Abstract
Background
Preclinical studies have shown that norepinephrine can directly stimulate tumor cell migration and that this effect is mediated by the beta-adrenergic receptor.
Patients and methods
We retrospectively reviewed 722 patients with non-small-cell lung cancer (NSCLC) who received definitive radiotherapy (RT). A Cox proportional hazard model was utilized to determine the association between beta-blocker intake and locoregional progression-free survival (LRPFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS).
Results
In univariate analysis, patients taking beta-blockers (n = 155) had improved DMFS (P < 0.01), DFS (P < 0.01), and OS (P = 0.01), but not LRPFS (P = 0.33) compared with patients not taking beta-blockers (n = 567). In multivariate analysis, beta-blocker intake was associated with a significantly better DMFS [hazard ratio (HR), 0.67; P = 0.01], DFS (HR, 0.74; P = 0.02), and OS (HR, 0.78; P = 0.02) with adjustment for age, Karnofsky performance score, stage, histology type, concurrent chemotherapy, radiation dose, gross tumor volume, hypertension, chronic obstructive pulmonary disease and the use of aspirin. There was no association of beta-blocker use with LRPFS (HR = 0.91, P = 0.63).
Conclusion
Beta-blocker use is associated with improved DMFS, DFS, and OS in this large cohort of NSCLC patients. Future prospective trials can validate these retrospective findings and determine whether the length and timing of beta-blocker use influence survival outcomes.
Keywords: beta-blockers, distant metastasis, non-small-cell lung cancer, radiation therapy
introduction
Definitive radiotherapy (RT) has an important role in the treatment of locally advanced or otherwise inoperable non-small-cell lung cancer (NSCLC). However, despite significant advances in the development of systemic therapy and RT technologies such as intensity-modulated radiotherapy, four-dimensional computed tomography (CT)-based treatment planning [1], and proton beam therapy [2] in recent years, the prognosis of this disease remains poor, with a 5-year overall survival rate of ∼15% [3]. The mechanisms underlying the development of metastasis, the major cause of death from NSCLC, are complex, but may involve chronic stress conditions and prolonged exposure to catecholamine stress hormones [4–7]. Specifically, norepinephrine has been shown to directly stimulate tumor cell migration, and this effect is mediated by beta-adrenergic receptors [8–12].
Several retrospective clinical studies have suggested that beta-blockers (beta-adrenergic receptor antagonists), which are typically prescribed for hypertension or heart disease, may also have antitumor activity, reducing metastasis, tumor recurrence, and cancer-specific mortality for patients with breast cancer and increasing survival time for patients with melanoma [13–16]. However, little is known about the effects of beta-blockers on lung cancer progression or metastasis in vivo. A large retrospective cohort study that included 436 patients with lung cancer showed no correlation between the use of beta-blockers and overall survival; however, no details were given on patient demographics or treatment regimens [17], making the conclusions of the paper less clear. To further investigate a possible link between beta-blockers and survival outcomes in lung cancer, we retrospectively assessed the use of beta-blockers among a large number of patients who underwent RT, with or without chemotherapy, as definitive treatment of lung cancer at a single institution. With only modest benefits being shown in a series of phase III clinical trials for advanced NSCLC, including high radiation dose or additional chemotherapy [18–20], it is clinically urgent to find novel agents and/or pathways that could block the growth and prevent the development of micrometastases in NSCLC patients. Our hypothesis is that the use of beta-blockers reduces the rates of disease progression and improves overall survival in locally advanced NSCLC. If the hypothesis is proven true, this information could be used to further develop improved systemic therapy in the context of this aggressive malignancy.
patients and methods
data sources
Patients in this retrospective review were selected from a large clinical database and treated with definitive RT for NSCLC from 1998 through 2010 at MD Anderson Cancer Center. The patient database contained detailed patient demographic data, comprehensive tumor details, RT data, chemotherapy data, outcome, and mortality data. This study was approved by the appropriate institutional review board, and patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act.
