Abstract
Objective: To compare various visualization techniques for the detection of non-solid nodules in low-dose lung cancer screening computed tomography (CT) scans. Methods: An enriched sample of 216 male lung cancer screening subjects aged 60.4 ± 6.0 years was used. Two blinded independent readers searched for non-solid nodules on 5-mm multiplanar reconstructions, 1-mm slices and 7-mm maximum intensity projections (trial protocol). The reference standard was a consensus diagnosis of all non-solid nodules reported at least once. Results: Twenty-three individuals (10.6%) had in total 34 non-solid nodules. Interobserver agreement was good (Cohen kappa 0.89–0.95). For both observers, we found no differences between the 3 viewing techniques (P > 0.13). Conclusion: In low-dose lung cancer screening CT scans, we were unable to find a viewing technique superior to that used in the trial by experienced observers who focused on non-solid nodule detection.
Keywords: Computed tomography, lung cancer, ground glass nodule
Introduction
Non-solid lung nodules are a relatively uncommon finding in the Dutch-Belgian NELSON lung cancer screening study. In the first round of this study, only 2.0% of the total of 8673 nodules found in 7557 participants were pure non-solid nodules or part-solid nodules[1].
Data suggest that human perception is an important limiting factor in the detection of solid and non-solid nodules[2–6], and undetected non-solid lung nodules were responsible for more than half of the missed carcinomas in one study[7]. Perception errors in non-solid nodules are multifactorial and in part due to their rarity and lower density compared with solid nodules, the low-dose techniques used in lung cancer screening and partial volume effects when thick slices are used. It is our hypothesis that a narrow window width and a low window level to enhance small contrast differences in combination with 5-mm multiplanar reconstructions (MPR) to decrease noise will accommodate the detection of non-solid nodules. Optimizing detection is important as non-solid nodules have a higher chance of being malignant compared with solid nodules[8] and in the study by Li et al.[7] 27 of the 39 missed carcinomas were non-solid (69%).
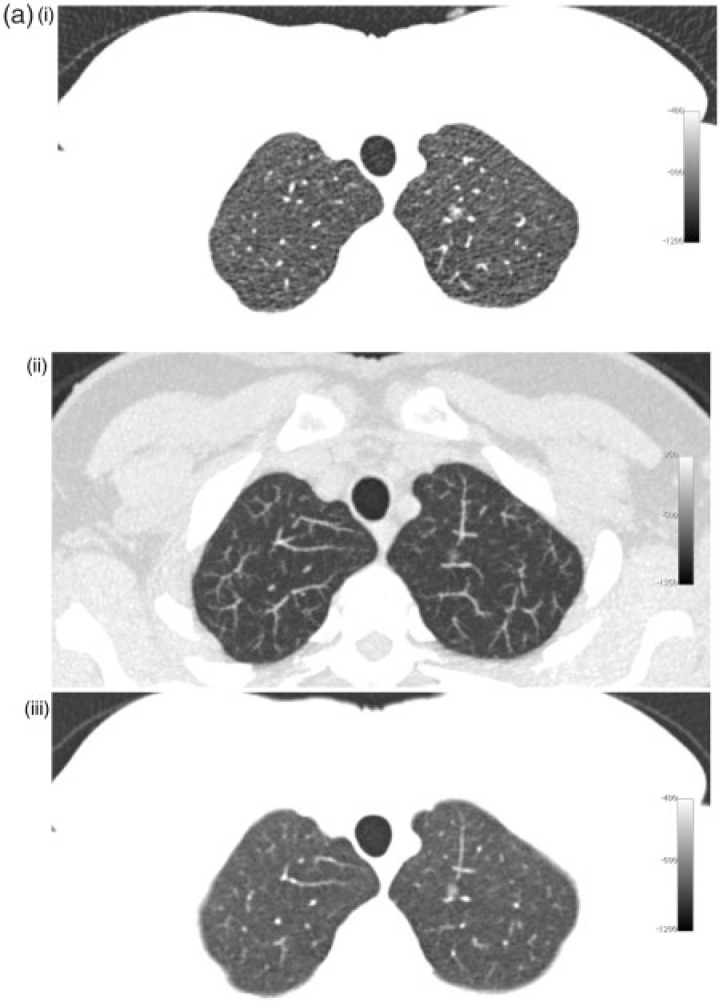
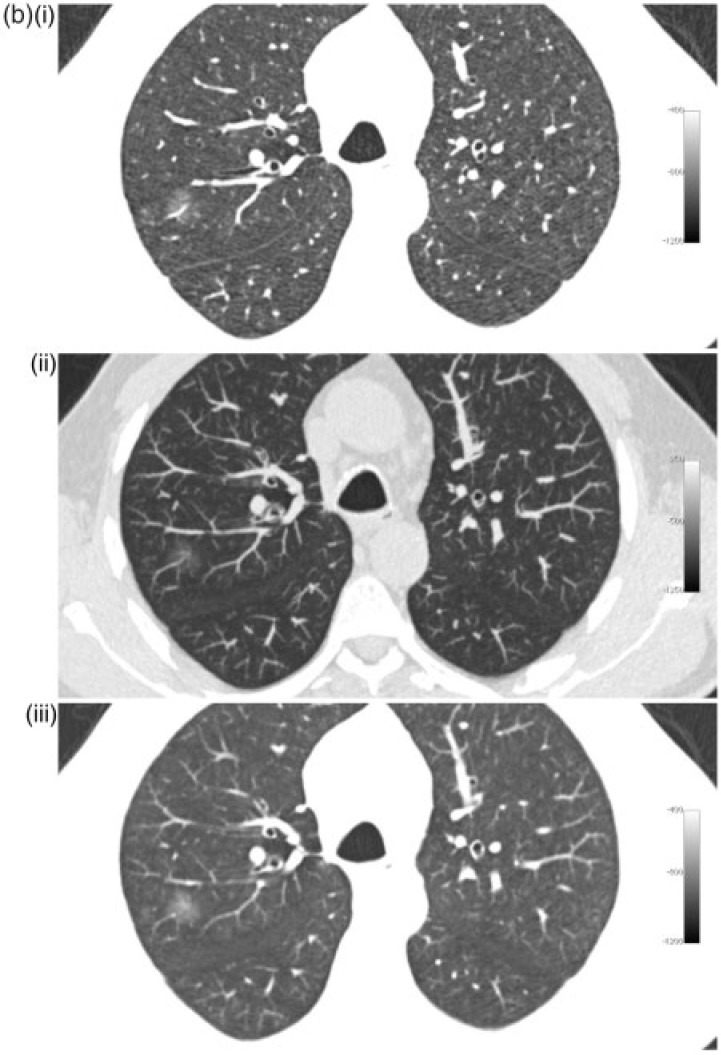
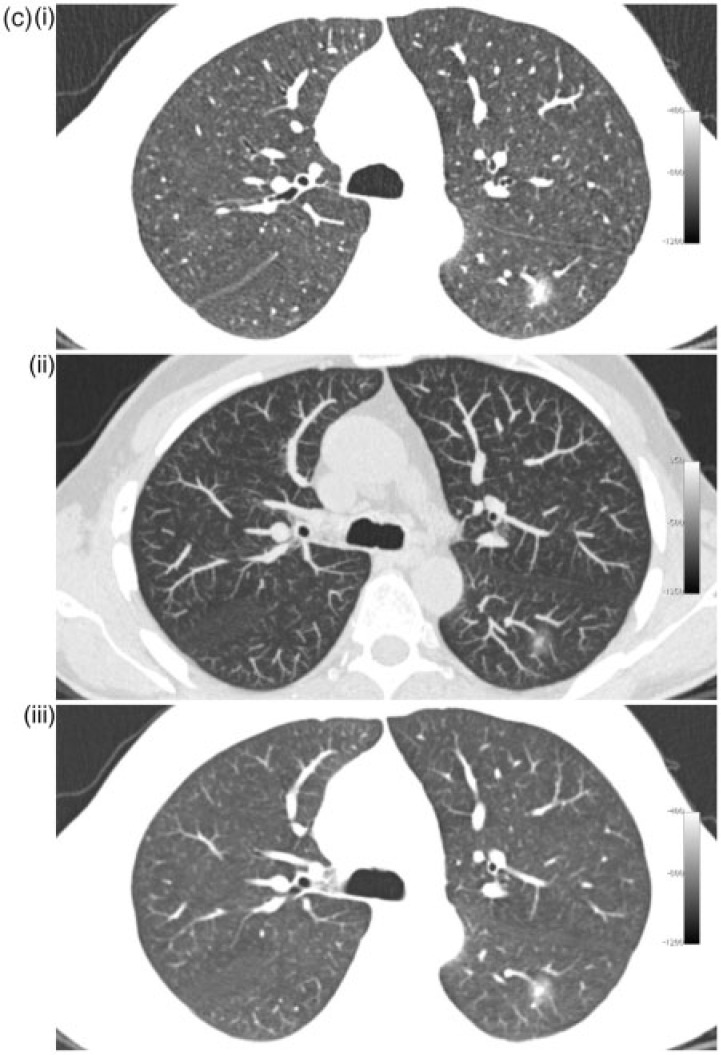
The purpose of the present study was to compare the detection rate of non-solid nodules larger than 5 mm by 2 readers on 5-mm MPR slices with window level/width of −800/800 HU, 1-mm slices with window level/width of −800/800 HU and 7-mm maximum intensity projection (MIP) images with window level/width −500/1500 on low-dose lung cancer screening CT scans from 216 subjects. These viewing techniques are illustrated with different nodules in Fig. 1.
