancer drugs: importance of radionuclide imaging for optimization of highly selective tumor targeting polymeric nanoparticles with or without drug. J Control Release. 2010;147(2):253–260. doi: 10.1016/j.jconrel.2010.07.096. [DOI] [PubMed] [Google Scholar]
- 24.Sui X, Wei W, Yang L, et al. Preparation, characterization and in vivo assessment of the bioavailability of glycyrrhizic acid microparticles by supercritical anti-solvent process. Int J Pharm. 2012;423(2):471–479. doi: 10.1016/j.ijpharm.2011.12.007. [DOI] [PubMed] [Google Scholar]