participants
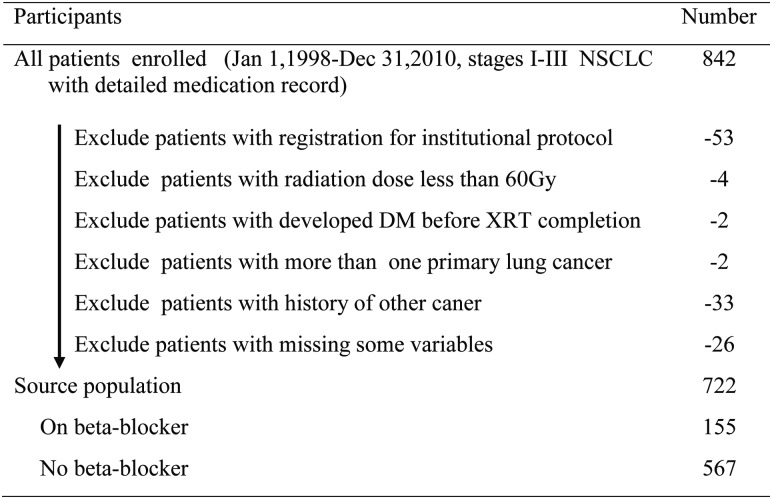
The inclusion criteria were as follows: (i) newly diagnosed and pathologically confirmed NSCLC, (ii) receipt of definitive RT with or without concurrent chemotherapy, (iii) receipt of at least 60 Gy [or, for proton therapy, Gy equivalent (GyE)] of radiation, and (iv) information on the use of beta-blockers before and during the entire RT course. Patients registered for an institutional protocol in which data reporting is currently prohibited were excluded, as were those with more than one primary lung cancer or a history of another malignancy (Figure 1).
Figure 1.
Study population selection.
Follow-up visits included an interval history and physical examination. Other imaging studies, such as a CT scan, positron emission tomography/CT scan, or brain magnetic resonance imaging were obtained at the discretion of the treating physician. Patients were evaluated weekly during RT, at 1–3 months after the completion of RT, every 3–4 months for 2–3 years, every 6 months till 5 years, and annually thereafter.
study covariates and outcomes
Information on medication use was retrieved from the review of medical and pharmacy records. Patients were included in the beta-blocker category if they used the medication throughout the duration of RT. In addition to beta-blocker use as a binary variable, the type of beta blockers, indication for intake, use of aspirin, and comorbidity of chronic pulmonary disease, all of which may affect the outcome of lung cancer and thus confound the analysis of beta-blocker use [21, 22] were tabulated. We analyzed the following study outcomes: locoregional progression-free survival (LRPFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS).
The time to distant metastasis (DM) was measured from the date of completion of RT to the date of first documented distant metastases. The time to locoregional progression (LRP) was measured from the date of completion of RT to the date of first documented primary recurrence and/or locoregional nodal recurrence. DFS was defined as the time from the date of completion of RT to the date of the documented recurrence, either local recurrence or distant metastasis. OS was defined as the time from the date of completion of RT to the date of death or last follow-up. Patients who died without disease recurrence were censored at the date of death. For DMFS, LRPFS, and DFS, death was a censoring time; for OS, death was an event time.
statistical methods
Patient and tumor characteristics were grouped according to beta-blocker use during RT, and between-group comparisons were made using Pearson's chi-square or Fisher's exact tests.
The Kaplan–Meier method was used to estimate the survival outcomes according to the use of beta-blockers or not, and the groups were compared with the log-rank statistic. Cox proportional hazards models were fitted to determine the association of beta-blocker intake with survival outcomes in both univariate analyses (UVA) and multivariate analyses (MVA). In MVA, confounders were included if they were significant at a 0.05 level or if they altered the coefficient of the primary variable (beta-blocker use) by >5% in cases in which the primary association was significant. The results are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). A P value of <0.05 was considered to indicate statistical significance; all tests were two-sided. All patients were included in UVA and MVA. Statistical analyses were carried out using Stata/SE v10.1 (Stata Corp LP, College Station, TX).
results
The final study population consisted of 722 patients, 155 of whom had taken beta-blockers during definitive RT and 567 who had not. Patient and tumor characteristics are listed in Table 1. The median age of the patients was 65 years (range 34–95 years), and most patients in both the groups had stage III disease. Patients taking beta-blockers were more likely to be older (P < 0.01), have poorer performance status (Karnofsky Performance Status scores ≤ 80) (P = 0.04), have hypertension (P < 0.01), and likely to take aspirin (P < 0.01). Patients taking beta-blockers also had less-advanced (lower-stage) disease (P = 0.04), but were less likely to have received concurrent chemotherapy (P = 0.02) and were given higher RT doses (P < 0.01). Other prognostic factors were not significantly different between the groups. The median follow-up time for surviving patients was 44 months (range 1–155 months).
Table 1.