Figure 1.
Different nodules with 1-mm (i), 7-mm MIP (ii) and 5-mm MPR slices (iii). (a) Small (6 mm) non-solid nodule in the left upper lobe. (b) Larger (11 mm) non-solid nodule in the right upper lobe. (c) Part-solid nodule in the apex of the left lower lobe.
Methods
Subjects
One of the investigators not involved in the readings selected 216 subjects participating in the Dutch-Belgium lung cancer screening trial[1]. Some had non-solid nodules according to the trial registration. This selection strategy was used to ensure that at least some non-solid nodules were included in the dataset, but the readers were blinded to the number of non-solid nodules. This study is a side study of the Dutch-Belgian multicentre randomized low-dose CT lung cancer screening trial (NELSON). The Minister of Health of the Netherlands and the ethics committees of all 4 participating hospitals have approved the NELSON study. Informed consent was obtained from all participants. The original approval and informed consent for the screening study included the ability to use data for future research, including the current side study (Current Controlled Trials number, ISRCTN63545820.)
CT data acquisition
A 16-section multidetector CT scanner (Mx8000 IDT or Brilliance 16P, Philips Medical Systems, Cleveland, OH) was used. Scanning of the entire chest was performed in a caudocranial direction. Scanning data were obtained in spiral mode, with 16 detector rows, 0.75-mm section thickness, and a pitch of 1.5. No contrast material was used. Low-dose settings were applied. Depending on body weight (<50 kg, 50–80 kg, or >80 kg), the peak voltage settings were 80–90 kVp, 120 kVp, and 140 kVp, respectively, to achieve volume CT dose index values of approximately 0.8 mGy, 1.6 mGy, and 3.2 mGy, respectively. The tube current–time product settings were adjusted accordingly. To minimize breathing artifacts, scans were made after appropriate instruction to the participants. Data were reconstructed at 1.0-mm section thickness, with a 0.7-mm reconstruction increment.
CT readings
Two independent readers (blinded for the number of subjects with nodules), who had 30 years and 9 years experience in chest CT interpretation, searched the CT scans for non-solid nodules in random order in 3 different ways with:
7-mm slab MIP projections with −500/1500 HU (these parameters were chosen because they were used in the original screening protocol)
1-mm axial images with −800/800 HU
5-mm MPR images with −800/800 HU
All images were viewed in axial projection. To avoid memory effects, we ensured that at least 2 weeks elapsed between reading sessions.
CT scans were recorded as negative when no non-solid nodules were found and this included non-specific ground glass opacities (small linear or triangular ground glass areas). Both pure non-solid and part-solid nodules were considered positive. As invasive adenocarcinomas in isolated non-solid nodules smaller than 5 mm are extremely rare[9], a lesion size limit exceeding 5 mm was used. After the independent readings, a consensus reading by the 2 readers was done for all non-solid nodules that were reported in any of the 6 readings from the 2 observers to define the final diagnosis of a non-solid nodule for this study. Furthermore, the trial management system was checked for any nodules that might have been missed in all 6 readings by the 2 observers.