Patient and tumor characteristics
| Characteristic | No. of patients (%) |
||
|---|---|---|---|
| Beta-blockers (N = 155) | No beta-blockers (N = 567) | P value | |
| Sex | 0.92 | ||
| Female | 69 (45) | 255 (45) | |
| Male | 86 (55) | 312 (55) | |
| Age, years | <0.01 | ||
| <65 | 53 (34) | 312 (55) | |
| ≥65 | 102 (66) | 255 (45) | |
| Race | 0.80 | ||
| Caucasian | 133 (86) | 482 (85) | |
| Non-Caucasian | 22 (14) | 85 (15) | |
| Karnofsky performance score | 0.04 | ||
| ≤80 | 121 (78) | 394 (69) | |
| >80 | 34 (22) | 173 (31) | |
| T Category | 0.89 | ||
| T1,2 | 82 (54) | 298 (53) | |
| T3,4 | 70 (46) | 261 (47) | |
| N Category | 0.03 | ||
| N0,1 | 34 (22) | 84 (15) | |
| N2,3 | 121 (78) | 483 (85) | |
| Clinical stage | 0.04a | ||
| I | 9 (6) | 13 (2) | |
| II | 8 (5) | 15 (3) | |
| IIIA | 62 (40) | 261 (46) | |
| IIIB | 76 (49) | 278 (49) | |
| Tumor histology | 0.47 | ||
| Squamous cell | 52 (34) | 208 (37) | |
| Non-squamous cell | 103 (66) | 359 (63) | |
| Smoking status | 0.49a | ||
| Never | 11 (7) | 38 (7) | |
| Previous | 113 (73) | 389 (69) | |
| Current | 31 (20) | 140 (25) | |
| Concurrent chemotherapy | 0.02 | ||
| No | 35 (23) | 84 (15) | |
| Yes | 120 (77) | 483 (85) | |
| Radiation dose, Gy | <0.01 | ||
| 60–63 | 69 (45) | 323 (57) | |
| >63 | 86 (55) | 244 (43) | |
| Gross tumor volume, cm3 | 0.12 | ||
| <119 | 86 (55) | 273 (48) | |
| ≥119 | 69 (45) | 294 (52) | |
| Hypertension | <0.01 | ||
| No | 50 (32) | 362 (64) | |
| Yes | 105 (68) | 205 (36) | |
| Chronic obstructive | |||
| Pulmonary disease | 0.21 | ||
| No | 112 (72) | 437 (77) | |
| Yes | 43 (28) | 130 (23) | |
| Aspirin | <0.01 | ||
| No | 90 (58) | 474 (84) | |
| Yes | 65 (42) | 93 (16) | |
aFisher's exact test.
Of the 722 patients in the study, 345(48%) were treated with three-dimensional conformal RT, 301 (42%) with intensity-modulated RT, and 76 (10%) with proton beam therapy. Complete dosimetric data [including total dose, gross tumor volume (GTV), and mean lung dose] were available for all patients. All patients underwent RT 5 days per week to a total dose of 60–87.4 Gy or GyE prescribed to cover 95% of the planning target volume regardless of which technique had been used. Treatment was given as induction chemotherapy followed by radiation (n = 43 [6%]), induction chemotherapy followed by concurrent chemotherapy and radiation (n = 252 [35%]), concurrent chemotherapy and radiation without induction treatment (n = 351 [49%]), or radiation alone (n = 76 [10%]).
Of the 155 patients taking beta-blockers during RT for NSCLC, 105 (68%) had a diagnosis of hypertension, and the other 50 (32%) had non-hypertensive disorders, most often coronary heart disease. The drugs used are shown in Table 2. The two most commonly prescribed drugs (given in 85% of cases) were metoprolol and atenolol.
Table 2.
Beta-blockers used to treat preexisting hypertension or coronary heart disease in patients with lung cancer
| Drug Categories | No. of patients |
|---|---|
| Selective beta-blockers | |
| Metoprolol | 89 |
| Atenolol | 43 |
| Bisoprolol | 2 |
| Nonselective beta-blockers | |
| Propranolol | 4 |
| Sotalol | 3 |
| Nadolol | 2 |
| Carvedilola | 11 |
| Labetalola | 1 |
| Total | 155 |
aAlso an alpha blocker.