Statistical analysis
After consensus reading, we analyzed the data on a per nodule basis. Interobserver agreement was determined by calculating the Cohen kappa coefficient. The McNemar test was used to compare the 3 viewing techniques with the consensus diagnosis. Statistical analysis was performed with SPSS version 18 software (SPSS, Chicago, IL). Data are given as mean ± standard deviation unless indicated otherwise. The significance level was set at P < 0.05.
Results
Subjects
The 216 subjects were all male with a mean age of 60.4 ± 6.0 years. All had at least 25 pack-years of smoking. Among the 216 subjects, 23 individuals were diagnosed with non-solid nodule by consensus, 19 had 1 nodule, 2 had 2 nodules, 1 had 4 nodules and 1 had 7 nodules, resulting in a total of 34 nodules. The size of these nodules was 11.5 ± 3.8 mm. The size was measured manually with calipers.
Observer agreement
Interobserver agreement was good for all 3 observations with a Cohen kappa between 0.86 and 0.95 (Table 1). In the consensus reading, by using the coordinates of the non-solid nodules, we confirmed that the observers indeed detected the same nodules.
Table 1.
Interobserver agreement of the detection of ground glass nodules in 34 nodules, 216 subjects
| Reconstruction technique | Window level/width (HU) | Slice thickness (mm) | Cohen kappa | Confidence interval |
|---|---|---|---|---|
| MIP | −500/1500 | 7 | 0.89 | 0.78–0.95 |
| MPR | −800/800 | 5 | 0.95 | 0.87–1.0 |
| Thin slices | −800/800 | 1 | 0.91 | 0.81–0.98 |
Data are given for 2 observers who evaluated 216 CT scans from 216 subjects.
Comparison of the 3 visualization techniques
For both observers, we found no differences between the 3 viewing techniques (Table 2). In the cases with multiple nodules, only one nodule was missed by one of the readers, therefore the analysis on a patient basis resulted in essentially the same result as our analysis on a per nodule basis.
Table 2.
Differences in ground glass nodule detection between the 3 visualization techniques for both observers in 34 nodules, 216 subjects
| Both negative (n) | Both positive (n) | First technique negative (n) | Second technique negative (n) | Difference between techniques (P value) | |
|---|---|---|---|---|---|
| Observer 1 | |||||
| MIP vs MPR | 191 | 29 | 2 | 3 | 1.0 |
| MIP vs thin | 191 | 30 | 2 | 2 | 1.0 |
| MPR vs thin | 193 | 31 | 1 | 0 | 1.0 |
| Observer 2 | |||||
| MIP vs MPR | 193 | 28 | 4 | 0 | 0.13 |
| MIP vs thin | 192 | 24 | 5 | 4 | 1.0 |
| MPR vs thin | 192 | 28 | 1 | 4 | 0.38 |
MIP images of 7-mm thickness at window level and width of −500/1500 HU. MPR of 5-mm thickness at window level and width of −800/800 HU. Thin slices from original 1-mm reconstruction at window level and width of −800/800 HU. Differences were tested with McNemar statistics.
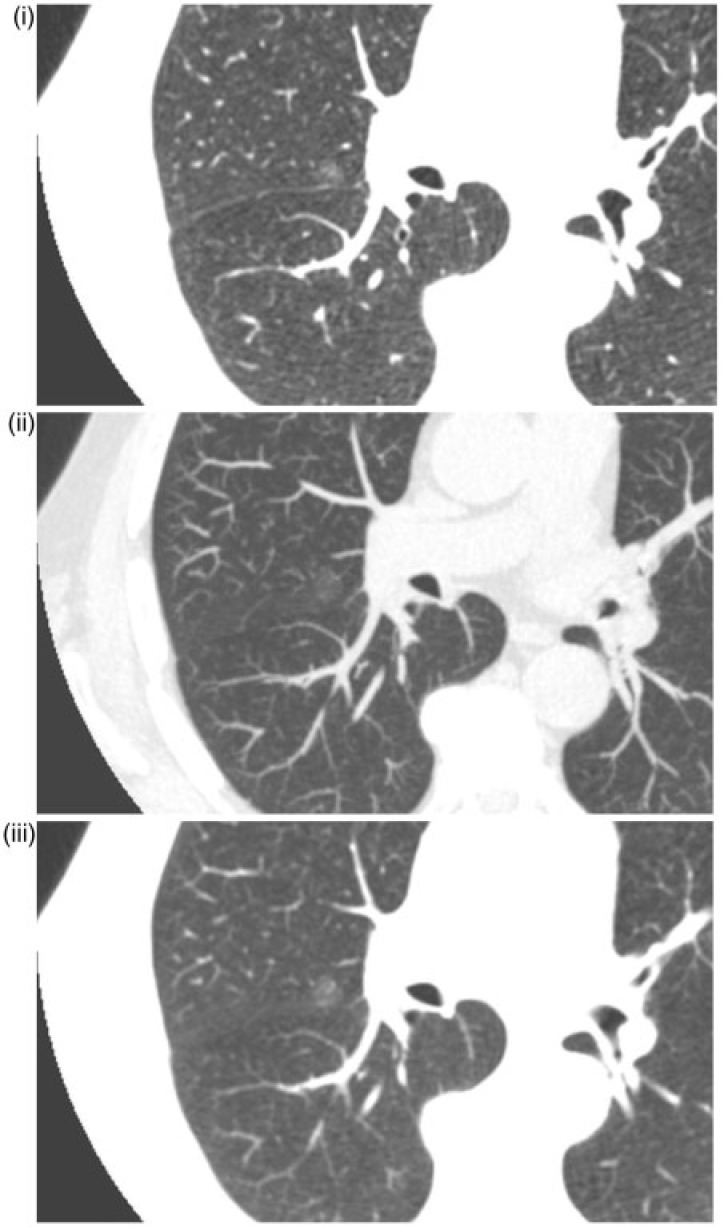
The size of the nodules that were missed in at least one reading was 9.0 ± 2.9 mm. Some misses could be explained by the fact the ground glass aspect of the lesion appeared solid on the MIP images. Fig. 2 illustrates an example of a non-solid nodule that was missed in one or more readings.
Figure 2.
Undetected right para-hilar non-solid nodule. (i) 1-mm slice; (ii) 7-mm MIP; nodule missed by one observer; (iii) 5-mm MPR.
Discussion
In this study, the aim was to determine whether visual detection of non-solid nodules on low-dose lung cancer screening CT scans can be improved by changing the visualization technique. In contrast to our hypothesis, we could not demonstrate that 5-mm MPRs (to reduce noise in low-dose CT images) in combination with narrow window settings (to enhance contrast between the non-solid nodule and the lung parenchyma) were superior to routine 7-mm MIP images and thin slices at narrow window settings.
For the detection of ground glass nodules, we still largely depend on visual detection. Currently available commercial CAD systems still focus primarily on the detection of solid nodules[10]. In addition, studies investigating optimization of the visibility of lung nodules primarily focus on solid nodules and not non-solid nodules[11]. In a study by Li et al.[7], non-solid nodules were found to be responsible for a large number of detection errors so there seems to be disagreement on how to optimize their detection. One explanation for the large number of non-solid nodules missed could be that their density and thus their contrast in relation to their surroundings is less compared with solid nodules. Furthermore, their delineation is less sharp than with small solid nodules. This effect is enhanced by a considerable partial volume effect, especially for the smaller nodules if a technique using 10-mm slices is used, as is the case in the series of Li et al.[7]. Also the use of low-dose CT techniques, which is customary in lung cancer screening programs, has a negative influence on the visibility of low-contrast lesions like non-solid nodules. Therefore, we argue that in order to improve the visibility and thus the detection percentage of these nodules, it seems indicated that the contrast difference of the lesion in relation to the surroundings needs to be improved and the slice thickness must be optimized. Contrast resolution can be improved by increasing the radiation dose. However, this is not an option in lung cancer screening programs. Contrast can also be increased by using a smaller viewing window, which has the effect of greater visibility of image noise. This latter effect is more pronounced in the noisier 1-mm images than in the thicker 5-mm MPR images. A further possibility to decrease noise, besides increasing the dose, is to increase the slice thickness, for example by postprocessing the images with MPR. This decrease in image noise will be at the expense of spatial resolution with a potentially negative effect on the detection of smaller lesions if the slice thickness is increased too much. Unexpectedly, in our study, we did not find a significant difference between the results of 3 different viewing methods with 2 experienced observers.