univariate analyses
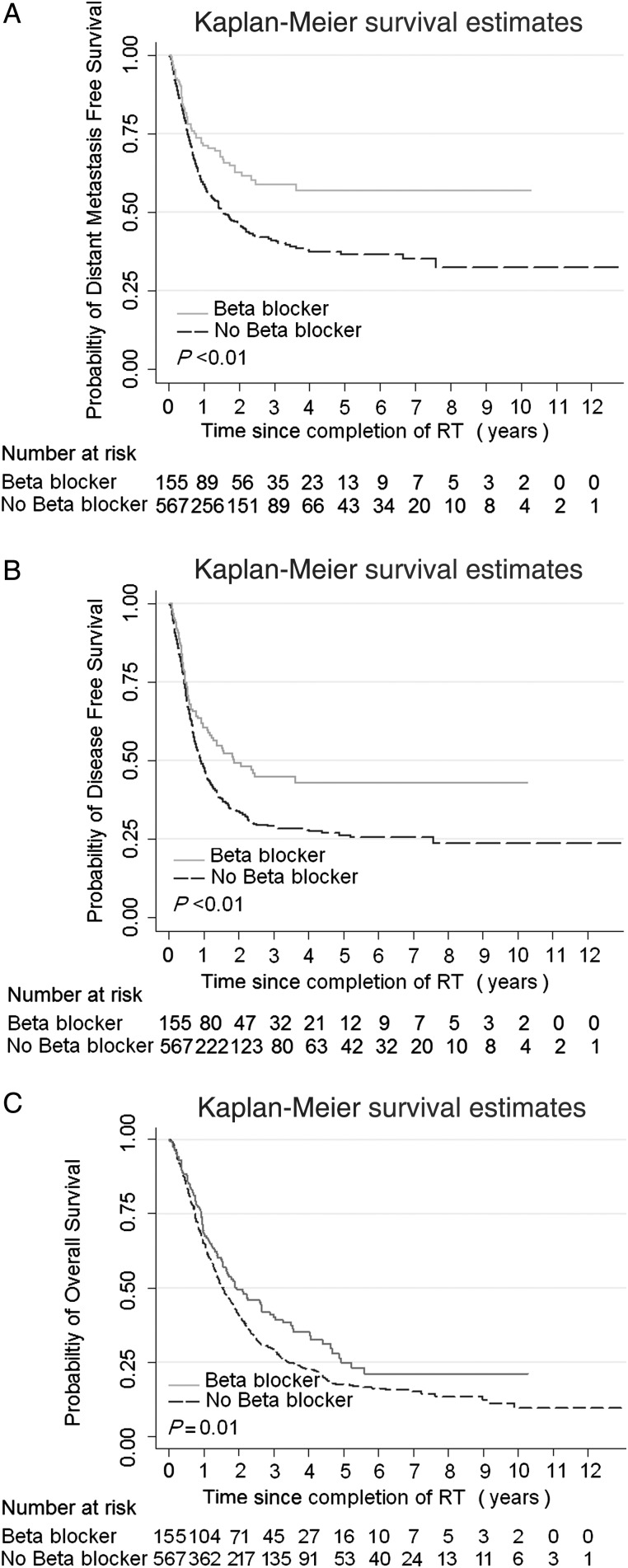
The Kaplan–Meier estimates of DMFS, DFS and OS according to use of beta-blockers (Figure 2) illustrate that the use of beta-blockers was associated with improved DMFS (P < 0.01, Figure 2A), DFS (P < 0.01, Figure 2B), and OS (P = 0.01, Figure 2C). The findings from UVA using Cox proportional hazards models of the influence of clinical characteristics on the survival outcome (Table 3) indicate that the use of beta-blockers was associated with better DMFS, DFS, and OS, but not LRPFS. Of other variables examined, younger age and advanced disease (T3, 4/N2, 3) were linked with reduced DMFS and DFS, and the poor performance status and advanced disease were linked with decreased OS. Notably, the use of concurrent chemotherapy was associated with improved OS (P < 0.01).
Figure 2.
Comparison of (A) distant metastasis-free survival (DMFS), (B) disease-free survival (DFS), and (C) overall survival (OS) in patients with non-small-cell lung cancer (NSCLC) who were or were not taking beta-blockers during definitive radiation therapy.
Table 3.
Univariable Cox proportional hazards model for all patients
| Variable | LRPFS |
DMFS |
DFS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Use of beta-blocker | ||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | ||||||||
| Yes | 0.85 | 0.61–1.19 | 0.33 | 0.60 | 0.45–0.81 | <0.01 | 0.66 | 0.52–0.85 | <0.01 | 0.76 | 0.61–0.94 | 0.01 |
| Sex | ||||||||||||
| Female | Ref. | Ref. | Ref. | Ref. | ||||||||
| Male | 1.02 | 0.77–1.33 | 0.91 | 0.88 | 0.71–1.09 | 0.36 | 0.89 | 0.73–1.07 | 0.21 | 1.06 | 0.90–1.26 | 0.69 |
| Age, years | ||||||||||||
| <65 | Ref. | Ref. | Ref. | Ref. | ||||||||
| ≥65 | 0.80 | 0.61–1.05 | 0.11 | 0.72 | 0.58–0.89 | <0.01 | 0.73 | 0.61–0.89 | <0.01 | 1.14 | 0.97–1.35 | 0.12 |
| Race | ||||||||||||
| Non-Caucasian | Ref. | Ref. | Ref. | Ref. | ||||||||
| Caucasian | 0.87 | 0.60–1.26 | 0.46 | 0.82 | 0.61–1.09 | 0.17 | 0.90 | 0.69–1.17 | 0.44 | 0.90 | 0.71–1.13 | 0.36 |
| KPS | ||||||||||||
| ≤80 | Ref. | Ref. | Ref. | Ref. | ||||||||
| >80 | 0.86 | 0.64–1.17 | 0.34 | 0.79 | 0.62–1.00 | 0.06 | 0.83 | 0.67–1.02 | 0.08 | 0.70 | 0.58–0.85 | <0.01 |
| T stage | ||||||||||||
| T1,2 | Ref. | Ref. | Ref. | Ref. | ||||||||
| T3,4 | 1.00 | 0.77–1.32 | 0.98 | 1.25 | 1.01–1.56 | 0.04 | 1.21 | 1.00–1.47 | 0.05 | 1.16 | 0.98–1.37 | 0.09 |
| N stage | ||||||||||||
| N0,1 | Ref. | Ref. | Ref. | Ref. | ||||||||
| N2,3 | 1.13 | 0.78–1.62 | 0.53 | 1.47 | 1.07–2.03 | 0.02 | 1.32 | 1.00–1.73 | 0.05 | 1.26 | 1.00–1.60 | 0.06 |
| Clinical disease stage | ||||||||||||
| I/II | Ref. | Ref. | Ref. | |||||||||
| III | 1.23 | 0.70–2.15 | 0.47 | 2.91 | 1.55–5.47 | <0.01 | 2.03 | 1.27–3.26 | <0.01 | 1.85 | 1.22–2.81 | <0.01 |
| Tumor histology | ||||||||||||
| Non-squamous | Ref. | Ref. | Ref. | Ref. | ||||||||
| Squamous | 1.42 | 1.08–1.86 | 0.01 | 0.72 | 0.57–0.91 | <0.01 | 0.96 | 0.78–1.17 | 0.66 | 1.24 | 1.04–1.47 | 0.02 |
| Smoking status | ||||||||||||
| Never | Ref. | Ref. | Ref. | Ref. | ||||||||
| Previous | 0.81 | 0.50–1.32 | 0.40 | 0.82 | 0.55–1.23 | 0.33 | 0.92 | 0.64–1.32 | 0.64 | 1.02 | 0.72–1.43 | 0.92 |
| Current | 0.71 | 0.41–1.23 | 0.23 | 0.99 | 0.64–1.54 | 0.98 | 0.98 | 0.66–1.45 | 0.90 | 1.10 | 0.76–1.60 | 0.60 |
| Concurrent chemotherapy | ||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | ||||||||
| Yes | 1.19 | 0.79–1.78 | 0.41 | 1.09 | 0.79–1.49 | 0.61 | 1.06 | 0.81–1.39 | 0.68 | 0.65 | 0.52–0.80 | <0.01 |
| Radiation dose, Gy | ||||||||||||
| 60–63 | Ref. | Ref. | Ref. | Ref. | ||||||||
| >63 | 1.31 | 1.00–1.72 | 0.05 | 1.00 | 0.80–1.24 | 0.98 | 1.04 | 0.86–1.26 | 0.65 | 0.95 | 0.80–1.13 | 0.56 |
| GTV, cm3 | ||||||||||||
| <119 | Ref. | Ref. | Ref. | Ref. | ||||||||
| ≥119 | 1.52 | 1.16–1.99 | <0.01 | 1.41 | 1.14–1.75 | <0.01 | 1.45 | 1.20–1.75 | <0.01 | 1.65 | 1.39–1.95 | <0.01 |
| Hypertension | ||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | ||||||||
| Yes | 0.80 | 0.61–1.05 | 0.11 | 0.82 | 0.66–1.02 | 0.07 | 0.82 | 0.67–0.99 | 0.04 | 0.90 | 0.76–1.06 | 0.20 |
| COPD | ||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | ||||||||
| Yes | 0.92 | 0.66–1.27 | 0.61 | 0.71 | 0.54–0.94 | 0.02 | 0.87 | 0.69–1.09 | 0.23 | 1.14 | 0.94–1.38 | 0.20 |
| Aspirin | ||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | ||||||||
| Yes | 1.06 | 0.77–1.45 | 0.73 | 0.67 | 0.50–0.89 | <0.01 | 0.78 | 0.61–0.99 | 0.04 | 0.93 | 0.76–1.15 | 0.52 |
LRPFS, locoregional progression-free survival; DMFS, distant metastasis-free survival; DFS, disease-free survival; OS, overall survival; KPS, Karnofsky performance score; GTV, gross tumor volume; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; CI, confidence interval; Ref, reference variable.