Our study has several limitations. First, the dataset is small. Nevertheless, we believe that our results show convincingly that there are no major differences between visualization techniques when observers focus on non-solid nodule detection. Second, the observers in our study had only one task, non-solid nodule detection. This does not fully represent the routine situation where a busy reader is reading lung cancer screening studies with solid nodules and other pathologies. It may be that the benefit of enhanced visualization of non-solid nodules is masked by the focus of the readers. Third, we have no real proof of the presence of non-solid nodules or the pathology of the lesions; consensus opinions after 6 readings of each scan by 2 experienced observers combined with the management system recordings were used to define the presence of non-solid nodules.
In conclusion, we found that in low-dose lung cancer CT screening, various viewing techniques using thin 1-mm slices, 5-mm MPR images and 7-mm MIP images at routine and narrow window settings have similar detection rates when experienced observers focus on non-solid nodule detection.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The NELSON-trial was sponsored by Netherlands Organisation for Health Research and Development (ZonMw); Dutch Cancer Society Koningin Wilhelmina Fonds (KWF); Stichting Centraal Fonds Reserves van Voormalig Vrijwillige Ziekenfondsverzekeringen (RvvZ); Roche Diagnostics, Siemens Germany; Rotterdam Oncologic Thoracic Steering committee (ROTS); G.Ph.Verhagen Trust, Flemish League Against Cancer, Foundation Against Cancer and Erasmus Trust Fund.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–2229. doi: 10.1056/NEJMoa0906085. . PMid:19955524. [DOI] [PubMed] [Google Scholar]
- 2.Seltzer SE, Judy PF, Adams DF, et al. Spiral CT of the chest: comparison of cine and film- based viewing. Radiology. 1995;197:73–78. doi: 10.1148/radiology.197.1.7568857. PMid:7568857. [DOI] [PubMed] [Google Scholar]
- 3.Rubin GD, Lyo JK, Paik DS, et al. Pulmonary nodules on multi-detector row CT scans: performance comparison of radiologists and computer-aided detection. Radiology. 2005;234:274–283. doi: 10.1148/radiol.2341040589. . PMid:15537839. [DOI] [PubMed] [Google Scholar]
- 4.Wormanns D, Ludwig K, Beyer F, Heindel W, Diederich S. Detection of pulmonary nodules at multirow-detector CT: effectiveness of double reading to improve sensitivity at standard-dose and low-dose chest CT. Eur Radiol. 2005;15:14–22. doi: 10.1007/s00330-004-2527-6. . PMid:15526207. [DOI] [PubMed] [Google Scholar]
- 5.Gruden JF, Ouanounou S, Tigges S, Norris SD, Klausner TS. Incremental benefit of maximum-intensity-projection images on observer detection of small pulmonary nodules revealed by multidetector CT. Am J Roentgenol. 2002;179:149–157. doi: 10.2214/ajr.179.1.1790149. [DOI] [PubMed] [Google Scholar]
- 6.Eibel R, Turk TR, Kulinna C, Herrmann K, Reiser MF. Mehrschicht-Spiral-CT der Lunge: Multiplanare Rekonstruktionen und Maximum-Intensitäts-Projektionen in der Detektion von Lungenrundherden. Rofo. 2001;173:815–821. doi: 10.1055/s-2001-16981. . PMid:11582561. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Sone S, Abe H, et al. Lung cancers missed at low-dose helical CT screening in a general population: comparison of clinical, histopathologic, and imaging findings. Radiology. 2002;225:673–683. doi: 10.1148/radiol.2253011375. . PMid:12461245. [DOI] [PubMed] [Google Scholar]
- 8.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. Am J Roentgenol. 2002;178:1053–1057. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 9.Godoy MCB, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology. 2009;253:606–624. doi: 10.1148/radiol.2533090179. . PMid:19952025. [DOI] [PubMed] [Google Scholar]
- 10.Peloschek P, Sailer J, Weber M. Pulmonary nodules: sensitivity of maximum intensity projection versus that of volume rendering of 3D multidetector CT data. Radiology. 2007;243:561–569. doi: 10.1148/radiol.2432052052. . PMid:17456878. [DOI] [PubMed] [Google Scholar]
- 11.Marten K, Engelke C. Computer-aided detection and automated CT volumetry of pulmonary nodules. Eur Radiol. 2007;17:888–901. doi: 10.1007/s00330-006-0410-3. . PMid:17047961. [DOI] [PubMed] [Google Scholar]