multivariate analyses
After adjustment for age, Karnofsky performance score, clinical stage, tumor histology, use of concurrent chemotherapy, radiation dose, GTV, hypertension, chronic obstructive pulmonary disease, and aspirin consumption, the use of beta-blockers was still associated with better DMFS (HR 0.67, 95% CI 0.50–0.91, P = 0.01), DFS (HR 0.74, 95% CI 0.58–0.95, P = 0.02), and OS (HR 0.78, 95% CI 0.63–0.97, P = 0.02) ,but not with LRPFS (HR = 0.91, 95% CI, 0.64–1.31, P = 0.63) (Table 4). When examining other clinical factors, only advanced stage, poorer performance status, larger GTV, and the lack of concurrent chemotherapy remained associated with reduced survival outcomes.
Table 4.
Multivariable Cox proportional hazards model for all patients
| Variable | LRPFS |
DMFS |
DFS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Beta-blocker use, yes versus no | 0.91 | 0.64–1.31 | 0.63 | 0.67 | 0.50–0.91 | 0.01 | 0.74 | 0.58–0.95 | 0.02 | 0.78 | 0.63–0.97 | 0.02 |
| Age, years, ≥65 versus <65 | – | – | – | – | – | – | 0.74 | 0.61–0.90 | <0.01 | – | – | – |
| KPS, >80 versus ≤80 | – | – | – | 0.76 | 0.59–0.97 | 0.03 | 0.77 | 0.62–0.96 | 0.02 | 0.72 | 0.59–0.87 | <0.01 |
| Clinical stage, III versus I, II | – | – | – | 2.39 | 1.26–4.53 | 0.01 | 1.66 | 1.02–2.69 | 0.04 | 1.97 | 1.28–3.05 | <0.01 |
| Tumor histology, | ||||||||||||
| Non-squamous cell versus squamous cell | 1.30 | 0.98–1.71 | 0.07 | 0.68 | 0.53–0.86 | <0.01 | – | – | – | – | – | – |
| Concurrent chemotherapy | ||||||||||||
| Yes versus no | – | – | – | – | – | – | – | – | – | 0.54 | 0.42–0.67 | <0.01 |
| Radiation dose, Gy | ||||||||||||
| 60–63 versus >63 | 1.46 | 1.10–1.94 | <0.01 | – | – | – | – | – | – | – | – | – |
| GTV, cm3 | ||||||||||||
| ≥119 versus <119 | 1.58 | 1.19–2.09 | <0.01 | 1.37 | 1.10–1.70 | <0.01 | 1.38 | 1.14–1.67 | <0.01 | 1.61 | 1.35–1.91 | <0.01 |
| Hypertension, Yes versus no | 0.84 | 0.63–1.13 | 0.26 | – | – | – | – | – | – | – | – | – |
| COPD, Yes versus no | – | – | – | 0.77 | 0.58–1.02 | 0.07 | – | – | – | – | – | – |
| Aspirin, Yes versus no | – | – | – | 0.74 | 0.55–0.99 | 0.05 | – | – | – | – | – | – |
LRPFS, locoregional progression-free survival; DMFS, distant metastasis-free survival; DFS, disease-free survival; OS, overall survival; KPS, Karnofsky performance score; GTV, gross tumor volume; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; CI, confidence interval.
discussion
The ultimate objective of this retrospective study was to assess whether the use of beta-blockers was associated with distant metastasis and subsequent survival outcomes for patients with NSCLC treated with definitive RT. In brief, we found that the use of beta-blockers did not affect LRP, but was associated with improved DMFS, DFS, and OS rates and that these correlations held even after adjusting for stage, histology, performance status, and treatment regimen used, suggesting that beta-blocker use was independently associated with improved survival. To our knowledge, our study represents the first analysis demonstrating a survival benefit associated with the use of beta-blockers during definitive RT for NSCLC.
Our findings are concordant with those of preclinical results of lung cancer [12, 23]. An in vitro study has shown that the beta-blocker propranolol can reverse the proliferation of NSCLC cells caused by nicotine through cooperative regulation of nicotinic and beta-adrenergic receptors [23]. Other such studies indicated that beta-adrenergic signaling can regulate several of the cellular processes involved in cancer progression, tumor cell proliferation, extracellular matrix invasion, angiogenesis, matrix metalloproteinase activation, and expression of inflammatory and chemotactic cytokines in several types of cancer, including lung, prostate, colon, stomach, breast, and ovary [12, 24, 25]. A mouse model study also showed that social stress induces the stimulation of NSCLC growth in vivo by increasing the beta-adrenergic neurotransmitter signaling that is mediated by nicotinic acetylcholine receptors and that gamma-aminobutyric acid can reverse this effect [26]. In our study, we proposed that beta-blockers abrogated the downstream activation of the beta-adrenergic signaling cascade in NSCLC cells and therefore, acted as a chemopreventive inhibitor during the process of metastasis development.
We did not find any association between the use of beta-blockers and LRPFS, suggesting that the drugs may be affecting the tumor metastatic cascade rather than affecting the primary tumor [6, 27, 28]. The choice of beta-blockers (selective versus nonselective) may also be important, although there was an insufficient number of patients in each arm to elucidate a difference between the two types of agents in our analysis. Most of the patients with outcome benefit in the current study were taking a selective (β1) beta-blocker, which is consistent with other findings, indicating that β1 is the primary beta-adrenergic system active in pulmonary adenocarcinoma [29]. Indeed, our results thus suggest that the β1 pathway is important in reducing the probability of distant dissemination and thus DFS and OS in clinical settings. However, it is also the case that even ‘selective’ β-blockers used clinically have cross activity and some β1-antagonists are more β2 selective in certain settings [30]. This mechanism warrants further exploration.
Our findings do not agree with those of Shah et al. [17], who found that hypertensive patients with a variety of solid tumor types, including lung cancer, did not show any benefit from the use of beta-blockers. Several reasons could explain this discrepancy. First, the patients in the prior analysis were selected from a primary care database, and no clinical variables were reported other than the prescription of beta-blockers. Moreover, that study also excluded patients with chronic obstructive pulmonary disease or coronary heart disease, both of which are common and clinically significant conditions in patients with lung cancer. Thus, although these prior results are provocative, we do not believe that they extend to the population studied here or that they invalidate our findings.
In addition to the constraints of any single-institution retrospective study, our study had the following limitations. First, data were missing or incomplete regarding the duration of beta-blocker use before and after treatment, the significance of which is unknown. Second, as noted above, patients in this study had received a variety of beta-blockers, which may have masked evidence of specific molecular mechanisms that would explain our findings. Third, most preclinical data on the effect of beta-blockers and lung cancer were conducted on models of adenocarcinoma, in contrast to our study which contained a heterogeneous mixture of adenocarcinoma (n = 255), squamous cell carcinoma (n = 268), and NSCLC, not otherwise specified (NOS) (n = 227). To account for this difference, we tested for an interaction between histology and outcomes with beta-blocker use and did not find any association (data not shown). Therefore, our clinical data suggest that the benefit of beta-blockers is not histology specific. Finally, although we assessed the use of beta-blockers in relation to lung cancer outcomes, we did not assess other variables such as the use of bisphosphonates, insulin, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers, which could affect NSCLC relapse and thus, could be confounding our results. Due to practical limitations, we were not able to assess the influence of all medications that patients were taking at the time of treatment, and these medication interactions can be the subject of future analyses, ideally in the clinical trial setting. However, strengths of this study worthy of note include that the database used is prospectively maintained and survival information is updated yearly, and that all patients received fairly homogenous radiation doses, consistent prescription constraints, and definitive RT at a single institution.
In conclusion, this analysis demonstrated that the incidental use of beta-blockers in this group of patients with NSCLC was associated with improved DMFS, DFS, and OS—but not with LRPFS—after definitive treatment that included RT. These findings are concordant with those of previous preclinical studies, suggesting that beta-blockers have specific effects on the metastatic cascade. Future prospective trials are needed to validate these retrospective findings and establish whether the length and timing of beta-blocker use influence survival outcomes.
funding
This work was supported in part by Cancer Center Support (Core) Grant CA016672 from the National Institutes of Health to The University of Texas MD Anderson Cancer Center.
disclosure
The authors have declared no conflicts of interest.
acknowledgments
We would like to sincerely thank Christine Wogan, M.S., for her valuable assistance in editing this manuscript.
references
- 1.Liao ZX, Komaki RR, Thames HD, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775–781. doi: 10.1016/j.ijrobp.2009.02.032. doi:10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Oshiro Y, Mizumoto M, Okumura T, et al. Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2012;7:370–375. doi: 10.1097/JTO.0b013e31823c485f. doi:10.1097/JTO.0b013e31823c485f. [DOI] [PubMed] [Google Scholar]
- 3.Korpanty G, Smyth E, Carney DN. Update on anti-angiogenic therapy in non-small cell lung cancer: are we making progress? J Thorac Dis. 2011;3:19–29. doi: 10.3978/j.issn.2072-1439.2010.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoni MH, Lutgendorf SK, Cole SW. The influence of bio-behavioural factors on tumour biology-pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. doi:10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi B, Benish M, Goldfarb Y, et al. Continuous stress disrupts immunostimulatory effects of IL-12. Brain Behav Immun. 2011;25:727–735. doi: 10.1016/j.bbi.2011.01.014. doi:10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Smith M, Lutgendorf SK, Sood AK, et al. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–1881. doi: 10.2217/fon.10.142. doi:10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armaiz-Pena GN, Lutgendorf SK, Cole SW, et al. Neuroendocrine modulation of cancer progression. Brain Behav Immun. 2009;23:10–15. doi: 10.1016/j.bbi.2008.06.007. doi:10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaker PH, Lutgendorf SK, Sood AK. The neuroendocrine impact of chronic stress on cancer. Cell Cycle. 2007;6:430–433. doi: 10.4161/cc.6.4.3829. doi:10.4161/cc.6.4.3829. [DOI] [PubMed] [Google Scholar]
- 9.Schuller HM. Beta-adrenergic signaling, a novel target for cancer therapy. Oncotarget. 2010;1:466–469. doi: 10.18632/oncotarget.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powe DG, Entschladen F. Targeted therapies: using β-blockers to inhibit breast cancer progression. Nat Rev Clin Oncol. 2011;8:511–512. doi: 10.1038/nrclinonc.2011.123. doi:10.1038/nrclinonc.2011.123. [DOI] [PubMed] [Google Scholar]
- 11.Glasner A, Avraham R, Rosenne E, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 Inhibitor. J Immunol. 2010;184:2449–2457. doi: 10.4049/jimmunol.0903301. doi:10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 12.Al-Wadei HAN, Ullah MF, Al-Wadei MH. Intercepting neoplastic progression in lung malignancies via the beta adrenergic (β-AR) pathway: implications for anti-cancer drug targets. Pharmacol Res. 2012;66:33–40. doi: 10.1016/j.phrs.2012.03.014. doi:10.1016/j.phrs.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Barron TI, Connolly RM, Sharp L, et al. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. doi:10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 14.Melhem-Bertrandt A, Chavez-MacGregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. doi:10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powe DG, Voss MJ, Zanker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemeshow S, Sorensen HT, Phillips G, et al. β-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:2273–2279. doi: 10.1158/1055-9965.EPI-11-0249. doi:10.1158/1055-9965.EPI-11-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah SM, Carey IM, Owen CG, et al. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72:157–161. doi: 10.1111/j.1365-2125.2011.03980.x. doi:10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82:1042–1044. doi: 10.1016/j.ijrobp.2011.12.032. doi:10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and US Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. doi:10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 20.Vokes EE, Herndon JEn, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: cancer and leukemia group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. doi:10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 21.Bosetti C, Rosato V, Gallus S, et al. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. doi:10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 22.Schroedl C, Kalhan R. Incidence, treatment options, and outcomes of lung cancer in patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2012;18:131–137. doi: 10.1097/MCP.0b013e32834f2080. doi:10.1097/MCP.0b013e32834f2080. [DOI] [PubMed] [Google Scholar]
- 23.Al-Wadei HA, Al-Wadei MH, Schuller HM. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for intervention. PLoS One. 2012;7:e29915. doi: 10.1371/journal.pone.0029915. doi:10.1371/journal.pone.0029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuller HM, Porter B, Riechert A. Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J Cancer Res Clin Oncol. 2000;126:624–630. doi: 10.1007/PL00008474. doi:10.1007/PL00008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28:4094–4099. doi: 10.1200/JCO.2009.26.9357. doi:10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Wadei HAN, Plummer HK, Ullah MF, et al. Social stress promotes and γ-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancer. Cancer Prev Res (Phila) 2011;5:189–196. doi: 10.1158/1940-6207.CAPR-11-0177. doi:10.1158/1940-6207.CAPR-11-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palm D, Lang K, Niggemann B, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by β-blockers. Int J Cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. doi:10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 28.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. doi:10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laag E, Majidi M, Cekanova M, et al. NNK activates ERK1/2 and CREB/ATF-1via beta-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int J Cancer. 2006;119:1547–1552. doi: 10.1002/ijc.21987. doi:10.1002/ijc.21987. [DOI] [PubMed] [Google Scholar]
- 30.Baker JG. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. doi:10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]